Abstract
This study evaluated the oncologic outcomes and complications of cervical cancer patients in terms of CT-based image-guided brachytherapy (IGBT) parameters. Of 68 cervical cancer patients treated with definitive radiotherapy/concurrent chemoradiotherapy, most received whole-pelvis external beam RT (EBRT) of 40 Gy in 20 fractions, pelvic EBRT with central shield of 10 Gy in 5 fractions, and CT-based IGBT of 18 Gy in 3 fractions prescribed to point A. Cumulative EBRT and IGBT doses were calculated as the total equivalent dose in 2 Gy fractions (EQD2). The median follow-up was 31 (3–52) months. The 2-year overall survival, local control, pelvic control, and disease-free survival rates of the 68 patients were 92%, 83%, 82% and 73%, respectively. The HR-CTV D90, length from the tandem axis to left/right margin of the HR-CTV (T-LR), and HR-CTV volume were significant IGBT parameters for predicting local/pelvic control. Patients who received an HR-CTV D90 of >60 Gy, compared with ≤60 Gy, had significantly better local/pelvic control. Furthermore, 70 Gy was a marginally significant HR-CTV D90 cut-off affecting local control. T-LR was an independent IGBT parameter predicting local/pelvic control on multivariate analysis. Three patients developed Grade 3 or higher treatment-related complications. The D2cm3 of organs at risk were not significant predictors of complications. Future challenges for further improving outcomes include additional interstitial needles for irregularly shaped HR-CTVs, and moderate dose escalation, especially for patients with poor tumor responses.
Keywords: cervical neoplasms, image-guided brachytherapy, computed tomography, high-risk clinical target volume
INTRODUCTION
Definitive radiotherapy (RT) consisting of external beam RT (EBRT) and intracavitary brachytherapy (ICBT) have played important roles for patients with cervical cancer [1–3]. Definitive RT and/or concurrent chemoradiotherapy (CCRT) are now the recommended treatments for Stage I to IVA cervical cancer patients, according to treatment guidelines [4, 5].
As with EBRT, 3D treatment planning has become widely used with ICBT [6]. In 3D image-guided brachytherapy (3D-IGBT), doses are prescribed and evaluated quantitatively for the target volume as well as organs at risk (OARs). As a result, individualized treatments can be planned using 3D-IGBT, in contrast to the standard two-dimensional (2D) ICBT treatment planning, in which doses are prescribed to point A. 3D-IGBT planning is based on 3D imaging modalities such as computed tomography (CT) and/or magnetic resonance imaging (MRI), performed with inserted applicators. Appropriate contouring of both the target volume and OARs is essential for appropriate delivery of 3D-IGBT. Of particular importance is the high-risk clinical target volume (HR-CTV), which serves as a reference for both prescription and evaluation of 3D-IGBT.
The Groupe Européen de Curiethérapie and European Society for Radiotherapy and Oncology (GEC-ESTRO) guidelines for 3D-IGBT for uterine cervical cancer [7, 8] and experts in this field [9] have recommended the use of T2-weighted MRI (T2WI) for the target volume delineation. However, in actual clinical practice, practical problems (such as accessibility to and limited operating time of the MRI machine) are associated with the use of MRI. Due to these practical limitations, most Japanese institutions utilize CT instead of MRI for 3D-IGBT [10], and excellent treatment results have been reported [11, 12]. The most critical issue with CT-based 3D-IGBT is inferior soft-tissue resolution on CT compared with T2WI MRI. Previous comparative studies demonstrated consistently larger HR-CTVs contoured with CT than with MRI, especially in a lateral direction [13, 14].
Dose–effect relationships in reference to the local control (LC) rate as well as the incidence and grade of complications were demonstrated in patients treated with MRI-based IGBT [15–21]. Murakami et al. also reported similar results with a series of CT-based IGBT [12]. These reports indicated that HR-CTV D90 is one of the most important predictors of LC [8, 12, 15, 20, 21]. To minimize the discrepancies in CT-based HR-CTV contouring and variation among physicians, consensus-based guidelines were recently developed by the Gynecological Tumor Committee Members of the Japanese Radiation Oncology Study Group (JROSG) [22].
In this study, we analyzed oncologic outcomes and complications according to various factors, including dose–volume histogram (DVH) parameters, of CT-based IGBT in cervical cancer patients treated with definitive RT/CCRT. For dose–response analyses of oncologic outcomes, we applied the CT-based HR-CTV definition proposed by the JROSG [22].
MATERIAL AND METHODS
Patients
Patient characteristics are listed in Table 1. Between November 2011 and June 2014, 90 consecutive cervical cancer patients were treated with definitive RT/CCRT at the University of the Ryukyus Hospital. From these, the following patients were excluded from the present analyses: 1 patient with condylomatous carcinoma, 1 patient with small-cell carcinoma, 8 patients treated with 2D-ICBT as part of their treatment, 9 patients with para-aortic node enlargement (>10 mm, as assessed by pretreatment CT), 2 patients who received IGBT using a cylinder applicator, and 1 patient whose CT images showed significant metallic artifacts. Therefore, the current study included 68 patients. Table 1 shows the characteristics of the patients analyzed in this study.
Table 1.
