Abstract
Platelets play a key role in mediating stent thrombosis, the major cause of ischemic events in the immediate period following percutaneous coronary intervention (PCI). For this reason, antiplatelet therapy, started at the time of PCI and continued for at least 30 days afterwards, is the cornerstone of antithrombotic therapy after PCI. However, the use of antiplatelet agents increase bleeding risk, with more potent antiplatelet agents further increasing bleeding risk. For this reason, balancing prevention of ischemic events with risk of bleeding is fundamental to the effective use of antiplatelet agents. In the past 5 years, potent and fast-acting P2Y12 inhibitors have been introduced, and have augmented the antiplatelet armamentarium available to interventional cardiologists. In this review, we review the preclinical and clinical data surrounding these new agents, and discuss the significant questions and controversies that still exist regarding the optimal antiplatelet strategy.
Keywords: Antiplatelet agents, P2Y12 receptor antagonists, glycoprotein IIb/IIIa inhibitors, percutaneous coronary intervention, bleeding, stent thrombosis
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is the evidence-based, guideline-recommended cornerstone of antithrombotic therapy for patients undergoing percutaneous coronary intervention (PCI) across a spectrum of indications. Fundamental to DAPT use is the balance of bleeding with the risk of recurrent myocardial infarction and stent thrombosis.
Until recently, the only available P2Y12 inhibitors were clopidogrel and ticlopidine, both prodrugs metabolized into active metabolites that irreversibly bind the adenosine P2Y12 receptor on platelet surfaces inhibiting platelet aggregation. Due to the requirement for first pass metabolism in the liver, neither drug achieves steady state levels of platelet inhibition for several hours following dosing, leaving a window of inadequate platelet inhibition during which post-PCI patients are theoretically vulnerable to acute stent thrombosis if they are not loaded with a P2Y12 inhibitor prior to PCI.
Over the past 5 years, 3 highly-potent, fast-acting P2Y12 inhibitors have been introduced to the market: prasugrel, ticagrelor, and cangrelor. Prasugrel and ticagrelor are oral agents, and cangrelor is an intravenous agent. These agents all achieve maximal platelet inhibition within 1 hour of loading dose in healthy volunteers. However, these drugs exhibit different pharmacokinetics in real-world patients undergoing PCI, especially patients with ST-segment elevation myocardial infarction (STEMI) and non-ST segment elevation acute coronary syndrome (NSTE-ACS), which may have important implications for their use as antiplatelet agents in urgent and primary PCI. These agents are additions to the antiplatelet armamentarium that also include aspirin and glycoprotein IIb/IIIa inhibitors, creating an array of combinations available for use in patients undergoing PCI.
This review will focus on the pharmacokinetic and pharmacodynamics properties of the newer and older P2Y12 inhibitors as well as glycoprotein IIb/IIIa inhibitors, and the clinical trial evidence supporting use of each of the available antiplatelet agents. Their effects on periprocedural major bleeding and early stent thrombosis will also be discussed, as will the optimal combinations and usage to achieve the best clinical outcomes.
The central role of platelets in outcomes after PCI: stent thrombosis and bleeding
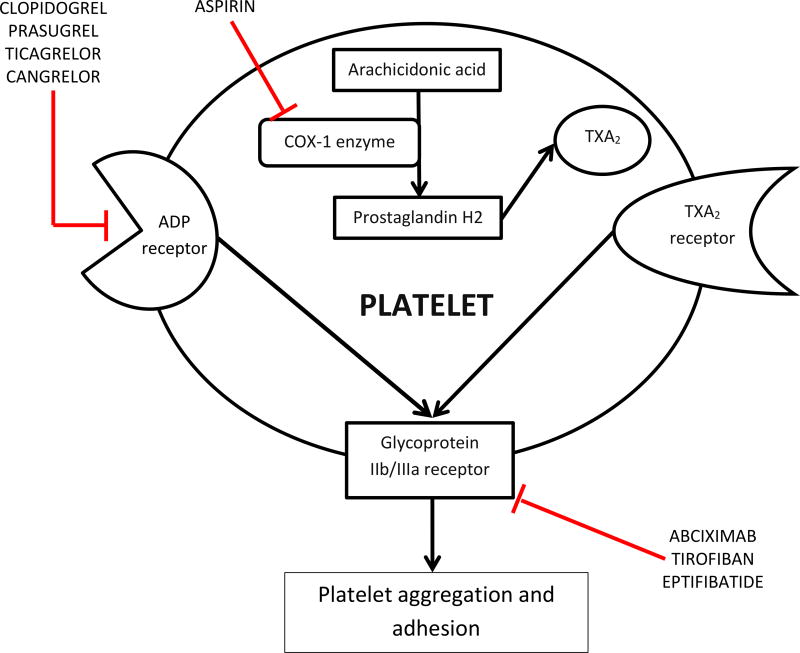
Platelets are integrally involved in the pathogenesis of adverse events after PCI. In particular, stent thrombosis is a platelet-mediated phenomenon, and the antithrombotic effect of antiplatelet therapy predisposes patients to bleeding complications. Inhibitors of platelet activation and aggregation are therapeutic options for patients undergoing PCI. Figure 1 shows potential receptor targets on the platelet surface, some of which are targets of available antiplatelet agents used for PCI.
Figure 1. Targets of antiplatelet agents used in patients undergoing PCI.
Cyclooxygenase-1, the ADP receptor, and glycoprotein IIb/IIIa receptors are the targets of available antiplatelet agents. The ADP receptor and cyclooxygenase-1 mediate processes that activate the glycoprotein IIb/IIIa receptor, which is the major molecule mediating platelet aggregation and adhesion.
ADP: adenosine diphosphate; COX-1: cyclooxygenase-1; TXA2: thromboxane A2
In the immediate post-PCI period, acute stent thrombosis and periprocedural bleeding are two events of concern. Both events are associated with increased mortality. Stent thrombosis can be grouped into acute (< 24 hours post PCI), subacute (24 hours to 30 days), late (30 days to 1 year), and very late (> 1 year). In an analysis of the RISK-PCI clinical trial, the 30-day risk adjusted mortality was more than 5-fold higher in patients with early (acute or subacute) stent thrombosis than those without, and 1-year risk adjusted mortality was more than 4-fold higher.1 In an analysis of the DESERT primary PCI registry, patients with stent thrombosis had a raw mortality rate of 23.6%, compared to 6% in those without stent thrombosis.2 This increase in mortality with stent thrombosis persists in patients without STEMI: In an analysis of the ACUITY trial, which enrolled patients undergoing urgent PCI for NSTE-ACS, 30-day mortality was 24.7% in patients with stent thrombosis, compared to 0.5% in those without.3
Similarly, post-PCI bleeding has long been identified as an independent predictor of mortality in patients undergoing elective, urgent, and emergent PCI. In a pooled analysis of 3 clinical trials enrolling patients undergoing PCI for stable coronary artery disease (CAD), NSTE-ACS, and STEMI, post-PCI major bleeding increased risk-adjusted 1-year mortality 4.2-fold, an increase in mortality greater than that of recurrent MI within 30 days.4 Analyses of the GRACE and CathPCI registries found similar links between major bleeding and mortality,5,6 with one CathPCI study demonstrating an additional in-hospital death for every 29 patients with PCI-related major bleeding.7
Interestingly, stent thrombosis carries a greater risk of death, but is rarer than bleeding leading to differences in attributable deaths for the two events. Acute stent thrombosis occurs in about 0.4% of PCIs8 and major bleeding occurs in about 1.7–2% in contemporary registries, depending on the definition.7,9
Importantly, both stent thrombosis and major bleeding are more common in patients with ACS than in those undergoing stenting for stable angina.4 The rate of early stent thrombosis increases along the continuum from elective to primary PCI: 0.4% in elective PCI, 1.4% in urgent PCI for NSTEMI, and 3.1% in primary PCI (Table 1).10
Table 1.
