Summary
Squamous cell carcinomas (SCCs) are heterogeneous tumors that are sustained by tumor propagating cancer cells (TPCs). SCCs frequently resist chemotherapy through mechanisms that are still unknown. Here, we combine H2BGFP based pulse-chasing with cell surface markers to distinguish quiescent from proliferative TPCs within SCCs. We find that quiescent TPCs resist DNA damage and exhibit increased tumorigenic potential in response to chemotherapy, whereas proliferative TPCs undergo apoptosis. Quiescence is regulated by TGFβ/SMAD signaling, which directly regulates cell cycle gene transcription to control a reversible G1 cell cycle arrest, independent of p21CIP function. Indeed, genetic or pharmacological TGFβ inhibition increases the susceptibility of TPCs to chemotherapy as it prevents entry into a quiescent state. These findings provide direct evidence that TPCs can reversibly enter a quiescent, chemoresistant state which underscores the need for combinatorial approaches to improve treatment of chemotherapy-resistant SCCs.
Keywords: Quiescence, resistance, squamous cell carcinoma, TGFβ, Cell cycle, Chemotherapy, tumor heterogeneity, cancer stem cells, tumor propagating cells, skin
eTOC Blurb
Heterogeneous tumors, such as squamous cell carcinomas, are often chemoresistant and comprised of subpopulations of poorly-characterized tumor propagating cancer cells (TPCs). Brown et al. demonstrate that TPCs reversibly enter a quiescent, chemoresistant state and inhibiting TGFβ signaling can increase their susceptibility to chemotherapy by preventing cell cycle withdrawal in tumors.

Introduction
Squamous cell carcinomas (SCCs) are heterogeneous tumors comprised of genetically and functionally distinct cancer cells (Leemans et al., 2011; Meacham and Morrison, 2013). This heterogeneity has hampered the development of targeted therapies for SCCs and patients are best treated with surgery plus adjuvant radiation and chemotherapy, which effectively reduces tumor burden (Bonner et al., 2006). However, many advanced SCCs will resist chemotherapy and progress after temporary disease containment by unknown mechanisms. Therefore, resistance must be rooted in specific properties of cancer stem cells, or heterogeneities that are inherent to tumor propagating cancer cells (TPCs) (Meacham and Morrison, 2013).
TPCs have been identified within the basal layer of benign papillomas and malignant SCCs, where they self-renew to sustain long-term tumor growth and differentiate into supra-basal cells without proliferative potential (Pierce and Wallace, 1971). Although all basal SCC cells located along the tumor-stroma interface express high levels of α6β4 and β1 integrins (Janes and Watt, 2006; Schober and Fuchs, 2011) and Epcam (Lapouge et al., 2012), they are functionally and phenotypically diverse. Basal cells differ in CD34 and Sox2 expression, which fluctuates with stage, grade and mutational status of the tumor. CD34 has been linked to increased tumor propagating potential in benign papillomas (Malanchi et al., 2008), but it is not always expressed in TPCs of poorly differentiated carcinomas (Schober and Fuchs, 2011). In contrast, Sox2 is a functional TPC marker, which cooperates with ΔNp63 to drive tumor cell proliferation, self-renewal and survival in mouse and human SCCs (Boumahdi et al., 2014; Siegle et al., 2014; Watanabe et al., 2014). Sox2 is de novo expressed in a few basal papilloma cells, but its expression rapidly expands in carcinomas to correlate with tumor propagating potential. Moreover, clonal lineage trace analyses and mathematical modeling propose that linear growth of benign papillomas depends on a few rapidly proliferating cells, which differentiate into slower cycling basal cells with limited proliferative potential that are poised to differentiate (Driessens et al., 2012). This linear mode of tumor growth converts to geometric growth kinetics once papillomas progress to malignant SCCs, which are sustained by a single, rapidly proliferating population of TPCs with limited differentiation potential.
Collectively, these studies suggest that SCC growth depends on rapidly proliferating TPCs, which raises the question of why chemotherapy would fail in SCC patients. One possible explanation is that SCCs could contain an unidentified population of quiescent (prolonged, but reversibly cell cycle arrested) TPCs. Alternatively, enzymatic and metabolic heterogeneities could also be responsible for therapy resistance. Indeed, increased Nrf2/Glutathione levels have been detected in a subset of TGFβ responsive, HrasG12V infected keratinocytes and their initiating tumors. This enzymatic mechanism was activated by the TGFβ target gene Cdkn1a (p21CIP), which protected Nrf2 from ubiquitination by Keap1 without effecting cell proliferation. Because increased Nrf2 and Glutathione levels can reduce reactive oxygen species (ROS) and detoxify the chemotherapeutic agent Cisplatin, it was proposed that TGFβ/p21CIP function elicits metabolic changes but no growth arrest to protect TPCs from chemotherapy (Oshimori et al., 2015).
However, high Nrf2 signaling and low ROS are signature characteristics of TPCs in malignant cancers, including SCCs, and their expression is not restricted to a small subset of therapy resistant tumor cells (Diehn et al., 2009). Indeed, Nrf2 signaling can be induced by many mechanisms (Ma, 2013), including activating Nrf2 and inactivating Keap1 mutations (Cancer Genome Atlas Network, 2015), oncogenic Ras (DeNicola et al., 2011), and ectopically expressed Sox2 in normal keratinocytes (Sendoel et al., 2017). Furthermore, identification of a SCC specific super enhancer driving Nrf2 transcription in HrasG12V initiated, Tgfbr2-deficient SCCs suggest TGFβ independent mechanisms (Yang et al., 2015). These data call into question whether the heterogeneous response to chemotherapy and progression after treatment can solely be explained by Nrf2/Glutathione expression, or whether these metabolic features require additional, unknown mechanisms that would endow a smaller subset of TPCs with the ability to resist treatment and initiate recurrent SCC growths.
Results
To address these questions, we developed an experimental approach to unbiasedly identify quiescent cells based on their slow proliferation rate in intact SCCs. We began by encoding a genetic system that previously identified slow cycling stem cells in a variety of tissues and organs (Tumbar et al., 2004; Wilson et al., 2008) within a lentivirus so that it can be applied to any cell type. Our reporter system expresses red fluorescent protein (RFP) to constitutively label the tumor parenchymal lineage, along with a Doxycycline (Dox) repressible Histone H2B green fluorescent protein (H2BGFP) that stably incorporates into nucleosomes (Figure 1A). Once H2BGFP transcription is turned off, proliferative cells incorporate only unlabeled histones as they replicate their genome, losing half their GFP intensity with every cell division.
Figure 1. Label retaining cells reside at the tumor-stroma interface in SCCs.
A, Schematic of proliferation reporter. B, Schematic of pulse-chase time course experiment. C, LR SCC cells (green, arrows) emerge at the tumor-stroma interface outlined by α6β4 (red). DAPI (blue) stains nuclei. Scale bars indicate 50μm. D, Flow cytometric analyses of live, RFP+, α6hiβ1hi SCC cells. E, Plating efficiency of LR and non-LR cells. Bar graphs show mean ±s.e.m. (n=7). F, Scatter plots show colony size distribution. Horizontal lines denote mean ±s.e.m. G, Tumor initiating potential in limit dilution transplantation assays. Mean ±s.e.m., (n=6). H, Tumor growth curves of LR and non-LR SCC cells. Points denote mean ±s.e.m., (n=3). See also Figures S1–2.
SCCs contain a subpopulation of slow cycling, label-retaining cells
To test our reporter system we infected primary murine SCC cultures (Schober and Fuchs, 2011), isolated transduced cells based on their RFP and H2BGFP expression and established single cell clones to avoid genetic heterogeneity and differences in reporter gene expression due to position effect variegation. We next transplanted these cells into the dermis of Nude recipient mice and allowed for tumors to expand to ~250mm3 before we chased H2BGFP expression in time course experiments (Figure 1B–D). H2BGFP levels declined gradually over time beginning in the highly proliferative, basal TPCs (Figure 1C). After 17 days of Dox treatment H2BGFP expression had faded in the majority of cells. Still, small clusters of label retaining (LR) H2BGFP expressing SCC cells remained detectable within the basal layer where they stained positive for α6β4 integrin and Sox2 (Figure S1A). This heterogeneous decline in H2BGFP expression was also seen by flow cytometry, where the majority of RFP+ parenchymal cells expressing Epcam and high levels of α6 and β1 integrin showed rapidly declining H2BGFP expression, expanding quickly to supra-basal RFP+α6loβ1lo cells. However, a small subset of cells within the α6hiβ1hi cohort retained the label at a brightness comparable to unchased controls (Figure 1D, Figure S1B–D). Cell cycle analyses verified the reduced proliferation rate of LR RFP+α6hiβ1hi SCC cells compared to non-LR RFP+α6hiβ1hi counterparts, which was similar to post mitotic α6loβ1lo SCC cells (Figure S1E). Mki67 staining (Figure S1F) and short-term EdU incorporation studies (Figure S1G) also confirmed proliferation differences between LR RFP+α6hiβ1hi and non-LR RFP+α6hiβ1hi SCC cells. Finally, qPCR analyses confirmed that H2BGFP transcription was turned off in both LR and non-LR SCC cells (Figure S1H) and TRE-GFP was, in contrast to TRE-H2BGFP, undetectable after a 12 day Dox chase (Figure S1I). This indicated that label retention was not due to a failure of Dox to reach all SCC areas, but was rather due to differences in cell proliferation. Together, our pulse-chase study revealed that the majority of α6hiβ1hi SCC cells proliferated rapidly to fuel tumor growth, while a small subset of these cells did not divide in weeks.
Quiescence is a dynamic property of TPCs in SCCs
To functionally test whether LR RFP+α6hiβ1hi SCC cells have tumor propagating potential, or whether they are post-mitotic and terminally differentiated, we measured their colony forming potential on 3T3 feeder layers and tested their tumor initiating potential in limited dilution transplantation experiments. The ability of LR RFP+α6hiβ1hi SCC cells to form colonies in culture (Figure 1E–F) and initiate tumor growths at rates comparable to their non-LR RFP+α6hiβ1hi counterparts (Figure 1G–H) supports the notion that SCCs can be sustained by both rapidly proliferating and slow-cycling TPCs.
To test whether LR and non-LR TPCs represent two independent lineages, or whether they signify dynamic states within the tumor, we compared the composition of daughter tumors that developed from LR RFP+α6hiβ1hi and non-LR RFP+α6hiβ1hi SCC cells to one another and their parent in serial transplantation experiments. The striking similarities in tumor composition suggested that label retention is a dynamic trait of TPCs (Figure S2A). Although similar dynamic expression changes had been described for CD34 in SCCs (Schober and Fuchs, 2011), co-labeling experiments revealed no direct correlation between label retention and CD34 expression (Figure S2B), suggesting that these markers describe distinct cell populations.
