Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease leading to progressive loss of memory and other cognitive functions. One of the well-known pathological markers of AD is the accumulation of amyloid-beta protein (Aβ), and its plaques, in the brain. Recent studies using Tg-5XFAD mice as a model of AD have reported that exposure to radiofrequency electromagnetic fields (RF-EMF) from cellular phones reduced Aβ plaques in the brain and showed beneficial effects on AD. In this study, we examined whether exposure to 1950 MHz RF-EMF affects Aβ processing in neural cells. We exposed HT22 mouse hippocampal neuronal cells and SH-SY5Y human neuroblastoma cells to RF-EMF (SAR 6 W/kg) for 2 h per day for 3 days, and analyzed the mRNA and protein expression of the key genes related to Aβ processing. When exposed to RF-EMF, mRNA levels of APP, BACE1, ADAM10 and PSEN1 were decreased in HT22, but the mRNA level of APP was not changed in SH-SY5Y cells. The protein expression of APP and BACE1, as well as the secreted Aβ peptide, was not significantly different between RF-EMF–exposed 7w-PSML, HT22 and SH-SY5Y cells and the unexposed controls. These observations suggest that RF-EMF exposure may not have a significant physiological effect on Aβ processing of neural cells in the short term. However, considering that we only exposed HT22 and SH-SY5Y cells to RF-EMF for 2 h per day for 3 days, we cannot exclude the possibility that 1950 MHz RF-EMF induces physiological change in Aβ processing with long-term and continuous exposure.
Keywords: 1950 MHz radiofrequency electromagnetic fields (RF-EMF), Alzheimer’s disease, Aβ processing, mouse hippocampal neuronal cell line, human neuroblastoma cell line, CHO cell–based 7w-PSML cell line
INTRODUCTION
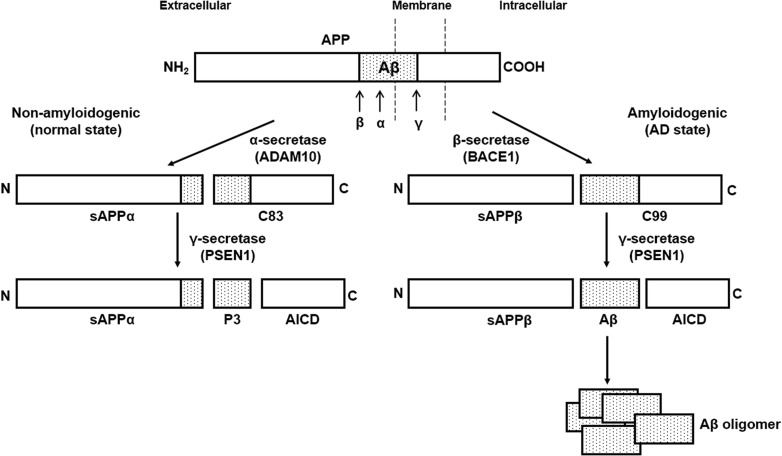
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorders, and it leads to progressive loss of memory and other cognitive functions (review by Kumar et al. [1]). One of the well-known pathological markers of AD is the accumulation of amyloid-beta protein (Aβ), and its plaques, in the brain. Aβ is processed from amyloid precursor protein (APP), a type I transmembrane protein. The pathways and enzymes for APP processing and Aβ formation were simplified and presented in Fig. 1. APP is newly synthesized in the endoplasmic reticulum (ER), and it traffics through the secretory pathway to the Golgi apparatus and to the plasma membrane. In the non-amyloidogenic pathway, APP is first cleaved by α-secretase [which is a member of the disintegrin and metalloproteinase (ADAM) family, notably ADAM10] and then by the γ-secretase complex, to produce an innocuous membrane-embedded peptide of 26 amino acids and secreted sAPPα (review by Jiang et al. [2]). sAPPα has been reported to activate the proliferation of adult neuroblasts (review by Chasseigneaux et al. [3]), but its physiological role is unknown [4]. The main subunit of the γ-secretase complex is presenilin 1 encoded by PSEN1, which is mutated in patients with AD [5]. APP can also be processed by β-secretase (encoded by beta-site amyloid precursor protein cleaving enzyme 1 [BACE1]) and γ-secretase, to release the neurotoxic Aβ peptide, which spontaneously forms oligomers, and then the larger amyloid plaques found in the brain of patients with AD (reviews by Jiang et al. [2], Thinakaran et al. [6] and Kumar et al. [1]). Diverse genetic and molecular evidence suggests that the abnormal accumulation of Aβ occurs in the early stage of the pathophysiological cascade that eventually leads to AD (reviews by Huang et al. [7], Palop et al. [8] and Bertram et al. [9]). However, what determines the processing of APP to Aβ, and how Aβ impairs neuronal function have not been clearly understood.
Fig. 1.