Patient characteristics (n = 68)
| Characteristics | No. of patients |
|---|---|
| Age | |
| Median (range) | 58 (32–89) |
| FIGO stage | |
| IB1 | 14 |
| IB2 | 8 |
| IIA1 | 1 |
| IIA2 | 1 |
| IIB | 23 |
| IIIB | 19 |
| IVA | 2 |
| Pelvic node metastasesa | |
| Yes | 23 |
| No | 45 |
| Pathology | |
| SCC | 56 |
| AC + ASC | 12 |
| Pretreatment tumor diameter (mm)b | |
| Median (range) | 46 (24–93) |
| Pre-IGBT tumor diameter (mm)b | |
| Median (range) | 30 (0–64) |
| Corpus invasionb | |
| Yes | 29 |
| No | 38 |
| Unknown | 1 |
| Pretreatment SCC antigen (ng/ml) | |
| Median (range) | 5.3 (0.5–178) |
SCC = squamous cell carcinoma, AC = adenocarcinoma, ASC = adenosquamous carcinoma, IGBT = image-guided intracavitary brachytherapy. aAssessed by MRI (T2-weighted images). bOver 10 mm in minimum diameter.
All patients underwent pretreatment pelvic MRI (T2WI) and chest-abdomen-pelvis CT. A second MRI (T2WI) was also performed prior to the first IGBT (within 1 week). One patient did not undergo pretreatment pelvic MRI, and one patient did not have the second (pre-IGBT) MRI.
This retrospective study was approved by the ethical review board of our institution.
Radiotherapy
Details of the radiotherapy are listed in Table 2. EBRT was delivered via a 3D conformal technique using a linear accelerator (Clinac iX, Varian Medical Systems, Palo Alto, CA, USA) with a 10-MV photon beam. Treatment planning was based on CT images with a 1.25-mm slice thickness using the Aquilion LB CT scanner (Toshiba Medical Systems, Otawara, Tochigi, Japan). An EBRT dose of 50 Gy in 25 fractions was prescribed. For the majority of patients (59 patients), an initial dose of 40 Gy in 20 fractions was delivered to the whole pelvis (WP) using a 4-field box technique; subsequently, 10 Gy in 5 fractions were delivered to the WP with a 4-cm-wide central shield (CS). For the patients whose tumors responded poorly to WP-EBRT and remained large after 40 Gy treatment, an additional WP-EBRT dose of 10 Gy without CS was delivered. One patient, whose tumor was still large after 50 Gy of WP-EBRT without CS, received additional EBRT of 6 Gy/3 fractions with reduced portals. One patient with Stage IB1 cancer received WP-EBRT of 20 Gy without CS and 30 Gy with CS; two patients received 45 Gy without CS; and one patient with active rheumatoid arthritis received 39.6 Gy of WP-EBRT.
Table 2.
Details of radiotherapy
| Overall treatment time (days) | |
| Median (range) | 48 (36–74) |
| EBRT | |
| Dose to central pelvis (Gy) | |
| Median (range) | 40 (20–56) |
| Dose to parametrium and pelvic wall (Gy) | |
| Median (range) | 50 (39.6–56) |
| IGBT | |
| Total dose prescribed at point A (Gy) | |
| Median (range) | 18 (12–24) |
| Total EQD2 (α/β = 10) at point A (Gy) | |
| Median (range) | 24 (14–32) |
| Total EQD2 (α/β = 10) of HR-CTV D90 (Gy) | |
| Median (range) | 31 (5–55) |
| Volume of HR-CTV at 1st IGBT (ml) | |
| Median (range) | 28 (10–128) |
| Distance from tandem axis to A/P edge of the HR-CTV (mm) | |
| Median (range) | 20 (10–44) |
| Distance from tandem axis to lateral edge of the HR-CTV (mm) | |
| Median (range) | 22 (14–39) |
| EBRT + IGBT | |
| Total EQD2 at point A (Gy) | |
| Median (range) | 64 (52–80) |
| Total EQD2 of HR-CTV D90 (Gy) | |
| Median (range) | 72 (55–95) |
EBRT = external beam radiotherapy, IGBT = image-guided intracavitary brachytherapy, EQD2 = the equivalent dose in 2 Gy fractions, HR-CTV = high-risk clinical target volume.
All patients received IGBT using tandem and ovoid applicators. Metallic or plastic applicators (tandem and ovoid) were utilized in the IGBT: 54 patients were treated using plastic applicators and 12 using metal applicators in all sessions; the remaining two patients were treated with both type of applicators. No patient was treated with a combination of standard intracavitary applicators and interstitial implants. IGBT was delivered using a high-dose-rate (HDR) 192Ir remote afterloading system (microSelectron, Elekta, Stockholm, Sweden). IGBT was planned based on CT images with a 1.25-mm slice thickness acquired using the Aquilion LB CT scanner with inserted applicators. The dose prescribed to point A was selected according to the Manchester System. For most cases, 18 Gy in 3 fractions were delivered. One patient received 24 Gy in 4 fractions (the same patient who received 20 Gy of WP-EBRT followed by 30 Gy of WP-EBRT with CS). Two patients who received 50 Gy WP-EBRT without CS also received IGBT of 12 Gy in 2 fractions. Two patients who received 45 Gy WP-EBRT without CS also received IGBT of 20 Gy in 4 fractions of IGBT. No patient was treated with a combination of standard intracavitary applicators (tandem and ovoid) and interstitial implants. IGBT was performed once a week. EBRT and IGBT were not administered on the same day. All IGBT planning for actual treatments as well as for the present study were performed using the Oncentra MasterPlan® (Nucletron, Veenendaal, The Netherlands). Although the OARs were routinely contoured at the time of their actual treatments, the HR-CTV was not contoured. In this study, two radiation oncologists (TK, TT) performed HR-CTV delineation by mutual agreement according to the guidelines of the JROSG [22]. In the actual treatments, the dwell time or position of the source was arranged with the objective of decreasing the dose to the OARs without decreasing the prescribed dose to point A, if the minimum dose to the most irradiated 2 cm3 (D2cm3) of the OARs seemed high. In 17 cases whose D2cm3 to the OARs was still high, even after the optimization, the prescribed dose to point A was decreased to 4–5.5 Gy per fraction. These arrangements were performed according to the physicians’ discretion. In the present analyses, the minimum dose delivered to 90% (D90) of the HR-CTV and other DVH parameters were re-calculated using the same dwell position and time of the source at the time of the actual treatment.