To investigate whether TPCs can interconvert between quiescent and proliferative states in intact tumors, we further stratified our TPC populations by anti-CD71 (transferrin receptor) staining, which declines along with Mki67 and Pyronin-Y as keratinocytes (Tani et al., 2000; Tumbar et al., 2004) and SCC cells (Bragado et al., 2012) withdraw from the cell cycle (Figure S2C). Indeed, post-mitotic α6loβ1lo SCC cells (Figure S2D–F) expressed CD71 at low levels, thereby presenting an internal control for gate selection, while RFP+α6hiβ1hi cells separated into CD71hi and CD71lo subpopulations (Figure 2A). This separation, when combined with H2BGFP label retention in pulse chase experiments, allowed us to assess each cell’s proliferation status at the experimental end point (CD71 levels), along with its proliferative history (H2BGFP levels). With this approach, we found that most LR RFP+α6hiβhi cells expressed low levels of CD71, while the non-LR RFP+α6hiβ1hi cohort separated into CD71hi and CD71lo populations (Figure 2A). As expected, CD71hi cells incorporated significantly more EdU (Figure 2B) and they resided more frequently in the S-G2/M phase of the cell cycle (Figure 2C) compared to CD71lo cells.
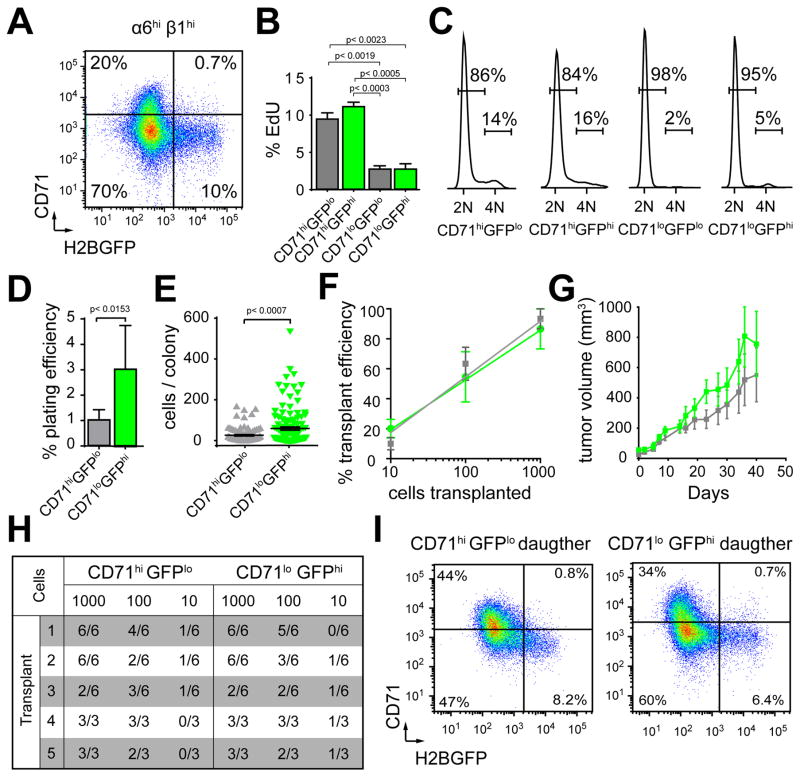
Figure 2. Quiescent and proliferative SCC cells maintain long-term tumor growth.
A, Scatter plot of CD71 and H2BGFP expression. B, 8hr EdU incorporation correlates with CD71. Bar graphs show mean ±s.e.m. (n=3). C, Cell cycle analyses validate proliferation rates of SCC subpopulations. D, Colony formation on 3T3 feeders. Bar graphs show mean ±s.e.m. (n=6, p<0.05, Student’s t-test). E, Scatter plots show colony size distribution. Horizontal lines denote mean ±s.e.m., (p=Mann-Whitney non-parametric t-test). F. Tumor initiating potential in limit dilution transplantation assays. Mean ±s.e.m., (n=5). G, Tumor growth curves starting at the time of tumor detection. Points denote mean ±s.e.m., (n=5). H, Table summarizes limit dilution transplantation assays with 5 serial passages. I, Flow cytometry analyses of live, RFP+, α6hiβ1hi cells following serial transplantation. Scatter plots show similar composition of daughter tumors and their parent (A). See also Figure S2 and Table S1.
To test the proliferative potential of the quiescent (GFPhiCD71loRFP+α6hiβ1hi) and proliferative (GFPloCD71hiRFP+α6hiβ1hi) cohorts we tested their colony forming and tumor initiating potential. Our experiments revealed that both fractions formed colonies on 3T3 feeders (Figure 2D,E) and they were indistinguishable in their long-term tumor initiating potential and growth rates, as measured by serial, limit dilution transplantations (Figure 2F–I). Serial transplants also showed that the composition of daughter tumors was similar to one another (Figure 2I) and their parent (Figure 2A) irrespective of their origin. Furthermore, the abundance of quiescent cells in SCCs does not depend on tumor size (Figure S2G) and quiescent cells can be consistently detected in multiple clones derived from independent SCCs (Figure S2H). Additionally, histological analyses of serial transplants suggests that quiescence has no effect on tumor grade and differentiation (Table S1). Together, these data are consistent with the idea that cellular quiescence is a reversible, dynamic property of TPCs, and it is regulated by phenotypic, rather than genetic mechanisms.
Quiescent TPCs selectively resist chemotherapy
To functionally test whether quiescent TPCs are able to resist chemotherapy in a pre-clinical model, we treated our labeled SCCs with 5-Fluorouracil (5FU). 5FU is a cytotoxic agent, which inhibits thymidylate synthase and incorporates into DNA and RNA. 5FU is widely used for the treatment of actinic keratosis, SCC in situ, head and neck SCCs (HNSCCs), and colorectal cancer (Longley et al., 2003). To visualize physiologically active tumor cells, we introduced a luciferase reporter into our TPCs before engrafting them onto mice (Ray et al., 2007; Schober and Fuchs, 2011). Next, we established SCCs of ~250mm3, which we treated daily with either PBS (Vehicle) or 2% Effudex (5FU) for 14 days. Measurements of tumor volume (Figure 3A) and luciferase activity (Figure 3B) revealed a moderate and slow response of SCCs to 5FU. However, a 10 day chase before the experimental endpoint revealed that therapy profoundly affected the composition of SCCs, resulting in a significant accumulation of quiescent TPCs at the expense of proliferative cells (Figure 3C, D). However, 5FU had no effect on H2BGFP transcription, before or after Dox (Figure S3A) and it had minimal effect on tumor grade and differentiation (Figure S3B).
Figure 3. Quiescent SCC cells resist cytotoxic therapy.
A, Tumor growth curves of vehicle (gray) and 5FU (red) treated SCCs. Points denote mean ±s.e.m., (n=3). B, Luciferase activity measurements indicate physiologically active tumor cells. Scale bar denotes increasing signal intensity from blue to red. C, Scatter plots of vehicle and 5FU treated SCCs. H2BGFP was chased for 10 days. D, Box plots show relative changes in tumor composition after 5FU (red). (n=3). E, Cell cycle profiles of TPC populations after vehicle (gray) or 5FU (red). F, Stacked bar graphs show relative increase of sub-G1 populations in GFPlo cohorts of 5FU treated SCCs. Bar graphs denote mean ±s.e.m. (n=3, p<0.05, Student’s t-test). G, Flow cytometric analyses of Casp3 activation on vehicle (gray) or 5FU (red) treated SCCs. Bar graphs denote mean ±s.e.m. (n=3, p<0.05, Student’s t-test). H, Representative image of γH2AX (red) on vehicle (left) or 5FU (right) treated SCCs. Asterisks denote γH2AX+ apoptotic cells. Arrows denote LR H2BGFPhi (green) cells. β4 (white) outlines the tumor-stroma interface and DAPI (blue) stains nuclei. Scale bar indicates 50μm. I, Dot plots show percentage of γH2AX+ cells in LR and non-LR populations after 5FU (red). J, Kaplan-Meier graphs show tumor initiation potential after transplantation of 100 purified TPCs, isolated from vehicle (left) or 5FU (right) treated SCCs. CD71hiGFPlo cells in gray, CD71hiGFPhi cells in light green, CD71loGFPlo cells in teal and CD71loGFPhi cells in dark green. (n=18, 3 independent experiments with 6 replicates each, p<0.05, Chi square test). See also Figure S3.
The accumulation of quiescent TPCs during treatment could be explained by a therapy induced proliferation arrest, or by the selective death of proliferative but not quiescent TPCs. To discriminate between these mechanisms, we acquired cell cycle profiles of proliferative and quiescent TPCs from 5FU treated and control SCCs (Figure 3E,F). GFPloCD71hiRFP+α6hiβ1hi cells featured a prominent sub-G1 population and a reduced S/G2-M fraction in 5FU treated SCCs. GFPloCD71loRFP+α6hiβ1hi cells showed a prominent sub-G1 population as well, while GFPhiCD71loRFP+α6hiβ1hi cells were more abundant and devoid of sub-G1 cells. Likewise, active Casp3 increased significantly upon 5FU treatment in all but GFPhiCD71loRFP+α6hiβ1hi TPCs (Figure 3G). Short-term 5FU exposure (Figure S3C) also increased the fraction of LR cells over time (Figure S3D), while non-LR cells stained positive for active Casp3 (Figure S3E, F).
The ability of quiescent TPCs to survive 5FU suggested that cells may either evade damage, repair damage more efficiently, or activate anti-apoptotic mechanisms to survive genotoxic stress. To discriminate between these possibilities, we stained tumor sections before and after 5FU with the DNA damage marker γH2AX. Confocal immunofluorescence microscopy detected elevated γH2AX staining primarily in non-LR TPCs and rarely in LR-TPCs (Figure 3H,I). These data suggest that quiescent cells either do not get damaged or are efficiently repaired. Indeed, we found 5FU was unable to compromise the colony forming potential of GFPhiCD71loRFP+α6hiβ1hi cells (Figure S3G) and it significantly enhanced their tumor initiating potential upon transplantation (Figure 3J). Collectively, our data suggest that chemotherapy effectively debilitates proliferative TPCs in tumors, while their quiescent counterparts survive and even increase their tumor propagating potential. A similar ability to survive radiation induced DNA-damage was previously reported for quiescent hair follicle stem cells (HFSCs) (Sotiropoulou et al., 2010). Therefore, quiescent TPCs would need to be sensitized to chemotherapy, or targeted with orthogonal approaches that are specific to the tumor and not employed by normal tissue stem cells in order to achieve successful therapeutic outcomes.
Cellular quiescence is governed by TGFβ dependent signature genes
To rationally design such combinatorial approaches, we began to transcriptionally define TPCs in their quiescent and proliferative states. We isolated GFPhiCD71loRFP+α6hiβ1hi and GFPloCD71hiRFP+α6hiβ1hi cells by flow cytometry, extracted their total RNA and determined transcript levels by next generation sequencing. Differential gene expression analyses revealed a transcriptional signature that distinguished quiescent from proliferative TPCs (Figure 4A, Table S2). Comparisons with slow cycling, LR interfollicular epidermal stem cells (IFSC) (Sada et al., 2016) and HFSCs (Blanpain et al., 2004; Greco et al., 2009; Tumbar et al., 2004) revealed that the behavior of quiescent cells in normal and malignant tissue is regulated by distinct transcriptional programs that are dependent on their respective cellular contexts (Figure 4B).
