Abstract
Introduction
Egg allergy is one of the most common food allergies in children. Egg white, including ovomucoid (OVM or Gal d 1) and ovalbumin (OVA or Gal d 2), is the major source of allergens. The aim of this study was to assess the role of Gal d 1 and Gal d 2 in predicting the risk of anaphylaxis caused by eggs in children, and to compare this new diagnostic tool with established methods of allergen-specific IgE detection.
Material and methods
One hundred and forty-eight children were divided into 2 groups according to a positive (group A, 33 children) or negative (group B, 115 children) history of anaphylaxis after ingestion/contact with eggs. All patients underwent an allergological evaluation by measurements of specific IgE against egg white: Gal d 1 and Gal d 2.
Results
Higher levels of Gal d 1, Gal d 2 and IgE against egg white were detected in group A compared to group B (p < 0.001). Although the area under the curve was similar for Gal d 1 and Gal d 2, egg white specific IgE showed a better sensitivity (85%) for a cut-off value ≥ 0.975 kUA/l, while Gal d 1 and Gal d 2 demonstrated a better specificity (90% and 80%, respectively) for cut-off values ≥ 1.460 kUA/l and ≥ 2.310 kUA/l, respectively.
Conclusions
Egg white specific IgE showed a similar ability as Gal d 1 and Gal d 2 in differentiating children at risk for egg anaphylaxis, although Gal d 1 and Gal d 2 showed a better specificity.
Keywords: egg allergy, ovomucoid, ovalbumin, anaphylaxis, oral food challenge, children
Introduction
Food allergy is a frequent problem during childhood [1, 2]. Egg allergy is one of the most common food allergies [1], affecting 1.8% to 2% of children younger than 5 years [3], and it is often associated with severe clinical manifestations, including anaphylaxis [4].
Many potentially allergenic egg proteins exist [4]; in particular, egg white contains 23 different glycoproteins, and most of them have been purified. Five major allergenic proteins from domestic chicken eggs (Gallus domesticus) have been identified and defined as Gal d 1–5 [5, 6]. Egg white is the main source of egg allergens, which include ovomucoid (OVM or Gal d 1, 11%), ovalbumin (OVA or Gal d 2, 54%), ovotransferrin (Gal d 3, 12%) and lysozyme (Gal d 4, 3.4%) [7]. Ovomucoid is the most important clinically relevant egg protein, although it represents only 10% of the total egg white proteins, and it is considered the dominant allergen in egg [7–9]. This is probably related to its ability to maintain allergenicity even after extensive heating [8]. In contrast, OVA is the most abundant protein contained in the egg white.
Growing evidence suggests that higher OVM specific IgE levels are associated with the persistence of egg allergy [4]. In addition, it has been suggested that the quantification of OVM IgE antibodies can be useful in helping physicians in deciding whether to perform an oral food challenge (OFC) [5].
The gold standard for the diagnosis of egg allergy is the double-blind placebo-controlled OFC, which is both resource and time expensive, and potentially hazardous [1, 4, 10]. As a potential alternative tool, allergen-specific IgE levels with greater than 95% predictive risk values for a positive food challenge have been identified for some foods, by using the ImmunoCAP (Phadia, Uppsala, Sweden). For eggs this value is ≥ 7 kUA/l, with a lower cut-off of ≥ 2 kUA/l for infants [11, 12]. The predictive values for allergen-specific IgE levels might differ from one immunoassay to the other, affecting management decisions [11–14]. In addition, a positive skin test and/or an increased serum IgE level against non-specific food might indicate sensitization to that food, but they are not predictive of anaphylaxis [11–13].
Therefore, the aim of the study was to assess the value of the two main egg allergens, Gal d 1 and Gal d 2, in predicting the risk of developing anaphylaxis caused by eggs in children, and to compare this new diagnostic tool with established methods of allergen-specific IgE detection.
