Abstract
Deep venous thrombosis (DVT) is a significant medical problem with an incidence of 1 in 1,000 adults and greatly reduces quality of life through post-thrombotic syndrome. Treatment choice for DVT can be influenced by the age of the clot. While new endovascular catheter techniques treat venous clots to potentially prevent post-thrombotic syndrome, they require improved imaging techniques to accurately determine clot age. This review investigates experimental and clinical evidence of elastography techniques for aging DVT. Strain elastography and shear wave elastography are the most common techniques to age thrombus. These elastography techniques can distinguish between acute and chronic clots by characterizing tissue stiffness. When clot age cannot be determined with ultrasound duplex analysis, elastography may offer a helpful adjunct. However, further investigation is required to validate accuracy and reproducibility for clinical implementation of this novel technique.
Keywords: Deep venous thrombosis (DVT), elastography, shear wave, ultrasound (US)
Introduction
Deep venous thrombosis (DVT) represents a significant clinical problem that affects 1 in 1,000 adults (1). Sequela of DVT includes sudden death from pulmonary embolism (PE), complications of anticoagulation, and reduced quality of life from post thrombotic syndrome (PTS) (2). Autopsy studies have demonstrated that only 19% of all pulmonary emboli are clinically suspected, and many PEs and DVTs are missed at the time of death (3). Additionally, not only do these ailments greatly harm patient prognosis and quality of life, but they also have grave economic consequences. DVT with PE costs between $13.5 and $69.5 billion dollars in total healthcare expenditure in the United States. Although duplex ultrasonography can reliably diagnose DVT with sensitivity and specificities greater than 95%, it is not able to reliably differentiate acute and chronic thrombi (4). Although some clinicians use size of the vein, collateral status, and thrombi echogenicity to help assess thrombus age, none can reliably distinguish acute and chronic thrombus (5-7). Aging of DVT remains largely qualitative and non-standardized, and purely based on patient reporting of symptoms; knowing the age of the clot is critical as it guides treatment.
Although the methods for aging DVT clots are imprecise, clot age has the utmost clinical significance. Thrombus age influences initial treatment choice for DVT, including anticoagulant type and the applicability of catheter-directed therapy (CDT). Specifically, acute clots, which are higher risk for PE, require immediate therapeutic anticoagulation with heparin. Conversely, treatment of chronic clots is less aggressive with slower-acting, oral anticoagulants. Recently developed CDTs can reduce risk of PTS, but are currently only indicated for thrombus occurring within 14 days of presentation (1,8,9). Thrombolysis and thrombectomy are only effective at removing acute thrombi because as the clot ages, it adheres to the vessel endothelium. There is an unacceptable level of risk of vessel wall damage in older clots (10-13). The benefits of CDT are preservation of valvular function and the vessel lumen, neither of which are preserved with conventional anti-coagulation therapy. Age differentiation of clots would also be useful in correctly diagnosing re-thrombosis of a previously thrombosed vein, a clinical dilemma that can often be unclear.
Since clot age clearly influences the treatment approach and prognosis for patients, measuring this quantity is extremely clinically desirable. As a clot ages, its mechanical properties also change; therefore, clot stiffness should be a surrogate marker for the chronicity of a clot. Clots progressively harden as it is transformed from being composed predominantly of platelets to fibrin. As clots mature, the fibrin fibers cross link causing further hardening. Additionally, there is replacement with dense connective tissue (Figure 1). Occasionally, these are palpated as a rope-like structure in the patient’s thigh or calf.
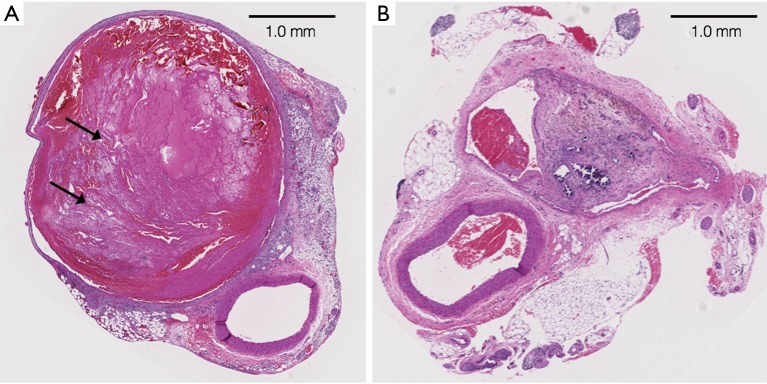
Figure 1.