We also evaluated the oncologic outcomes according to the maximum distance between the tandem axis and the left/right margin of the HR-CTV (T-LR). Based on a dosimetric study performed by Tamaki et al. [23], the patients were stratified according to a T-LR cut-off of 30 mm and an anterior–posterior distance cut-off of 20 mm for directions, as those were estimated as the dosimetric edge of a total equivalent dose in 2 Gy fractions (EQD2) of 60 Gy.
Chemotherapy
Forty-seven patients were treated with concurrent chemoradiotherapy (CCRT). A weekly cisplatin dose of 40 mg/m2 was administered to patients with squamous cell carcinoma (SCC). Tri-weekly doses of 50 mg/m2 cisplatin combined with weekly doses of 50 mg/m2 paclitaxel was administered to patients with adenocarcinoma (AC) and adenosquamous carcinoma (ASC).
Follow-up
During the treatment periods, the radiation oncologists and gynecologic oncologists performed pelvic examinations and blood tests at least once a week. After treatment completion, patients were followed up every 1–3 months by the radiation and/or gynecologic oncologists. The follow-up included a pelvic examination and Pap smear. CT scans of the abdomen and pelvis and chest X-rays (or CT scans) were performed every 6–12 months. Pelvic MRIs were not performed regularly.
A local recurrence was defined as disease progression in the uterine cervix and/or corpus and/or parametrium. Pelvic recurrence was defined as local recurrence and/or pelvic node progression. Distant metastasis was defined as development of recurrence outside the pelvis. For cases with a recurrence diagnosed pathologically, the date of the event was defined as the first date that the recurrence was suspected clinically by pelvic examination and/or diagnostic images.
Adverse events, including cystitis, proctitis and enterocolitis, were evaluated according to the National Cancer Institute Common Toxicity Criteria version 4.0 (CTCAEv4.0). Events that occurred in the sigmoid colon were considered enterocolitis. Five patients were lost to follow-up and were censored (at 3, 9, 13, 15, 18 months). The median follow-up of the patients who were not censored was 32 months (8–52 months).
Statistical analysis
Cumulative outcomes [overall survival (OS), local control (LC), pelvic control (PC) and disease-free survival (DFS)] were estimated using the Kaplan–Meier method. The outcomes were measured from the date of treatment initiation to the date the event occurred. Differences in outcomes were compared by a log-rank test. For univariate analysis, all variables were dichotomized at a median value. For multivariate analysis, we evaluated those variables with a P-value of <0.10 in the univariate analyses. All statistical analyses were performed using IBM SPSS Statistics version 22.0 for Windows (IBM Japan, Tokyo, Japan).
Dose parameters of IGBT, including the dose of point A, D2cm3 to the OARs, and HR-CTV D90 were calculated for each session. The cumulative dose of IGBT plus WP-EBRT converted to EQD2 was calculated using the linear–quadratic model with α/β = 10 for tumors and α/β = 3 for OARs. The WP-EBRT dose with CS was not included in the calculation.
RESULTS
Oncologic outcomes
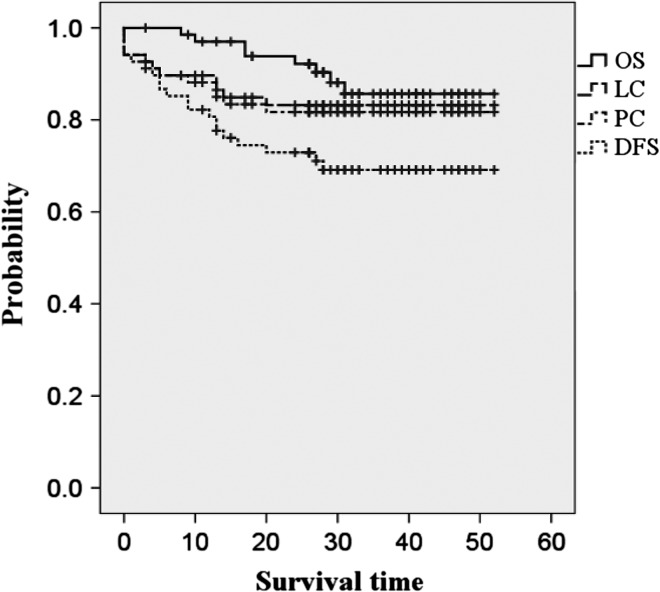
Of the 68 patients, 20 developed recurrence: pelvic recurrence alone in 6 patients, distant metastases alone in 8, and both pelvic recurrence and distant metastasis in 6. Among the pelvic recurrence cases, 11 had local recurrence. Eight patients died: 4 due to cervical cancer, 3 due to other diseases, and 1 due to an unknown cause. Among all 68 patients, the 2-year OS, LC, PC and DFS rates were 92%, 83%, 82% and 73%, respectively (Fig. 1).
Fig. 1.
Kaplan–Meier estimates for overall survival (OS), local control (LC), pelvic control (PC) and disease-free survival (DFS) in the 68 patients.
The oncologic outcomes according to the patient-related and the tumor-related variables are shown in Table 3. The 2-year LC, PC and DFS rates were significantly poorer for patients with AC/ASC than those with SCC. A large pretreatment tumor diameter had negative effects on LC and PC, but the associations did not reach statistical significance. On the other hand, tumor diameter assessed immediately before the first IGBT had significantly negative impacts on LC, PC and DFS. Neither pelvic node status nor uterine corpus invasion had an effect on the oncologic outcomes.
