In the title compound, ethyl 2-cyano-2-(1,3-dithian-2-ylidene)acetate, the six-membered 1,3-dithiane ring has a twist-boat conformation. In the crystal, the molecule stack in layers up the a axis; there are no significant intermolecular interactions present.
Keywords: crystal structure; 1,3-dithian-2-ylidene; twist-boat conformation
Abstract
The title compound, C9H11NO2S2, contains a 1,3-dithiane ring which has a twist-boat conformation. The dihedral angle between the mean planes of the ethyl acetate group and the dithiane ring is 17.56 (13)°. In the crystal, molecules stack in layers up the a-axis direction, however, there are no significant intermolecular interactions present.
Chemical context
The derivatives of compounds such as α-oxo-ketene dithioacetals may undergo various transformations, in addition to the reactions involving the carbonyl group, C=C double bond, or the sulfur atoms. The emphasis in recent years has focused on the development of new and efficient intermediates. Some examples include (a) the preparation of highly regioselective compounds in a one-step reaction [the first example to be reported was the regiospecific synthesis of poly-substituted phenols from 1,5-dielectrophiles, via the five carbon atoms that are available in the structures of acenoyl ketene dithioacetals (Bi et al., 2005 ▸)]; (b) the synthesis of complex molecules based on new efficient and cost-effective reactions because they allow more than one transformation into a single synthetic sequence (Dömling et al., 2012 ▸; Tietze et al., 2006 ▸); (c) the preparation of trifluoromethyl-containing organic compounds of particular interest in the pharmaceutical and agrochemical fields due to their lipophilicity, hydrophobic properties and stable metabolic character (Furuya et al., 2011 ▸). Muzard and co-workers have been involved in the chemistry of trifluoromethylketene dithioacetals, especially perfluoroketene dithioacetals, and have reported in their work the preparation of trifluoromethylketene dithioacetals (Muzard & Portella, 1993 ▸).
The functionalization of ketene dithioacetals provides more powerful tools for the development of new intermediates (Wang et al., 2011 ▸; Gao et al., 2010 ▸; Hu et al., 2012 ▸). Of such constructions on the skeleton of the ketene dithioacetals, especially those involving the formation of the C—C bonds using carboelectrophiles such as aldehydes, have provided an effective link between these compounds and a variety of organic compounds with other functional groups. Minami et al. (1996 ▸) reported in their work the synthesis of α-hydroxyphosphonoketene dithioacetals from aldehydes. In addition, Kouno et al. (1998 ▸) have shown that phosphorus enyne-containing groups and dithiolanes could be prepared by cross-coupling of dithioacetal cyclic α-(iodopropane) with the corresponding alkyne phosphonoketene.
The direct formation of the C—C bond has been carried out by reacting α-cyano ketene dithioacetal and Morita–Baylis–Hillman (MBH) alcohols resulting from the reaction of acrylonitrile and aryl aldehydes. This reaction led to the corresponding 1,4-pentadiene derivatives (Zhao et al., 2007 ▸).
New synthetic pathways of various intermediates characterized by several functional groups have been created by transforming the α-acetylcetaldithioacetal functional group into α-hydroxy, α-chloro and α-bromo (Liu et al., 2003 ▸) and α-ethynyl ketene (Dong et al., 2005 ▸). The creation of new pathways to access such multi-functionalized compounds has also been achieved by reactions involving cleavage of the C—S bond (Dong et al., 2011 ▸). It should be noted here that the functionalization of the alkylthio group of these compounds has led to products useful in a wide range of applications (Mahata et al., 2003 ▸)
Fiala et al. (2007 ▸) have studied the inhibitive action of some synthesized ketene dithioacetal derivatives towards the corrosion of copper in aerated nitric acid solutions. They concluded that these compounds are good inhibitors of copper corrosion in this medium. The inhibitory properties of the title compound with respect to the corrosion of a transition metal in an acid medium were investigated in a separate study.