Material and methods
The study population included 148 children (99 males and 49 females, mean age ± SD: 6.51 ±3.64 years) who experienced allergic reactions to egg proteins and with documented IgE-mediated egg allergy. Children were recruited at the Allergological and Respiratory Unit of the Pediatric Department, University of Chieti, Italy, between July 2010 and November 2013.
An allergological assessment was performed in all patients and included: skin prick tests for main food allergens; total IgE; specific IgE against the main food allergens (cow’s milk, tomato, peanut and cod) and specific IgE against hen’s egg and against the main egg white allergens: OVM or Gal d 1 and OVA or Gal d 2.
The study population was divided into two groups, on the basis of the presence (group A) or absence (group B) of a reported history of anaphylaxis after ingestion and/or contact with egg or egg derivatives.
The diagnosis of anaphylaxis was considered to be highly likely when any one of the following 3 clinical criteria was fulfilled [11]:
-
Acute onset of an illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (generalized hives, pruritus or flushing, and swollen lips-tongue-uvula) AND at least 1 of the following:
Respiratory compromise (dyspnea, wheeze-bronchospasm, stridor, reduced peak expiratory flow (PEF), hypoxemia);
Reduced blood pressure (BP) or associated symptoms of end-organ dysfunction (hypotonia [collapse], syncope, incontinence).
-
Two or more of the following that occur rapidly after exposure to a likely allergen for that patient (minutes to several hours):
Involvement of the skin–mucosal tissue (generalized hives, itch-flush, swollen lips-tongue-uvula);
Respiratory compromise (dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia);
Reduced BP or associated symptoms (hypotonia [collapse], syncope, incontinence);
Persistent gastrointestinal symptoms (cramping abdominal pain, vomiting).
Reduced BP after exposure to a known allergen for that patient (minutes to several hours): low systolic BP (age-specific) or greater than 30% decrease in systolic BP.
Most children with a history of anaphylaxis were often hospitalized for this severe reaction; only a few of them were managed by local pediatricians. In all the cases the diagnosis was made according to the World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis [15].
Children in group A were not subjected to OFC for the high risk of severe anaphylaxis [16], given that they experienced more than one episode of moderate to severe anaphylactic reactions.
According to current guidelines, OFC may be deferred if there is a high likelihood of a severe reaction to the food as predicted by the food reaction history, whether immediate or delayed, levels of serum food-specific IgE antibody, and/or results of quantitative skin prick testing and the patient’s age [16]. Oral food challenge is relatively contraindicated in conditions that increase the risk of severe anaphylaxis, such as a recent convincing anaphylactic reaction to a specific food or unstable asthma. It would not be recommended to perform an OFC for a patient with recent anaphylaxis to the trigger food [16]. Patients with a convincing history of anaphylaxis to a specific food and evidence of sensitization to that food should not undergo OFC because of their high risk of anaphylaxis [11].
Children in group B experienced mild to moderate allergic reactions to egg proteins, mainly gastrointestinal symptoms (such as abdominal pain, diarrhea or vomiting) and skin symptoms (such as urticaria and atopic dermatitis), but they did not have any history of anaphylactic reaction to eggs. All patients from group B had a positive OFC (Sampson’s score ≥ 3), performed by administering pasteurized whisked hen’s egg by titration steps, as recommended by the American Academy of Allergy, Asthma & Immunology in the PRACTALL consensus report for double-blind, placebo-controlled OFC [17].
Written informed consent was obtained from all parents and oral consent from all children, and the study was performed in accordance with the Declaration of Helsinki (1964). The study was approved by the Ethics Committee of the University of Chieti.
Statistical analysis
Data were analyzed using SPSS (version 17.0 SPSS, Inc, Chicago, III). The one-sample Kolmogorov-Smirnov test was performed to estimate the distribution of each variable. The Mann-Whitney U test or independent t-test was used for comparisons of continuous parameters.
Receiver operating characteristic (ROC) analysis was performed to assess the performance of specific IgE antibodies against hen’s egg white and its major allergens (Gal d 1 and Gal d 2) in relation to a positive history for anaphylaxis. Results are presented as area under the curve (AUC) with a 95% confidence interval (95% CI). An optimal cut-off point for hen’s egg white specific IgE as for its major allergens was obtained using the Youden index (maximum (sensitivity + specificity –1)) [18].