A simple diagram of APP processing and Aβ secretion in brain cells. The processing of amyloid precursor protein (APP) occurs in two distinct pathways: amyloidogenic and non-amyloidogenic. In the non-amyloidogenic pathway, APP is cleaved by α-secretase (ADAM10) at the site within the Aβ domain, releasing a soluble APPα (sAPPα) fragment and a C-terminal fragment (C83). The C83 fragment is further cleaved by γ-secretase (PSEN1) to release the APP intracellular domain (AICD) and a 3 kDa (p3) fragment. The amyloidogenic pathway involves the sequential cleavage of APP by β-secretase (BACE1) and γ-secretase (PSEN1). β-secretase generates a soluble APPβ (sAPPβ) fragment and a C-terminal fragment (C99), which is cleaved by γ-secretase (PSEN1) to release AICD and amyloid-β (Aβ) peptide. Secreted Aβ forms oligomers and aggregates, generating the toxic amyloid plaques observed in the AD patient’s brain (drawn by J. Park, and review by Hicks et al. [33]).
In neural cells, the endogenous expression of APP is usually very low, and the amount of secreted Aβ is insufficient to be detected, which has been a technical barrier to studying APP processing and its mechanism [10]. 7w-PSML is a stably transfected CHO-based cell line that expresses both the wild-type human APP and mutant presenilin-1 (M146L), which is an efficient model for the detection of APP, and its cleaved and secreted form, Aβ peptide [11–13]. Thus, 7w-PSML has been used as a cellular model system for AD for studying Aβ processing in vitro: the conversion of monomeric Aβ into a toxic aggregated state [12, 14], the Aβ generation through endocytosis of APP [11], and the role of neuronal membrane cholesterol in excessive Aβ peptide production [10].
Previously, 900 and 1800 MHz RF-EMF radiations, allocated to cellular phones, were reported to induce negative effects on brain function, such as brain tumors (reviews by Lagorio et al. [15] and Hardell et al. [16]) and memory impairments [17]. However, several recent studies using transgenic (Tg)-FAD mice as a model of AD have shown that RF-EMFs have beneficial effects on neurodegenerative disorders: 918 MHz EMF enhanced memory and decreased brain Aβ aggregation in AD 3X Tg-mice [18]. RF-EMF with a frequency range from 800 to 2450 MHz improved cognitive impairment in AD 2X or 3X Tg-mice [19, 20], and enhanced brain mitochondrial function, through the disaggregation of Aβ oligomers in Tg-5XFAD and normal mice [21]. In addition, 1950 MHz RF-EMF directly affected Aβ pathology by reducing Aβ plaques and BACE1 expression in Tg-5XFAD mice [22]. These studies demonstrated the effects of RF-EMF radiation on AD pathology, mainly with FAD mouse models, but could not elucidate the cellular mechanism involved in these RF-EMF–derived physiological effects.
In this study, we investigated the effects of RF-EMF on Aβ processing in HT22 mouse hippocampal neuronal cells, SH-SY5Y human neuroblastoma cells and 7w-PSML cells, to examine the mechanism for the physiological outcomes of RF-EMF at a cellular level.
MATERIALS AND METHODS
Cell culture
HT22 (immortalized mouse hippocampal neuronal) cells and SH-SY5Y (human neuroblastoma) cells were kindly provided by Dr Inhee Mook-Jung (Seoul National University College of Medicine, Seoul, Korea). These two cell lines have been used as well-established in vitro cellular models for neurodegenerative disorders such as AD and Parkinson’s disease; HT22 cells have functional cholinergic properties related to the cognitive defects of AD [23], and SH-SY5Y cells have synaptic structures, functional axonal vesicle transport, and express neurospecific proteins (review by Carolindah et al. [24]). CHO cell–based 7w-PSML cells [which overexpress wild-type human APP and the mutant presenilin-1 (M146L) [13]] were provided by Dr David Kang (Florida State University, USA). These cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (v/v) fetal bovine serum (FBS; Sigma-Aldrich, MO, USA) and 10 ml/l penicillin–streptomycin (GIBCO, NY, USA). All cells were grown at 37°C in a humidified atmosphere containing 5% CO2.
The RF-EMF radiation system for cell exposure
We used a radial transmission line (RTL) exposure system as an in vitro multifrequency radiation exposure system for this study [25, 26]. A typical Code–Division Multiple Access (CDMA) signal at 837 MHz and a Wideband Code–Division Multiple Access (WCDMA) signal at 1950 MHz were applied to the RTL after amplification. The signals were modulated by real CDMA and WCDMA signals for cell exposure. The specific absorption rate (SAR) distribution inside the exposed medium was defined by numerical simulations using the finite-difference time-domain (FDTD) method (XFDTD 6.5, Remcom, State College, PA). The simulated and measured SAR values for 1 W input power in the entire sample was 0.105 ± 0.019 W/kg for the CDMA frequency and 0.262 ± 0.055 W/kg for the WCDMA frequency.