Herein, we report on the synthesis and crystal structure of ethyl 2-cyano-2-(1,3-dithian-2-ylidene)acetate (I). We also examined the effect of the substitution of the methyl group of methyl 2-cyano-2-(1,3-dithian-2-ylidene)acetate (II) (Hamdouni et al., 2017 ▸) by the ethyl group of the title compound.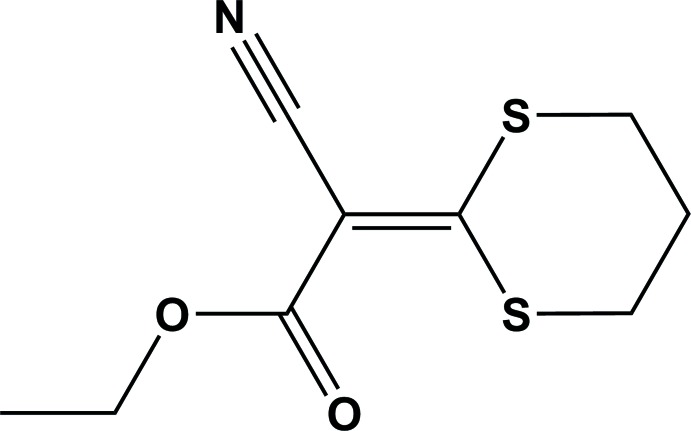
Structural commentary
The molecular structure of the title compound (I), is illustrated in Fig. 1 ▸. The mean planes of the ethyl acetate group [C1/C2/O1/O2/C8/C9; maximum deviation of 0.051 (2) Å for atom O2] and the dithiazane ring (S1/S2/C1–C4) are inclined to one another by 17.56 (13)°. The dithiane ring (S1/S2/C4–C7) has a twist-boat conformation [puckering parameters: amplitude (Q) = 0.909 (2) Å, θ = 89.88 (19)°, and φ = 331.65 (16)°].
Figure 1.
The molecular structure of the title compound (I), with the atom labelling. Displacement ellipsoids are drawn at the 50% probability level.
The C—S bond lengths differ as expected, with the Csp 2—S bonds [S1—C4 = 1.747 (2) and S2—C4 = 1.736 (2) Å] being shorter that the Csp 3—S bonds [S1—C5 = 1.805 (3) and S2—C7 = 1.817 (3) Å]. The C2=C4 bond length is 1.378 (3) Å. All the bond lengths and angles agree well with those reported for similar compounds, for example in methyl 2-cyano-2-(1,3-dithian-2-ylidene)acetate, compound (II) mentioned above.
Supramolecular features
In the crystal of (I), molecules stack in layers up the a-axis direction (Fig. 2 ▸); however, there are no significant intermolecular interactions present.
Figure 2.
A view along the b axis of the crystal packing of the title compound (I).
Database survey
A search of the Cambridge Structural Database (Version 5.38, update May 2017; Groom et al., 2016 ▸) for the 2-(1,3-dithian-2-ylidene) skeleton yielded eight hits. They include a number of 1,2-bis(dithian-2-ylidenes), such as dimethyl 1,2-bis(dithian-2-ylidene)-ethane-1,2-dicarboxylate (ZIGVOA; Benati et al., 1995 ▸). Since that update, the structure of the methyl analogue, (II), of the title compound has been reported by our group (Hamdouni et al., 2017 ▸). The two structures differ essentially in the orientation of the twist-boat dithiazane ring, as shown by the structural overlap of the two molecules in Fig. 3 ▸. The puckering parameters for (I) are Q = 0.909 (2) Å, θ = 89.88 (19)° and φ = 331.65 (16)°, while those for (II) are Q = 0.632 (3) Å, θ = 106.5 (3)° and φ= 114.3 (3)°. The mean planes of the ethyl acetate group [C1/C2/O1/O2/C8/C9; maximum deviation of 0.051 (2) Å for atom O2] and the dithiazane ring (S1/S2/C1–C4) in compound (I) are inclined to one another by 17.56 (13)°. The corresponding dihedral angle in compound (II) is 11.60 (12)°. In the crystals, the molecules stack along [100] in (I) and [010] in (II), and there are no significant intermolecular interactions present in either.
Figure 3.
Structural overlap of compounds (I) and (II); the latter is shown in red.