Table 3.
Treatment outcomes according to patient- and tumor-related factors
| Factors | n | 2-yr OS (%) | 2-year LC (%) | 2-year PC (%) | 2-year DFS (%) |
|---|---|---|---|---|---|
| Age (years) | |||||
| <60 | 35 | 94 | 86 | 83 | 73 |
| 60≦ | 33 | 90 | 81 | 81 | 66 |
| NS | NS | NS | NS | ||
| FIGO stage | |||||
| I | 22 | 100 | 86 | 86 | 86 |
| II | 25 | 87 | 83 | 79 | 71 |
| III | 19 | 89 | 83 | 83 | 63 |
| IVA | 2 | 100 | 50 | 50 | 50 |
| NS | NS | NS | NS | ||
| Pelvic node metastasesa | |||||
| Yes | 23 | 96 | 82 | 78 | 74 |
| No | 45 | 90 | 84 | 84 | 72 |
| NS | NS | NS | NS | ||
| Pathology | |||||
| SCC | 56 | 94 | 87 | 85 | 80 |
| AC+ASC | 12 | 83 | 67 | 67 | 42 |
| NS | P = 0.036 | P = 0.059 | P = 0.004 | ||
| Pretreatment tumor diameter (mm)b | |||||
| <= 40 | 21 | 95 | 91 | 91 | 76 |
| > 40 | 40 | 92 | 79 | 77 | 72 |
| NS | NS | NS | NS | ||
| Pre-IGBT tumor diameter (mm)b | |||||
| ≦ 30 | 32 | 100 | 97 | 94 | 88 |
| 30< | 30 | 85 | 67 | 68 | 58 |
| P = 0.021 | P = 0.002 | P = 0.008 | P = 0.007 | ||
| Corpus invasionb | |||||
| Yes | 29 | 88 | 78 | 78 | 67 |
| No | 38 | 95 | 87 | 84 | 76 |
| NS | NS | NS | NS | ||
| Pretreatment SCC antigen (ng/ml) | |||||
| <6.9 | 27 | 96 | 100 | 96 | 92 |
| 6.9≦ | 29 | 93 | 74 | 74 | 68 |
| NS | P = 0.006 | P = 0.030 | P = 0.037 | ||
OS = overall survival rate, LC = local control rate, PC = pelvic control rate, DFS = disease-free survival rate, SCC = squamous cell carcinoma, AC = adenocarcinoma, ASC = adenosquamous carcinoma, IGBT = image-guided brachytherapy. aOver 10 mm in shortest diameter as assessed by CT/MRI. bAssessed by MRI T2WI.
Table 4 shows the oncologic outcomes according to treatment-related factors, including DVH parameters. A prolonged overall treatment time (OTT) had adverse effects on LC, PC and DFS. Regarding the cumulative dose, patients were divided into two groups according to the total point A dose and HR-CTV D90 (EQD2). We compared the outcomes of patients treated with >60 Gy versus ≤60 Gy based on the reports of Murakami et al. [12]. Although the total point A EQD2 had no effects on outcomes, the cumulative HR-CTV D90 EQD2 was a significant predictor for all oncologic outcomes. Patients who received an HR-CTV dose of >60 Gy had significantly superior OS, LC, PC and PFS compared with those receiving ≤60 Gy. Oncologic outcomes were compared further using different cumulative HR-CTV D90 cut-off values. We found that patients who received >70 Gy achieved superior LC compared with those who received ≤70 Gy, with marginal significance. The maximum diameter from the tandem axis to the anterior/posterior margin of the HR-CTV adversely affected the LC, with marginal significance. Patients with a T-LR >30 mm had a significantly inferior LC, PC and DFS compared with those with a T-LR ≤30 mm. Patients with an HR-CTV >45 ml demonstrated poorer LC and PC compared with those with an HR-CTV ≤45 ml.
Table 4.
Treatment outcomes according to treatment factors
| Factors | n | 2-year OS (%) | 2-year LC (%) | 2-year PC (%) | 2-year DFS (%) |
|---|---|---|---|---|---|
| OTT (days) | |||||
| ≤48 | 37 | 97 | 94 | 91 | 86 |
| >48 | 31 | 85 | 71 | 71 | 58 |
| P = 0.068 | P = 0.008 | P = 0.023 | P = 0.002 | ||
| Chemotherapy administration | |||||
| CCRT | 47 | 91 | 85 | 83 | 76 |
| RT alone | 21 | 95 | 80 | 80 | 66 |
| NS | NS | NS | NS | ||
| Total EQD2 at point A (Gy) | |||||
| >60 | 61 | 93 | 81 | 80 | 72 |
| ≤60 | 7 | 86 | 100 | 100 | 86 |
| NS | NS | NS | NS | ||
| Total EQD2 of HR-CTV D90 (Gy) | |||||
| >60 | 58 | 95 | 88 | 86 | 77 |
| ≤60 | 10 | 78 | 58 | 58 | 47 |
| P = 0.031 | P = 0.005 | P = 0.009 | P = 0.003 | ||
| >70 | 36 | 94 | 91 | 88 | 83 |
| ≤70 | 32 | 86 | 74 | 74 | 61 |
| NS | P = 0.053 | NS | P = 0.047 | ||
| Length from tandem axis to anterior/posterior edge of HR-CTV (mm) | |||||
| ≤20 | 40 | 95 | 89 | 87 | 77 |
| >20 | 28 | 88 | 74 | 74 | 67 |
| NS | P = 0.077 | NS | NS | ||
| Length from tandem axis to left/right edge of HR-CTV (mm) | |||||
| ≤30 | 60 | 93 | 88 | 86 | 76 |
| 30< | 8 | 86 | 45 | 45 | 45 |
| NS | P = 0.001 | P = 0.001 | P = 0.003 | ||
| HR-CTV Volume (ml) | |||||
| ≤45 | 55 | 92 | 88 | 86 | 76 |
| 45< | 13 | 92 | 62 | 62 | 62 |
| NS | P = 0.009 | P = 0.017 | NS | ||
OS = overall survival rate, LC = local control rate, PC = pelvic control rate, DFS = disease-free survival, OTT = overall treatment time, CCRT: concurrent chemoradiotherapy, RT: radiotherapy, EQD2 = the equivalent dose in 2 Gy fractions.