The exposure system was warmed up for at least 30 min, for equilibration, before the RF-EMF exposure. The 100-mm culture dishes were placed at 13.6 cm from the conical antenna, which was located at the center of the exposure chamber, and cells were then exposed to RF-EMF radiation in the culture dishes. This system has already been used in other published cellular studies [25, 26]. The variation in RF-EMF exposures on different culture dishes was negligible, when multiple dishes were exposed at the same time: the variation in the average SAR values for WCDMA frequency was ~7.7% of the single dish exposure when three culture dishes were used, and ~2.0% when six dishes were exposed [25, 26].
Cells were exposed to RF-EMF radiation of a single signal (WCDMA signal at 1950 MHz) at 6 W/kg for 2 h/day for 3 days, with the same fixed timetable for the same intervals. During the exposure period, the temperature was maintained within a range of 37 ± 0.3°C by circulating water within the cavity, and a 5% CO2 concentration was also maintained in the chamber. After exposure to RF-EMF radiation, the cells were immediately transferred to a cell culture incubator. RF-EMF-treated and untreated cells and each culture medium were collected immediately after the last exposure to RF-EMF radiation, for further experiments.
RNA isolation and quantitative real-time PCR
Cells were harvested and their total RNA was isolated using the RNeasy Mini kit (QIAGEN, Germany). cDNA was synthesized with purified total RNA using the PrimeScript RT reagent kit (Takara Bio Inc., Shiga, Japan). Quantitative real-time PCR was performed using the CFX96 instrument (Bio-Rad, CA, USA), with the primers in Table 1. The relative expression of genes was normalized to that of the control gene encoding β–actin. The normalized fold expression of mRNA levels in RF-EMF–exposed and –unexposed cells was analyzed using the 2−ΔΔCT method as described previously [27]. Real-time PCR products were confirmed by electrophoresis on a 2.5% agarose gel.
Table 1.
The primers used for quantitative real-time PCR
| Gene | Oligonucleotide primer | Product size (bp) |
|---|---|---|
| Human/Mouse | 5′-CATCTTCACTGGCACACCGT-3′ (forward) | 115 |
| APP | 5′-CAAACTCTACCCCTCGGAAC-3′ (reverse) | |
| Human/Mouse | 5′-AATTCTGCTCCTCTCCTGGGC-3′ (forward) | 211 |
| ADAM10 | 5′-CCTCTTCATTCGTAGGTTGA-3′ (reverse) | |
| Human/Mouse | 5′-TGTGGAGATGGTGGACAACCTG-3′ (forward) | 168 |
| BACE1 | 5′-TGCCTCTGGTAGTAGCGATG-3′ (reverse) | |
| Human/Mouse | 5′-GAGCTGACATTGAAATATGG-3′ (forward) | 218 |
| PSEN1 | 5′-ACAATGACACTGATCATGATGGC-3′ (reverse) | |
| Human β-actin | 5′-TCCCTGGAGAAGAGCTACGA-3′ (forward) | 194 |
| 5′-AGCACTGTGTTGGCGTACAG-3′ (reverse) | ||
| Mouse β-actin | 5′-CGCCACCAGTTCGCCATGGA-3′ (forward) | 105 |
| 5′-TACAGCCCGGGGAGCATCGT-3′ (reverse) |
Protein analysis
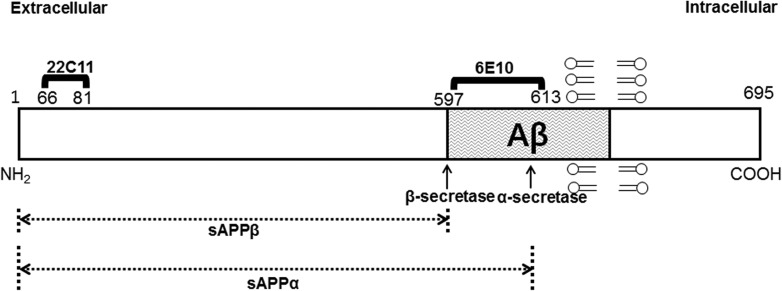
After the cells were exposed to the RF-EMF, the cell culture medium was collected to precipitate secreted peptides by TCA precipitation. Precipitated samples were separated by the 4–20% gradient precast Tris-Glycine SDS gel (KomaBiotech, Seoul, Korea), to detect secreted Aβ peptides. RF-EMF–exposed and unexposed cells were harvested and lysed as described previously by Park et al. [28]. Cell extracts were analyzed by western blot by 7.5% or 10% SDS-PAGE with anti-Alzheimer Precursor Protein A4 (clone 22C11; Millipore, MA, USA), anti-β-amyloid 1–16 (clone 6E10; BioLegend, CA, USA), anti-BACE1 (Millipore, MA, USA), and anti-β-actin (Cell Signaling Technology, Inc., MA, USA) antibodies. To detect the differentially processed forms of APP in cells, we used two different APP antibodies, anti-22C11 and anti-6E10, which detect different regions of the human APP protein (Fig. 2). Anti-22C11 binds to membrane APP (amino acids 66–81 in the N-terminal domain of APP) and can precipitate the full-length APP as well as its N-terminal fragments, soluble APPα (sAPPα) and APPβ (sAPPβ) (Fig. 2). Anti-6E10 reacts not only with APP and the processed sAPPα, but also with Aβ by binding between amino acids 597–613 (Fig. 2) [29, 30]. An enhanced chemiluminescence reagent (Amersham Biosciences, Buckinghamshire, UK) was used for the blot analysis. The relative band intensity of the samples from RF-EMF–exposed and unexposed cells to the loading control (β-actin) was measured using GelQuantNet (BiochemLabSolutions, CA, USA), and the average result from three independent experiments was plotted.