Incidence of major bleeding and stent thrombosis by PCI indication
| Elective PCI | Urgent PCI for NSTE-ACS | Primary PCI for STEMI | |
|---|---|---|---|
| Major Bleeding | 0.7% | 1.8% | 2.6% |
| Stent Thrombosis | 0.4% | 1.4% | 3.1% |
Beyond type of presentation, the strongest risk factors for PCI-related major bleeding include increasing age, female sex, pre-existing anemia, and renal failure.6,11 In clinical trials evaluating different antithrombotic strategies, like ACUITY, HORIZONS-AMI, and REPLACE-2, randomization to heparin plus routine glycoprotein IIb/IIIa inhibitors (as compared to bivalirudin) was also a very strong predictor of bleeding.4
The strongest predictor of stent thrombosis across multiple studies is premature cessation of DAPT. Other risk factors include presentation with STEMI, diabetes mellitus, complex CAD, and procedural factors like stent length, stent diameter, successful restoration of coronary artery flow, and lack of P2Y12 inhibitor treatment prior to stenting.1–3,8,12,13 This fact highlights the central role of periprocedural antiplatelet therapy in both PCI-related major bleeding and early stent thrombosis.
Antiplatelet agents
Ticlopidine
Ticlopidine is an oral thienopyridine molecule that requires metabolism in the liver into a biologically active form that irreversibly binds to, and inhibits, the P2Y12 subunit of the platelet ADP receptor. Due to this need for first pass metabolism, when given to healthy volunteers at a standard dose (250mg twice daily), ticlopidine required 3 to 4 days to achieve maximal platelet inhibition.14 However, despite favorable effects on platelets, ticlopidine’s use is limited by its side effects, which include diarrhea, rash, and agranulocytosis.15 Of these, the most serious is agranulocytosis, which develops in up to 2.4% of patients treated with ticlopidine, with the incidence increasing with longer duration of treatment.15
Clopidogrel
Clopidogrel is another oral thienopyridine that irreversibly inhibits the P2Y12 subunit of the ADP receptor on platelets. Clopidogrel is also a prodrug that is converted to its active form by the CYP2C19 enzyme in the liver, with less than 15% of the prodrug being converted to active metabolite.16 In healthy subjects treated with aspirin plus a loading dose of 300mg clopidogrel, inhibition of an ex vivo model of arterial thrombosis occurs within 1.5 hours, though the peak effect is delayed by 6 hours, consistent with clopidogrel’s need to undergo first pass metabolism prior to taking effect.17 Pharmacodynamic studies conducted in patients with stable CAD undergoing PCI similarly showed that platelet reactivity remained high 4 hours following a loading dose of 300mg.18
Prasugrel
Prasugrel is another oral thienopyridine P2Y12 inhibitor that requires first pass metabolism into an active metabolite. However, unlike clopidogrel and ticlopidine, in which a majority of the absorbed drug is converted into molecules that have no effect on platelet aggregation, prasugrel is efficiently converted into its active metabolite, leading to a much higher likelihood of response and faster onset of action than clopidogrel.19 In preclinical studies in healthy volunteers, prasugrel reaches peak platelet inhibition within 1 hour following administration, and reaches an equivalent level of platelet inhibition to clopidogrel’s maximal platelet inhibition within 15 minutes.20 These findings were similar in patients with stable CAD and those undergoing PCI, with maximal platelet inhibition reached within 2 hours after a loading dose of prasugrel 60mg.21,22
Ticagrelor
Unlike prasugrel, clopidogrel, and ticlopidine, ticagrelor is an oral pentotriazolo-pyrimidine. Its development was precipitated by the discovery that ATP reversibly inhibits the P2Y12 subunit of the ADP receptor at a site distinct from the thienopyridines.19 Ticagrelor undergoes first pass metabolism in the liver, but both ticagrelor and its major metabolite inhibit platelet aggregation. Ticagrelor’s rapidity of onset and potency was demonstrated in the ONSET/OFFSET trial, which randomized stable patients with CAD to a loading dose of ticagrelor or clopidogrel followed by maintenance dosing, and measured platelet function.23 By 1 hour after loading, ticagrelor had already inhibited platelets more effectively than clopidogrel’s peak effect, and 98% of patients had > 50% platelet inhibition after 2 hours. Other pre-clinical studies demonstrated similar effects.24
Cangrelor
Like ticagrelor, cangrelor is an ATP analog that is biologically active without any need for first pass metabolism and reversibly inhibits the P2Y12 subunit of the ADP receptor.19 Unlike the previously discussed agents, cangrelor is administered intravenously. Since cangrelor does not require either gastrointestinal absorption or first pass metabolism, it inhibits platelets immediately upon the start of its infusion. Unlike the oral P2Y12 inhibitors, which have plasma half-lives between 7 and 8.5 hours, cangrelor’s plasma half-life is 3 minutes, and its antiplatelet effect lasts less than 30 minutes after the infusion is stopped.19
Glycoprotein IIb/IIIa inhibitors: tirofiban, eptifibatide, and abciximab
Unlike P2Y12 receptor antagonists, which inhibit platelet activation upstream of platelet aggregation, glycoprotein IIb/IIIa receptor antagonists exert their antiplatelet effect via blockade of the glycoprotein IIb/IIIa receptor, which is involved directly in binding fibrin and allows for aggregation of adjacent platelets. First studied in the mid-1990s, these drugs, which include tirofiban, eptifibatide, and abciximab, inhibit platelet aggregation nearly completely within 15 minutes of intravenous bolus, theoretically making them ideal antiplatelet agents for use in PCI.25 Abciximab, a fragment of a human-murine monoclonal antibody, irreversibly binds to and inactivates platelets; thus, even though abciximab has a short plasma half-life, it exerts its antiplatelet effect as long as abciximab-bound platelets remain in circulation. In contrast, tirofiban and eptifibatide are reversible inhibitors of the glycoprotein IIb/IIIa receptor with short plasma half-lives, with platelet aggregation returning to normal within 4 hours of cessation of an infusion.26
Clinical data
Ticlopidine
Pivotal randomized trials conclusively demonstrated that coronary stents reduce the incidence abrupt closure and restenosis compared with balloon angioplasty alone.27–29 The disadvantages of stenting include neointimal hyperplasia (leading to in-stent restenosis) and stent thrombosis.29 Subsequent clinical trials employed aggressive antiplatelet treatment with aspirin and dipyridamole, and anticoagulation with heparin and warfarin. Despite these aggressive measures, stent thrombosis continued to complicate up to 3.5% of cases.27,28
The development of the potent antiplatelet agent ticlopidine and demonstration of its efficacy in registry studies and small clinical trials30,31 led to the STARS trial, in which 1653 patients who underwent successful coronary stenting were randomized to aspirin alone, aspirin plus ticlopidine, or aspirin plus warfarin (Table 2).32 Compared to aspirin alone, aspirin plus ticlopidine reduced the incidence of 30-day death, target vessel revascularization, angiographically-evident stent thrombosis, or MI by 85% (0.5% vs. 3.6%; RR 0.15, 95% CI 0.05–0.43; p < 0.001). The rate of stent thrombosis was also reduced with ticlopidine (0.5 vs. 2.9% ; RR 0.19, 95% CI 0.06–0.57; p = 0.005). Compared with the combination of aspirin and warfarin, treatment with aspirin plus ticlopidine reduced the risk of the primary outcome to a slightly smaller degree (0.5% vs. 2.7%; RR 0.20, 95% CI 0.07–0.61; p = 0.01); all clinical events in both groups were stent thromboses. The combination of aspirin and ticlopidine increased the rate of hemorrhagic complications 3-fold compared with aspirin alone (5.5% vs. 1.8%, RR 3.06, p = 0.002) but had a similar rate of hemorrhagic complications to the combination of aspirin plus warfarin. This trial established that stent thrombosis is a platelet-mediated phenomenon, and dual antiplatelet therapy was essential to reduce adverse outcomes after PCI with stenting.
Table 2.