Induced thrombus within the rat IVC (hematoxylin and eosin stain). (A) Three-day old thrombus in the rat IVC; the vein is markedly expanded with characteristic lines of Zahn (arrows) indicating the acute state of the thrombus; (B) 30-day old thrombus in the rat IVC; the vein is markedly fibrotic with dense connective tissue. IVC, inferior vena cava.
The current gold standard for assessment of DVT is venous duplex ultrasonography, combining color flow Doppler imaging with compression ultrasonography. However, no conventional modality, including ultrasound (US), magnetic resonance imaging (MRI), or computed tomography (CT), can directly provide information about tissue rigidity, a characteristic associated with clot age. In contrast, elasticity imaging techniques, such as elastography, can non-invasively estimate the mechanical properties of tissues, such as stiffness, and thus can estimate clot age in DVT patients. Age differentiation of clot would also be useful in correctly diagnosing re-thrombosis of a previously thrombosed vein, a clinical decision that can often be unclear.
Elastography is a non-invasive medical imaging modality for assessing the elastic mechanical properties of soft tissue, both quantitatively and qualitatively. This technique, introduced approximately 25 years ago, relies on the principle that pathological tissues are stiffer than normal tissues (14-16). Specifically, elastography determines the tissue’s stiffness, or resistance to applied forces, by measuring a ratio of stress to strain (i.e., the amount of deformation of an object given an external force) called the Young’s modulus, expressed in units of pressure. Elastography techniques have been widely used in liver imaging employing both MR and US based methods, particularly for cirrhosis assessment. While initially developed to non-invasively assess liver fibrosis, physicians have recently adopted the technique to characterize a variety of other tissues, including the brain, breast, prostate, thyroid, and muscles. However, the application of elastography to the evaluation of DVT stems from the need to differentiate between acute and chronic thrombi.
Few promising imaging techniques have emerged to estimate thrombus age in an objective, accurate and cost-effective manner that would be feasible in a clinical setting. This review aims to collate and evaluate experimental and clinical evidence on these imaging system and deformation techniques for indirect elasticity measurement. A summary of different studies reviewed can be seen in Table 1.
Table 1. Summary of animal and clinical studies on venous clot aging using elastography techniques.
| Authors | Elastography technique | Experimental setting | Thrombus model | Thrombus ages | Outcomes |
|---|---|---|---|---|---|
| Emelianov et al. (17) | Strain | In vivo | Rat IVC | 2, 6, and 9 days | Older clots were consistently harder (higher Young’s modulus) and more homogenous than younger ones |
| Rubin et al. (18) | Strain | Clinical | Lower extremity DVT | 25 days and 3 years | Chronic clot was more homogenous with strain 10× smaller than vessel wall; subacute clot had strain 3–4× greater than the vessel wall |
| Xie et al. (19) | Strain | In vivo | Rat IVC | 3, 4, 5, 6, 7, and 10 days | Strain magnitude progressively decreases as clot ages; model developed that could estimate clot age accurate within 0.8 days |
| Aglyamov et al. (20) | Strain | In vivo and ex vivo | Rat IVC explanted in gelatin | 2 and 9 days | Elasticity reconstruction may prove to be a practical adjunct to triplex scanning to detect, diagnose, and stage DVT |
| Xie et al. (21) | Strain | In vivo and ex vivo | Rat IVC | 3, 6, 10, 12, and 14 days | Strong agreement between mechanical measurement of Young’s modulus and that measured by ultrasound |
| Geier et al. (22) | Strain | Ex vivo | Explanted porcine iliac vein | 1, 3, 6, 9, 12, and 15 days | The most pronounced changes in clot elasticity occurred between the 6th and 12th days, P<0.01 |
| Rubin et al. (23) | Strain | Clinical | Lower extremity DVT | Acute (mean =5.7 days) and chronic (>8 months) | Median normalized strain magnitude for the acute cases was 2.75, with an interquartile range of 2.40 to 3.71, whereas the median normalized strain magnitude for the chronic cases was 0.94, with interquartile range of 0.48 to 1.36 |
| Yi et al. (24) | Strain | Clinical | Lower extremity DVT | Various | The strain ratio of the chronic thrombosis group and the subacute thrombosis group were higher than that of the acute thrombosis group (P<0.001, <0.05) |
| Mfoumou et al. (25) | Shear wave | In vivo and ex vivo | Rabbit IJ | Every 10 minutes for 2 hours and up to 2 weeks | Stagnant blood in the region of interest underwent clotting and progressive hardening with thrombus aging; transition points observed around day 7 |
| Liu et al. (26) | Shear wave | In vivo and ex vivo | Rabbit IVC | Every 10 minutes for 2 hours and up to 2 weeks | Strain elastography measurements consistent with histological analysis; days 4 and 7 after thrombus induction may represent the transition points for acute, sub-acute and chronic thrombi in rabbit models |
IVC, inferior vena cava; DVT, deep venous thrombosis; IJ, internal jugular.