Fig. 2.
A representation of regions of human APP differentially processed and detected with two different antibodies. A schematic structure of human APP protein (695 amino acid form) with the regions that respectively react to two different antibodies: anti-22C11 and anti-6E10 (adapted from [29, 30]).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., CA, USA). Data are represented with the mean ± standard error of the mean (S.E.M.) of at least three independent experiments. We applied t-tests to assess statistically significant differences. P < 0.05 (*), P < 0.01 (**), and P < 0.0001 (****) indicated statistical significance compared with control. Non-statistically significant results (n.s) represented P > 0.05.
RESULTS
The effect of 1950 MHz EMF exposure on the expression of genes associated with Aβ processing in mouse and human neural cells
In order to determine the molecular mechanism of the effect of RF-EMF on Aβ processing at the cellular level, we first investigated whether exposure to RF-EMF could affect the expression of the genes critical for Aβ processing in neural cells; amyloid precursor protein (APP), α-secretase (ADAM10), β-secretase (BACE1) and γ-secretase (PSEN1). HT22 (mouse hippocampal neuronal) cells and SH-SY5Y (human neuroblastoma) cells were exposed to RF-EMF radiation (single 1950 MHz W-CDMA signal at 6 W/kg) for 2 h per day for 3 days. The mRNA level of each gene was measured by quantitative real-time PCR in the RF-EMF–exposed cells and was compared with that of the unexposed control cells. The mRNA expression of APP was reduced 0.64-fold in RF-EMF–exposed HT22 cells, when compared with that of unexposed HT22 cells, and the mRNA levels of ADAM10, BACE1 and PSEN1 slightly decreased in RF-EMF–exposed HT22 cells (Fig. 3A and B). In SH-SY5Y cells exposed to RF-EMF, there was no decrease in the mRNA expression of APP, but the mRNA expression level of ADAM10 and BACE1 was slightly decreased and that of PSEN1 was marginally increased compared with those of unexposed cells (Fig. 3C and D). These results showed that RF-EMF exposure decreased the expression of APP mRNA and slightly diminished the expression of genes involved in the processing of APP, ADAM10, BACE1 and PSEN1 in mouse HT22 cells. In human SH-SY5Y cells, RF-EMF exposure induced marginal changes in the expression of genes involved in Aβ processing, but not on the expression of APP.
Fig. 3.
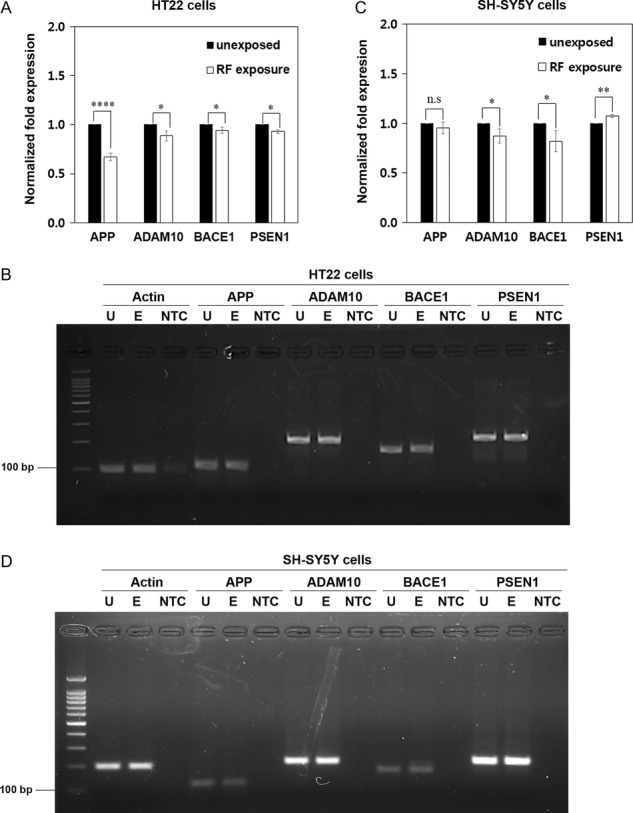
The effect of 1950 MHz EMF exposure on the expression of APP and genes associated with Aβ processing in mouse and human neural cells. (A–D) HT22 (A, B) and SH-SY5Y (C, D) cells were exposed to radio frequency–electromagnetic field (RF-EMF) radiation (1950 MHz at 6 W/kg) for 2 h per day for 3 days. (A, C) The mRNA expression of APP, ADAM10, BACE1 and PSEN1 was analyzed in RF-EMF–treated and –untreated cells by quantitative real-time PCR and normalized to β-actin mRNA expression. Data are represented as mean ± S.E.M. from three independent experiments. P < 0.05 (*); P < 0.01 (**); P < 0.0001(****); P > 0.05 (non-significant, n.s). (B, D) Quantitative real-time PCR products in HT22 (B) and SH-SY5Y (D) were confirmed by electrophoresis on a 2.5% agarose gel. U = unexposed cells, E = RF-EMF–exposed cells, NTC = No Template Control.