Major clinical trials evaluating antiplatelet agents in PCI
| Trial | Number of patients |
Patient population |
IIb/IIIa inhibitor |
P2Y12 inhibitor | P2Y12 inhibitor treatment prior to PCI |
30-day ischemic events |
30-day major bleeding |
|
|---|---|---|---|---|---|---|---|---|
| STARS32 | Ticlopidine arm | 546 | Elective PCI | N/A | ticlopidine 250mg BID | No | 0.5% | 5.5% |
| Placebo arm | 557 | N/A | None | N/A | 3.6% | 1.8% | ||
| CLASSICS33 | Clopidogrel 300/75mg arm | 345 | Elective PCI | 0% | clopidogrel 300mg followed by 75mg daily | No | 1.2% | 1.5% |
| Clopidogrel 75mg arm | 335 | 0% | Clopidogrel 75mg daily | No | 1.5% | 1.2% | ||
| Ticlopidine arm | 340 | 0% | Ticlopidine 250mg BID | No | 0.9% | 1.2% | ||
| PCI-CURE37 | Clopidogrel pretreatment arm | 1313 | NSTE-ACS | 20.9% | Clopidogrel 300mg followed by 75mg daily | Median 6 days prior to PCI | 4.5% | 1.6% |
| Placebo arm | 1345 | 26.6% | None until after PCI | None | 6.4% | 1.4% | ||
| PCI-CLARITY39 | Clopidogrel pretreatment arm | 933 | Fibrinolytic-treated STEMI | 31.1% | Clopidogrel 300mg followed by 75mg daily | Median 3 days prior to PCI | 3.6% | 0.5% |
| Placebo arm | 930 | 33.5% | None until after PCI | None | 6.2% | 1.1% | ||
| CREDO59 | Clopidogrel pretreatment arm | 1053 | Elective PCI | 47.4% | Clopidogrel 300mg followed by 75mg daily | 3–24 hours prior to PCI | 6.8% | 4.8% |
| Placebo arm | 1063 | 43.4% | None until after PCI | None | 8.3% | 3.8% | ||
| CURRENT OASIS 744 | High dose clopidogrel arm | 8650 | 37% STEMI, 63% NSTE-ACS | 41% | 600mg clopidogrel | 3.1 hours in NSTE-ACS, 0.5 hours in STEMI | 3.9% | 1.6% |
| Low dose clopidogrel arm | 8703 | 40% | 300mg clopidogrel | 3.3 hours in NSTE-ACS, 0.5 hours in STEMI | 4.5% | 1.1% | ||
| ESPIRIT72 | Eptifibatide arm | 1040 | 50% elective, 50% NSTE-ACS within 180 days | 100% | Clopidogrel | NR | 6.5% | 1.30% |
| Placebo arm | 1024 | 0.04% | Clopidogrel | NR | 10.8% | 0.4% | ||
| CHAMPION PHOENIX54 | Clopidogrel arm | 5470 | 11% STEMI, 25% NSTE-ACS, 57% elective | 3.50% | clopidogrel 600mg | 63% prior to PCI | 5.9% | 0.10% |
| Cangrelor arm | 5472 | 2.30% | cangrelor, followed by clopidogrel 600mg | 63% prior to PCI | 4.7% | 0.10% | ||
| TRITON TIMI 3846 | Clopidogrel arm | 26% STEMI, 74% NSTE-ACS | 55% | 300mg clopidogrel | No | 7.2% | 1.80% | |
| Prasugrel arm | 54% | 60mg prasugrel | No | 5.6% | 2.40% | |||
| PLATO48 | Clopidogrel arm | 9235 | 49% STEMI, 51% NSTE-ACS | 35% | 300–600mg clopidogrel | 2.4 hours in NSTE-ACS, 0.5 hours in STEMI | 5.4% | 5.8% |
| Ticagrelor arm | 9186 | 35% | 180mg ticagrelor | 2.4 hours in NSTE-ACS, 0.5 hours in STEMI | 4.8% | 5.8% | ||
| ACCOAST58 | Prasugrel pretreatment arm | 1389 | NSTE-ACS | 3.7% | 30mg prasugrel pretreatment, then 30mg at the time of PCI | 4.3 hours prior to PCI | 7.7% | 1.7% |
| No pretreatment arm | 1372 | 3.9% | 60mg prasugrel at the time of PCI | No | 7.3% | 0.7% | ||
| ATLANTIC60 | Pre-hospital ticagrelor arm | 908 | STEMI | 30% | Pre-hospital ticagrelor 180mg | 63 minutes prior to PCI | 4.5% | 1.3% |
| In-hospital ticagrelor arm | 950 | 27% | In-hospital ticagrelor, 180mg | 28 minutes prior to PCI | 4.4% | 0.8% | ||
Clopidogrel
Despite ticlopidine’s superior efficacy to aspirin alone, concerns persisted about its side effects, especially agranulocytosis, which was sometimes irreversible. Preclinical studies reported that clopidogrel was as efficacious as ticlopidine without similar safety concerns. The CLASSICS trial was thus designed to compare the safety of several different regimens of aspirin plus clopidogrel to aspirin plus ticlopidine in a cohort of patients that had undergone successful stenting.33 The primary endpoint of the study – major bleeding, neutropenia, thrombocytopenia, or treatment discontinuation due to an adverse drug event at 28 days – occurred in 9.1% of the ticlopidine treated cohort, compared to 4.6% of the clopidogrel cohort (RR 0.50, 95% CI 0.31–0.51, p = 0.005). This endpoint was driven almost entirely by discontinuation of ticlopidine for gastrointestinal reasons; only 1 of the 340 patients in the ticlopidine arm developed neutropenia, and bleeding risk was similar between the two groups. Although the study was not powered to detect a difference in cardiovascular events, patients treated with ticlopidine and clopidogrel had similar rates of major adverse cardiovascular events at 28 days.33
After other clinical trials demonstrated comparable efficacy between clopidogrel and ticlopidine for the prevention of cardiovascular events,15,34 and the long-term safety of clopidogrel was confirmed in the 19,000-patient CAPRIE study,35 ticlopidine largely fell out of use due to its side effect profile.
Initially, trials of DAPT in patients undergoing stenting largely enrolled either populations with stable CAD or unselected populations. PCI-CURE was a substudy of patients undergoing PCI in the CURE trial, which randomized patients with NSTE-ACS to clopidogrel or placebo, started immediately and continued for 3–12 months.36 Since the standard of care for patients undergoing stenting required DAPT for 4 weeks following stenting, all patients in PCI-CURE received clopidogrel following stenting; however, only the group randomized to clopidogrel received clopidogrel pre-treatment prior to stenting.37 Thus, PCI-CURE’s 30-day outcomes represent the findings of a clinical trial comparing clopidogrel pre-treatment to no clopidogrel pre-treatment. The clopidogrel group was loaded with 300mg clopidogrel and treated with 75mg daily for a median of 6 days prior to PCI. Clopidogrel pre-treatment reduced the rate of the primary endpoint – 30-day cardiovascular death, MI, or urgent target vessel revascularization – by 30% (4.5% vs. 6.4%, RR 0.70, 95% CI 0.50–0.97; p = 0.03); this reduction in the rate of the primary endpoint appeared as early as 2 days after PCI.37 Major bleeding rates from the time of PCI to 30 days were comparable between the two groups (1.6% with clopidogrel vs. 1.4% with placebo).
In the CLARITY trial, the benefit of clopidogrel plus aspirin for prevention of cardiovascular events after stenting was extended to patients with STEMI.38,39 In CLARITY, patients with STEMI treated with fibrinolytics received clopidogrel (300mg loading dose, followed by 75mg daily) or placebo, prior to undergoing coronary angiography 2 to 8 days (median 3 days) after presentation.38 PCI-CLARITY was a substudy enrolling patients undergoing PCI after coronary angiography. As in PCI-CURE, P2Y12 treatment as an adjunct to PCI had already been established as the standard of care, so 75% of patients in both the clopidogrel and placebo groups received a loading dose of clopidogrel or ticlopidine at the time of stenting, and 90% received maintenance P2Y12 inhibitor therapy.39 Thus, PCI-CLARITY represents the results of a trial comparing clopidogrel pretreatment to no clopidogrel pretreatment in STEMI. Similar to PCI-CURE, pretreatment with clopidogrel reduced the risk of 30-day major adverse cardiovascular events (cardiovascular death, recurrent MI, or stroke) following PCI by 46% (3.6% vs. 6.2%, OR 0.54, 95% CI 0.35–0.85, p =0.008).39 The rate of TIMI major bleeding and overall bleeding was similar between the two groups (major bleeding: 0.5% with clopidogrel vs. 1.1% with placebo; overall bleeding 2% vs. 1.9%).