All p-values < 0.05 were considered significant.
Results
The study population was divided into two groups, on the basis of a positive (group A) or negative (group B) reported history of anaphylaxis after ingestion and/or contact with egg or egg derivatives: group A: 33 children, 22 males, 11 female, mean age: 6.6 ±3.4 years; group B: 115 children, 77 males, 38 females, mean age: 6.5 ±3.8 years. Groups A and B were comparable for age at the time of assessment (Table I).
Table I.
Major egg allergens and specific egg IgE levels in children with and without anaphylaxis
| Parameter | Children with anaphylaxis (Group A) | Children without anaphylaxis (Group B) | P-value |
|---|---|---|---|
| Number of patients | 33 | 115 | |
| Gender (M/F) | 22/11 | 77/38 | 0.98 |
| Age [years] | 6.6 ±3.4 | 6.5 ±3.8 | 0.94 |
| IgE egg white [kUA/l] | 4.05 (1.56–16.35) | 0.63 (0.38–2.27) | < 0.001 |
| IgE egg yolk [kUA/l] | 1.32 (0.39–4.29) | 0.19 (0.09–0.67) | < 0.001 |
| Gal d 1 [kUA/l] | 1.66 (0.12–5.42) | 0.17 (0.06–-0.45) | < 0.001 |
| Gal d 2 [kUA/l] | 2.46 (1.12–8.79) | 0.54 (0.24–2.00) | < 0.001 |
| Recombinants’ sum [kUA/l] | 4.14 (1.44–24.49) | 0.74 (0.45–2.52) | < 0.001 |
Data are expressed as median (interquartile range) or mean ± standard deviation.
Serum levels of egg white specific IgE were higher in group A than in group B (4.05 vs. 0.63 kUA/l, p < 0.001). Similarly, levels of IgE against Gal d 1 and Gal d 2 were higher in group A than in group B (Gal d 1 = 1.66 vs. 0.17 kUA/l and Gal d 2 = 2.46 vs. 0.54 kUA/l; p < 0.001) (Table I). Also the sum of the main egg white allergens (Gal d 1 + Gal d 2) was higher in group A than B (p < 0.001) (Table I).
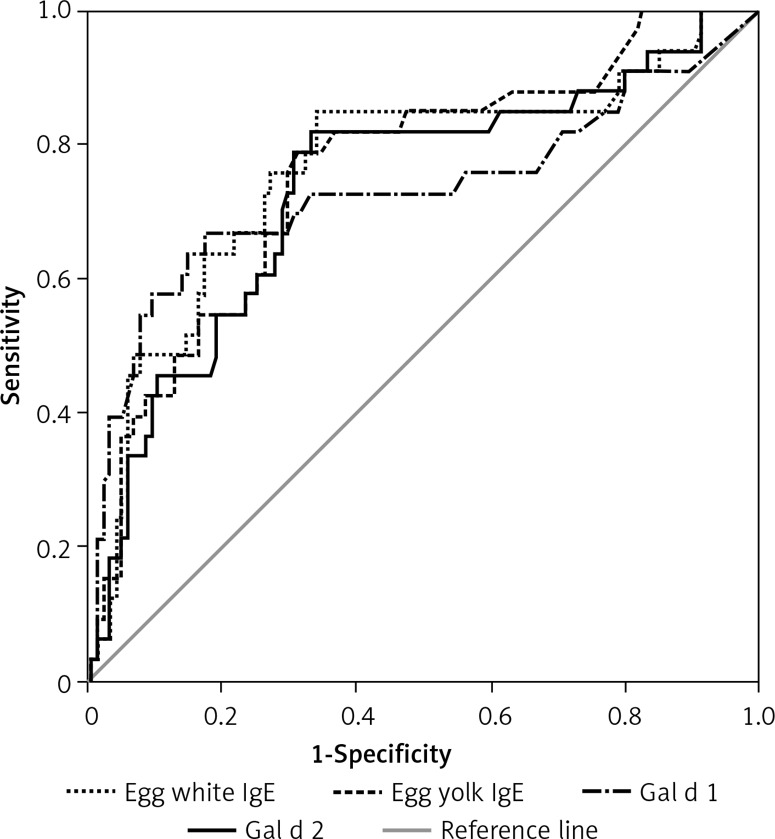
The receiver operating characteristic (ROC) analysis showed that the Gal d 1 ImmunoCAP test was similar to Gal d 2 in its ability to differentiate children at risk for egg anaphylaxis, with an AUC of 0.728 for Gal d 1 (95% CI: 0.610–0.846) and of 0.732 for Gal d 2 (95% CI: 0.629–0.836) (Figure 1). A similar result was obtained for egg white specific IgE, with an AUC of 0.763 (95% CI: 0.660–0.866) (Figure 1).