1950 MHz EMF exposure does not affect the processing and secretion of Aβ in 7w-PSML cells
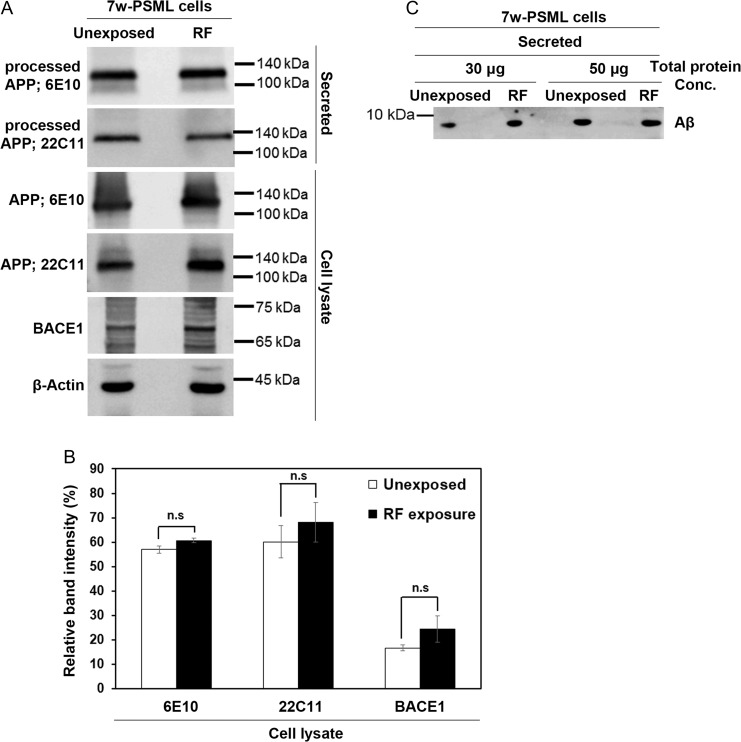
Several studies have discovered that exposure to RF-EMF induces the reduction of APP, BACE1 and Aβ peptide in the AD transgenic mouse model [20, 22]. In order to understand whether RF-EMF exposure could affect the processing of APP into Aβ, in neural cells, we first examined the expression of BACE1 and several processed forms of APP in 1950 MHz EMF–exposed 7w-PSML cells.
7w-PSML cells were exposed to 1950 MHz EMF for 2 h every day for 3 days, and both the cells and culture medium were collected. Secreted peptides in the culture medium were precipitated by TCA precipitation, and analyzed by the 4–20% gradient precast Tris-Glycine SDS-PAGE. Expression of APP and BACE1 was also detected by western blot analysis with the lysates of RF-EMF–exposed cells. No significant difference in the expression of APP and BACE1 was detected in RF-EMF–exposed cells, when compared with the unexposed controls (Fig. 4A). To confirm this result, we normalized the relative band intensity of APP and BACE1 to β-actin as a loading control, but could not detect a meaningful difference between RF-EMF–exposed cells and unexposed controls (Fig. 4B). In addition, RF-EMF exposure did not affect the secreted level of sAPP (α or β) or Aβ peptide in 7w-PSML cells (Fig. 4A and C). These results showed that 1950 MHz EMF exposure does not significantly change the pattern of Aβ processing in 7w-PSML cells.
Fig. 4.
1950 MHz EMF exposure does not directly affect the processing and secretion of Aβ in 7w-PSML cells. (A) 7w-PSML cells were exposed to RF-EMF radiation (1950 MHz at 6 W/kg) for 2 h per day for 3 days. Secreted peptides in the culture medium were precipitated by TCA. APP and BACE1 in cell lysates, and sAPP (α or β) in precipitated samples, were detected by western blot analysis. β-actin was used as a loading control. (B) The relative band intensity of APP and BACE1 in cell lysates to the loading control was calculated by GelQuantNet software from the results of three independent experiments, including (A). Data are represented as mean ± S.E.M. from three independent experiments. P > 0.05 (non-significant, n.s). (C) Total 30 and 50 μg proteins of the TCA precipitated medium were separated by a 4–20% gradient precast Tris-Glycine SDS gel, and Aβ peptides were detected with anti-6E10 by western blot analysis.