After DANAMI-2 and PRAGUE-2 (amongst other, smaller trials) demonstrated the superiority of primary PCI to fibrinolytics in STEMI,40,41 and multiple trials demonstrated the superiority of a routine invasive strategy in NSTE-ACS to a delayed, provisional strategy42, the practice of clopidogrel preloading followed by delayed PCI tested in PCI-CURE and PCI-CLARITY became inconsistent with contemporary clinical practice. Since treatment with P2Y12 inhibitors at the time of PCI had been established as the standard of care, no trial has tested the efficacy of P2Y12 inhibitors versus placebo given immediately prior to PCI in patients with ACS. Despite this fact, however, future trials of oral P2Y12 inhibitors in ACS compared either higher loading doses of clopidogrel to lower doses given within 8 hours of PCI or new P2Y12 inhibitors to clopidogrel.
In the ARMYDA-2 trial, patients undergoing elective PCI who were randomized to a 600mg loading dose of clopidogrel, given 4 to 8 hours prior to PCI, had a lower risk of death, periprocedural MI, or target vessel revascularization compared with the group randomized to the 300 mg loading dose (4% vs. 12%; RR 0.48).43 Similarly, in CURRENT OASIS-7, patients with ACS (37% with STEMI) were randomized to a loading dose of 600mg of clopidogrel followed by 150mg daily or a loading dose of 300mg followed by 75mg daily. Among patients undergoing PCI, higher-dose clopidogrel reduced a primary endpoint of death, MI, or stroke by 14% (3.9% vs. 4.5%, RR 0.86, 95% CI 0.76–0.99, p = 0.039), with most of that reduction driven by reduction in recurrent MI. Outcomes were consistent in the NSTE-ACS and STEMI subgroups, despite the fact that patients undergoing PCI for STEMI underwent PCI at a median time of 0.5 hours following clopidogrel loading. Those patients treated with higher-dose clopidogrel also had a reduction in the rate of stent thrombosis occurring within 2 days of PCI from 0.4% to 0.2%.44 A subsequent meta-analysis of 7 studies investigating clinical outcomes in patients receiving 600mg or 300mg clopidogrel loading doses prior to PCI demonstrated a 34% relative risk reduction in major adverse cardiac events in patients treated with a higher loading dose with no increase in major bleeding events.45
Prasugrel
In the TRITON TIMI-38 trial, patients with moderate- to high-risk ACS (including both STEMI and NSTE-ACS) were randomized after diagnostic angiography to 300mg clopidogrel or 60mg prasugrel, with the loading dose given any time between randomization and 1 hour after leaving the cardiac catheterization lab.46. Since patients were not preloaded with a P2Y12 inhibitor, 55% received a glycoprotein IIb/IIIa inhibitor. Compared with clopidogrel, prasugrel reduced the incidence of death, nonfatal stroke, or nonfatal MI by 19% (9.9% vs. 12.1%, HR 0.81, 95% CI 0.73–0.90, p < 0.001) after 15 months. However, this reduction in ischemic events came at a cost of an increased risk of major bleeding, including fatal bleeding (2.4% vs. 1.8%, HR 1.32, 95% CI 1.03–1.68, p = 0.03; fatal bleeding 0.4% vs 0.1%, HR 4.19, 95% CI 1.58–11.11, p=0.002). Prasugrel reduced the incidence of stent thrombosis by > 50% (1.1 vs. 2.4%, RR 0.48, 95% CI 0.36–0.64, p < 0.001), appearing as early as 3 days following stent implantation (0.33% vs. 0.67%) and occurring regardless of stent type (drug-eluting or bare metal). The role of prasugrel in PCI for stable angina has not been studied.
Ticagrelor
In the PLATO trial, patients with ACS (STEMI and moderate- to high-risk NSTE-ACS) were randomized to either ticagrelor or clopidogrel. Patients were given a loading dose of either clopidogrel or ticagrelor; in patients that underwent PCI, the median time from loading dose to PCI was 15 minutes for STEMI and 4 hours for NSTE-ACS.47 Roughly 35% of patients in both arms received glycoprotein IIb/IIIa inhibitors. Ticagrelor, compared to clopidogrel, reduced the rate of 12-month death, nonfatal MI, or stroke by 16% (9.0% vs. 10.7%, RR 0.84, 95% CI 0.75–0.94, p = 0.0025) with no increase in major bleeding (7.9% in both arms). In addition, ticagrelor reduced cardiovascular and all-cause mortality (4.0% vs. 5.1%, p=0.001; 10.2% vs. 12.3%, p<0.001, respectively).48 Definite stent thrombosis within 30 days was also reduced in the ticagrelor arm compared to the clopidogrel arm (1.3% vs. 2.0%, RR 0.64, 95% CI 0.46–0.88, p = 0.0054).48 However, there was no difference in the rate of definite or probable stent thrombosis seen in the first 24 hours after stent implantation (0.36% vs. 0.37%).49
Glycoprotein IIb/IIIa inhibitors
The efficacy of routine glycoprotein IIb/IIIa inhibitors for reducing early cardiovascular events following stent implantation was first conclusively established in the EPISTENT trial, which randomized patients with stable angina undergoing coronary stenting to abciximab or placebo, in addition to aspirin, ticlopidine, and heparin. The incidence of 30-day death, MI, or urgent target vessel revascularization was reduced by 52% with abciximab compared to placebo (5.3% vs. 10.8%, HR 0.48, 95% CI 0.33–0.69, p < 0.001) without an increase in bleeding complications.50 Although subsequent trials of glycoprotein IIb/IIIa inhibitors mostly demonstrated similar reductions in ischemic complications, all have also demonstrated an increase in bleeding events.
In a meta-analysis comparing glycoprotein IIb/IIIa inhibitors to placebo or usual care in patients undergoing PCI, use of glycoprotein IIb/IIIa inhibitors significantly reduced 30-day mortality by 21% (0.92% vs. 1.33%, RR 0.79, 95% CI 0.64–0.97), and a combined endpoint of death or recurrent MI by 34% (5.05% vs. 7.04%, RR 0.66, 95% CI 0.60–0.72).51 The 30-day survival benefit was attenuated in patients with ACS (RR 0.71 in stable CAD, 0.79 in patients with NSTEMI, and 0.88 in patients with STEMI), and in those pre-treated with clopidogrel (RR 0.83). The reduction in ischemic events and mortality came at the cost of increased bleeding; glycoprotein IIb/IIIa inhibitor use increased the 30-day major bleeding rate by 39% (3.03% vs. 2.23%, RR 1.39, 95% CI 1.21–1.61), an effect that persisted regardless of whether the patient had ACS or was pretreated with clopidogrel. Overall, treatment of 1000 patients with glycoprotein IIb/IIIa inhibitors would prevent 20 non-fatal MIs and 4 deaths at a cause of 8 excess bleeding events.51 The availability of the newer more potent oral antiplatelet agents and the trials demonstrating better net clinical adverse outcomes with the direct thrombin inhibitor bivalirudin (and use of glycoprotein IIb/IIIa inhibitors for ischemic or angiographic bailout) has drastically diminished the role of glycoprotein IIb/IIIa inhibitors in clinical practice.