Strain elastography (SE)
US has been the gold standard for the diagnosis of DVT, so ultrasonography-related techniques have been the most investigated for determining the age of venous thrombosis. The most common US elastography technique for investigating venous thrombosis is SE. SE, also known as quasi-static elastography or real-time elastography, uses deformation induced by manually compressing the transducer against the tissue of interest and measuring the displacement (27). Tissue stiffness can be visually represented using measurements of this relative displacements (28-30). Several commercial systems have been developed to perform these real-time calculations, similar to the Doppler imaging mode. These systems can determine the relative stiffness of the lesion compared to the background tissue. SE often does not require any modification to conventional US hardware probes, but does require post-processing and software modifications (31).
During SE, the technologist applies slightly varying pressure to acquire information derived from B-mode images (14). Variations in tissue images assumed from the external stress are then compared to the original images (31). The tissue displacement is estimated along multiple lines in the axis of the probe, and this deformation data determines strain. An elastogram is then mapped in color scale over the grayscale images to visually represent the relative stiffness (Figure 2). SE has several major limitations, including that it requires fine control of the stress applied to the tissue and cannot easily provide quantitative data. Moreover, the stress applied is entirely operator-dependent, leading to low reproducibility.
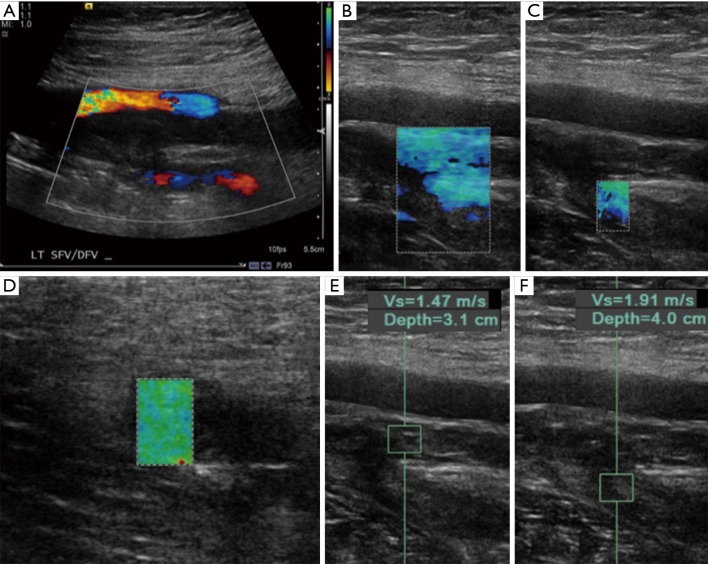
Figure 2.
Ultrasound imaging in a 35-year-old male who reported left leg pain that started 10 hours ago as he was walking on his way to work. (A) Color Doppler imaging of the lower extremity demonstrating absence of flow in the superficial femoral vein and diminished flow in the deep femoral artery; (B-D) strain elastography over the superficial femoral artery and deep femoral artery branch with overlaid strain map. The green and blue color overlays indicate that these areas are relatively soft as described previously by Itoh et al. in their grading system; (E,F) corresponding shear wave elastography qualitative measurements in the region of echogenic thrombus. Measurements reported represent the velocity of the shear waves. Although no criteria have been developed for the diagnosis of acute versus chronic clot, these numbers are also relatively low suggesting a soft thrombus. This patient was subsequently taken to the interventional radiology suite for catheter-assisted thrombolysis using an EKOS catheter.
Experimental studies
Initial investigations into assessing clot age focused on US characteristics of the clot (32). Histological evaluation of clots of varying age have demonstrated marked increases in fibroblasts and collagen production as clots age. After approximately two weeks, there are typically signs of advanced organization within the clot with organized collagen filaments, focal calcifications within the thrombus, and fibrous thickening of the venous intima (22).