1950 MHz EMF exposure has no direct effect on Aβ processing and secretion in HT22 and SH-SY5Y cells
We then investigated whether exposure to 1950 MHz EMF alters the endogenous protein expression of APP and BACE1 as well as of processed Aβ in HT22 and SH-SY5Y cells. HT22 and SH-SY5Y cells were exposed to 1950 MHz EMF radiation for 2 h every day for 3 days, and the expression of APP and BACE1 were analyzed by western blot. The endogenous expression of APP and BACE1 did not change in RF-EMF–exposed HT22 cells, compared with in the unexposed controls (Fig. 5A). The normalized band intensity of APP and BACE1 relative to a loading control β-actin in cell lysates of HT22 showed a marginal decrease of APP in RF-EMF–exposed cells, although it was not statistically meaningful (Fig. 5B). No significant difference in the amount of secreted Aβ peptide was observed between RF-EMF–exposed and unexposed HT22 cells (Fig. 5C). In SH-SY5Y cells, we also could not detect any meaningful variation in the expression of APP and BACE1 as well as of the secreted Aβ in RF-EMF–exposed cells and unexposed controls (Fig. 5D–F). These observations demonstrated that short-term exposure to 1950 MHz EMF does not change the expression of proteins for APP processing and Aβ secretion in either HT22 or SH-SY5Y cells.
Fig. 5.
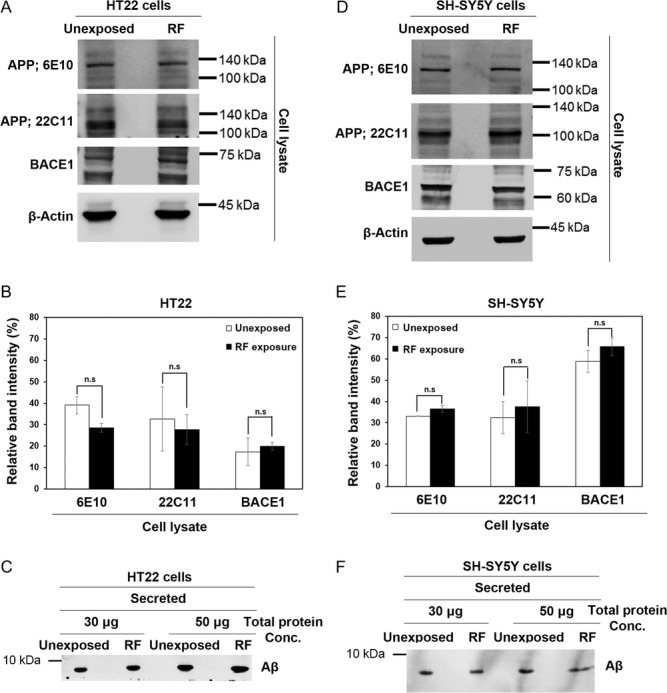
1950 MHz EMF exposure has no significant effect on the APP protein expression and Aβ secretion in either HT22 or SH-SY5Y cells. (A–F) HT22 (A, B, C) and SH-SY5Y (D, E, F) cells were exposed to 1950 MHz at 6 W/kg for 2 h per day for 3 days. (A, D) Expression of APP and BACE1 in cell extracts of (A) HT22 and (D) SH-SY5Y was analyzed by western blot. β-actin was used as a loading control. (B, E) The relative band intensity of APP and BACE1 in cell lysates of (B) HT22 and (E) SH-SY5Y to the loading control was calculated by GelQuantNet software from the results of three independent experiments, including (A) and (D). Data are represented as mean ± S.E.M. from three independent experiments. P > 0.05 (non-significant, n.s). (C, F) Cell culture medium was precipitated with TCA, and secreted peptides were analyzed by a 4–20% gradient precast Tris-Glycine SDS gel. Secreted Aβ peptide was detected by western blot analysis with anti-6E10 in (C) HT22 and (F) SH-SY5Y cell media.
DISCUSSION
Previously, researchers have focused on elucidating the negative effect of RF-EMF radiation emitted by cellular phones on the brain, such as brain tumor development [31, 32]. However, several recent studies have reported the beneficial effect of RF-EMF radiation on AD using AD transgenic mouse models [19–21]. In order to evaluate the cellular mechanism of the effect of RF-EMF on AD, in this study, we examined the effect of 1950 MHz EMF radiation (single WCDMA signal at SAR 6 W/kg, 2 h per day for 3 days) on the endogenous expression of APP and major proteins for APP processing, as well as the secretion of Aβ in HT22 and SH-SY5Y neural cells. In addition, we also investigated the effect of RF-EMF radiation in the 7w-PSML cell as it is an efficient model for the detection of APP and Aβ peptide [11–13]. We showed in this study that 1950 MHz EMF radiation for 2 h every day for 3 days did not induce significant changes in the level of APP and processing and secretion of Aβ in mouse and human neural cells as well as in 7w-PSML cells.