Cangrelor
The newest intravenous antiplatelet agent is the P2Y12 inhibitor cangrelor. Its benefit in patients undergoing PCI was established by the CHAMPION series of clinical trials. CHAMPION PLATFORM and CHAMPION PCI, the first two trials comparing cangrelor to clopidogrel and adequately powered to detect clinical outcomes, did not demonstrate a reduction in death, MI, or ischemia-driven revascularization (7.0% vs. 8.7%, RR 0.87, 95% CI 0.71–1.07, p =0.17 in CHAMPION PLATFORM; 7.5% vs. 7.1%, RR 1.05, 95% CI 0.88–1.24, p = 0.67 in CHAMPION PCI).52,53 However, cangrelor did reduce the incidence of acute stent thrombosis in both studies, and investigators hypothesized that cangrelor’s failure to significantly reduce the primary endpoint in both studies could be explained by a definition of periprocedural MI that failed to distinguish true reinfarction in patients presenting with MI.
Subsequently, in the CHAMPION PHOENIX trial, investigators randomized patients undergoing PCI (57% elective, 25% urgent for NSTE-ACS, 18% primary PCI for STEMI) to cangrelor started prior to PCI, or clopidogrel given as a loading dose prior to PCI. Glycoprotein IIb/IIIa inhibitors were used infrequently. Patients in the cangrelor arm had a 22% lower rate of 48-hour death, MI, ischemia-driven revascularization, or stent thrombosis (4.7% vs. 5.9%, OR 0.78, 95% CI 0.66–0.93, p = 0.005) with much of the difference accruing in the first 2 hours following PCI.54 Similarly, cangrelor treatment reduced the rate of stent thrombosis (0.8% vs. 1.4%, OR 0.62, 95% CI 0.43–0.90, p = 0.01), with much of the benefit in so-called intra-procedure stent thrombosis. The rate of 48-hour major bleeding was low, and similar in both groups (0.1%), and the benefits of cangrelor were similar in patients with and without STEMI.54
Controversies: Preloading and platelet function testing
Preloading with P2Y12 inhibitors prior to PCI
Given the rapid onset of action of prasugrel and ticagrelor in healthy volunteers, both drugs should have demonstrated early benefit in the prevention of acute stent thrombosis. However, neither prasugrel nor ticagrelor work as rapidly in patients with ACS as they do in healthy volunteers.55 In the RAPID trial, investigators randomized 50 patients with STEMI, all treated with bivalrudin monotherapy, to prasugrel or ticagrelor and measured platelet activity at 2, 4, 8, and 12 hours.56 After 2 hours, high residual platelet activity was found in 44% of prasugrel-treated patients and 60% of ticagrelor-treated patients; 35% of ticagrelor-treated patients still had high residual platelet activity 4 hours after the loading dose. This delayed onset of action in ACS patients may be partially explained by the frequent use of morphine sulfate to treat angina pain since the absorption of both ticagrelor and prasugrel is delayed when morphine is administered.57
Given the delayed onset of antiplatelet effect with oral P2Y12 inhibitors, especially in patients with ACS, and the high risk of bleeding with glycoprotein IIb/IIIa inhibitors, early administration of oral P2Y12 inhibitors may theoretically provide a benefit. Earlier administration of clopidogrel, prasugrel, or ticagrelor would allow their antiplatelet activity to take effect prior to stent deployment, limiting the need for the adjunctive antiplatelet activity of glycoprotein IIb/IIIa inhibitors. Moreover, in patients with STEMI, early, potent platelet inhibition could limit continued intracoronary thrombosis, potentially restoring patency to the infarct-related artery and limiting total ischemic time. These hypotheses were tested in a series of clinical trials examining the effect of early P2Y12 inhibitor administration.37,58–60 However, despite the theoretical benefits of P2Y12 inhibitor preloading, none of these trials has demonstrated a conclusive benefit to this strategy.
Though PCI-CURE and PCI-CLARITY showed that clopidogrel preloading reduced 30-day ischemic events in patients with ACS, patients enrolled in PCI-CURE and PCI-CLARITY underwent PCI at a median of 6 and 3 days following the start of clopidogrel loading, respectively, a time course inconsistent with modern clinical practice, but better aligned with clopidogrel’s pharmacokinetics.37,39 CREDO, which randomized patients with stable CAD undergoing elective PCI in the next 3 to 24 hours to clopidogrel or placebo pretreatment, may be a better approximation of modern clinical practice.59 In CREDO, clopidogrel pretreatment had no significant effect on the 28-day rate of cardiovascular death, MI, or urgent target vessel revascularization (6.8% with pretreatment vs. 8.3% without pretreatment, RR 0.81, 95% CI 0.58–1.14, p = 0.23). However, consistent with clopidogrel pharmacokinetics, a pre-specified subset analysis in patients pretreated with clopidogrel at least 6 hours prior to stent implantation demonstrated a marginally significant 39% reduction in the primary endpoint (RR 0.61, 95% CI 0.37–1.02, p = 0.051).59
A meta-analysis of 9 trials comparing clopidogrel pretreatment to no clopidogrel pretreatment (including CREDO, PCI-CURE, and PCI-CLARITY) reported that pretreatment reduced the incidence of major cardiovascular events by 23% (9.8% vs. 12.4%, OR 0.77%, 95% CI 0.66–0.89, p < 0.001).61 In subset analyses, pretreatment reduced cardiovascular events in patients with ACS, but not those with stable angina. Importantly, most of the trials included evaluated a preloading strategy with a delay of 4–6 hours or more from loading to PCI.
Despite the fact that the theoretical advantages of P2Y12 pretreatment should be accentuated with the new oral P2Y12 inhibitors prasugrel and ticagrelor due to their favorable pharmacokinetics compared to clopidogrel, trials examining preloading with these agents have also failed to demonstrate a clear clinical benefit.
In the ACCOAST trial, investigators examined the potential benefits and harms of pretreatment with prasugrel in patients with NSTE-ACS. Patients were randomized to receive either 30mg prasugrel at the time of diagnosis followed by another 30mg after angiography but prior to PCI, or to receive 60mg of prasugrel after angiography but prior to PCI (the strategy employed in TRITON-TIMI 38).58 Pretreatment with prasugrel did not significantly reduce the incidence of the primary endpoint, 7-day cardiovascular death, MI, stroke, urgent revascularization, or glycoprotein IIb/IIIa inhibitor bailout (10% vs. 9.8%, HR 1.02, 95% CI 0.84–1.25, p = 0.81), but did increase the incidence of 7-day non-CABG-related TIMI major bleeding by 3-fold (1.3% vs. 0.5%, HR 2.95, 95% CI 1.39–6.28, p = 0.003).
In the ATLANTIC trial, investigators attempted an alternative strategy to achieve earlier platelet inhibition with P2Y12 inhibitors, randomizing patients with STEMI to ticagrelor loading in the ambulance en route to the hospital or upon hospital arrival.60 The group receiving ticagrelor in the ambulance received it 31 minutes earlier than the group receiving it at the hospital and 63 minutes prior to PCI. Although the primary endpoint of infarct artery patency upon arrival at the cath lab did not differ between the two arms, the rate of acute stent thrombosis was lower in the pre-hospital ticagrelor arm compared to the in-hospital ticagrelor arm (0% vs. 0.8%, p = 0.008). TIMI major bleeding at 30 days was 1.3% in both groups. In a pharmacodynamic substudy involving 37 patients, there were no significant differences between the 2 groups in mean platelet inhibition at any time. However, platelet inhibition was numerically greater in the pre-hospital group than the in-hospital group at the end of PCI and at 1 hour post-PCI.60 These data suggest that preloading with ticagrelor may reduce stent thrombosis. Confirmatory studies are needed, especially since the pre-hospital group had higher mortality than the in-hospital group (3.3% vs. 2.0%, OR 1.68, 95% CI 0.94–3.01).
Another strategy employed by investigators to quickly reduce platelet activity in patients with STEMI was employed in the MOJITO trial, in which 82 patients undergoing primary PCI for STEMI were randomized to a loading dose of crushed or whole ticagrelor tablets.62 Though the study was not powered to detect clinical endpoints, this strategy led to reduced platelet activity in the group receiving crushed ticagrelor at 1 hour after PCI (35% vs. 63%). Ticagrelor’s package insert notes that tablets may be “crushed, mixed with water and drunk.”