Emelianov et al. originally hypothesized that fibrin organization changes in clots as they mature, and these structural changes leads to measurable changes in elasticity, so US elastography was used to evaluate clot age (17). Clots were surgically induced in rats, which were split into equal groups kept for 2, 6, or 9 days before undergoing US evaluation. In this study, US was used to estimate strain during variable compression of induced clots of different ages. The group demonstrated chronic clots had a higher Young’s modulus and a more homogenous echotexture than acute ones, suggesting chronic clots are stiffer. They also demonstrated that the detection of a clot did not require excessive pressure applied by the transducer, a practice frequently used to prove the absence of a clot. Since focused pressure during US can be painful for patients, this improved technique can detect clots in patients without causing such severe pain.
Other studies by Aglyamov et al. and Geier et al. using similar animal models had similar findings showing US elastography could be used to assess the thrombus age (20,22). In both studies, manual compression of the transducer by the operator with a series of images obtained at varying compression levels induced strain in the target vein. Next, a predefined algorithm used the degree of compression and the changes in shape and speckle movement with each compression to compute the Young’s modulus. In each of these studies, the strain values normalized to either the surrounding tissue or the vessel wall most accurately differentiated clot age. Geier et al. reported that the most pronounced changes in clot elasticity occurred between the 6th and 12th days (22).
Although these animal models confirmed the hypothesis that clot stiffness is time-dependent and that US elastography techniques can assess this change, none of these studies had provided an actual method for estimating clot age from the strain measurements. Therefore, Xie et al. built upon this premise with a staging model that estimated clot age from measured thrombus strain normalized to the vessel wall (19). Applied to induced clots in different rats, this model estimated the clot age within 0.8 days of the true age; these results suggest that a similar model could be developed for human clots. Xie also demonstrated agreement between Young’s modulus measurements by US and mechanical measurements of explanted thrombosed vessels (21). In this rat model, the least accurate estimate was 1.7 days for a 10-day-old clot.
Clinical studies
One of the big obstacles in applying the techniques developed with the inferior vena cava (IVC) rat models to the human deep venous system is deforming the target vein. Usual deformations performed reliably in rats, such as pushing the IVC against the hard spine, do not exist in humans. Deformations against the femur or tibia are not reliable due to the obliquity of the forces applied. Nonetheless, several applications of these US elastography techniques to human patients have successfully demonstrated its ability to age clots. Rubin et al. initially applied US elastography imaging in patients with DVTs of known age from the start of the symptoms. In this study, the transducer applied manual strain similarly to how normal duplex studies apply compression for DVTs; the relative movement of the speckle within the clot was compared to the vessel wall during compression. In a patient with a 3-year-old chronic clot, US revealed a homogenous echogenicity and relative increased hardness compared to the vessel wall. In the subacute 25-day old clot, this clot exhibited a much more heterogeneous strain profile, and the majority of the clot was softer than the vessel wall. These findings were analogous to those demonstrated in a prior rat model (17). Additionally, estimating the strain required much less forceful pushing of the transducer than conventional compression techniques. The reduced force suggests that this technique applies to patients who cannot tolerate deep compression.
Although the initial study by Rubin et al. only had two patients, a similar study design was implement in a larger prospective human study consisting of 54 patients, of which 26 had acute DVT (mean age =5.7 days) and 28 had chronic DVT (mean age >8 months) compared acute and chronic thrombi (23). This expanded study demonstrated similar results, with a significant statistical difference between acute and chronic clots. The median normalized strain magnitude for the acute cases was 2.75, while chronic cases of thrombus measured a strain of 0.94. Although these findings were demonstrated in human patients, the two populations had widely different ages: the average age of acute clots was 5.7 days, and chronic clots were older than 8 months. Moreover, this study did not address differences in acute and subacute clots. Therefore, the study only demonstrated that the performance of US elasticity was equal to that of using thrombus echogenicity at differentiating clot age.
The most recent US elastography for clot age differentiation was performed by Yi et al. in 2017 (24). This prospective study included 127 patients with DVT and used strain elastography to assess clot age. This study used a five-point scoring scale initially developed by Itoh et al. for scoring the elasticity of thyroid and breast nodules, with a score of 1 indicating iso-elasticity (green on imaging) and a high degree of deformability, and a score of 5 indicating that the entire lesion and surrounding tissue are stiff (blue on imaging). This study confirmed the ability of US elastography to measure differences in clot age. The strain ratio of the chronic thrombosis group and the subacute thrombosis group were higher than that of the acute thrombosis group (P<0.001, <0.05), and the strain ratio of the chronic thrombosis group was higher than that of the acute and subacute thrombosis group (P<0.05).