We exposed these cells with 1950 MHz EMF radiation for 2 h a day, because the Tg-5xFAD mice, which showed ameliorated Aβ pathology by RF-EMF, were exposed to 1950 MHz EMF for 2 h every day [22]. We wanted to examine the cellular effect of RF-EMF under the same conditions as those used for Tg mice. Also, the RF-EMF system we used was originally designed for a maximum of 2 h exposure per day, based on the assumption that most people on average use mobile phones for a total of 2 h a day. With our observations, we could suggest that the RF exposure conditions applied in this study may not have a significant effect on the expression and processing of APP or Aβ in mouse and human neural cells.
However, in the RF-EMF–exposed HT22 cells, we repeatedly observed that the mRNA expression of APP and genes for APP processing seems to be decreased with statistically meaningful significance (Fig. 3A), and the expression of APP was marginally reduced, although the secreted Aβ was not much changed (Fig. 5A–C). Thus, with these results, it would be difficult to define whether 1950 MHz EMF radiation has a meaningful effect on decreasing the expression of APP and the secretion of Aβ in mouse neural cells, or not. However, we cannot exclude the possibility that 1950 MHz RF-EMF exposures to mouse neural cells induce the minute decline observed in the mRNA expression of APP and genes for APP processing as well as the marginal decrease in APP, which may lead to the beneficial effect of RF-EMF radiation on AD in AD transgenic mouse models.
In RF-EMF–exposed human SH-SY5Y cells, the mRNA of the genes involved in APP processing was slightly changed, but the mRNA and protein expression of APP, as well as the secreted Aβ, was not decreased with statistically meaningful significance. These observations suggest that the mouse neural cells might be more sensitive to RF-EMF than the human neural cells, although we need more results on the effect of RF-EMF on other mouse and human neural cells to compare with. The limitation of available mouse and human neural cell lines would be a technical barrier in studying the different effects of RF-EMF on the expression and processing of APP.
Why could we not observe a significant change in the level of APP as well as in the secretion of Aβ in mouse and human neural cells by RF-EMF radiation, while RF radiation to the AD transgenic mouse models showed reduced expression of APP and BACE1, as well as Aβ depositions in the brain? [18, 22]. We believe that a major reason for the discrepancy in the effect of RF is the exposure period. We exposed HT22 and SH-SY5Y cells to RF-EMF for a short period (2 h per day for 3 days) only, because 3 days was the limitation of the cell culture span. However, the AD mouse models were exposed to RF-EMF for 8 months [22]. Thus, we could conclude that a short-term exposure of RF-EMF to neural cells does not induce significant changes in the expression or processing of APP at the cellular level. However, minute changes caused by RF-EMF radiation, accumulated for a long period of exposure in the AD mouse model, may result in obvious physiological outcomes, such as reduced Aβ deposits. Further studies would be necessary to understand whether long-term and/or continuous exposure or extended exposure time to RF-EMF radiation affects Aβ processing at the cellular level.
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning [No. NRF-2016M3A9C6918275] and by Korea Mobile EMF consortium. J. Park was partially supported by the Graduate School of YONSEI University Research Scholarship Grants of 2017.
ACKNOWLEDGEMENTS
The authors thank Dr David Kang (at Florida State University) for providing 7w-PSML cells, and Dr Inhee Mook-Jung (Seoul National University College of Medicine, Seoul, Korea) for providing HT22 cells and SH-SY5Y cells. K.S. conceived the idea, designed the experiments, and wrote the manuscript. J.P. performed the experiments and wrote the manuscript. J.H.K. and N.K. provided the 1950 MHz EMF radiation device for exposure of the cells.