Figure 1.
Receiver operating characteristic curve for levels of specific hen’s egg IgE and its major IgE allergens
A cut-off value for egg white specific IgE ≥ 0.975 kUA/l showed a Youden index of 0.51 with a sensitivity of 85% and a specificity of 66%, while a cut-off value of Gal d 1 ≥ 1.460 kUA/l was associated with a better specificity (90%) and a lower sensitivity (55%) (Youden index = 0.45). A cut-off value of Gal d 2 ≥ 2.310 kUA/l showed a better specificity (80%) than egg white specific IgE, but lower than Gal d 1, and a similar sensitivity (55%) of Gal d 1 vs. egg white specific IgE (Table II).
Table II.
Sensitivity and specificity of specific IgE against hen’s egg and its major allergens
| Variable | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|
| Cut-off for Gal d 1 IgE ≥ 1.460 kUA/l |
55 | 90 | 0.45 |
| Cut-off for Gal d 2 IgE ≥ 2.310 kUA/l |
55 | 80 | 0.35 |
| Cut-off for recombinants’ sum ≥ 3.335 kUA/l |
64 | 81 | 0.45 |
| Cut-off for egg white IgE ≥ 0.975 kUA/l |
85 | 66 | 0.51 |
| Cut-off for egg yolk IgE ≥ 0.370 kUA/l |
79 | 68 | 0.47 |
The odds ratio for the development of egg anaphylaxis according to a cut-off value of egg white specific IgE ≥ 0.975 kUA/l was 10.6 (95% CI: 3.81–29.68), similar to the odds ratio of 11.35 (95% CI: 4.50–28.61) related to a cut-off value ≥ 1.46 kUA/l of Gal d 1, whereas the odds ratio related to Gal d 2 was lower (4.55, 95% CI: 2.01–10.33) (Table III).
Table III.
Anaphylaxis risk according to levels of specific IgE against hen’s egg and its major allergens
| Variable | Anaphylaxis | Odds ratio | |
|---|---|---|---|
| No (group B) | Yes (group A) | ||
| Cut-off for Gal d 1 IgE ≥ 1.460 kUA/l |
11/115 (9.6%) | 18/33 (54.5%) | 11.35 (4.50–28.61) |
| Cut-off for Gal d 2 IgE ≥ 2.310 kUA/l |
24/115 (20.9%) | 18/33 (54.5%) | 4.55 (2.01–10.33) |
| Cut-off for recombinants’ sum ≥ 3.335 kUA/l |
22/115 (19.1%) | 21/33 (63.6%) | 7.40 (3.17–17.27) |
| Cut-off for egg white IgE ≥ 0.975 kUA/l |
40/115 (34.7%) | 28/33 (84.8%) | 10.60 (3.81–29.68) |
| Cut-off for egg yolk IgE ≥ 0.370 kUA/l |
37/115 (32.1%) | 26/33 (78.8%) | 7.93 (3.16–19.93) |
Data are n (%) or odds ratio (95% CI).