Figure 4. Differential gene expression identifies regulators of TPC quiescence in SCC.
A, Dendrogram and heat map show differentially expressed transcripts. Blue denotes transcripts upregulated in proliferative (P)-TPCs and red denotes transcripts upregulated in quiescent (Q)-TPCs. B, Venn-Diagram visualizes overlap in transcripts that are upregulated in Q-TPCs, LR-IFSCs, and Q-HFSCs. C, IPA prediction of upstream regulators. z-score denotes pathway activation (positive, red) or inhibition (negative, blue) in Q-TPCs. D–F, qPCR validation of Trp53, Cdkn1a and Tgfbr2 knockdown. Bar graphs show mean fold change. Error bars denote ±s.e.m. (n=3, p<0.05, Student’s t-test). Scatter plots show relative changes in tumor composition. Horizontal lines denote mean ±s.e.m., (p=Mann-Whitney non-parametric t-test). G, Scatter plot analyses of CD71 and H2BGFP in live, RFP+, α6hiβ1hi Tgfbr2wt and Tgfbr2ko SCCs. H, Cell cycle analyses of Tgfbr2wt and Tgfbr2ko SCC cells in vitro before and after 24hr TGFβ1. I, Western blot analyses of SCC cells treated for 0, 1, and 8hr with TGFβ1. See also Figures S4–5 and Tables S2–4.
To better understand how differences between quiescent and proliferative TPCs are governed, we performed upstream regulator predictions with Ingenuity Pathway Analysis (IPA), which suggested Trp53, Tgfβ, and Cdkn1a activities as culprits for quiescence in SCC TPCs (Figure 4C). Furthermore, elevated KDM5B, which linked slow melanoma growth to drug-resistance and oxidative phosphorylation (Roesch et al., 2010; 2013), and differential activation of ERK (z=-1) and p38-MAPK (z=0,7) signaling, which had been linked to dormancy (Sosa et al., 2011), were also found, although with lower statistical significance. In contrast, Nrf2-mediated oxidative stress response genes and Glutathione metabolism regulators, which can protect a cell from cytotoxic stress and chemotherapeutic agents (Ma, 2013; Traverso et al., 2013) were indistinct (z=0) between quiescent and proliferative TPCs. However, Nrf2 and Glutathione pathway components were generally elevated in SCC TPCs, compared to normal skin epithelial stem and progenitor cells (Figure S4A,B; Table S3) (Schober and Fuchs, 2011) suggesting functions that are not specific to quiescent TPCs.
To test whether Trp53, Cdkn1a, and Tgfbr2 govern cellular quiescence in TPCs, we transduced cells with shRNAs directed against Trp53, Cdkn1a and Tgfbr2. shScr transduced cells were used as control. Knockdown efficiencies were tested by qPCR (Figure 4D–F), before the cells were transplanted intra-dermally into Nude recipient mice. Once tumors reached ~250mm3 we chased H2BGFP expression for 12 days to measure changes in the composition of SCCs by flow cytometry (Figure 4D–F). Surprisingly, the relative population sizes were unaffected in Trp53 (Figure 4D) and Cdkn1a (Figure 4E) depleted TPCs, while shTgfbr2 transduced TPCs showed a significant reduction in the quiescent (GFPhiCD71loRFP+α6hiβ1hi), along with an increase in the proliferative (GFPloCD71hiRFP+α6hiβ1hi) TPC cohorts (Figure 4F).
To independently validate these data we generated SCCs in conditional Tgfbr2ko epidermis (Guasch et al., 2007; Schober and Fuchs, 2011), isolated TPCs, transduced the cells with our proliferation reporter, and transplanted single cell clones expressing RFP and H2BGFP (derived from independent primary SCCs) into the dermis of Nude recipient mice. Pulse-chase experiments revealed a quick decline in H2BGFP expression, consistent with the rapid expansion of Tgfbr2ko SCCs (Figure S4C–E). No GFPhiCD71loRFP+α6hiβ1hi cells were detected after a 12 day chase (Figure 4G). Although Tgfβ signaling was critical for TPC quiescence, it was dispensable for their differentiation into SCC cells without proliferative potential (Figure S4F,G). As expected, Tgfbr2ko SCC cultures were unable to respond to active TGFβ1, while Tgfbr2wt SCC cultures reversibly withdrew from the cell cycle to resume proliferation following TGFβ1 inhibition (Figure 4H; Figure S4E,H). Tgfβ signaling is thus required and sufficient for the reversible cell cycle withdrawal of TPCs in tumors and their cultures, while their permanent cell cycle exit during tumor cell differentiation does not depend on Tgfbr2 function.
Mechanistically, TGFβ1 stimulation lead to increased Smad2/3 phosphorylation, p21 and p53 stabilization (Figure 4I), elevated Cdkn1a, Atf3, Fn1, Id1, Id2, Id3, Fos and Mmp11 transcript levels, and declining Cyclin D1, CD71, Cdc6, Cdc25b, and Ccna2 expression in Tgfbr2wt but not in Tgfbr2ko TPCs (Figure S4I). Immunostaining for antigens that are commonly expressed and deregulated in SCCs showed no direct correlation with cellular quiescence (Figure S4J), consistent with our differential gene expression data (Figure 4A, Table S2). Though Tgfβ1 and Tgfβ3 transcript levels are higher in the quiescent population, Tgfβ ligands were diffusely detected throughout our tumors regardless of genotype (Figure S4J).
Surprised that TGFβ1 induced Trp53 and Cdkn1a expression (Figure 4I), but shTrp53 and shCdkn1a had no significant effect on TPC quiescence, we wondered whether these genes were already mutated in our tumors. Therefore, we retrieved all nonsynonymous mutations from our RNA-seq datasets and defined copy number variations that were inherent to our tumors (Table S4). Both analyses uncovered a mutational spectrum similar to the genomic landscape of DMBA induced SCCs (McCreery et al., 2015; Nassar et al., 2015). We identified not only the oncogenic HRasQ61L allele, which is the signature mutation most commonly found in DMBA initiated SCCs, but we also uncovered Trp53N128Y and Trp53R277S alleles (Figure S5A). Still, exposure of these TPC cultures to Nutlin-3a stabilized Trp53 and Cdkn1a (Figure S5B) and caused a G1 arrest (Figure S5C), while apoptosis rates were not increased (Figure S5D). To fully ablate Trp53 function, we used CRISPR-Cas9 to generate Trp53KO clones, where p53 protein was no longer detectable upon Nutlin-3a treatment (Fig S5E). Nevertheless, Trp53KO clones could still cell cycle arrest upon TGFβ1 treatment (Figure S5F) and tumor composition was unaffected (Figure S5G). Similar results were obtained when Cdkn1a was deleted with CRISPR-Cas9 (Figure S5H–J).
Unable to simply explain TGFβ dependent TPC quiescence based on elevated Cdkn1a or Trp53 function, or by mutations in known DNA damage response genes, we thought to globally define the TGFβ controlled transcriptome in quiescent TPCs (Figure 5A). To do this, we performed Smad2/3 chromatin immunoprecipitation followed by Next-generation sequencing (ChIP-seq) on TGFβ1 stimulated TPCs, which identified 7,525 primary Smad3 and/or Smad2:Smad3:Smad4 consensus motif containing peaks, primarily located at distal transcriptional enhancers (Figure 5B). Next, we performed assays for transposase accessible chromatin (ATAC) (Buenrostro et al., 2013) on TPCs that we isolated directly from SCCs. Intersection of our Smad bound elements with ATAC-seq peaks allowed us to refine our list to 3,701 Smad2/3 bound open chromatin segments that could modulate the expression of 4006 candidate genes (Figure 5C). To determine which of these candidate genes are bona fide TGFβ-Smad2/Smad3 targets in TPCs, we intersected them with transcripts that were differentially expressed between proliferative and quiescent TPCs. Together, our analyses uncovered 1,130 TGFβ regulated signature genes that govern cellular quiescence of TPCs in SCC (Figure 5C).
Figure 5. Chromatin accessible regions bound by Smad2/3 are enriched for cell cycle regulators in quiescent TPCs.
A, Schematic representation of integrated ChIP, ATAC, and RNA-seq analyses. B, Global distribution of Smad2/3 bound regulatory elements. C, Venn diagram shows overlap of genes with Smad2/3 bound open chromatin in close proximity and transcripts that are differentially expressed between Q- and P-TPCs. D, Scatter plot visualizes GO data of Smad2/3 regulated genes with transcripts that are differentially expressed between Q- and P-TPCs. Color labels denote statistical significance and circle sizes visualize GO-term frequency (more general terms are larger). E, Bar graphs show differential fold change of Cdc25b expression between Q- and P-TPCs in two independent SCCs. F, Histograms visualizing Smad2/3 binding and chromatin accessibility around the Cdc25b locus in SCC TPCs. G, ChIP-qPCR of Smad2/3 at the Cdc25b enhancer after 1hr TGFβ1 (blue). Bar graphs show mean fold change. Error bars denote ±s.e.m. (n=3, p<0.05, Student’s t-test). H, Histograms show chromatin accessibility at Smad2/3 bound regulatory elements. I, Histograms show genome wide chromatin accessibility at the TSS. J, Enrichment of transcription factor motifs at Smad2/3-bound enhancer sequences. See also Table S5.
Gene ontology (GO) analyses of these Smad2/3 bound signature genes revealed significant enrichments in cell proliferation, tissue morphogenesis, adhesion, growth and cell migration (Figure 5D, Table S5). Intriguingly, >70 of these TGFβ-Smad2/Smad3 dependent target genes were known cell cycle regulators including Cdc25b, Wee1, Cdc6, Cdc7, Cdc42, Cdt, Ccnb2, Ccnd1 and Mki67. Cdc25b expression was elevated in proliferative over quiescent TPCs (Fig 5E) and the Cdc25b genomic locus revealed a Smad2/3 ChIP-seq peak with a centrally distributed Smad motif at its peak summit (Figure 5F). ChIP-qPCR experiments confirmed the significant enrichment of Smad2/3 at this enhancer in TGFβ1 stimulated, but not control or Tgfbr2ko TPCs (Figure 5G). Furthermore, ATAC-seq tracks of TPCs in their distinctive states revealed the highest chromatin accessibility around this Smad2/3 bound motif in the quiescent state (GFPhiCD71loRFP+α6hiβ1hi) (Figure 5F), while chromatin of proliferative (GFPloCD71hiRFP+α6hiβ1hi) and Tgfbr2ko TPCs was less readily accessible. Similarly, global analyses of ATAC-seq signals at all Smad2/3 bound elements suggest a higher accessibility of Smad2/3 bound enhancers in quiescent, compared to proliferative TPCs (Figure 5H). In contrast, transcriptional start sites (TSSs) were equally accessible in quiescent and proliferative TPCs and were more accessible in Tgfbr2ko TPCs (Figure 5I). Although Tgfβ signaling enhances chromatin accessibility at Smad2/3 bound enhancers, these regions remained relatively open, even when Tgfβ signaling was inactive. This finding is consistent with the proposal that Smad proteins bind lineage determining transcription factors, which recruit them onto chromatin to cause context specific transcriptional responses (Mullen et al., 2011). Indeed, de novo discovery of transcription factor motifs at Smad2/3 bound loci show a significant enrichment for Atf3, AP-1/c-Jun and Trp63 (Figure 5J), which have previously been reported to physically interact with Smads (Kang et al., 2003; Yan et al., 2002).