As shown in Tables 3 and 4, those variables with P-values <0.1 in the univariate analyses were assessed in a Cox proportional regression analysis. According to the results of the multivariate analysis, AC/ASC and a wider tumor diameter (T-LR >30 mm) remained statistically significant factors associated with poor LC (Table 5).
Table 5.
Multivariate analysis for local conrol (n = 61)
| Risk | P value | HR | 95% CI | |
|---|---|---|---|---|
| Pathology | AC/ASC | 0.035 | 11.5 | 1.2–111.2 |
| Pretreatment SCC antigen (ng/ml) | ≥6.9 | 0.078 | 6.1 | 0.8–45.3 |
| Pre-IGBT tumor diameter (mm) | >30 | 0.053 | 9 | 1.0–82.7 |
| OTT (days) | >48 | 0.77 | 1.2 | 0.3–5.7 |
| Length from T to A or P of HR-CTV (mm) | >20 | 0.48 | 1.6 | 0.4–6.1 |
| Length from T to L or R of HR-CTV (mm) | >30 | 0.047 | 9.7 | 1.0–90.5 |
| HR-CTV (ml) | >45 | 0.17 | 0.2 | 0.0–1.9 |
| Total EQD2 at HR-CTV D90 (Gy) | ≤60 | 0.56 | 0.6 | 0.1–3.3 |
AC = adenocarcinoma, ASC = adenosquamous carcinoma, IGBT = image-guided brachytherapy, OTT = overall treatment time, Length from T to A or P of HR-CTV = length from tandem to anterior or posterior border of high-risk clinical target volume, Length from T to L or R of HR-CTV = length from tandem to left or right border of high-risk clinical target volume, EQD2 = the equivalent dose in 2 Gy fractions.
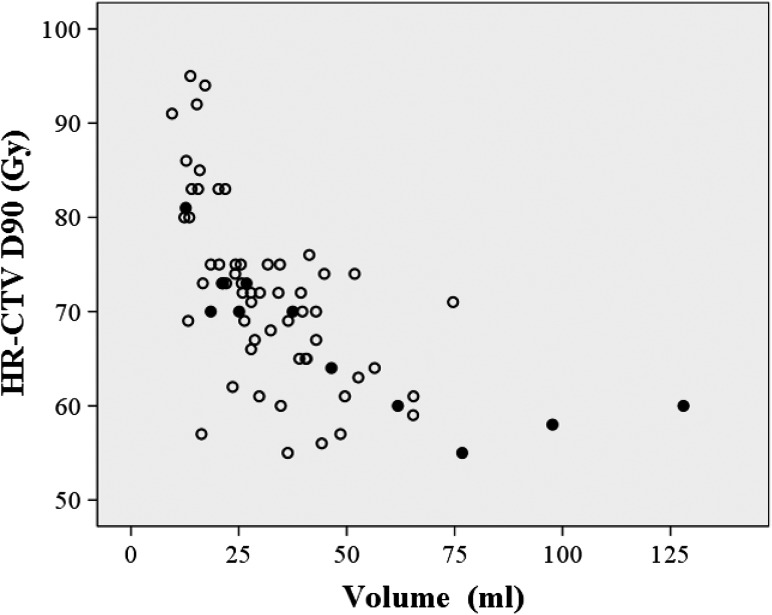
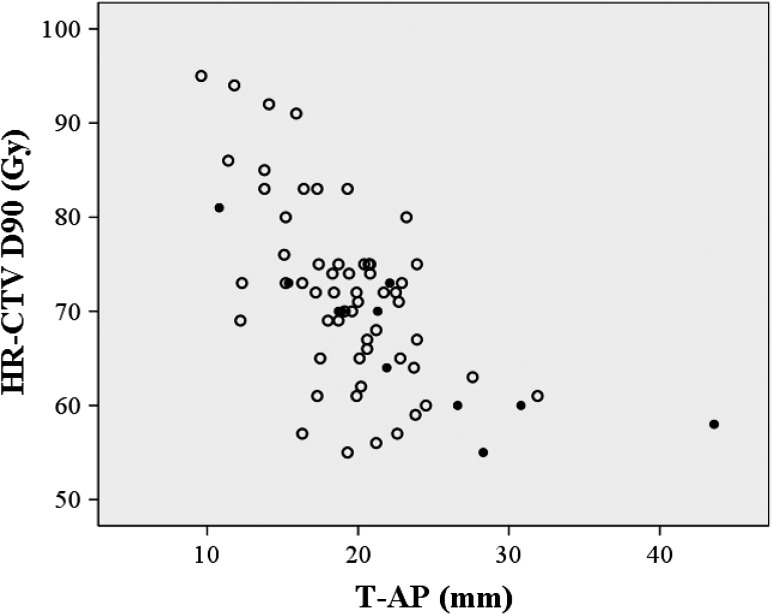
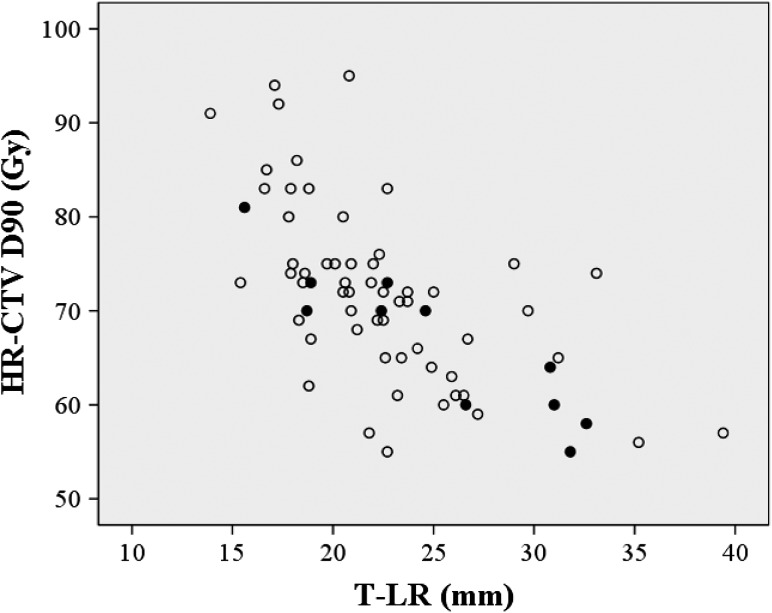
Figure 2 shows local failures as a function of the HR-CTV and HR-CTV D90. A negative correlation between the volume and the dose was observed. In Figs 3 and 4, the local failures are plotted according to the length from the tandem to the anterior–posterior or lateral border of the HR-CTV and HR-CTV D90. These plots revealed that patients with a large HR-CTV and low HR-CTV D90 tended to have frequent local failures.
Fig. 2.
Local failure as a function of the HR-CTV (volume) and HR-CTV D90 (dose). Open circles: patients with LC, Filled circles: patients with local failure.
Fig. 3.
A scattergram of HR-CTV D90, length from tandem axis to the antero-posterior margin of the HR-CTV (T-AP), and local control. Open circles: patients with LC; filled circles: patients with local failure.
Fig. 4.
A scattergram of HR-CTV D90, length from tandem axis to the lateral edge of the HR-CTV (T-LR), and local control. Open circles: patients with LC; filled circles: patients with local failure.
Complications
The cumulative EQD2 values of D2cm3 (EBRT plus ICBT) to the OARs were as follows: 69 (50–96) Gy for the bladder, 53 (38–72) Gy for the rectum, 61 (34–80) Gy for the sigmoid and 56 (32–78) Gy for the small intestine. The late complications (G1/G2/G3/G4) occurred as follows: cystitis (2/1/0/0), proctitis (1/8/0/0) and enterocolitis (3/4/4/2). There were no significant differences in the average cumulative EQD2 of the D2cm3 (EBRT plus IGBT) between patients with and those without complications with respect to each OAR. Among four cases with Grade 3 enterocolitis, only one was considered as actual radiation enteritis. The other two had peritoneal cancer dissemination, and one suffered a paralytic ileus that was potentially caused by opioid administration as part of her terminal care. The two cases of Grade 4 enterocolitis were sigmoid colon perforations requiring surgery. The cumulative EQD2 values of the D2cm3 of the sigmoid colon in these two patients were 43 Gy and 66 Gy, respectively.