Discussion
The international literature supports the role of molecular diagnosis based on major egg allergens as a useful tool to identify clinical phenotypes of children with egg allergy. In particular, egg specific IgE molecules, identifying sequential or conformational epitopes of Gal d 1 and Gal d 2, can distinguish different clinical phenotypes of egg allergy. It has been shown that egg-allergic patients, with IgE antibodies reacting against sequential epitopes, tend to have persistent allergy, whereas those with IgE antibodies primarily to conformational epitopes tend to have transient allergy [5, 19].
Several studies have supported the hypothesis that high levels of specific IgE antibodies against Gal d 1 have a greater predictive value for persistent egg allergy [4, 7, 8, 19], whereas their reduction is associated with the development of tolerance [7, 20].
The data obtained from the present study suggest that Gal d 1 and Gal d 2 IgE levels show similar ability to differentiate children at risk for egg anaphylaxis, as indicated by a similar AUC.
These data are in line with the results obtained by Ott and colleagues, who evaluated the utility of microarray-based IgE detection in the diagnostic workup of food allergy, comparing this new diagnostic tool with established methods of allergen-specific IgE detection. They investigated 130 children with suspected allergy to cow’s milk or hen’s egg, performing serum IgE measurements, skin prick tests allergen microarray assays and OFC with cow’s milk and hen’s egg. They obtained ROC curves by plotting the true positive rate (the rate of correctly identified positive double-blind, placebo-controlled food challenges results) against the false positive rate for all possible IgE cut-off points. The AUC was similar for Gal d 1, Gal d 2 and fluorescence enzyme immunoassays for hen’s egg [21].
Although egg white specific IgE levels showed a higher sensitivity (85%), a better specificity was related to a cut-off value of 1.460 kUA/l for Gal d 1 (90%) and of 2.310 kUA/l for Gal d 2 (80%). These results suggest that both Gal d 1 and Gal d 2 can be useful in identifying children at high risk of developing anaphylaxis; however, Gal d 1 had the highest positive predictive value.
In the present study, children with values of Gal d 1 higher than 1.46 kUA/l had a risk almost 11 times greater of developing anaphylaxis to egg and egg-containing foods than children with values below that cut-off. However, a lower cut-off for egg white specific IgE (≥ 0.975 kUA/l) showed a similar odds ratio (10.60) for developing egg anaphylaxis.
From another point of view, it has been suggested that quantification of OVM antibodies could be useful in guiding the physician in deciding whether to perform an OFC. Recent published data suggested that a concentration of IgE antibodies against OVM higher than 11 kUA/l (positive decision point) indicates a high risk of reacting to heated (as well as less heated or undercooked) egg, while concentrations lower than 1 kUA/l (negative decision point) were associated with a lower risk of reaction to heated egg, even if the patients might well react to less heated or undercooked egg [1]. In contrast, in our study a cut-off point for Gal d 1 ≥ 1.46 kUA/l was associated with a high odds ratio for developing anaphylaxis.
However, different studies have reported discordant cut-off values for Gal d 1 associated with a good specificity in predicting serious allergic reactions, likely due to different sample sizes and study design. For example, Bartnikas et al. proposed a cut-off for Gal d 1 IgE of 0.35 kUA/l to identify patients who would pass a baked egg challenge (approximately 90% rate of passing), and they were able to determine cut-offs with a specificity higher than 95%: OVM IgE of 3.38 kUA/l [22].
Whereas Ott et al. supported the role of allergen microarrays as a new tool to diagnose symptomatic hen’s egg allergy [21], our data strengthen the potential role of molecular diagnosis based on major egg white allergens, in particular related to anaphylactic risk.
Some limitations of the present study need to be acknowledged. Firstly, we did not include a control group. Secondly, our group A was not challenged for the high risk of severe anaphylaxis, but all subjects had a reported history of anaphylaxis after ingestion and/or contact with egg or egg derivatives. However, in all the cases the diagnosis was made after hospitalization or by pediatricians according to the World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis.
On the other hand, strengths of this study were the large sample size (148 children) and the fact that the two groups (A and B) were comparable for age at the time of assessment.