TGFβ inhibition sensitizes SCCs to chemotherapy
To investigate the functional links between TGFβ induced quiescence and drug resistance within tumors, we treated Tgfbr2ko SCCs with either vehicle or 5FU for 14 days. While vehicle-treated tumors expanded quickly, 5FU treated tumors ceased to grow (Figure 6A). A similar decline in tumor growth was seen in Tgfbr2wt SCCs when 5FU was combined with function blocking anti-TGFβ antibodies (1D11), but not with IgG controls (Figure S6A). Bioluminescence imaging showed the accelerated formation of de-cellularized plugs at the center of 5FU treated Tgfbr2ko SCCs (Figure 6B) and quantification of our measurements revealed a significant decline in the density of physiologically active SCC cells in Tgfbr2ko, but not in progressive Tgfbr2wt tumors (Figure S6B). To assess how 5FU affected Tgfbr2ko SCCs, we processed tumor remnants, extracted the few surviving cells, and analyzed their composition by flow cytometry. 5FU was unable to induce a growth arrest in Tgfbr2ko SCCs and their composition remained unchanged (Figure 6C). Instead, it compromised the cells’ integrity resulting in a significantly larger sub-G1 population (Figure 6D,E) along with higher apoptosis rates (Figure S6C,D), when directly compared to Tgfβ responsive SCCs (Figure 3). 5FU treatment had only modest effects on the degree of differentiation in Tgfbr2ko SCCs (Figure S6E). Still, colony formation (Figure 6F), growth potential in culture (Figure 6G), and tumor initiating potential (Figure 6H) had been severely compromised in 5FU treated Tgfbr2ko TPCs compared to vehicle controls. Together, these data suggest that TGFβ dependent quiescence protects cells from chemotherapy and that TGFβ inhibition significantly enhances their responsiveness to treatment in SCCs.
Figure 6. Tgfbr2ko tumors are highly responsive to chemotherapy.
A, Tumor growth curves of Tgfbr2ko SCCs treated daily with vehicle (gray) or 5FU (red). Points denote mean ±s.e.m. (n=4). B, Luciferase activity measurements indicate physiologically active tumor cells. Arrows indicate de-cellularized plug. Scale bar denotes increasing signal intensity from blue to red. C, Scatter plot analyses of CD71 and H2BGFP in Tgfbr2ko SCCs after vehicle or 5FU. D, Cell cycle profiles after vehicle (gray) or 5FU (red). E, Stacked bar graphs show mean percentage of cells in different stages of the cell cycle in vehicle (gray) and 5FU (red) treated Tgfbr2ko SCCs. F, Colony formation after vehicle (gray) or 5FU (red) on 3T3 feeders. Bar graphs show mean ±s.e.m. (n=3, p<0.05, Student’s t-test). G, Scatter plots show colony size distribution. Horizontal lines denote mean ±s.e.m., (p=Mann-Whitney non-parametric t-test). H, Kaplan-Meier curves show tumor initiating potential after transplantation of 100 purified SCC cells, isolated from vehicle (gray) or 5FU (red) treated tumors (n=18, 3 experiments with 6 replicates each, p<0.05, Chi square test). I, Growth analyses of Tgfbr2wt and Tgfbr2ko SCC cells treated with vehicle or 5FU with or w/o TGFβ1. Bar graphs show mean ±s.e.m. (n=3, p<0.05, Student’s t-test). J, Stacked bar graphs of cell cycle phases of Tgfbr2wt and Tgfbr2ko SCC cells cultured with vehicle or 5FU with or w/o TGFβ1 over time. Bars show mean percentage ±s.e.m. (n=3, p<0.05, Student’s t-test). K, Growth analyses of 5FU treated Tgfbr2wt SCC cells with (red) or w/o (light red) TGFβ1 for 5 days. Cells were released and regrown for 5 days. Bar graphs show mean ±s.e.m. (n=3, p<0.05, Student’s t-test). L, Immunofluorescence microscopy images of γH2AX (red) on Tgfbr2wt SCC cells treated with vehicle or 5FU with or w/o TGFβ1. DAPI (blue) stains nuclei. Box plots of quantifications of γH2AX after 5FU with or w/o TGFβ1. (n=25, p<0.05, Student’s t-test). M, Western blot analyses of Tgfbr2wt and Tgfbr2ko SCC cells treated for 0, 24, and 48hr with 5FU with or w/o TGFβ1. See also Figure S6–7.
To model TGFβ mediated regulation of quiescence and chemotherapy resistance, we established cultures of Tgfbr2wt and Tgfbr2ko TPCs (Figure 6I). Both cultures expanded geometrically in the absence of TGFβ ligands, but Tgfbr2wt cells arrested at G1 upon TGFβ1 exposure, while Tgfbr2ko cells did not (Figure 6I,J). 5FU (red) induced a rapid decline of proliferative cultures (Figure 6I) along with an emerging sub-G1 population (Figure 6J) and increased apoptosis rates (Figure S6F). In contrast, growth arrested cells remained inert and able to expand when 5FU and TGFβ1 had been withdrawn (Figure 6K). Similar protective effects by TGFβ1 induced cell cycle withdrawal were also seen with other chemotherapeutic agents, including the DNA cross-linking compound Cisplatin, and the microtubule stabilizing agent Paclitaxel (Figure S6G–J).
To directly address whether TGFβ1 induced growth arrest is sufficient to circumvent DNA damage by 5FU (Figure 3H,I), we measured γH2AX expression in 5FU treated cells that we cultured with and without active TGFβ1 (Figure 6L,M). Time course experiments revealed increased γH2AX along with active Casp3 expression in proliferative (−TGFβ1), but not quiescent (+TGFβ1) TPCs after 5FU (Figure 6L,M, Figure S6K,L). Instead, pro-survival factor Bcl2 increased and pro-apoptotic factors Puma and Noxa declined (Figure S6M), similar to what had been observed in HFSCs (Sotiropoulou et al., 2010). Interestingly, Bcl2 expression in Tgfbr2ko cells was not sufficient to evade apoptosis upon 5FU treatment as Puma and Noxa were augmented (Figure S6N).
Recent data linked canonical TGFβ to Nrf2 mediated stress response signaling and drug resistance in a HRasG12V induced squamous papilloma model (Oshimori et al., 2015), so we tested whether TGFβ1 stimulation further enhanced this mechanism in TPCs of moderately to poorly differentiated SCCs, where it was already active (Figure S4A,B). Western blot and qPCR analyses on extracts from our TPCs revealed that TGFβ activation was unable to further stabilize Nrf2 protein and activate its target genes (Figure S7A, B). In order to uncouple TGFβ signaling from cellular quiescence, we lowered serum levels in culture, which also caused a protective, reversible cell cycle arrest without Smad2/3 phosphorylation (Figure S7C–H). p21 expression was slightly elevated along with a significant reduction in Mki67 expression (Figure S7H, I), and Nrf2 target genes did not get activated (Figure S7J). We conclude, in contrast to previous proposals, that cell cycle withdrawal is critical to chemotherapy resistance, but it may synergize with metabolic mechanisms to achieve enhanced chemotherapy resistance.
Progressive HNSCC patients display active TGFβ signaling
Given the histological and mutational similarities including deregulated TGFβ signaling in progressed mouse and human SCCs (Cammareri et al., 2016; Cancer Genome Atlas Network, 2015; Lu et al., 2006; McCreery et al., 2015; Nassar et al., 2015), we compared transcriptomes of HPV-negative HNSCCs that showed either a complete response or resistance to carboplatin or cisplatin (P) followed by paclitaxel (T) (Cancer Genome Atlas Network, 2015). Unsupervised cluster and principle component analyses revealed clear differences between patients presenting with either progressive disease or a complete response (Figure 7A). We uncovered 3316 genes (p<0.05), which are differentially expressed between progressive and responsive disease specimens (Figure 7B). Interestingly, genes that are upregulated in progressive HNSCCs overlap significantly with our quiescent TPC signature genes (Figure 7C, p<0.0001), while genes enriched in responsive HNSCCs do not (Figure 7C, p=1.0). In addition, upstream regulator analyses identified TGFβ1 signaling as one of the most significantly activated in progressive HNSCCs (Figure 7D, Table S6). Together, these analyses suggest close ties between chemotherapy resistance and TGFβ mediated cellular quiescence in mouse and human SCCs.
Figure 7. TGFβ signaling is activated in HNSCC patients with progressive disease.
A, Multi-Dimensional Scaling plot of HPV- HNSCC patients with complete response (blue) or progressive disease (red) after Cisplatin (P), Carboplatin (P) or Paclitaxel (T). B, Volcano plot of differentially expressed genes in responsive and progressive HNSCCs. Red denotes statistically significant transcript changes. C, Venn-Diagram visualizes overlap in transcripts upregulated in Q-TPCs (green), P-TPCs (gray) and patients with progressive HNSCCs (red) or responsive HNSCCs (blue). D, IPA prediction of upstream regulators. z-score indicates pathway activation (positive, red) or inhibition (negative, blue) in patients with progressive disease. E, Stacked bar graphs of cell cycle phases of HNSCC25 cells cultured with vehicle or 5FU with or w/o TGFβ1 in low serum conditions. Bars show mean percentage ±s.e.m. (n=3, p<0.05, Student’s t-test). F, Dose response curves of HNSCC25 cells pretreated for 24hr with DMSO or TGFβ1 before addition of 5FU for 72hr. Fold change was measured by relative luminance units (RLUs). Points denote mean ±s.e.m. (n=3, nonlinear regression). G, Quantification of activity area fold change from dose response assays of HNSCC25 cells in 10, 5, 0.5, and 0.1% serum conditions with or w/o TGFβ1. Bar graphs show mean ±s.e.m. (n=3, p<0.05, Student’s t-test). See also Figure S7 and Table S6.
To test this hypothesis, we measured cell proliferation rates and chemotherapeutic drug activity in human HNSCC cells in the absence or presence of active TGFβ. As seen in mouse SCCs, exposure of HNSCC25 cells to active TGFβ1 resulted in a significant increase in G1 arrested cells (Figure 7E, Figure S7K), which were effectively protected from 5FU as indicated by a significantly smaller sub-G1 population and a shift towards higher drug concentrations in dose-response assays (Figure 7E,F). Interestingly, TGFβ mediated cell cycle withdrawal and drug resistance improved significantly with decreasing serum levels, while Smad2/3 phosphorylation was serum independent (Figure 7G, Fig S7K,L). These data suggest, consistent with our pathway predictions (Figure 4C) and published data from prostate stem cells (Salm, 2005), that quiescence is defined by low receptor tyrosine kinase and high TGFβ signaling. Taken together, our experiments uncover TGFβ mediated quiescence as an innate drug resistance mechanism that protects a subset of TPCs from chemotherapeutic agents in progressive mouse and human SCCs, where their effective targeting improves significantly when chemotherapy is paired with TGFβ inhibition.