DISCUSSION
This study demonstrated significantly better oncologic outcomes in cervical cancer patients who received a cumulative EQD2 of the HR-CTV D90 >60 Gy than in those who received ≤60 Gy in CT-based IGBT. The result is consistent with the results of a previously published study on CT-based IGBT [12]. Based on these findings, a cumulative EQD2 of the HR-CTV D90 should be >60 Gy for cervical cancer patients treated with CT-based IGBT. In the present series, IGBT doses were prescribed to point A irrespective of the tumor volume. The cumulative dose to point A had no impact on any oncologic outcome evaluated in this study. This finding suggests that ICBT dose prescription and evaluation using a 3D dose–volume parameter, i.e. HR-CTV D90, are essential for predicting the oncologic outcomes of cervical cancer patients treated with definitive RT/CCRT.
Some factors can lead to a decrease in the HR-CTV D90. We observed a negative correlation between the D90 and the actual HR-CTV in this study. A large HR-CTV could be one reason for the low HR-CTV D90 in patients whose ICBT doses were prescribed to point A. The present study demonstrated that a large HR-CTV had a negative effect on LC and PC. All three patients with an HR-CTV >75 ml had an HR-CTV D90 of ≤60 Gy and developed local failure. In this study, patients with AC/ASC had a poorer LC and PC compared with those with SCC, and 33% of the patients with AC/ASC received a cumulative HR-CTV D90 of ≤60 Gy. Poor and delayed responses to radiation may have affected the findings and be a reason for the unfavorable LC in the patients with AC/ASC. To overcome this negative effect, one potential solution is escalation of the EBRT dose to decrease the HR-CTV prior to IGBT. In our series, most patients received 40 Gy in 20 fractions from EBRT. Additionally, 10–16 Gy were delivered in patients with a poor response to the standard EBRT dose. Despite these efforts, we could not achieve favorable results in these patients. Therefore, EBRT dose escalation with the intention of decreasing the HR-CTV is not reasonable. Using the dose prescription with HR-CTV D90 instead of point A is considered the proper approach for delivering the appropriate dose to the entire tumor volume, especially for patients with large tumors at the time of ICBT. In this approach, simultaneous monitoring of the doses to OARs is mandatory [16, 17, 19, 24, 25]. The American Brachytherapy Society (ABS) guidelines [25] suggest that the D2cm3 dose constraints are 70–75 Gy for the rectum and sigmoid and ~90 Gy for the bladder. In our study, the D2cm3 for the bladder and rectum were within these tolerable limits. No patients suffered Grade 4 complications of the bladder or rectum. Therefore, dose-adaptive IGBT to maintain an HR-CTV D90 of >60 Gy for patients with a large HR-CTV might be feasible and appropriate for the majority of patients. However, two patients in our study experienced a sigmoid colon perforation (Grade 4), despite receiving a cumulative D2cm3 to the sigmoid colon of <70 Gy. No consensus has been reached regarding dose constraints for the sigmoid colon [16]. We have to pay attention not only to DVH parameters, e.g. D2cm3, but also to other factors, such as patient comorbidities, to minimize toxicity to the sigmoid colon.