Shear wave elastography
Although many techniques investigated for age characterization of clot rely on US to apply technician-driven compression to the target tissue, other elastography techniques do not require such compression. Shear wave elasticity imaging (SWEI) is an imaging technique for quantitative elastic evaluation of tissues. By generating shear waves, SWEI creates response to deformation forces, such as steady vibration. The relationship between shear waves and Young’s modulus is beyond the scope of this text; however, in brief, elasticity of the tissue is proportional to the square of the shear velocity. Acoustic radiation force (ARF) provides the deformation forces, and in response to the deformation, shear waves propagate away from the focal point at relatively slow speeds measurable by Doppler US. The Young’s modulus of the tissue can then be derived from the shear wave properties in the tissue (33). Since shear waves propagate at right angles to the source of deformation, specialized transducers must create ARFs at a 90-degree angle to the pulse-echo receiver.
Mfoumou et al. (25) were the first to apply SWEI for the measurement of time-dependent hardening of blood clots in vivo. In this study, SWEI evaluated induced thrombus in the rabbit internal jugular vein every 10 minutes for the first 2 hours and every day for 2 weeks. Even within the first 2 hours, SWEI demonstrated that stagnant blood in the region of interest underwent clotting and progressive hardening with thrombus aging, evident. The mean Young’s modulus varied from 1.0±0.6 kPa (at 10 min), to 5.3±1.6 kPa (at 2 hours), and finally to 25.0±6.8 kPa (at 14 days) post-surgery. Ex vivo evaluation of the clotted segment performed at 2 weeks confirmed the in vivo measurements. A transition point in elasticity around day 7 for the rabbit thrombus suggested a definable shift from acute to chronic thrombus in the rabbit model. Similar shifts had been seen in mouse and rat models (19-22). Whether the timing of these shifts is the same in human clot transformation is not known, but these results provide a tangible starting point for future studies in humans.
A similar study conducted by Liu et al. evaluated IVC clots from rabbits ranging from 2 hours to 2 weeks of age using SWEI and selected histological analysis of clots of different age (26). This study evaluated several areas in each clot—the “head”, “body”, and “tail”—corresponding to the “epicenter” of the clot and regions surrounding this epicenter. Similar to the study of Mfoumou et al., the Young’s modulus increased with thrombus maturity. Significant increases occurred on different days for each part: The head showed significant increases on days 4 and 6, the body showed significant increases on days 4 and 7, and the tail showed significant increases on days 3 and 6. The histological changes were concordant with the transition points within the Young’s modulus curve. In this study, they speculated that these days represented the transition points between acute, sub-acute, and chronic thrombi. Since heterogeneity existed between transition times of various locations, the authors suggested that the body of the clot was the best for evaluation.
MRI elastography
Similar to US, elastography techniques can also be applied to MRI. Although several techniques involving MRI have been developed for aging thrombus, no investigations have applied MRI elastography to thrombus imaging most likely because of limited availability of MR imaging and the need for fast, cost-effective exams for thrombosis cases. Although US methods more efficiently image veins in the extremities relevant for DVT, MRI elastography techniques could be useful in regions not as easily accessible or with limited compressibility (e.g., the IVC, iliac veins, portal/mesenteric veins, and dural sinuses). Nonetheless, no investigations of MR elastography have been done in these areas.
Conclusions
Accurate estimate of the stage and maturity of DVT is useful in determining proper therapeutic management. Acute thrombus is unstable and carries a significant rate of PE necessitating treatment with anticoagulation (34). With a shift toward CDT for acute thrombus treatment for extensive, proximal DVT, it is increasingly important for noninvasive studies to confidently differentiate between acute and chronic thrombus (35). Since thrombus hardens over time, several studies have demonstrated that two US-based elastography techniques can assess thrombus age in animal models. For instance, Xie and his team have built a model to prospectively estimate thrombus age in animal studies. However, US elastography techniques are still unable to prospectively estimate the age of thrombus in patients with DVT. Further investigations to accurately characterize DVT clot maturation in humans is necessary to develop a similar model that can be used in clinical applications. There is a progressive enthusiasm towards developing advanced elastography techniques that will accurately diagnose the age of thrombus in DVT. Ultimately, while elastography has the potential to determine the highly clinical relevant characteristic of clot age and classify acute or chronic DVT, further studies with large cohorts of human participants must validate the accuracy and reproducibility of data generated using animal models and establish more cost-effective and clinically relevant treatment protocols based on these novel techniques.