Discussion
Differences in cell proliferation rates between tumor cells led to speculations that quiescent TPCs resist chemotherapy and initiate recurrent tumors after remission (Meacham and Morrison, 2013). Although this hypothesis is intriguing, it has been technically challenging to directly identify quiescent TPCs based on their slow proliferation rate within intact tumors, test their ability to initiate new tumor growths after treatment, and determine the molecular mechanisms that govern their behavior. Classic nucleotide incorporation experiments can measure differences in proliferation rates between distinctive cell populations, but cell surface markers used to distinguish these populations can vary greatly within tumors (Lapouge et al., 2012; Quintana et al., 2010; Schober and Fuchs, 2011). Additionally, detection of incorporated nucleotides requires cell fixation and permeabilization, which are incompatible with functional and transcriptomic studies. Lipid membrane dyes are an alternative approach that is amendable to live cell tracing, but their use in tumor initiation studies requires ex vivo labeling of large cell numbers prior to their transplantation. Moreover, as secondary tumor growth inevitably requires proliferation of tumor initiating cells, dye retention after engraftment can be interpreted as either continued dormancy or as a loss of tumor propagating potential.
In an attempt to circumvent these technical limitations, which have so far hampered the unbiased identification of slow cycling cells, we employed the Dox repressible H2BGFP expression system (Tumbar et al., 2004). This technique presents a powerful alternative approach to discriminate between proliferative and quiescent cells in pulse-chase experiments as the marker is genetically encoded, allowing for robust expression, and the separation of rapidly proliferating from slow cycling cells in established tumors. These features allow for highly reproducible, functional assays and serial transplantation experiments where cells are not required to retain their label as they initiate new tumor growths, making this a fundamentally different approach from membrane dye retention studies. To apply this system to a broad spectrum of existing and newly emerging tumor models, we encoded it on a lentiviral backbone, which allows for the rapid transduction of any tumor cell, along with a constitutively expressed fluorescent marker to simultaneously trace the fates of both proliferative and quiescent progeny in tumor propagation studies.
Focusing on SCC as a paradigm, we identified LR cells that did not proliferate in weeks, yet were highly capable of establishing secondary tumors in serial transplantation studies. By employing an additional marker to separate actively cycling (CD71hi) from cell cycle arrested (CD71lo) cells, we were able to gain a clearer view of the dynamic nature of fate decisions within the SCC hierarchy. We found that TPCs can periodically transition between proliferative and quiescent states in SCCs. This ability and the lack of exhaustion between cell populations in serial transplants argues against a stable hierarchical model, in which label retention is due to a subset of continuous, but slowly cycling tumor cells. Although it is formally possible that these state transitions are of a stochastic nature, their defined transcriptional signature and their dependence on TGFβ/Tgfbr2 function in tumors supports a governance by cell extrinsic cues. This contrasts with previously described stochastic state transition models, where marker gene expression and proliferation differences are thought to be stochastically defined (Gupta et al., 2011; Quintana et al., 2010; Roesch et al., 2010). Instead, our data on quiescent and proliferative TPCs in SCCs are more in line with a “zoned stem cell model” (Li and Clevers, 2010), in which well-defined regulatory circuits sustain co-existing proliferative and quiescent stem cell pools, which are responsible for tissue growth and injury repair, respectively. Consistent with this model, we find that quiescent cells are relatively rare in primary SCC transplants, but they become enriched and very potent in their tumor initiating potential in response to chemotherapy, while still sustaining proliferative heterogeneity in their serial transplants. Additionally, our results are compatible with clonal repopulation dynamics observed in response to chemotherapy in colorectal cancer (Kreso et al., 2013). Therefore, it will be important to test if molecular mechanisms that define quiescence and therapy resistance in our SCC models apply to other cancer types.
Our transcriptomic studies revealed that the dynamic interconversion between quiescent and proliferative TPC states in SCC is likely governed by a complex network of pro- and anti-proliferative cancer signaling modules. Amongst these, TGFβ signaling has surfaced as a mechanism that is required and sufficient for the reversible cell cycle arrest of TPCs within the basal layer, while it is dispensable for the irreversible cell cycle withdrawal that accompanies their differentiation into post-mitotic cells without proliferative potential. The broad expression of TGFβ in SCCs suggests that paracrine TGFβ sources might govern cellular quiescence (Guasch et al., 2007; Oshimori et al., 2015). However, Tgfβ1 and Tgfβ3 are upregulated along with their potential activators Ltbp3, Ltbp4 and Thbs1 in quiescent TPCs, which could also sustain this pathway in an autocrine manner. Finally, α6β4 integrin can prevent Smad2 entry into the nucleus (Owens et al., 2003) and local fluctuations in α6β4 expression could modulate Tgfβ-Smad2/3 signaling to stimulate the transition of TPCs between proliferative and quiescent states.
Regardless of the stimulus, Tgfβ mediated quiescence in SCCs is to our surprise independent of its transcriptional target p21CIP. p21CIP is a well-known cell cycle regulator that permits epidermal cells to stratify and differentiate into post-mitotic supra-basal cells when stimulated by Notch activity (Di Cunto et al., 1998; Rangarajan et al., 2001). Although redundancies with other CDK inhibitors could be at play, our transcriptomic analyses suggest a model where Smad2/3 can directly repress cell cycle gene transcription. These transcriptional responses are likely guided by lineage specific transcription factor interactions (Mullen et al., 2011; Xi et al., 2011), which might explain the context dependency of TGFβ signaling responses, as exemplified by reduced, but not arrested proliferation rates of IFSCs, and the accelerated proliferation of HFSCs at regeneration (Derynck et al., 2001; Guasch et al., 2007; Oshimori and Fuchs, 2012). Consistent with this notion, TGFβ signaling provokes a prolonged, but reversible cell cycle arrest in quiescent TPCs, while transcripts associated with epithelial to mesenchymal transition (Zeb2, Vimentin), or metabolism (Nrf2/Glutathione) are not enriched within those cells. Taken together, these data underscore that TGFβ signaling might elicit spatially and temporally distinct effects on different cells during skin carcinogenesis amounting to a high degree of phenotypic heterogeneity within these tumors (Oshimori et al., 2015). Our research identified yet another role for TGFβ signaling in progressed SCCs, where it controls quiescence mediated drug resistance.
Although distinct from previously described skin epithelial and SCC signatures, the transcripts that define quiescent TPCs overlap significantly with transcripts that are over-represented in chemotherapy resistant human HNSCC patient specimens. Amongst these overlapping transcripts, we identified the TGFβ target genes ID1 and FN1, along with the G1 cell cycle regulators DOC1R, RACK1 and CDK5. This signature may thus provide prognostic markers to prospectively distinguish responsive from progressive disease in order to personalize treatment options. Furthermore, because quiescent TPCs increase their tumor initiating potential following chemotherapy by still unknown mechanisms, it is imperative to either target these cells with combinatorial therapies that mobilize them to increase their susceptibility to chemotherapy, or to employ orthogonal approaches to cure patients with progressive cancers (Luo et al., 2009). While it seems counterintuitive to accelerate the proliferation rate of cancer cells, it may be more deleterious to allow quiescent cells to remain arrested in the G1 phase of the cell cycle where they have only error prone repair mechanisms at their disposal, which could initiate new mutations in response to treatment (Blanpain et al., 2011; Mohrin et al., 2010). In conclusion, the general applicability of our approach provides an opportunity to better define quiescence as an innate drug resistance mechanism in solid cancers and to uncover prognostic markers that can stratify patients based on quiescent cells within their tumors to better personalize their treatment programs.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Markus Schober (markus.schober@nyumc.org).
Experimental Model and Subject Details
Mice
Female 6-week-old Nude (NU/NU [088] Charles River) mice were used for orthotypic transplantations and xenograft studies and were not used in previous procedures. Mice were randomly assigned to experimental groups. Tumors were detected by palpation, measured using a digital caliper and tumor volume was calculated (VTumor. π/6xlxw2, where l=length in mm and w=width in mm). Mice were injected with 50μg/g EdU (Invitrogen, A10044) (or BrdU [Invitrogen, B23151] 10μg/g) intraperitoneally 8hrs before analysis. EdU incorporation was determined by flow cytometry using the Click-iT EdU PacBlue Cell Proliferation Assay Kit for flow cytometry (Invitrogen, C35002) following the manufacturer’s instructions. For the tet-off-H2BGFP system mice were placed on doxycycline-containing (200mg) diet after tumors were established, while control mice remained on a normal diet. Mice were treated with 5mg/kg TGFβ neutralizing antibody, 1D11 (R&D, MAB1835) or IgG1 control antibody 11711 (R&D, MAB002) by i.p. injection two days before the start of topical 2% 5FU solution (Taro Pharmaceuticals, NDC 51672-4062-1). Mice were then treated every two days during the 14 day 5FU treatment. Mice received topical 2% 5FU solution each day during the 14 day treatment. Animals were imaged using the IVIS Lumina XR In Vivo Imaging System Series III. Images were then analyzed by Living Image Software and plotted as average radiance (p/s/cm2/sr). Mice were housed and cared for under standard conditions by NYU’s Division of Comparative Medicine, an ALAAC-accredited mouse facility. All animal experiments were performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee at New York University Langone Medical Center.
Cell lines
Primary murine squamous cell carcinoma cell lines (SCC1-5) were established from tumors that initiated after DMBA treatment (Schober and Fuchs, 2011; Siegle et al., 2014). SCC1 and SCC2 are Tgfbr2wt and SCC3-5 are Tgfbr2ko. SCC1-5 were cultured in E media (Nowak and Fuchs, 2009) supplemented with 15% chelexed FBS (Corning, 35-010-CV). Human 293FT (Invitrogen, R70007) was maintained in DMEM (Gibco, 11995-065) supplemented with 10% FBS (Corning, 35-010-CV) and penicillin-streptomycin (Gibco, 15140-122). Human SCC-25 (ATCC, CRL-1628) was maintained in P media (DMEM:F12 [3:1] [Gibco, 90-5010EA], sodium bicarbonate [Gibco, 11810-033], L-glutamine [Gibco, 21051-032] and penicillin-streptomycin [Gibco, 15140-122]) with 10% FBS (Corning, 35-010-CV). Mouse 3T3 (ATCC, CRL-1658) was maintained in P media (Nowak and Fuchs, 2009) supplemented with bovine calf serum (F media; Hyclone, SH30072.03). All cell lines were kept at 37°C in 7% CO2. Cell lines were tested for mycoplasma contamination using the Mouse Essential Virus Panel, (Charles River Research Animal Diagnostic Services) and authenticated by STR analysis by manufacturers.