A second factor that can negatively affect LC and PC is an asymmetrical tumor shape. The typical dose distribution of ICBT using tandem and ovoid applicators is ‘pear shaped’. With significantly asymmetrical tumors, it is difficult to encompass the entire HR-CTV within the typical dose distribution and to achieve an adequate D90. The definitive RT method used in Japan involves some specific features, with use of CS in WP-EBRT being one of the most important. CS causes different EBRT dose distribution patterns compared with those in the absence of CS. Tamaki et al. demonstrated a difference in the cumulative (EBRT plus ICBT) dose distribution between EBRT with CS and that without CS [23]. They demonstrated different dose distribution patterns, especially in the anterior–posterior directions. Based on these findings, we also evaluated the distances of the tandem axis to the anterior–posterior and to the lateral margin of the HR-CTV. We selected diameters of 20 mm and 30 mm as thresholds for the anterior–posterior and lateral diameters, respectively. These values were also selected based on those reported by Tamaki et al., who showed that a dose of 60 Gy encompassed a diameter of 4.4 cm in the anterior–posterior direction and of 6.9 cm in the lateral direction in patients who received 40 Gy EBRT without CS, 10 Gy EBRT with CS, and 18 Gy of ICBT prescribed to point A. [23]. Murakami et al. revealed that the distance between the tandem axis and the lateral margin of HR-CTV (35 mm) was a significant indicator for PFS, as well as HR-CTV D90 [12]. In our study, the length from the tandem axis to the lateral edge of the HR-CTV was also found to be a significant predictor of LC and PC. For significantly asymmetrical shaped tumors, a hybrid approach using additional interstitial sources would be a good idea to overcome this issue without increasing the dose to the OARs [26]. Ohno et al. reported the 5-year follow-up results in cervical cancer patients treated with CT-based IGBT [11]. The HR-CTV D90 values in their series were as follows: 69 ± 12 Gy for small tumors (<40 mm), 68 ± 6 Gy for medium-sized tumors (40–60 mm) and 67 ± 6 Gy for large tumors (>60 mm). The 5-year LC rates were excellent: 96% for small tumors, 91% for medium-sized tumors and 94% for large tumors. A combination of ICBT and interstitial brachytherapy was performed in 18% of the patients in their series. Based on these findings, coverage of the HR-CTV within an adequate dose constraint is important in addition to achieving an adequate HR-CTV D90 (e.g. 60 Gy).
The retroEMBRACE study demonstrated that a mean dose escalation from 78 Gy to 93 Gy resulted in increased LC in all patient categories [20]. The ABS guidelines recommend a cumulative EQD2 of 80–90 Gy to maximize LC [25]. In contrast, a threshold dose of 60 Gy, as demonstrated in Japanese series, including ours, seems to be unnaturally low. We consider two possible reasons for this. The first is the difference in EBRT delivery. In most Japanese institutions, including ours, CS is utilized in conjunction with the EBRT. We summed the EQD2 of 3D-IGBT and EBRT without CS EQD2 to give the cumulative HR-CTV D90, as done in previously published studies. However, Tamaki et al. revealed that this calculation method leads to underestimation of the cumulative dose (HR-CTV D90) [23]. Although CS of 4 cm width was utilized in our series, the actual doses delivered to the HR-CTV might be higher than those estimated using this simple calculation. The second reason involves the images utilized for IGBT. As Murakami pointed out [12], potential overestimation of the HR-CTV contoured on CT compared with MRI might have caused the dose discrepancy between the global and the Japanese series. Overestimation of the HR-CTV can lead to underestimation of the HR-CTV D90 [13, 14]. In the present study, HR-CTV was contoured on CT images in accordance with the published consensus-based guidelines [22]. The aim of the guidelines is to minimize variation in the HR-CTV among physicians and the discrepancy between CT-based and MR-based HR-CTVs. The guidelines recommend performing MRI immediately before the first ICBT as a reference, to further minimize discrepancies. In this study, some patients developed local recurrence despite receiving >60 Gy CT-based HR-CTV D90, and a cut-off dose of 70 Gy was found to have marginal significance on LC. Based on these findings, moderate dose escalation of the HR-CTV D90 (to ~70 Gy) might further improve LC with CT-based IGBT if the HR-CTV is contoured properly. A prospective study is needed to investigate the feasibility and efficacy of the dose-escalation strategy.
The present study showed that a prolonged OTT negatively affected oncologic outcomes. Tenderup et al. also demonstrated this finding in their 3D-IGBT series [21]. However, OTT was not found to be an independent factor in the multivariate analysis of this study. This suggested that the poorer outcomes observed were caused by other factors, e.g. the response to prior EBRT, residual tumor volume at the time of ICBT, and EBRT boost application.