Acknowledgements
Funding: Dr. Rahmi Oklu gratefully acknowledges funding from the National Institutes of Health (No. EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Behravesh S, Hoang P, Nanda A, et al. Pathogenesis of Thromboembolism and Endovascular Management. Thrombosis 2017;2017:3039713. 10.1155/2017/3039713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AT, Hamilton M, Mitchell SA, et al. Comparison of the Novel Oral Anticoagulants Apixaban, Dabigatran, Edoxaban, and Rivaroxaban in the Initial and Long-Term Treatment and Prevention of Venous Thromboembolism: Systematic Review and Network Meta-Analysis. PLoS One 2015;10:e0144856. 10.1371/journal.pone.0144856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med 1989;82:203-5. 10.1177/014107688908200407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas MG, Sumner DS. Duplex scanning for deep vein thrombosis: has it replaced both phlebography and noninvasive testing? Semin Vasc Surg 1996;9:3-12. [PubMed] [Google Scholar]
- 5.Fowlkes JB, Strieter RM, Downing LJ, et al. Ultrasound echogenicity in experimental venous thrombosis. Ultrasound Med Biol 1998;24:1175-82. 10.1016/S0301-5629(98)00089-1 [DOI] [PubMed] [Google Scholar]
- 6.Hammers LW, Cohn SM, Brown JM, et al. Doppler color flow imaging surveillance of deep vein thrombosis in high-risk trauma patients. J Ultrasound Med 1996;15:19-24. 10.7863/jum.1996.15.1.19 [DOI] [PubMed] [Google Scholar]
- 7.Murphy TP, Cronan JJ. Evolution of deep venous thrombosis: a prospective evaluation with US. Radiology 1990;177:543-8. 10.1148/radiology.177.2.2217798 [DOI] [PubMed] [Google Scholar]
- 8.Oklu R, Wicky S. Catheter-directed thrombolysis of deep venous thrombosis. Semin Thromb Hemost 2013;39:446-51. 10.1055/s-0033-1334142 [DOI] [PubMed] [Google Scholar]
- 9.Wicky S, Pinto EG, Oklu R. Catheter-Directed Thrombolysis of Arterial Thrombosis. Semin Thromb Hemost 2013;39:441-5. 10.1055/s-0033-1334482 [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner HR. Blood flow and thrombogenesis. Correlation of blood platelets, blood coagulation factors and the vascular wall. Internist (Berl) 1984;25:75-81. [PubMed] [Google Scholar]
- 11.Kistner RL, Sparkuhl MD. Surgery in acute and chronic venous disease. Surgery 1979;85:31-43. [PubMed] [Google Scholar]
- 12.Smith GW. Iliofemoral venous thrombectomy. Indications, technique, and results in forty-five cases. Circulation 1968;37:847-53. 10.1161/01.CIR.37.5.847 [DOI] [PubMed] [Google Scholar]
- 13.Stiegler H, Arbogast H, Nees S, et al. Thrombectomy, lysis, or heparin treatment: concurrent therapies of deep vein thrombosis: therapy and experimental studies. Semin Thromb Hemost 1989;15:250-8. 10.1055/s-2007-1002715 [DOI] [PubMed] [Google Scholar]
- 14.Ophir J, Céspedes I, Ponnekanti H, et al. Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues. Ultrasonic Imaging 1991;13:111-34. 10.1177/016173469101300201 [DOI] [PubMed] [Google Scholar]
- 15.Lerner RM, Huang SR, Parker KJ. "Sonoelasticity" images derived from ultrasound signals in mechanically vibrated tissues. Ultrasound Med Biol 1990;16:231-9. 10.1016/0301-5629(90)90002-T [DOI] [PubMed] [Google Scholar]
- 16.Muthupillai R, Lomas DJ, Rossman PJ, et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 1995;269:1854-7. 10.1126/science.7569924 [DOI] [PubMed] [Google Scholar]
- 17.Emelianov SY, Chen X, O'Donnell M, et al. Triplex ultrasound: elasticity imaging to age deep venous thrombosis. Ultrasound Med Biol 2002;28:757-67. 10.1016/S0301-5629(02)00516-1 [DOI] [PubMed] [Google Scholar]