Platelet function testing
When given a loading dose of clopidogrel, healthy individuals with polymorphisms of the gene encoding a CYP2C19 enzyme with reduced function have 34% less exposure to clopidogrel’s active metabolite and 9% less of a reduction in platelet activity than individuals without CYP2C19 polymorphisms.63 In patients with ACS (STEMI and NSTE-ACS) randomized to clopidogrel in TRITON-TIMI 38, a reduced function CYP2C19 allele was associated with an increased risk of cardiovascular death (2% vs. 0.4%; OR 4.79) and stent thrombosis (2.7% vs. 0.8%; OR 3.33).63 Nearly all of the increased risk of stent thrombosis was concentrated in the first few days following PCI. This finding has also been demonstrated in multiple registry studies,64 including the ADAPT-DES registry, in which patients with high on-clopidogrel platelet reactivity had a 3-fold increased risk of early stent thrombosis, and high on-treatment platelet reactivity explained 60% of early stent thrombosis events.65
Recognizing this heterogeneity in clopidogrel responsiveness among different patients and the higher incidence of stent thrombosis and adverse cardiovascular events among clopidogrel non-responders,64 there have been a series of clinical investigations exploring a strategy involving platelet function testing and escalation of antiplatelet therapy based on the results. This strategy theoretically ensures that clopidogrel non-responders receive an effective dose of antiplatelet therapy, while avoiding the use of more potent antiplatelet regimens and their attendant higher bleeding risk in patients whose platelet function is effectively inhibited by standard dosing of clopidogrel.
The ARCTIC trial randomized patients undergoing PCI for stable angina to standard therapy or to platelet reactivity testing after diagnostic angiography; all patients were supposed to receive a loading dose of clopidogrel at least 6 hours prior to angiography.66 If platelet reactivity was high despite the loading dose of clopidogrel, then these patients received glycoprotein IIb/IIIa inhibitors at the time of PCI, and either a larger loading dose of clopidogrel (≥ 600mg) or a loading dose of 60mg prasugrel. High platelet reactivity was identified in 34.5% of patients in the platelet function testing arm; 80.2% of these patients received another loading dose of clopidogrel, 3.3% received a loading dose of prasugrel, and 87.1% received glycoprotein IIb/IIIa inhibitors.66 Patients with high platelet reactivity, either at the time of stenting or 2–4 weeks after, also received higher maintenance doses of clopidogrel. Despite more aggressive antiplatelet therapy in the platelet function-testing guided arm, this group had a numerically higher incidence of the primary efficacy endpoint, 1-year death, MI, stent thrombosis, stroke, or urgent revascularization (34.6% vs. 31.1%, HR 1.13, 95% CI 0.98–1.29, p = 0.10).
Other trials have approached the question of adjusting antiplatelet therapy based on platelet function testing through different means, but have nevertheless failed to demonstrate any benefit with this strategy. The TRIGGER-PCI trial, which randomized patients who had undergone successful drug-eluting stent implantation for stable angina and had high platelet reactivity to either continued clopidogrel or more aggressive antiplatelet therapy with prasugrel, was stopped early due to a low rate of outcome events.67 The GRAVITAS trial, which had a similar design, but randomized patients with high on-clopidogrel platelet reactivity to higher-dose clopidogrel, also failed to demonstrate that more aggressive antiplatelet therapy reduced cardiovascular events in clopidogrel non-responders.68 In TRANSLATE-POPS, hospitals were randomized to either no-cost platelet function testing or standard care.69 In hospitals randomized to receive no-cost platelet function testing, 66.9% of patients underwent platelet function testing prior to discharge compared to 1.4% at control hospitals, and patients undergoing coronary stenting more frequently had their antiplatelet agent adjusted prior to discharge (14.8% vs. 10.5%, OR 1.68, 95% CI 1.18–2.64, p = 0.004). However, cardiovascular events and major bleeding at 30-day follow-up were similar in both groups (OR 0.94, 95% CI 0.66–1.34 for cardiovascular events; OR 0.86, 95% CI 0.55–1.34 for major bleeding).
Based on these trials, the use of routine genotype or platelet function testing to guide antiplatelet therapy cannot be recommended.
Guidelines recommendations
In patients undergoing PCI for stable angina, the ACCF/AHA/SCAI and ESC guidelines recommend clopidogrel 600mg (class I, Level of Evidence B in the ACC/AHA guidelines, LOE A in the ESC guidelines) (Table 3).70,71 The ESC guidelines add that clopidogrel loading should preferably occur at least 2 hours prior to the procedure (class I, LOE A).71 The ACCF/AHA/SCAI guidelines note that it is reasonable to use glycoprotein IIb/IIIa inhibitors in patients undergoing PCI for stable angina that have not been pre-loaded with clopidogrel (class IIa, LOE B) or in patients that have been preloaded with clopidogrel (class IIb, LOE B).70 The ESC guidelines recommend reserving glycoprotein IIb/IIIa inhibitors only for bailout (class IIa, LOE C).71
Table 3.
Guideline recommendations
| Recommendation | ACC/AHA/SCAI | ESC/EACTS |
|---|---|---|
| Stable angina | ||
| A loading dose of clopidogrel (600mg) should be given to patients undergoing PCI with stenting | Class I, LOE B | Class I, LOE A |
| Clopidogrel should be loaded once anatomy is known and decision is made to proceed with PCI, preferably 2 hours or more prior to the procedure | Class I, LOE A | |
| It is reasonable to administer a glycoprotein IIb/IIa inhibitor in patients undergoing elective PCI treated with UFH and not adequately pre-treated with clopidogrel | Class IIa, LOE B | |
| It may be reasonable to administer a glycoprotein IIb/IIIa inhibitor to patients undergoing elective PCI treated with UFH and adequately pretreated with clopidogrel | Class IIb, LOE B | |
| Glycoprotein IIb/IIIa inhibitors should be considered only for bailout | Class IIa, LOE C | |
| NSTE-ACS | ||
| A loading dose of clopidogrel (600mg), prasugrel (60mg) or ticagrelor (60mg) should be given to patients undergoing PCI with stenting | Class I, LOE B | |
| A loading dose of prasugrel (60mg) should be given to patients in whom coronary anatomy is known and are proceeding to PCI if no contraindications | Class I, LOE B | |
| A loading dose of ticagrelor (180mg) should be given to patients at moderate-to-high risk of ischemic events if no contraindications | Class I, LOE B | |
| Clopidogrel should be used only when prasugrel and ticagrelor are unavailable or contraindicated | Class I, LOE B | |
| Prasugrel should not be used in patients in whom coronary anatomy is not known | Class III, LOE B | Class III, LOE B |
| It is useful to administer a glycoprotein IIb/IIIa bolus at the time of PCI to patients with high-risk features treated with UFH and not adequately pretreated with clopidogrel | Class I, LOE A | |
| It is reasonable to administer a glycoprotein IIb/IIIa inhibitor at the time of PCI to patients with high-risk features treated with UFH | Class IIa, LOE B | |
| Glycoprotein IIb/IIIa inhibitors should be considered for bail-out situation or thrombotic complications | Class IIa, LOE C | |
| Pretreatment with glycoprotein IIb/IIIa inhibitors is not recommended | Class III, LOE A | |
| STEMI | ||
| A loading dose of clopidogrel (600mg), prasugrel (60mg) or ticagrelor (60mg) should be given to patients undergoing PCI with stenting | Class I, LOE B | |
| A loading dose of prasugrel (60mg) should be given to patients in whom coronary anatomy is known and are proceeding to PCI if no contraindications | Class I, LOE B | |
| A loading dose of ticagrelor (180mg) should be given to patients at moderate-to-high risk of ischemic events if no contraindications | Class I, LOE B | |
| Clopidogrel should be used only when prasugrel and ticagrelor are unavailable or contraindicated | Class I, LOE B | |
| It is reasonable to administer a glycoprotein IIb/IIIa inhibitor in patients treated with UFH not pretreated with clopidogrel | Class IIa, LOE A | |
| It is reasonable to administer a glycoprotein IIb/IIIa inhibitor in patients treated with UFH pretreated with clopidogrel | Class IIa, LOE C | |
| Glycoprotein IIb/IIIa inhibitors should be considered for bailout or evidence or no-reflow, or evidence of a thrombotic complication | Class IIa, LOE C | |
ACC: American College of Cardiology; AHA: American Heart Association; SCAI: Society for Coronary Angiography and Intervention; ESC: European Society of Cardiology; EACTS: European Association for Cardio-Thoracic Surgery; LOE: Level of Evidence; PCI: percutaneous intervention; UFH: unfractionated heparin; NSTE-ACS: Non-ST segment elevation acute coronary syndrome; STEMI: ST-segment elevation myocardial infarction
In patients undergoing PCI for an ACS indication, the ACCF/AHA/SCAI guidelines recommend a loading dose of either 600mg clopidogrel, 60mg prasugrel, or 180mg ticagrelor (class I, LOE B).70 The ESC guidelines recommend either prasugrel or ticagrelor, with clopidogrel reserved for situations in which the newer P2Y12 agents are not available or contraindicated (class I, LOE A for NSTE-ACS, LOE B for STEMI).71 The ACCF/AHA/SCAI guidelines recommend the use of glycoprotein IIb/IIIa inhibitors in patients with high-risk NSTE-ACS treated with heparin and not adequately pre-treated with clopidogrel (class I, LOE A), and note that glycoprotein IIb/IIIa inhibitors are reasonable in these patients even if they are adequately pre-treated with clopidogrel (class IIa, LOE B).70 In contrast, the ESC guidelines recommend considering glycoprotein IIb/IIIa inhibitors during PCI for NSTE-ACS only for bailout, or with thrombotic complications (class IIa, LOE C).71 In all patients undergoing primary PCI for STEMI, the ACCF/AHA/SCAI guidelines allow for the consideration of glycoprotein IIb/IIIa inhibitors (class IIa, LOE A in patients not pretreated with clopidogrel, LOE C in patients pretreated with clopidogrel).70 The ESC guidelines recommend reserving glycoprotein IIb/IIIa inhibitors for bailout, thrombotic complications, or no-reflow (class IIa, LOE C).71
Neither set of guidelines issues formal recommendations with regard to platelet function testing, and as of this writing, a set of recommendations regarding cangrelor has not been developed.