There are some potential limitations in this study. It was a retrospective, single-center study involving a limited number of patients and a limited follow-up period. The actual EBRT and IGBT dose delivered were selected according to the physicians’ discretion. Although we adopted the consensus-based guidelines for CT-based HR-CTV, we still experienced some difficulties with target volume delineation in some cases.
In conclusion, the CT-based HR-CTV D90 was found to be an important prognostic indicator of LC in patients with cervical cancer treated with definitive RT/CCRT whose ICBT doses were prescribed to point A. The ICBT dose prescription with the CT-based HR-CTV D90 is essential for achieving a cumulative dose of >60 Gy, especially for patients with a large HR-CTV at the time of ICBT. To further improve the outcomes, application of additional interstitial needles for irregularly shaped HR-CTVs of certain diameters and moderate escalation of the HR-CTV D90 (to ~70 Gy) is encouraged in CT-based IGBT.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING
This work was supported by the JSPS KAKENHI (grant number JP16K10398).
REFERENCES
- 1. Landoni F, Maneo A, Colombo A et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- 2. Nakano T, Kato S, Ohno T et al. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer 2005;103:92–101. [DOI] [PubMed] [Google Scholar]
- 3. Toita T, Kato S, Niibe Y et al. Prospective multi-institutional study of definitive radiotherapy with high-dose-rate intracavitary brachytherapy in patients with nonbulky (<4-cm) stage I and II uterine cervical cancer (JAROG0401/JROSG04–2). Int J Radiat Oncol Biol Phys 2012;82:e49–56. [DOI] [PubMed] [Google Scholar]
- 4. Ebina Y, Yaegashi N, Katabuchi H et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 2015;20:240–8. [DOI] [PubMed] [Google Scholar]
- 5.NCCN clinical practice guidelines in oncology (NCCN guidelines®) Cervical Cancer version I.2017—October 10, 2016. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. (27 August 2017, date last accessed).
- 6. Grover S, Harkenrider MM, Cho LP. Image guided cervical brachytherapy: 2014 survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2016;94:598–604. [DOI] [PubMed] [Google Scholar]
- 7. Haie-Meder C, Pötter R, Van Limbergen E et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group* (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45. [DOI] [PubMed] [Google Scholar]
- 8. Pötter R, Haie-Meder C, Van Limbergen E et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 9. Harkenrider MM, Alite F, Silva SR et al. Image-based brachytherapy for the treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2015;92:921–34. [DOI] [PubMed] [Google Scholar]
- 10. Ohno T, Toita T, Tsujino K et al. A questionnaire-based survey on 3D image-guided brachytherapy for cervical cancer in Japan: advances and obstacles. J Radiat Res 2015;56:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohno T, Noda SE, Okonogi N et al. In-room computed tomography–based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res 2017;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakami N, Kasamatsu T, Wakita A et al. CT based three dimensional dose–volume evaluations for high-dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer 2014;14:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viswanathan AN, Dimopoulos J, Kirisits C et al. Computed tomography versus magnetic resonance imaging–based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys 2007;68:491–8. [DOI] [PubMed] [Google Scholar]
- 14. Viswanathan AN, Erickson B, Gaffney DK et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2014;90:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimopoulos JC, Pötter R, Lang S et al. Dose–effect relationship for local control of cervical cancer by magnetic resonance image–guided brachytherapy. Radiother Oncol 2009;93:311–5. [DOI] [PubMed] [Google Scholar]
- 16. Georg P, Lang S, Dimopoulos JC et al. Dose–volume histogram parameters and late side effects in magnetic resonance image–guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2011;79:356–62. [DOI] [PubMed] [Google Scholar]
- 17. Georg P, Pötter R, Georg D et al. Dose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image–guided adaptive cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys 2012;82:653–7. [DOI] [PubMed] [Google Scholar]
- 18. Jastaniyah N, Yoshida K, Tandrup K et al. A volumetric analysis of GTVD and CTVHR as defined by the GEC ESTRO recommendations in FIGO stage IIB and IIIB cervical cancer patients treated with IGABT in a prospective multicentric trial (EMBRACE). Radiother Oncol 2016:120;404–11. [DOI] [PubMed] [Google Scholar]
- 19. Mazeron R, Fokdal LU, Kirchheiner K et al. Dose–volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: results from the prospective multicenter EMBRACE study. Radiother Oncol 2016;120:412–9. [DOI] [PubMed] [Google Scholar]
- 20. Sturdza A, Pötter R, Fokdat LU et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016;120:428–33. [DOI] [PubMed] [Google Scholar]
- 21. Tanderup K, Fokdal LU, Sturdza A et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol 2016;120:441–6. [DOI] [PubMed] [Google Scholar]
- 22. Ohno T, Wakatsuki M, Toita T et al. Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res 2017;58:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamaki T, Ohno T, Noda SE et al. Filling the gap in central shielding: three dimensional analysis of EQD2 dose in radiotherapy for cervical cancer with the central shielding technique. J Radiat Res 2015;56:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato S, Tran DN, Ohno T et al. CT-based 3D dose–volume parameter of the rectum and late rectal complication in patients with cervical cancer treated with high-dose-rate intracavitary brachytherapy. J Radiat Res 2010;51:215–21. [DOI] [PubMed] [Google Scholar]
- 25. Viswanathan AN, Beriwai S, De Los Santos JF et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High dose-rate brachytherapy. Brachytherapy 2012;11:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wakatsuki M, Ohono T, Yoshida D et al. Intracavitary combined with CT-guided interstitial brachytherapy for locally advanced uterine cervical cancer: introduction of the technique and a case presentation. J Radiat Res 2011;52:54–58. [DOI] [PubMed] [Google Scholar]