- 18.Rubin JM, Aglyamov SR, Wakefield TW, et al. Clinical application of sonographic elasticity imaging for aging of deep venous thrombosis: preliminary findings. J Ultrasound Med 2003;22:443-8. 10.7863/jum.2003.22.5.443 [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Kim K, Aglyamov SR, et al. Staging deep venous thrombosis using ultrasound elasticity imaging: Animal model. Ultrasound Med Biol 2004;30:1385-96. 10.1016/j.ultrasmedbio.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 20.Aglyamov S, Skovoroda AR, Rubin JM, et al. Model-based reconstructive elasticity imaging of deep venous thrombosis. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:521-31. 10.1109/TUFFC.2004.1320825 [DOI] [PubMed] [Google Scholar]
- 21.Xie H, Kim K, Aglyamov SR, et al. Correspondence of Ultrasound Elasticity Imaging to Direct Mechanical Measurement in Aging DVT in Rats. Ultrasound Med Biol 2005;31:1351-9. 10.1016/j.ultrasmedbio.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geier B, Barbera L, Muth-Werthmann D, et al. Ultrasound elastography for the age determination of venous thrombi. Evaluation in an animal model of venous thrombosis. Thromb Haemost 2005;93:368-74. [DOI] [PubMed] [Google Scholar]
- 23.Rubin JM, Xie H, Kim K, et al. Sonographic elasticity imaging of acute and chronic deep venous thrombosis in humans. J Ultrasound Med 2006;25:1179-86. 10.7863/jum.2006.25.9.1179 [DOI] [PubMed] [Google Scholar]
- 24.Yi X, Wei X, Wang Y, et al. Role of real-time elastography in assessing the stage of thrombus. Int Angiol 2017;36:59-63. [DOI] [PubMed] [Google Scholar]
- 25.Mfoumou E, Tripette J, Blostein M, et al. Time-dependent hardening of blood clots quantitatively measured in vivo with shear-wave ultrasound imaging in a rabbit model of venous thrombosis. Thromb Res 2014;133:265-71. 10.1016/j.thromres.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Li N, Wen C. Effect of pathological heterogeneity on shear wave elasticity imaging in the staging of deep venous thrombosis. PLoS One 2017;12:e0179103. 10.1371/journal.pone.0179103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varghese T. Quasi-Static Ultrasound Elastography. Ultrasound Clin 2009;4:323-38. 10.1016/j.cult.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlsen J, Ewertsen C, Sletting S, et al. Ultrasound Elastography in Breast Cancer Diagnosis. Ultraschall Med 2015;36:550-62; quiz 63-5. 10.1055/s-0035-1553293 [DOI] [PubMed] [Google Scholar]
- 29.Eisenbrey JR, Dave JK, Forsberg F. Recent technological advancements in breast ultrasound. Ultrasonics 2016;70:183-90. 10.1016/j.ultras.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 30.Gkali CA, Chalazonitis AN, Feida E, et al. Breast Elastography: How We Do It. Ultrasound Q 2015;31:255-61. 10.1097/RUQ.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 31.Jeong WK, Lim HK, Lee HK, et al. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography 2014;33:149-60. 10.14366/usg.14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons RE, Sigel B, Feleppa EJ, et al. Age determination of experimental venous thrombi by ultrasonic tissue characterization. J Vasc Surg 1993;17:470-8. 10.1016/0741-5214(93)90146-D [DOI] [PubMed] [Google Scholar]
- 33.Meng W, Zhang G, Wu C, et al. Preliminary results of acoustic radiation force impulse (ARFI) ultrasound imaging of breast lesions. Ultrasound Med Biol 2011;37:1436-43. 10.1016/j.ultrasmedbio.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 34.Siragusa S, Cosmi B, Piovella F, et al. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med 1996;100:269-77. 10.1016/S0002-9343(97)89484-3 [DOI] [PubMed] [Google Scholar]
- 35.Oklu R, Ghasemi-Rad M, Irani Z, et al. Aspiration thrombectomy using the penumbra catheter. J Vasc Interv Radiol 2015;26:454-5. 10.1016/j.jvir.2014.11.028 [DOI] [PubMed] [Google Scholar]