Summary
The introduction in the past 5 years of the potent and fast-acting P2Y 12 inhibitors prasugrel, ticagrelor, and cangrelor have provided interventional cardiologists with new agents for inhibiting platelet activity and preventing thrombotic events following stent implantation. However, significant questions still exist regarding the optimal antiplatelet strategy. Increased potency of antiplatelet effect is associated with an increased bleeding risk, particularly in vulnerable populations like the elderly and those with prior stroke or transient ischemic attack. It remains unclear whether pretreatment is beneficial in patients undergoing PCI. Genotype or platelet reactivity testing has not been proven to be beneficial. As PCI continues to evolve, these issues will be important to resolve in order to improve outcomes.
Key Points.
Balancing the risks of stent thrombosis and major bleeding is key to the effective use of antiplatelet agents during and after PCI
The new oral P2Y12 inhibitors prasugrel and ticagrelor have onsets of action considerably faster than that of clopidogrel and reduce the incidence of recurrent ischemic events when administered to patients with acute coronary syndrome (ACS) undergoing PCI
Despite the theoretical benefits of P2Y12 inhibitor preloading and platelet function testing-guided selection of antiplatelet agents, clinical trials have failed to demonstrate the benefit of these strategies.
Acknowledgments
This manuscript was prepared without external funding
Footnotes
Disclosures:
Alex Fanaroff MD – none
Sunil V. Rao MD – consultant Merck
References
- 1.Mrdovic I, Savic L, Lasica R, et al. Usefulness of the RISK-PCI score to predict stent thrombosis in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a substudy of the RISK-PCI trial. Heart and vessels. 2013 Jul;28(4):424–433. doi: 10.1007/s00380-012-0276-z. [DOI] [PubMed] [Google Scholar]
- 2.De Luca G, Dirksen MT, Spaulding C, et al. Time course, predictors and clinical implications of stent thrombosis following primary angioplasty. Insights from the DESERT cooperation. Thrombosis and haemostasis. 2013 Oct;110(4):826–833. doi: 10.1160/TH13-02-0092. [DOI] [PubMed] [Google Scholar]
- 3.Palmerini T, Dangas G, Mehran R, et al. Predictors and implications of stent thrombosis in non-ST-segment elevation acute coronary syndromes: the ACUITY Trial. Circulation. Cardiovascular interventions. 2011 Dec 1;4(6):577–584. doi: 10.1161/CIRCINTERVENTIONS.111.963884. [DOI] [PubMed] [Google Scholar]
- 4.Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC. Cardiovascular interventions. 2011 Jun;4(6):654–664. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) European heart journal. 2003 Oct;24(20):1815–1823. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 6.Rao SV, Dai D, Subherwal S, et al. Association between periprocedural bleeding and long-term outcomes following percutaneous coronary intervention in older patients. JACC. Cardiovascular interventions. 2012 Sep;5(9):958–965. doi: 10.1016/j.jcin.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chhatriwalla AK, Amin AP, Kennedy KF, et al. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. Jama. 2013 Mar 13;309(10):1022–1029. doi: 10.1001/jama.2013.1556. [DOI] [PubMed] [Google Scholar]
- 8.D'Ascenzo F, Bollati M, Clementi F, et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. International journal of cardiology. 2013 Jul 31;167(2):575–584. doi: 10.1016/j.ijcard.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Amin AP, House JA, et al. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. Jama. 2010 Jun 2;303(21):2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 10.Cook S, Windecker S. Early stent thrombosis: past, present, and future. Circulation. 2009 Feb 10;119(5):657–659. doi: 10.1161/CIRCULATIONAHA.108.842757. [DOI] [PubMed] [Google Scholar]
- 11.Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. Journal of the American College of Cardiology. 2010 Jun 8;55(23):2556–2566. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal J, Sumaya W, Tatman V, et al. Incidence and predictors of stent thrombosis: a single-centre study of 5,833 consecutive patients undergoing coronary artery stenting. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2013 May 20;9(1):62–69. doi: 10.4244/EIJV9I1A10. [DOI] [PubMed] [Google Scholar]
- 13.Aoki J, Lansky AJ, Mehran R, et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2009 Feb 10;119(5):687–698. doi: 10.1161/CIRCULATIONAHA.108.804203. [DOI] [PubMed] [Google Scholar]
- 14.Di Perri T, Pasini F, Frigerio C, et al. Pharmacodynamics of ticlopidine in man in relation to plasma and blood cell concentration. European journal of clinical pharmacology. 1991;41(5):429–434. doi: 10.1007/BF00626364. [DOI] [PubMed] [Google Scholar]
- 15.Moussa I, Oetgen M, Roubin G, et al. Effectiveness of clopidogrel and aspirin versus ticlopidine and aspirin in preventing stent thrombosis after coronary stent implantation. Circulation. 1999;99(18):2364–2366. doi: 10.1161/01.cir.99.18.2364. [DOI] [PubMed] [Google Scholar]
- 16.Yousuf O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nature reviews. Cardiology. 2011 Oct;8(10):547–559. doi: 10.1038/nrcardio.2011.96. [DOI] [PubMed] [Google Scholar]
- 17.Cadroy Y, Bossavy J-P, Thalamas C, Sagnard L, Sakariassen K, Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation. 2000;101(24):2823–2828. doi: 10.1161/01.cir.101.24.2823. [DOI] [PubMed] [Google Scholar]
- 18.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Is a 300 mg clopidogrel loading dose sufficient to inhibit platelet function early after coronary stenting? A platelet function profile study. The Journal of invasive cardiology. 2004 Jun;16(6):325–329. [PubMed] [Google Scholar]
- 19.Ferri N, Corsini A, Bellosta S. Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs. 2013;73(15):1681–1709. doi: 10.1007/s40265-013-0126-z. [DOI] [PubMed] [Google Scholar]
- 20.Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. American heart journal. 2007;153(1):66. e69–66. e16. doi: 10.1016/j.ahj.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Braun O, Johnell M, Varenhorst C, et al. Greater reduction of platelet activation markers and platelet-monocyte aggregates by prasugrel compared to clopidogrel in stable coronary artery disease. Thrombosis and haemostasis. 2008;100(4):626–633. [PubMed] [Google Scholar]
- 22.Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel Compared With High Loading-and Maintenance-Dose Clopidogrel in Patients With Planned Percutaneous Coronary Intervention The Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 Trial. Circulation. 2007;116(25):2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 23.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease the ONSET/OFFSET study. Circulation. 2009;120(25):2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 24.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. European heart journal. 2006;27(9):1038–1047. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 25.Harrington RA, Kleiman NS, Kottke-Marchant K, et al. Immediate and reversible platelet inhibition after intravenous administration of a peptide glycoprotein IIb/IIIa inhibitor during percutaneous coronary intervention. The American journal of cardiology. 1995;76(17):1222–1227. doi: 10.1016/s0002-9149(99)80345-2. [DOI] [PubMed] [Google Scholar]
- 26.Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. American heart journal. 2000;139(2):s38–s45. doi: 10.1067/mhj.2000.103742. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. New England Journal of Medicine. 1994;331(8):489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 28.Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. New England Journal of Medicine. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 29.Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. New England Journal of Medicine. 1991;324(1):13–17. doi: 10.1056/NEJM199101033240103. [DOI] [PubMed] [Google Scholar]