Method Details
Cell culture
For stable cell line generation, lentivirus was produced by Lipofectamine 2000 (Invitrogen, 11668019) transfection of 293FT cells (Invitrogen, R70007) with pLKO-PGK-tTA-P2A-RFP and pLKO-TRE-H2BGFP plasmids. For short hairpin RNAs, lentivirus was produced by Lipofectamine 2000 transfection of 293FT cells with pLKO shRNA-carrying plasmid (pLKO.1, Addgene 10879) and helper plasmids pMD2.G and psPAX2 (Addgene 12259 and 12260, respectively). For list of shRNAs used in lentiviral infections see Table S7. Transfected 293FT cells were maintained in media consisting of 75% DMEM and 25% Opti-MEM (Gibco, 11058-021). Viral supernatant was collected 36h and 60h after transfection, and filtered through a 0.22-μm filter. For viral infections, 100,000 cells were plated per well in a 6-well plate and incubated with lentiviral supernatant in the presence of 31μg/ml of polybrene (Sigma, 107689-10G) and spun at 1,100g for 30min at 37°C. Lentiviral infect ed SCC cells were expanded and then FACS-sorted in order to then generate single cell clones. Cloned out cells were then used for functional assays. SCC1 and SCC2 were each cloned out following infection and cell sorting into Clone 1 (c.1) and Clone 2 (c.2) and are Tgfbr2wt. SCC3-5 are each single Tgfbr2ko clones selected after infection and cell sorting. For CRISPR-Cas9 knockouts, Trp53, Cdkn1a and dTomato sgRNAs were cloned into the pLKO U6-puro vector. Trp53 and dTomato sgRNAs were a gift from the Papagiannakopoulos lab at NYULMC. After sgRNA transduction and puromycin (2μg/mL; Sigma, P8833-25MG) selection for 2 days, cells were subsequently infected with lentiCas9-Blast (Addgene 52962), and selected with Blasticidin (5μg/mL; Gibco, R210-01) for 3 days. Single cell clones were then selected and validated by Western blot analysis. For list of sgRNA sequences, see Table S7. For tumor growth assays, FACS isolations were performed on a BD FACSAria II equipped with 488, 633, 405, 562 and 355 nm lasers. Cells were gated on live, single cells, which were RFP+ and α6hiβ1hi and then sorted based on CD71 and H2BGFP expression. Sorted cells were suspended in 50% Matrigel (BD, 356237) diluted with F media (Nowak and Fuchs, 2009) at a concentration of 10,000 cells per injection and injected intradermally into Nude (Charles River, NU/NU [088]) recipient mice. Growth curves of cultured cells were measured by crystal violet staining and reported as p<0.05 calculated by Student’s t-test. Dose response curves were measured by Promega CellTiter-Glo 2.0 Reagent after 72hrs of treatment according to manufacturer instructions (G9242.) Luminance was measured for 1000ms and reported as relative luminance units (RLUs). Cultured cells were treated with 50ng/mL Doxycycline from Sigma (D9891-5G), 5ng/mL Human Transforming Growth Factor- Beta 1 from Corning (354039), 100μM 5-fluorouracil from Sigma (F6627), 10μM Cisplatin from Tocris (2251), 5μM Paclitaxel from Selleckchem (S1150) and 10μM Nutlin-3a from Enzo (ALX-430-128-M001).
Colony Formation Assays
For colony formation assays, 1,000 sorted tumor cells were plated per well on top of 3T3 fibroblast feeder layers (ATCC, CRL-1628) in a 6-well plate. At 6 days, colonies were imaged and numbers of cells per colony were counted. Colonies were then grown for an additional 6 days (12 days total) and fixed for 10min with 4% formaldehyde (Thermo Scientific, 28908). Colonies were then rinsed with PBS, stained with Rhodamine B (Sigma, R6626) for visualization and the total number of colonies were counted (single blinded) to calculate plating efficiency. Outliers were excluded using the Grubbs’ test in Prism 7 for colony formation experiments. No other data was excluded. Statistical significance was calculated by either the Student’s t-test for plating efficiency measurements, or the Mann–Whitney test for colony size distributions (cells/colony).
RNA-sequencing analysis and data sources
Total RNA was extracted from 1x105 FACS sorted SCC cells from two biological replicates (SCC1 and SCC2) using the Absolutely RNA Nanoprep Kit (Agilent Technologies, 400753) according to manufacturer’s instructions. RNA quality was defined on an Agilent 2100 Bioanalyzer before we prepared Poly(A)+ selected, multiplexed, paired end libraries with the Illumina TruSeq RNA preparation kit. Multiplexed libraries have been sequenced on an Illumina HiSeq 2100 Genome Analyzer using the 50-base pair paired end read method. Mouse assembly version mm10/NCBIm38 build and ENSEMBL annotations release 69 were used for the RNA-sequencing alignment, transcriptome quantification and differential expression analysis. Human reference genome GRCh37 from the Ensembl database was used to align raw HNSCC patient datasets from TCGA (Cancer Genome Atlas Network, 2015). More specifically, bowtie version 2.1.0 (https://www.ncbi.nlm.nih.gov/pubmed/19261174) was used for alignment of sequenced reads. Transcriptome quantification and differential expression analysis was performed using the Cufflinks protocol (https://www.ncbi.nlm.nih.gov/pubmed/22383036) tophat version 2.0.9 (with parameters --no-coverage-search and --no-novel-juncs) and cufflinks version 2.2.0 (default parameters). Differential gene expression analyses were performed between quiescent (CD71lo/GFPhi) and proliferative (CD71hi/GFPlo) populations isolated from two independent tumors. Differential gene expression data have been visualized in volcano plots. Fold change and P-value of differentially expressed gene sets have been imported into QIAGEN’s Ingenuity Pathway Analysis (IPA® QIAGEN Redwood City, www.qiagen.com/ingenuity) for pathway analyses and upstream regulator predictions. Area proportional Venn diagrams were generated with BioVenn (https://www.ncbi.nlm.nih.gov/pubmed/18925949). Gene ontology analyses were performed with the Database for Annotation, Visualization and Integrated Discovery (DAVID), version 6.7 (https://www.ncbi.nlm.nih.gov/pubmed/17576678) and visualized with REVIGO (https://www.ncbi.nlm.nih.gov/pubmed/21789182).
RNA-seq variant detection
Sequencing results from two biological replicate SCCs (SCC1 and SCC2) were demultiplexed and converted to FASTQ format using Illumina Bcl2FastQ software. The reads were aligned to the mouse reference genome (build mm10/GRCm38) using the splice-aware STAR aligner (https://www.ncbi.nlm.nih.gov/pubmed/23104886). PCR duplicates were removed using Sambamba (https://www.ncbi.nlm.nih.gov/pubmed/25697820). The Genome Analysis Toolkit (GATK) (http://www.ncbi.nlm.nih.gov/pubmed/21478889) was used to split reads into exon segments and hard-clip any sequences overhanging into the intronic regions. GATK was then used for further local indel realignment and base-quality score recalibration. Single-nucleotide variants and small indels were detected with LoFreq (https://www.ncbi.nlm.nih.gov/pubmed/23066108). ANNOVAR was used to annotate variants with functional consequence on genes and presence in dbSNP (http://www.ncbi.nlm.nih.gov/pubmed/20601685).
Whole genome copy number analysis
Sequencing results from two biological replicates (SCC1 and SCC2) were demultiplexed and converted to FASTQ format using Illumina Bcl2FastQ software. Reads were adapter and quality trimmed with Trimmomatic (https://www.ncbi.nlm.nih.gov/pubmed/24695404) and then aligned to the mouse reference genome (build mm10/GRCm38) using the Burrows-Wheeler Aligner with the BWA-MEM algorithm (https://www.ncbi.nlm.nih.gov/pubmed/19451168). Low confidence mappings (mapping quality <10) and duplicate reads were removed using Sambamba (https://www.ncbi.nlm.nih.gov/pubmed/25697820). Further, local indel realignment and base-quality score recalibration were performed using the Genome Analysis Toolkit (GATK) (http://www.ncbi.nlm.nih.gov/pubmed/21478889). Copy number profiles were calculated using Control-FREEC (https://www.ncbi.nlm.nih.gov/pubmed/22155870). ANNOVAR was used to annotate variants with genomic context (http://www.ncbi.nlm.nih.gov/pubmed/20601685).
ChIP-seq, ATAC-seq and data analyses
For ChIP-seq analyses 12x106 SCC cells were harvested, fixed for 10min at RT in suspension with 1% formaldehyde, quenched with 2.5M glycine, pelleted at 1,250g at 4C and washed twice with ice cold 1x PBS. Chromatin was prepared according to the ChIP-IT High Sensitivity protocol (Active Motif, 53040). Cell pellets were re-suspended in 10ml of chromatin prep buffer supplemented with 10μl PIC and 10μl PMSF and lysed in a chilled dounce homogenizer. Nuclei were sonicated at 25% amplitude and pulsed for 30 seconds on and 30 seconds off for 30 cycles. Immunoprecipitation was performed overnight at 4°C on 30μg of sheared chromatin using 4μg Smad2/3 (D7G7) XP Rabbit mAB (#8685, Cell Signaling). Chromatin samples were washed five times before reversing crosslinks. DNA was then concentrated in a DNA purification column (Active Motif, 103928) and eluted in DNA Purification Elution Buffer (Active Motif, 103498) for ChIP-seq analysis. Sample quality and DNA concentration was assessed by Qubit. Libraries were generated using the Rubicon ThruPLEX DNA-seq kit. Multiplexed libraries were sequenced on an Illumina HiSeq 2500 Genome Analyzer using the 50-base pair single read method. ChIP peak distribution was determined by ChIPseeker (https://www.ncbi.nlm.nih.gov/pubmed/25765347). For ChIP-qPCR analysis, immunoprecipitated chromatin was eluted in 200μl of DNA purification and elution buffer. Input, IgG and Smad2/3 immunoprecipitated samples were analyzed by qPCRs using specific primers for the analyzed regions. For list of primers see Table S7. qRT–PCR was performed with FastStart Universal SYBR Green Master (Roche, 10802200). As a negative control, primers designed to non-specific (NS) regions nearby target genes that were devoid of Smad2/3 peaks. Standard curves were done to validate the efficiency of the primers and calculate the ng of DNA in each ChIP-qPCR reaction. The enrichment of target sequences in ChIP material was calculated relative to the negative control, and normalized to their relative amplification in the IgG sample. ATAC-seq samples were prepped as described in Buenrostro JD, et al., Nature Methods, 2013. 5x104 TPCs were isolated by FACS and washed once with cold 1x PBS. Cells were pelleted lysed with 50μl of cold lysis buffer (10mM Tris-HCl, pH 7.4, 10 mM NaCl, 3mM MgCl2, 0.1% IGEPAL CA-630) and spun at 500g for 6min at 4C to collect their nuclei. Pelleted nuclei were incubated for 30min at 37°C with Tn5 transposase (Illumina Cat #FC-121-1030) before transposed DNA fragments were purified with a Qiagen MinElute Kit for ATAC-seq library generation. To reduce GC and size bias during the PCR amplification step, the number of PCR cycles were optimized by qPCR and amplification was stop before saturation, at a cycle number corresponding to a quarter of the maximum fluorescent intensity. Multiplexed libraries were sequenced on an Illumina HiSeq 2500 Genome Analyzer using the 50-base pair paired end read method. Mouse assembly version mm10/NCBI m38 build were used for sequence alignments with bowtie (https://www.ncbi.nlm.nih.gov/pubmed/19261174) version 2.1.0. Sequences with MAPQ scores <30 were removed with samtools (https://www.ncbi.nlm.nih.gov/pubmed/19505943) and duplicates were removed with picard-tools version 1.88 (http://broadinstitute.github.io/picard). IGV viewer files were generated with igvtools count -z 5 -w 25 -e 250 (https://www.ncbi.nlm.nih.gov/pubmed/21221095). ChIP-seq peaks were called with MACS version 2.1.0 (https://www.ncbi.nlm.nih.gov/pubmed/18798982) in comparison to input controls with the parameters -q 0.001 --nomodel --extsize 400. Motif discovery was performed with MEME-ChIP (https://www.ncbi.nlm.nih.gov/pubmed/21486936) using 200bp sequence surrounding peak summits. Motif containing ChIP-seq peaks were analyzed with the GREAT: Genomic Regions Enrichment of Annotations Tool (https://www.ncbi.nlm.nih.gov/pubmed/20436461), using default parameters. ATAC-seq peaks were called with PeaKDEck (https://www.ncbi.nlm.nih.gov/pubmed/24407222) and the parameter (-sig 0.0001). DeepTools was used to normalize the data and generate histograms to visualize the average enrichment of ATAC signal over Smad2 bound regions and annotated TSSs (transcriptional start sites) on mm10 were used to validate normalization of the datasets (https://www.ncbi.nlm.nih.gov/pubmed/24799436). Area proportional Venn diagrams were generated with BioVenn (https://www.ncbi.nlm.nih.gov/pubmed/18925949).