Synthesis and crystallization
The title compound was prepared according to a method proposed by Thuillier & Vialle (1962 ▸). Potassium carbonate, K2CO3, (42 g, 0.3 mol) and the corresponding active methylene compound, ethyl 2-cyanoacetate, (0.1 mol) were taken in 50 ml of DMF. The reaction mixture was stirred magnetically, then carbon disulfide (9 ml, 0.15 mol) was added at all once at room temperature. The stirring was maintained for 10 min before the dropwise addition of 1,3-dibromopropane (0.12 mol) over a period of 20 min. After stirring at room temperature for 7 h, ice-cold water (500 ml) was added to the reaction mixture. The yellow precipitate that formed was filtered, dried and then purified by recrystallization from ethanol (yield 93%, m.p. 368 K). The title compound exhibited the following characteristics: molar mass is M w = 229 g mol−1. FT–IR (cm−1): 1700 (C=O), 1246–1004 [C—O (ester)], 2206 (C≡N), 1437 (C=C). 1H NMR (CDCl3, δ p.p.m., 250 MHz): 1.35 (t, 3H, CH3—CH2), 2.30 (m, 2H, CH2), 3.00 (t, 2H, CH2S), 3.10 (t, 2H, CH2S), 4.30 (q, 2H, CH2O). 13C NMR (CDCl3, δ p.p.m., 250 MHz):14.22 (s, CH3—CH2—O), 23.36 (s, S—CH2—CH2—CH2—S), 28.99 (s, S—CH2—CH2—CH2—S), 61.26 (s, CH3–CH2), 120.55 (s, CN), 76.69 (s, O=C—C=C), 165.56 (s, O—-C=O). MS: m/z 229.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. The H atoms were included in calculated positions and treated as riding atoms: C—H = 0.96–0.97 Å with U iso(H) = 1.5U eq(C-methyl) and 1.2U eq(C) for other H-atoms.
Table 1. Experimental details.
| Crystal data | |
| Chemical formula | C9H11NO2S2 |
| M r | 229.31 |
| Crystal system, space group | Monoclinic, I2/a |
| Temperature (K) | 293 |
| a, b, c (Å) | 15.826 (3), 8.0772 (6), 18.431 (2) |
| β (°) | 111.830 (16) |
| V (Å3) | 2187.1 (5) |
| Z | 8 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.46 |
| Crystal size (mm) | 0.48 × 0.27 × 0.13 |
| Data collection | |
| Diffractometer | Agilent Xcalibur Eos |
| Absorption correction | Multi-scan (CrysAlis PRO; Agilent, 2013 ▸) |
| T min, T max | 0.334, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4539, 2132, 1667 |
| R int | 0.035 |
| (sin θ/λ)max (Å−1) | 0.617 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.049, 0.138, 1.08 |
| No. of reflections | 2132 |
| No. of parameters | 127 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.46, −0.34 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989017017893/su5404sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017017893/su5404Isup2.hkl
CCDC reference: 1811267
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Mr F. Saidi, Engineer at the Laboratory of Crystallography, University Constantine 1, for assistance in collecting the X-ray data on the Xcalibur diffractometer.
supplementary crystallographic information
Crystal data
| C9H11NO2S2 | F(000) = 960 |
| Mr = 229.31 | Dx = 1.393 Mg m−3 |
| Monoclinic, I2/a | Mo Kα radiation, λ = 0.71073 Å |