- 30.Serruys PW, Emanuelsson H, Van Der Giessen W, et al. Heparin-Coated Palmaz-Schatz Stents in Human Coronary Arteries Early Outcome of the Benestent-II Pilot Study. Circulation. 1996;93(3):412–422. doi: 10.1161/01.cir.93.3.412. [DOI] [PubMed] [Google Scholar]
- 31.Schömig A, Neumann F-J, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. New England Journal of Medicine. 1996;334(17):1084–1089. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- 32.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. New England Journal of Medicine. 1998;339(23):1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand ME, Rupprecht H-J, Urban P, Gershlick AH Investigators C. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting the clopidogrel aspirin stent international cooperative study (CLASSICS) Circulation. 2000;102(6):624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 34.Taniuchi M, Kurz HI, Lasala JM. Randomized comparison of ticlopidine and clopidogrel after intracoronary stent implantation in a broad patient population. Circulation. 2001;104(5):539–543. doi: 10.1161/hc3001.093435. [DOI] [PubMed] [Google Scholar]
- 35.Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) The Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Fox K, Tognoni G, et al. Effects of clopidogrel in addition to aspirin in patients with acutecoronary syndromes without ST-segment elevation. New England Journal of Medicine. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 37.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. The Lancet. 2001;358(9281):527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 38.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. New England Journal of Medicine. 2005;352(12):1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 39.Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. Jama. 2005;294(10):1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 40.Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. New England Journal of Medicine. 2003;349(8):733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 41.Widimsky P, Budesinsky T, Vorac D, Groch L, Zelizko M, Formanek P. PRAGUE Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction: final results of the randomised national multicentre trial-PRAGUE-2. European heart journal. 2003;24(1):94–104. doi: 10.1016/s0195-668x(02)00468-2. [DOI] [PubMed] [Google Scholar]
- 42.Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. Jama. 2005;293(23):2908–2917. doi: 10.1001/jama.293.23.2908. [DOI] [PubMed] [Google Scholar]
- 43.Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2005 Apr 26;111(16):2099–2106. doi: 10.1161/01.CIR.0000161383.06692.D4. [DOI] [PubMed] [Google Scholar]
- 44.Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet (London, England) 2010 Oct 9;376(9748):1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 45.Siller-Matula JM, Huber K, Christ G, et al. Impact of clopidogrel loading dose on clinical outcome in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Heart (British Cardiac Society) 2011 Jan;97(2):98–105. doi: 10.1136/hrt.2010.195438. [DOI] [PubMed] [Google Scholar]
- 46.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 47.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 48.Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. The Lancet. 2010;375(9711):283–293. doi: 10.1016/S0140-6736(09)62191-7. [DOI] [PubMed] [Google Scholar]
- 49.Steg PG, Harrington RA, Emanuelsson H, et al. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective randomized PLATO trial. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.002589. CIRCULATIONAHA. 113.002589. [DOI] [PubMed] [Google Scholar]
- 50.Topol EJ Investigators E. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. The Lancet. 1998;352(9122):87–92. doi: 10.1016/s0140-6736(98)06113-3. [DOI] [PubMed] [Google Scholar]
- 51.Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. The Cochrane Library. 2010 doi: 10.1002/14651858.CD002130.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. New England Journal of Medicine. 2009;361(24):2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 53.Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. New England Journal of Medicine. 2009;361(24):2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 54.Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. New England Journal of Medicine. 2013;368(14):1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 55.Michelson AD, Frelinger AL, Braunwald E, et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. European heart journal. 2009;30(14):1753–1763. doi: 10.1093/eurheartj/ehp159. [DOI] [PubMed] [Google Scholar]
- 56.Parodi G, Valenti R, Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. Journal of the American College of Cardiology. 2013;61(15):1601–1606. doi: 10.1016/j.jacc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 57.Parodi G, Bellandi B, Xanthopoulou I, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circulation: Cardiovascular Interventions. 2015;8(1):e001593. doi: 10.1161/CIRCINTERVENTIONS.114.001593. [DOI] [PubMed] [Google Scholar]
- 58.Montalescot G, Bolognese L, Dudek D, et al. Pretreatment with prasugrel in non–ST-segment elevation acute coronary syndromes. New England Journal of Medicine. 2013;369(11):999–1010. doi: 10.1056/NEJMoa1308075. [DOI] [PubMed] [Google Scholar]
- 59.Steinhubl SR, Berger PB, Mann JT, III, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. Jama. 2002;288(19):2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 60.Montalescot G, van't Hof AW, Lapostolle F, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. New England Journal of Medicine. 2014;371(11):1016–1027. doi: 10.1056/NEJMoa1407024. [DOI] [PubMed] [Google Scholar]
- 61.Bellemain-Appaix A, O’Connor SA, Silvain J, et al. Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Jama. 2012;308(23):2507–2516. doi: 10.1001/jama.2012.50788. [DOI] [PubMed] [Google Scholar]
- 62.Parodi G, Xanthopoulou I, Bellandi B, et al. Ticagrelor crushed tablets administration in STEMI patients: The MOJITO study. Journal of the American College of Cardiology. 2015;65(5):511–512. doi: 10.1016/j.jacc.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 63.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. The New England journal of medicine. 2009 Jan 22;360(4):354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 64.Aradi D, Storey RF, Komócsi A, et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. European heart journal. 2014;35(4):209–215. doi: 10.1093/eurheartj/eht375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. The Lancet. 2013;382(9892):614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 66.Collet J-P, Cuisset T, Rangé G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. New England Journal of Medicine. 2012;367(22):2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 67.Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. Journal of the American College of Cardiology. 2012;59(24):2159–2164. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 68.Price MJ, Berger PB, Teirstein PS, et al. Standard-vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. Jama. 2011;305(11):1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 69.Wang TY, Henry TD, Effron MB, et al. Cluster-Randomized Clinical Trial Examining the Impact of Platelet Function Testing on Practice The Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome Prospective Open Label Antiplatelet Therapy Study. Circulation: Cardiovascular Interventions. 2015;8(6):e001712. doi: 10.1161/CIRCINTERVENTIONS.114.001712. [DOI] [PubMed] [Google Scholar]
- 70.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013 Oct 1;82(4):E266–355. doi: 10.1002/ccd.23390. [DOI] [PubMed] [Google Scholar]
- 71.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. European heart journal. 2014:ehu278. doi: 10.1093/eurheartj/ehu422. [DOI] [PubMed] [Google Scholar]
- 72.Investigators E. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet (London, England) 2000;356(9247):2037. doi: 10.1016/S0140-6736(00)03400-0. [DOI] [PubMed] [Google Scholar]