Immunofluorescence and imaging
Unfixed tumors were embedded in OCT (Tissue Tek, 4583). Frozen sections were cut to a thickness of 10μm on a Leica cryostat and mounted on SuperFrost Plus slides (VWR, 48311-703). Slides were air-dried for 10min, then fixed for 10min with 4% formaldehyde (Thermo Scientific, 28908), rinsed with PBS, permeabilized with 0.1% Triton X-100 in PBS for 15min, then blocked for 1hr in gelatin block (5% normal donkey serum, 1% BSA, 2% gelatin, 0.2% Triton X-100 in PBS) and incubated in primary antibody diluted in blocking buffer at 4°C overnight. After washing wit h PBS, secondary antibodies and Hoechst 33342 (1:2000; Anaspec Inc, AS-83218) were diluted in blocking buffer and incubated with the slides for 1hr at room temperature (RT). After washing, slides were mounted in ProLong Diamond (Invitrogen, P36965) or Vectashield (Vector Laboratories, H-1400). For BrdU (Invitrogen, B23151) detection on frozen sections the same procedure was followed as outlined above, except after primary and secondary antibody detection, sections were re-fixed in 4% formaldehyde for 10min. After washing, antigen retrieval was performed using 4N HCl/1% triton X-100 in PBS for 10min at RT. Cells were then re-blocked for 1hr at RT with gelatin block, washed and then incubated with anti-BrdU (1:200; Abcam, Ab6326) at 4°C overnight. After washing with PBS, secondary antibody, conjugated to Alexa 568 (1:1000, Invitrogen, A10037) and Hoechst 33342 (Anaspec Inc, AS-83218) were diluted in blocking buffer and incubated on sections for 1hr at RT. After washing, slides were mounted in ProLong Diamond (P36965, Invitrogen). For primary mouse antibodies the Mouse on Mouse (M.O.M.) Detection kit from Vector (BMK-2202) was used. Following fixation and staining with non-mouse antibodies as outlined above, slides were incubated for 1hr in M.O.M. Blocking Reagent and washed twice for 2min with PBS. Slides were then incubated for 5min in M.O.M. Dilutent, excess Dilutent was removed and primary antibody diluted in M.O.M. Dilutent was incubated for 30min at RT. Slides were then washed twice for 2min in PBS and secondary antibody was incubated for 10min at RT with Hoescht 33342. Slides were then washed twice in PBS and mounted in Prolong Diamond (Invitrogen, P36965). H&E stains were performed by the Histopathology core at NYULMC. Blinded slides were scored by a pathologist for differentiation status before and after 5FU treatment. Imaging was performed on a Nikon Eclipse TiE Microscope or a Zeiss LSM780 Confocal Microscope. Images have been analyzed in NIS-Elements or Zen software, respectively. For proliferation, apoptosis and DNA damage quantifications, pictures were acquired at 20x magnification and deconvolved, before Ki67, active Casp3 or γH2AX expression was detected in cells using the NIS element software. Quantifications were performed single-blinded. Statistical significance was calculated using the Student’s t test. Antibodies used for immunofluorescence were CD104 (1:1000; BD Biosciences, 555721), CD49f (1:200; Biolegend, 313618), γH2AX (1:1000; Abcam, Ab11174), Sox2 (1:1000; Abcam, Ab92494), pKi67 (1:1000; Novocastra, NCL-Ki67p), BrdU (1:200; Abcam, Ab6326), Caspase-3 (1:1000; R&D, MAB835), Epcam (1:200; Biolegend, 118203), E-cadherin (1:250; BD Biosciences, 610182), TGFβ1,2,3 (25μg/mL; R&D, MAB1835), Keratin 14 (1:1000; Biolegend, 905301), Keratin 17 (1:1000; Abcam, 53707), Vimentin (1:100; Cell Signaling, D21H3), Alexa 568 donkey anti-rabbit IgG (H+L) (1:1000; Invitrogen, A10042), Alexa 568 donkey anti-mouse IgG (H+L) (1:1000; Invitrogen, A10037), Dylight 649 goat anti-rat IgG (1:1000; Biolegend, 405411) and Streptavidin Alexa 594 (1:1000; Invitrogen, S11227).
Quantitative reverse-transcription PCR
mRNA was isolated using Qiazol (Qiagen, 79306) and Direct-zol RNA Mini Prep (Zymo Research, R2052). Samples were quantified using a Nanodrop spectrophotometer (Thermo Scientific). Complementary DNA was synthesized from 1.5μg of total RNA using SuperScript VILO with random primers (Invitrogen). qRT–PCR was performed with FastStart Universal SYBR Green Master (Roche, 10802200) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems). Measurements were recorded in triplicate on biological replicates. Statistical significance was calculated using the Student’s t-test and reported as p<0.05, mean±s.e.m. Differences between samples and controls were calculated based on the 2−ΔΔCT method and normalized to Rplp0.
Western blotting
Cell lysates were prepared using RIPA buffer (150mM sodium chloride, 0.1% Triton-X 100, 0.5% SDS and 50mM Tris pH 8 in ddH2O) with complete Mini EDTA-free protease inhibitor tablets (Roche, 04693159001). Protein concentrations were determined following the instructions of Pierce BCA Protein Assay Kit (Pierce, 23225). Lysates were boiled with 5x Laemmli buffer (6% SDS,15% β-mercaptoethanol, 30% glycerol, 0.006% bromophenol blue, 0.188M Tris–HCl) for 10 min at 95°C. Protein ladder used was Full-Range Rainbow Molecular Weight Markers (GE Healthcare, RPN800). 30μg of protein was loaded per lane. Gel electrophoresis was performed using a 10 or 12% Bis-Tris gel run for 75–150min at 120V, gel was transferred for 1h at 4°C at 100V to a 0.45 μm nitrocellulose membrane (Whatmann) and transfer was assessed by Ponceau S staining (0.1% (w/v) Ponceau S in 5% (v/v) acetic acid). Membranes were blocked with 5% non-fat dry milk in TBST, then incubated with primary antibodies diluted in blocking buffer or antibody dilutent (5% (w/v) BSA in TBST (TBS with 0.1% Tween-20)) overnight at 4°C with gentle agitation. Membranes were rinsed with TBST before incubating with horseradish peroxidase-conjugated secondary antibodies diluted in blocking buffer for 1h at RT. Membranes were washed with TBST, then with TBS before incubating with Supersignal West Pico Chemiluminscent substrate (Life Technologies, 34080) and exposed to X-ray film (GeneMate, F-9024-8_10) using a Kodak X-Omat 2000A Processor. Antibodies used for western blotting were pSmad2/3 (1:1000; Cell Signaling, 8828S), Smad2/3 (1:1000; BD Biosciences, 610842), p53 (1:500; Cell Signaling, 2524S), phospho-p53 (Ser15) (1:1000; Cell Signaling, 1608), p21 (1:500; Santa Cruz, Sc-6246), Cyclin D1 (1:1000; Cell Signaling, 2978S), Vinculin (1:10000; Sigma, V9131), γH2AX (1:2000; Abcam, Ab11174), Caspase-3 (1:1000; Cell Signaling, 9664S), Bcl2 (1:1000; Cell Signaling, 50E3), Nrf2 (1:1000; Cell Signaling, 12721), GAPDH (1:10000; Santa Cruz, SC-32233), HRP donkey anti-rabbit IgG (H+L) (1:2000; Jackson, 711-035-152), HRP donkey anti-mouse IgG (H+L) (1:2000; Jackson, 711-035-151).
Tumor isolation and flow cytometry
Cells were injected intradermally into Nude (Charles River, NU/NU [088]) recipient mice and grown up to ~250mm3 before being randomly assigned to experimental groups. No specific strategy of randomization was used. Tumors from murine allografts were isolated as previously described (Schober and Fuchs, 2011; Siegle et al., 2014). Briefly, after separating tumors from normal skin, blood vessels and connective tissue, tumors were minced and incubated with 0.25% collagenase (Sigma, C2670) in HBSS (Gibco, 14170-112) for 45min at 37°C shaking. During the last 10m in of collagenase digestion, 62.5U/ml DNaseI (Worthington, LS002138) was added. The cell suspension was filtered through 70 and 40μm cell strainer, where tissue remaining in filters was digested an additional 10min with 0.25% trypsin-EDTA (Gibco, 25200-056) at 37°C shaki ng and strained again. Cell suspensions were diluted in wash buffer (2% chelexed FBS in DPBS) and pelleted at 300g for 10min. For flow cytometry, cell suspensions were stained for 30min on ice with CD71-APC (1:200; eBioscience, 17-0711), CD29-AF700 (1:200; Biolegend, 102218) CD49f-PerCP-Cy5.5 (1:200; Biolegend, 313618) and Epcam (1:200; Biolegend, 118203). DAPI (40,6-diamidino-2-phenylindole; Invitrogen, D1306) was used for live/dead cell exclusion. EdU incorporation was determined by flow cytometry using the Click-iT EdU PacBlue Cell Proliferation Assay Kit for flow cytometry (Invitrogen, C35002) following the manufacturer’s instructions. Chicken anti-GFP antibody (1:1000; Abcam, Ab13970) followed by Alexa GFP goat anti-chicken IgG (H+L) (1:200; Invitrogen, A11039) was used to visualize H2BGFP+ cells following the Click-iT labeling reaction. For in vivo Caspase-3 assays, 250,000 cells isolated from tumors were stained first with CD29-AF700, CD49f-PerCp Cy5.5, CD71-APC, CD45-Biotin (1:200; BD Pharmingin, 553078), CD140a-Biotin (1:200; eBioscience, 13-1401-82) and CD31-Biotin (1:200; eBioscience, 13-0311-85) for 30min on ice. Cells were washed and then stained with Streptavidin AF594 secondary (1:200; Invitrogen, S11227) for 30min on ice. Next cells were fixed using the Cytofix/Cytoperm kit from BD (554714). Cells were then incubated for 30min on ice in Caspase-3 antibody (1:20; BD Biosciences, 560627), washed and resuspended in 1x Perm/Wash buffer. FACS analyses were performed on a BD LSRII equipped with 488, 642, 407, 355 and 562nm lasers. Flow cytometry data analysis was carried out using FlowJo. Statistical significance of tumor growth curves was calculated using the Student’s t-test, while the Mann-Whitney test was used to calculate significant changes in tumor cell composition.