In conclusion, egg white specific IgE levels showed similar ability compared to Gal d 1 and Gal d 2 to differentiate children at risk for egg anaphylaxis, although Gal d 1 and Gal d 2 demonstrated a better specificity. Therefore, molecular diagnosis based on major egg white allergens can be a new good diagnostic tool that can help clinicians to better characterize egg allergy and especially anaphylactic risk in egg allergic children. However, often this tool is available only in specialized centers, and it is certainly more expensive. Therefore, measuring egg white specific IgE levels can be the first approach for a patient with suspected hen’s egg allergy, leaving the molecular diagnosis as a second step of diagnostic evaluation.
Future studies are required to confirm the values of Gal d 1 and Gal d 2 and their cut-off values in identifying egg allergic children at risk of anaphylaxis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ando H, Movérare R, Kondo Y, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122:583–8. doi: 10.1016/j.jaci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Ambroszkiewicz J, Rowicka G, Chelchowska M, Gajewska J, Strucińska M, Laskowska-Klita T. Biochemical markers of bone metabolism in children with cow’s milk allergy. Arch Med Sci. 2014;10:1135–41. doi: 10.5114/aoms.2013.36906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard SA, Sampson HA, Sicherer SH, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130:473–80. doi: 10.1016/j.jaci.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marriage DE, Erlewyn-Lajeunesse M, Unsworth DJ, Henderson AJ. Unscrambling egg allergy: the diagnostic value of specific IgE concentrations and skin prick tests for ovomucoid and egg white in the management of children with hen’s egg allergy. ISRN Allergy. 2012;2012:627545. doi: 10.5402/2012/627545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am. 2011;58:427–43. doi: 10.1016/j.pcl.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine RG, Laske N, Hill DJ. The diagnosis and management of egg allergy. Curr Allergy Asthma Rep. 2006;6:145–52. doi: 10.1007/s11882-006-0053-0. [DOI] [PubMed] [Google Scholar]
- 7.Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994;93:1047–59. doi: 10.1016/s0091-6749(94)70054-0. [DOI] [PubMed] [Google Scholar]
- 8.Benhamou AH, Caubet JC, Eigenmann PA, et al. State of the art and new horizons in the diagnosis and management of egg allergy. Allergy. 2010;65:283–9. doi: 10.1111/j.1398-9995.2009.02251.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159:2026–32. [PubMed] [Google Scholar]
- 10.Urisu A, Ando H, Morita Y, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100:171–6. doi: 10.1016/s0091-6749(97)70220-3. [DOI] [PubMed] [Google Scholar]
- 11.Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125:S161–81. doi: 10.1016/j.jaci.2009.12.981. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA, Burks AW. Adverse reactions to foods. In: Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FER, editors. Middleton’s allergy: principles and practice. 7th ed. St Louis: Mosby Inc; 2009. pp. 1139–67. [Google Scholar]
- 13.Simons FE, Frew AJ, Ansotegui IJ, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120:S2–24. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121:1219–24. doi: 10.1016/j.jaci.2007.12.1150. [DOI] [PubMed] [Google Scholar]
- 15.Simons FE, Ardusso LR, Bilò MB, et al. World Allergy Organization. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–83. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–74. doi: 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cut points obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–5. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Järvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62:758–65. doi: 10.1111/j.1398-9995.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- 20.Montesinos E, Martorell A, Félix R, Cerdá JC. Egg white specific IgE levels in serum as clinical reactivity predictors in the course of egg allergy follow up. Pediatr Allergy Immunol. 2010;21:634–9. doi: 10.1111/j.1399-3038.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 21.Ott H, Baron JM, Heise R, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008;63:1521–8. doi: 10.1111/j.1398-9995.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 22.Bartnikas LM, Sheehan WJ, Larabee KS, Petty C, Schneider LC, Phipatanakul W. Ovomucoid is not superior to egg white testing in predicting tolerance to baked egg. J Allergy Clin Immunol Pract. 2013;1:354–60. doi: 10.1016/j.jaip.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]