| a = 15.826 (3) Å | Cell parameters from 1541 reflections |
| b = 8.0772 (6) Å | θ = 3.7–28.9° |
| c = 18.431 (2) Å | µ = 0.46 mm−1 |
| β = 111.830 (16)° | T = 293 K |
| V = 2187.1 (5) Å3 | Needle, pale yellow |
| Z = 8 | 0.48 × 0.27 × 0.13 mm |
Data collection
| Agilent Xcalibur Eos diffractometer | 2132 independent reflections |
| Graphite monochromator | 1667 reflections with I > 2σ(I) |
| Detector resolution: 8.02 pixels mm-1 | Rint = 0.035 |
| ω scans | θmax = 26.0°, θmin = 3.4° |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2013) | h = −17→19 |
| Tmin = 0.334, Tmax = 1.000 | k = −9→9 |
| 4539 measured reflections | l = −22→20 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.138 | H-atom parameters constrained |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0673P)2 + 0.4223P] where P = (Fo2 + 2Fc2)/3 |
| 2132 reflections | (Δ/σ)max < 0.001 |
| 127 parameters | Δρmax = 0.46 e Å−3 |
| 0 restraints | Δρmin = −0.34 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S2 | 0.12671 (5) | 0.08363 (8) | 0.29431 (4) | 0.0555 (3) | |
| S1 | 0.11241 (5) | 0.40461 (8) | 0.37170 (4) | 0.0589 (3) | |
| O1 | 0.14349 (14) | −0.1692 (2) | 0.40827 (11) | 0.0641 (5) | |
| O2 | 0.12483 (13) | −0.1107 (2) | 0.52062 (11) | 0.0592 (5) | |
| N1 | 0.1307 (2) | 0.2907 (3) | 0.55919 (14) | 0.0741 (7) | |
| C1 | 0.13376 (16) | −0.0706 (3) | 0.45311 (14) | 0.0477 (6) | |
| C2 | 0.13016 (16) | 0.1100 (3) | 0.44342 (13) | 0.0447 (6) | |
| C3 | 0.13080 (18) | 0.2096 (3) | 0.50818 (15) | 0.0512 (6) | |
| C4 | 0.12494 (15) | 0.1895 (3) | 0.37572 (14) | 0.0449 (6) | |
| C5 | 0.1624 (2) | 0.4577 (4) | 0.30133 (16) | 0.0620 (7) | |
| H5A | 0.166410 | 0.577323 | 0.299200 | 0.074* | |
| H5B | 0.224000 | 0.414405 | 0.319553 | 0.074* | |
| C6 | 0.1113 (2) | 0.3938 (3) | 0.21943 (16) | 0.0639 (7) | |
| H6A | 0.153558 | 0.381976 | 0.192819 | 0.077* | |
| H6B | 0.065809 | 0.474754 | 0.190915 | 0.077* | |
| C7 | 0.06477 (19) | 0.2289 (3) | 0.21774 (15) | 0.0615 (7) | |
| H7A | 0.053615 | 0.176859 | 0.167585 | 0.074* | |
| H7B | 0.006049 | 0.249883 | 0.221227 | 0.074* | |
| C8 | 0.1298 (2) | −0.2859 (3) | 0.54028 (17) | 0.0617 (7) | |
| H8A | 0.185483 | −0.334042 | 0.539292 | 0.074* | |
| H8B | 0.078396 | −0.344992 | 0.503306 | 0.074* | |
| C9 | 0.1279 (2) | −0.2958 (4) | 0.62053 (19) | 0.0731 (9) | |
| H9A | 0.131070 | −0.409675 | 0.636284 | 0.110* | |
| H9B | 0.179046 | −0.236653 | 0.656364 | 0.110* | |
| H9C | 0.072531 | −0.247544 | 0.620570 | 0.110* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S2 | 0.0705 (5) | 0.0497 (4) | 0.0437 (4) | 0.0033 (3) | 0.0182 (3) | −0.0037 (3) |
| S1 | 0.0862 (5) | 0.0418 (4) | 0.0494 (4) | 0.0049 (3) | 0.0259 (4) | 0.0024 (3) |
| O1 | 0.0886 (14) | 0.0476 (10) | 0.0518 (11) | 0.0075 (9) | 0.0211 (10) | −0.0022 (9) |
| O2 | 0.0821 (13) | 0.0399 (9) | 0.0561 (11) | 0.0057 (8) | 0.0262 (10) | 0.0067 (8) |
| N1 | 0.115 (2) | 0.0547 (14) | 0.0513 (14) | 0.0002 (13) | 0.0293 (15) | −0.0027 (12) |