Cell Cycle Analysis
Cells were stained live with 10μg/mL Hoechst 33342 (Anaspec Inc, AS-83218) in cell culture media for 45min at 37°C. Cell-cycle analysis was performed by flow cytometry and analyzed using FlowJo software. Bar graphs represented mean±s.e.m. and statistical significance was calculated with the Student’s t-test.
Quantification and Statistical Analysis
All experiments were carried out single blinded. All in vivo and in vitro experiments were repeated at least three independent times with biological replicates. All quantitative data were collected from experiments performed in at least triplicate, and expressed as mean±s.e.m., 95% confidence interval, min/max. Differences between groups were assayed using unpaired or paired two-tailed Student’s t-test, or Mann–Whitney test using Prism 7 (GraphPad Software). Box-and-whisker plots are used to describe the entire population without assumptions about the statistical distribution. Significant differences were considered when P<0.05 as indicated by asterisks. Outliers were excluded using the Grubbs’ test in Prism 7 for colony formation experiments. No other data was excluded. For dose response curves, IC50 was calculated with Prism 7 using nonlinear regression for dose response inhibition. Area under the curve was calculated using Prism 7 and then subtracted from the total area to get the activity area in dose response experiments. Imaging was performed on a Nikon Eclipse Ti epi-fluorescence microscope or a Zeiss LSM700 Confocal Microscope. Images were analyzed in NIS-Elements or Zen software, respectively. For proliferation, apoptosis and DNA damage quantifications, pictures were acquired at 20x magnification and deconvolved, before Ki67, active Casp3 or γH2AX expression was detected in cells using the NIS element software. Differential gene expression analyses were performed between quiescent (CD71lo/GFPhi) and proliferative (CD71hi/GFPlo) populations isolated from two independent tumors. Differential gene expression data have been visualized in volcano plots. Fold change and P-value of differentially expressed gene sets have been imported into QIAGEN’s Ingenuity Pathway Analysis (IPA® QIAGEN Redwood City, www.qiagen.com/ingenuity) for pathway analyses and upstream regulator predictions. Statistical and graphical data analyses were performed in R and Prism 7. Figures were prepared using Adobe Photoshop and Illustrator CC 2014.
Data and Software Availability
The accession number for the RNA, ATAC and ChIP-seq data reported in this paper is NCBI GEO: GSE74762.
Supplementary Material
Table S1, related to Figure 2. Tumor Histology
Table S2, related to Figure 4. Differential gene expression table between TPC populations
Table S3, related to Figure 4. Intersection between TPCs and HFSCs or EPCs
Table S4, related to Figure 4. Nonsynonymous mutation and copy number variation
Table S5, related to Figure 5. Gene ontology analyses of Smad2/3 bound signature genes
Table S6, related to Figure 7. Intersection between quiescent TPC and progressive HNSCC signature genes
Table S7, related to STAR Methods. List of Oligonucleotides
Highlights.
Functional identification of quiescent, therapy resistant TPCs in intact SCCs
Transcriptional signatures define quiescent and proliferative states of TPCs
Smad2 dependent transcripts elicit a reversible G1 arrest independent of p21CIP
TGFβ inhibition prevents quiescence mediated chemotherapy resistance in SCCs
Acknowledgments
We thank W. L. Carroll, G. David, E. Hernando-Monge, P. Manga, D. Rifkin and E. Gonzalez for discussions and comments on the manuscript. We thank D. Fenyo for help with Monte Carlo Simulations, T. Papagiannakopoulos for sgRNAs and E. Fuchs for generous support and reagents. We are grateful for the help received from the NYU Medical Center Flow Cytometry Core facility and NYU’s Division of Comparative Medicine for their expert handling and care of the mice. We thank A. Heguy and the NYU Genome Technology Center for Next Generation sequence analyses and the NYU Medical Center High-Performance Computing Facility for providing access to their computing cluster and storage. Core funding is partially supported by the Laura and Isaac Perlmutter Cancer Center support grant P30CA016087 from the National Cancer Institute. This research was supported by grants from the National Institutes of Health to M.S. R00-AR057260 and R01CA181111 and J.B T32 CA009161 (Levy).
Footnotes
Supplementary information includes 7 figures and 7 tables.
Author Contributions
Conceptualization, J.A.B. and M.S.; Methodology, J.A.B., N.H., A.S.P., M.S.; Formal Analysis, J.A.R., S.B., I.D., S.M., A.T., M.S.; Investigation, J.A.B., Y.Y., N.H., A.S.P., and A.B.; Writing-Original Draft, J.A.B., M.S.; Writing-Review and editing, J.A.B., M.S., A.S.P., S.B.; Visualization, J.A.B., M.S., A.S.P.; Funding Acquisition, J.A.B., M.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Mohrin M, Sotiropoulou PA, Passegué E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Bragado P, Estrada Y, Sosa MS, Avivar-Valderas A, Cannan D, Genden E, Teng M, Ranganathan AC, Wen HC, Kapoor A, et al. Analysis of marker-defined HNSCC subpopulations reveals a dynamic regulation of tumor initiating properties. PLoS ONE. 2012;7:e29974. doi: 10.1371/journal.pone.0029974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammareri P, Rose AM, Vincent DF, Wang J, Nagano A, Libertini S, Ridgway RA, Athineos D, Coates PJ, McHugh A, et al. Inactivation of TGFβ receptors in stem cells drives cutaneous squamous cell carcinoma. Nature Communications. 2016;7:12493. doi: 10.1038/ncomms12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK, Dotto GP. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:1–5. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, SCHOBER M, Pasolli HA, Stokes N, Cruz-Racelis, Dela J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch G, SCHOBER M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- Kang Y, Chen CR, Massagué J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Molecular Cell. 2003;11:915–926. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AMK, Ng K, Ma J, Wienholds E, Dunant C, et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339:543–548. doi: 10.1126/science.1227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Beck B, Nassar D, Dubois C, Dekoninck S, Blanpain C. Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness. Embo J. 2012;31:4563–4575. doi: 10.1038/emboj.2012.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- Lu S-L, Herrington H, Reh D, Weber S, Bornstein S, Wang D, Li AG, Tang C-F, Siddiqui Y, Nord J, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annual Review of Pharmacology and Toxicology. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- McCreery MQ, Halliwill KD, Chin D, DelRosario R, Hirst G, Vuong P, Jen KY, Hewinson J, Adams DJ, Balmain A. Evolution of metastasis revealed by mutational landscapes of chemically induced skin cancers. Nat Med. 2015;21:1514–1520. doi: 10.1038/nm.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegué E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AC, Orlando DA, Newman JJ, Lovén J, Kumar RM, Bilodeau S, Reddy J, Guenther MG, DeKoter RP, Young RA. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar D, Latil M, Boeckx B, Lambrechts D, Blanpain C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat Med. 2015;21:946–954. doi: 10.1038/nm.3878. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Fuchs E. Isolation and culture of epithelial stem cells. Methods Mol Biol. 2009;482:215–232. doi: 10.1007/978-1-59745-060-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimori N, Oristian D, Fuchs E. TGF-β Promotes Heterogeneity and Drug Resistance in Squamous Cell Carcinoma. Cell. 2015;160:963–976. doi: 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Romero MR, Gardner C, Watt FM. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- Pierce GB, Wallace C. Differentiation of malignant to benign cells. Cancer Res. 1971;31:127–134. [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. Embo J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Tsien R, Gambhir SS. Construction and Validation of Improved Triple Fusion Reporter Gene Vectors for Molecular Imaging of Living Subjects. Cancer Res. 2007;67:3085–3093. doi: 10.1158/0008-5472.CAN-06-2402. [DOI] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE, et al. Overcoming Intrinsic Multidrug Resistance in Melanoma by Blocking the Mitochondrial Respiratory Chain of Slow-Cycling JARID1B(high) Cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Jacob F, Leung E, Wang S, White BS, Shalloway D, Tumbar T. Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat Cell Biol. 2016;18:619–631. doi: 10.1038/ncb3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm SN. TGF- maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-{beta} and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USa. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendoel A, Dunn JG, Rodriguez EH, Naik S, Gomez NC, Hurwitz B, Levorse J, Dill BD, Schramek D, Molina H, et al. Translation from unconventional 5′ start sites drives tumour initiation. Nature. 2017;541:494–499. doi: 10.1038/nature21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, SCHOBER M. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nature Communications. 2014;5:4511. doi: 10.1038/ncomms5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MS, Avivar-Valderas A, Bragado P, Wen H-C, Aguirre-Ghiso JA. ERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17:5850–5857. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulou PA, Candi A, Mascre G, De Clercq S, Youssef KK, Lapouge G, Dahl E, Semeraro C, Denecker G, Marine J-C, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USa. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev. 2013;2013:972913–10. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, Fox C, Francis JM, Pedamallu CS, Deluca DS, et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014;124:1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Xi Q, Wang Z, Zaromytidou AI, Zhang XHF, Chow-Tsang LF, Liu JX, Kim H, Barlas A, Manova-Todorova K, Kaartinen V, et al. A poised chromatin platform for TGF-β access to master regulators. Cell. 2011;147:1511–1524. doi: 10.1016/j.cell.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang H, Boyd DD. ATF3 represses 72-kDa type IV collagenase (MMP-2) expression by antagonizing p53-dependent trans-activation of the collagenase promoter. J Biol Chem. 2002;277:10804–10812. doi: 10.1074/jbc.M112069200. [DOI] [PubMed] [Google Scholar]
- Yang H, Schramek D, Adam RC, Keyes BE, Wang P, Zheng D, Fuchs E. ETS family transcriptional regulators drive chromatin dynamics and malignancy in squamous cell carcinomas. Elife. 2015;4:e10870. doi: 10.7554/eLife.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to Figure 2. Tumor Histology
Table S2, related to Figure 4. Differential gene expression table between TPC populations
Table S3, related to Figure 4. Intersection between TPCs and HFSCs or EPCs
Table S4, related to Figure 4. Nonsynonymous mutation and copy number variation
Table S5, related to Figure 5. Gene ontology analyses of Smad2/3 bound signature genes
Table S6, related to Figure 7. Intersection between quiescent TPC and progressive HNSCC signature genes
Table S7, related to STAR Methods. List of Oligonucleotides