REFERENCES
- 1. Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 2015;67:195–203. [DOI] [PubMed] [Google Scholar]
- 2. Jiang ST, Li YF, Zhang X et al. Trafficking regulation of proteins in Alzheimer’s disease. Mol Neurodegener 2014;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chasseigneaux S, Allinquant B. Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J Neurochem 2012;120:99–108. [DOI] [PubMed] [Google Scholar]
- 4. Demars MP, Bartholomew A, Strakova Z et al. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res Ther 2011;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chavez-Gutierrez L, Bammens L, Benilova I et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. Embo J 2012;31:2261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem 2008;283:29615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang YD, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 2012;148:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palop JJ, Mucke L. Amyloid-β induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 2010;13:812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron 2010;68:270–81. [DOI] [PubMed] [Google Scholar]
- 10. Abad-Rodriguez J, Ledesma MD, Craessaerts K et al. Neuronal membrane cholesterol loss enhances amyloid pepticle generation. J Cell Biol 2004;167:953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem 1994;269:17386–9. [PubMed] [Google Scholar]
- 12. Walsh DM, Tseng BP, Rydel RE, et al. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 2000;39:10831–9. [DOI] [PubMed] [Google Scholar]
- 13. Jung ES, Hong H, Kim C, et al. Acute ER stress regulates amyloid precursor protein processing through ubiquitin-dependent degradation. Sci Rep 2015;5:8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Podlisny MB, Ostaszewski BL, Squazzo SL, et al. Aggregation of Secreted Amyloid Beta-Protein into Sodium Dodecyl Sulfate-Stable Oligomers in Cell-Culture. J Biol Chem 1995;270:9564–70. [DOI] [PubMed] [Google Scholar]
- 15. Lagorio S, Roosli M. Mobile Phone Use and Risk of Intracranial Tumors: A Consistency Analysis. Bioelectromagnetics 2014;35:79–90. [DOI] [PubMed] [Google Scholar]
- 16. Hardell L, Carlberg M, Soderqvist F, et al. Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol 2013;43:1833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ntzouni MP, Skouroliakou A, Kostomitsopoulos N, et al. Transient and cumulative memory impairments induced by GSM 1.8 GHz cell phone signal in a mouse model. Electromagn Biol Med 2013;32:95–120. [DOI] [PubMed] [Google Scholar]
- 18. Arendash GW, Sanchez-Ramos J, Mori T, et al. Electromagnetic Field Treatment Protects Against and Reverses Cognitive Impairment in Alzheimer’s Disease Mice. J Alzheimers Dis 2010;19:191–210. [DOI] [PubMed] [Google Scholar]
- 19. Banaceur S, Banasr S, Sakly M, et al. Whole body exposure to 2.4 GHz WIFI signals: Effects on cognitive impairment in adult triple transgenic mouse models of Alzheimer’s disease (3xTg-AD). Behav Brain Res 2013;240:197–201. [DOI] [PubMed] [Google Scholar]
- 20. Arendash GW, Mori T, Dorsey M et al. Electromagnetic treatment to old Alzheimer’s mice reverses β-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS One 2012;7:e35751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dragicevic N, Bradshaw PC, Mamcarz M et al. Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer’s transgenic mice and normal mice: a mechanism for electromagnetic field-induced cognitive benefit? Neuroscience 2011;185:135–49. [DOI] [PubMed] [Google Scholar]
- 22. Jeong YJ, Kang GY, Kwon JH et al. 1950 MHz electromagnetic fields ameliorate Aβ pathology in Alzheimer’s disease mice. Curr Alzheimer Res 2015;12:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Li LX, Suo WZ. HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci 2009;84:267–71. [DOI] [PubMed] [Google Scholar]
- 24. Carolindah MN, Rosli R, Adam A et al. An overview of in vitro research models for Alzheimer’s disease (AD). Regen Res 2013;2:8–13. [Google Scholar]
- 25. Hong MN, Kim BC, Ko YG et al. Effects of 837 and 1950 MHz radiofrequency radiation exposure alone or combined on oxidative stress in MCF10A cells. Bioelectromagnetics 2012;33:604–11. [DOI] [PubMed] [Google Scholar]
- 26. Lee JS, Kim JY, Kim HJ et al. Effects of combined radiofrequency field exposure on amyloid-beta–induced cytotoxicity in HT22 mouse hippocampal neurones. J Radiat Res 2016;57:620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 28. Park J, Lee H, Lee HJ et al. Non-thermal atmospheric pressure plasma efficiently promotes the proliferation of adipose tissue–derived stem cells by activating NO-response pathways. Sci Rep 2016;6:39298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sastre M, Turner RS, Levy E. X11 interaction with beta-amyloid precursor protein modulates its cellular stabilization and reduces amyloid beta-protein secretion. J Biol Chem 1998;273:22351–7. [DOI] [PubMed] [Google Scholar]
- 30. Caille I, Allinquant B, Dupont E et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 2004;131:2173–81. [DOI] [PubMed] [Google Scholar]
- 31. Khurana VG, Teo C, Kundi M et al. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol 2009;72:205–14. [DOI] [PubMed] [Google Scholar]
- 32. Hardell L, Carlberg M, Soderqvist F et al. Meta-analysis of long-term mobile phone use and the association with brain tumours. Int J Oncol 2008;32:1097–103. [PubMed] [Google Scholar]
- 33. Hicks DA, Nalivaeva NN, Turner AJ. Lipid rafts and Alzheimer’s disease: protein–lipid interactions and perturbation of signaling. Front Physiol 2012;3:189. [DOI] [PMC free article] [PubMed] [Google Scholar]