| C1 | 0.0463 (13) | 0.0469 (13) | 0.0407 (13) | 0.0027 (10) | 0.0057 (10) | 0.0025 (11) |
| C2 | 0.0471 (12) | 0.0443 (13) | 0.0349 (11) | 0.0026 (10) | 0.0064 (10) | −0.0013 (10) |
| C3 | 0.0616 (15) | 0.0447 (13) | 0.0413 (13) | 0.0019 (11) | 0.0122 (12) | 0.0057 (11) |
| C4 | 0.0417 (12) | 0.0435 (13) | 0.0422 (13) | 0.0018 (9) | 0.0073 (10) | 0.0005 (10) |
| C5 | 0.0682 (17) | 0.0556 (15) | 0.0593 (17) | −0.0084 (13) | 0.0203 (14) | 0.0065 (13) |
| C6 | 0.080 (2) | 0.0629 (17) | 0.0485 (15) | −0.0046 (14) | 0.0235 (14) | 0.0030 (14) |
| C7 | 0.0710 (17) | 0.0669 (17) | 0.0389 (13) | −0.0043 (14) | 0.0116 (13) | 0.0008 (13) |
| C8 | 0.0763 (19) | 0.0408 (13) | 0.0678 (19) | 0.0036 (12) | 0.0266 (15) | 0.0098 (12) |
| C9 | 0.098 (2) | 0.0544 (17) | 0.078 (2) | 0.0146 (15) | 0.0454 (19) | 0.0181 (15) |
Geometric parameters (Å, º)
| S2—C4 | 1.736 (2) | C5—H5B | 0.9700 |
| S2—C7 | 1.817 (3) | C6—C7 | 1.517 (4) |
| S1—C4 | 1.747 (2) | C6—H6A | 0.9700 |
| S1—C5 | 1.805 (3) | C6—H6B | 0.9700 |
| O1—C1 | 1.198 (3) | C7—H7A | 0.9700 |
| O2—C1 | 1.343 (3) | C7—H7B | 0.9700 |
| O2—C8 | 1.456 (3) | C8—C9 | 1.492 (4) |
| N1—C3 | 1.146 (3) | C8—H8A | 0.9700 |
| C1—C2 | 1.469 (3) | C8—H8B | 0.9700 |
| C2—C4 | 1.378 (3) | C9—H9A | 0.9600 |
| C2—C3 | 1.436 (3) | C9—H9B | 0.9600 |
| C5—C6 | 1.514 (4) | C9—H9C | 0.9600 |
| C5—H5A | 0.9700 | ||
| C4—S2—C7 | 100.12 (13) | C5—C6—H6B | 108.9 |
| C4—S1—C5 | 101.16 (13) | C7—C6—H6B | 108.9 |
| C1—O2—C8 | 116.8 (2) | H6A—C6—H6B | 107.7 |
| O1—C1—O2 | 124.3 (2) | C6—C7—S2 | 115.69 (19) |
| O1—C1—C2 | 125.9 (2) | C6—C7—H7A | 108.4 |
| O2—C1—C2 | 109.8 (2) | S2—C7—H7A | 108.4 |
| C4—C2—C3 | 118.1 (2) | C6—C7—H7B | 108.4 |
| C4—C2—C1 | 124.0 (2) | S2—C7—H7B | 108.4 |
| C3—C2—C1 | 117.9 (2) | H7A—C7—H7B | 107.4 |
| N1—C3—C2 | 179.1 (3) | O2—C8—C9 | 106.2 (2) |
| C2—C4—S2 | 122.55 (18) | O2—C8—H8A | 110.5 |
| C2—C4—S1 | 117.99 (18) | C9—C8—H8A | 110.5 |
| S2—C4—S1 | 119.43 (14) | O2—C8—H8B | 110.5 |
| C6—C5—S1 | 114.9 (2) | C9—C8—H8B | 110.5 |
| C6—C5—H5A | 108.5 | H8A—C8—H8B | 108.7 |
| S1—C5—H5A | 108.5 | C8—C9—H9A | 109.5 |
| C6—C5—H5B | 108.5 | C8—C9—H9B | 109.5 |
| S1—C5—H5B | 108.5 | H9A—C9—H9B | 109.5 |
| H5A—C5—H5B | 107.5 | C8—C9—H9C | 109.5 |
| C5—C6—C7 | 113.3 (2) | H9A—C9—H9C | 109.5 |
| C5—C6—H6A | 108.9 | H9B—C9—H9C | 109.5 |
| C7—C6—H6A | 108.9 | ||
| C8—O2—C1—O1 | 1.6 (4) | C7—S2—C4—C2 | 153.6 (2) |
| C8—O2—C1—C2 | −178.2 (2) | C7—S2—C4—S1 | −24.31 (17) |
| O1—C1—C2—C4 | 9.4 (4) | C5—S1—C4—C2 | 153.59 (19) |
| O2—C1—C2—C4 | −170.8 (2) | C5—S1—C4—S2 | −28.43 (18) |
| O1—C1—C2—C3 | −171.5 (2) | C4—S1—C5—C6 | 65.6 (2) |
| O2—C1—C2—C3 | 8.2 (3) | S1—C5—C6—C7 | −32.9 (3) |
| C3—C2—C4—S2 | 178.22 (18) | C5—C6—C7—S2 | −37.1 (3) |
| C1—C2—C4—S2 | −2.7 (3) | C4—S2—C7—C6 | 65.9 (2) |
| C3—C2—C4—S1 | −3.9 (3) | C1—O2—C8—C9 | 174.2 (2) |
| C1—C2—C4—S1 | 175.18 (18) |
References
- Agilent (2013). CrysAlis PRO. Agilent Technologies, Yarnton, England.
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Benati, L., Calestani, G., Montevecchi, P. C. & Spagnolo, P. (1995). J. Chem. Soc. Chem. Commun. pp. 1999–2000.
- Bi, X., Dong, D., Liu, Q., Pan, W., Zhao, L. & Li, B. (2005). J. Am. Chem. Soc. 127, 4578–4579. [DOI] [PubMed]
- Dömling, A., Wang, W. & Wang, K. (2012). Chem. Rev. 112, 3083–3135. [DOI] [PMC free article] [PubMed]
- Dong, D., Liu, Y., Zhao, Y., Qi, Y., Wang, Z. & Liu, Q. (2005). Synthesis, 85–91.
- Dong, Y., Wang, M., Liu, J., Ma, W. & Liu, Q. (2011). Chem. Commun. 47, 7380–7382. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Fiala, A., Chibani, A., Darchen, A., Boulkamh, A. & Djebbar, K. (2007). Appl. Surf. Sci. 253, 9347–9356.
- Furuya, T., Kamlet, A. S. & Ritter, T. (2011). Nature, 473, 470–477. [DOI] [PMC free article] [PubMed]
- Gao, X., Di, C.-A., Hu, Y., Yang, X., Fan, H., Zhang, F., Liu, Y., Li, H. & Zhu, D. (2010). J. Am. Chem. Soc. 132, 3697–3699. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hamdouni, N., Boudjada, A., Meinnel, J., Fiala, A., Brahim Ladouani, H. & Lemallem, S. E. (2017). IUCrData, 2, x171018.
- Hu, Y., Qin, Y., Gao, X., Zhang, F., Di, C.-A., Zhao, Z., Li, H. & Zhu, D. (2012). Org. Lett. 14, 292–295. [DOI] [PubMed]
- Kouno, R., Okauchi, T., Nakamura, M., Ichikawa, J. & Minami, T. (1998). J. Org. Chem. 63, 6239–6246. [DOI] [PubMed]
- Liu, Q., Che, G., Yu, H., Liu, Y., Zhang, J., Zhang, Q. & Dong, D. (2003). J. Org. Chem. 68, 9148–9150. [DOI] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Mahata, P. K., Venkatesh, C., Syam Kumar, U. K., Ila, H. & Junjappa, H. (2003). J. Org. Chem. 68, 3966–3975. [DOI] [PubMed]
- Minami, T., Okauchi, T., Matsuki, H., Nakamura, M., Ichikawa, J. & Ishida, M. (1996). J. Org. Chem. 61, 8132–8140. [DOI] [PubMed]
- Muzard, M. & Portella, C. (1993). J. Org. Chem. 58, 29–31.
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Thuillier, A. & Vialle, J. (1962). Bull. Soc. Chim. Fr. pp. 2187–2193.
- Tietze, L. F., Brasche, G. & Gericke, K. (2006). Domino Reactions in Organic Synthesis. Weinheim: Wiley-VCH.
- Wang, H., Zhao, Y.-L., Ren, C.-Q., Diallo, A. & Liu, Q. (2011). Chem. Commun. 47, 12316–12318. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zhao, Y.-L., Chen, L., Liu, Q. & Li, D.-W. (2007). Synlett, pp. 37–42.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989017017893/su5404sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017017893/su5404Isup2.hkl
CCDC reference: 1811267
Additional supporting information: crystallographic information; 3D view; checkCIF report





