Abstract
Thrombosis is a prevailing vascular disorder that has been historically studied in vivo with conventional animal models. Here we review recent advances in methods and techniques that allow for engineering of biomimetic in vitro models of thrombosis, usually combined with microfluidic devices, termed thrombosis-on-a-chip systems, to reproduce such vascular pathology outside living organisms. These human cell-based thrombosis-on-a-chip platforms recapitulate the important characteristics of native thrombosis in terms of vascular structures, extracellular matrix properties, cellular composition, and pathophysiology, making them enabling in vitro models to study this important class of vascular disorders as well as to develop personalized treatment regimens.
Keywords: Interventional radiology, thrombosis, thrombosis-on-a-chip, soft lithography, bioprinting, blood vessels
Introduction
Thrombosis is a prevailing vascular disorder that may readily occur in both arteries and veins (1-4). Thrombosis is often the result of vascular injuries, featured by obstruction, to different degrees, of vascular lumens by coagulated blood and thus disturbance/cessation of blood flows at the site surrounding the thrombus (5,6). In healthy vessels, the endothelium functions to prevent coagulation, through secretion of anti-thrombotic agents such as nitric oxide and expression of binding sites for similar molecules such as antithrombin (5,7,8). Therefore, injuries of the endothelium usually lead to the activation of primary homeostasis (platelet binding and aggregation) and the coagulation cascade (fibrin formation and crosslinking) (Figure 1A) (5,7,8,10). While acute thrombosis may be resolved via administration of thrombolytic agents such as tissue plasminogen activator (tPA) that degrade fibrin (11-14), this is not always effective. The invasion and propagation of fibroblasts and smooth muscle cells from the site of endothelial injury into the mass of thrombus usually result in its fibrosis and matrix organization, turning the thrombus to a permanent clot that cannot be resolved (Figure 1B) (15-20). Subsequently, the vessel wall stiffens and blood pressure builds up (21,22), further developing post-thrombotic syndrome, clinically manifesting as symptoms including edema, pain, and ulceration, among others (15,18).
Figure 1.

Thrombosis and vascular injury. (A) Mechanism of thrombus formation and development. After the endothelium damage, platelets adhere to the endothelial layer via vWF. This leads to the activation and finally aggregation of platelets. Simultaneously, coagulation cascade results in cleavage of fibrinogen to fibrin through thrombin, to eventually form a thrombus. (B) Formation of fibrotic thrombus through fibroblast migration into the blood clot from the surrounding tissue via the damaged endothelium. Collagen type I is then expressed by the invading fibroblasts, leading to fibrosis. Adapted with permission from Ref. (9), copyright 2016 Royal Society of Chemistry. vWF, von Willebrand factor.
While animals have been historically used as models to study thrombosis (23-25), these in vivo models typically differ from the human system, leading to biased understanding and inaccurate predictions of treatment effects in humans. In addition, imaging and characterizing thrombosis in animals are cumbersome, and they cannot achieve high throughput that is needed in cases such as screening of therapeutics. Here, we discuss some recent advances in methods and techniques used for generating in vitro biomimetic models of human thrombosis, termed thrombosis-on-a-chip systems, which to a large extent recapitulate the important pathophysiology of their in vivo counterparts in terms of vascular structures, extracellular matrix properties, and cellular composition. These miniaturized, usually transparent devices also allow for convenient analyses at much increased throughputs than animals. We finally conclude with future perspectives. It should be noted that, only hydrogel-based thrombosis-on-a-chip models will be illustrated in the current Review, as they typically present better biomimetic features than those fabricated with silicone elastomers and plastics.
Thrombosis-on-a-chip models generated using soft lithography
Soft lithography is a long-established technique for patterning a variety of (bio)materials at microscales (26). It is generally conducted through a replica molding process, i.e., a master mold with a desired pattern is first fabricated, on top of which the secondary material is cast and crosslinked; this secondary material is then detached from the master mold to achieve the replication of the pattern in the reverse mode. As such, the interconnected microfluidic channels inside a bulk material can be readily produced to mimic the perfusable vascular structure (27,28). Soft lithography is easy to operate, capable of high-resolution patterning, and highly reproducible, but is sometimes limited by the reliance on the need for the master molds with pre-designed primary patterns.
In a prominent example, Zheng and colleagues utilized a vascular network generated with soft lithography to study angiogenesis and thrombosis (29). In their procedure, a polydimethylsiloxane (PDMS) stamp containing a surface pattern of interconnected ridges was cast with a bath of collagen solution; upon gelation, the PDMS stamp was removed to expose the patterned grooves at the bottom of the collagen hydrogel, followed by attachment to another layer of flat collagen at the bottom to form a closed vascular structure within the collagen matrix (Figure 2A). Subsequently, endothelial cells were seeded into this hollow vascular pattern, leading to the formation of a monolayer of endothelium on the interior surface of the microchannels (Figure 2A,B).
Figure 2.
Thrombosis-on-a-chip model generated using soft lithography. (A) Schematics of the soft lithography fabrication procedures of the vascular chip: (i) molding the microstructured collagen in top jig; (ii) molding flab collagen slab in bottom jig; (iii) assembling the jigs mechanically and seeding cells through the inlet and outlet reservoirs; and (iv) culturing the microvessel network with gravity-driven flow. (B) Confocal projection view of endothelialized microfluidic vessels (overall network, left) and orthogonal views of the corner. Scale bars: 100 µm. (C) Time sequences of whole blood perfusion through a vascular chip, either quiescent (control vessels, i) or (ii) stimulated, at a flow rate of 10 µL min-1 at time points of 5, 50, 100, 150, and 250 s after initiation of perfusion. The platelets are in green, labeled for CD41a to platelet-specific glycoprotein IIb (integrin αIIb); flow direction is indicated with arrow. Scale bars: 100 µm. (D) Platelets and leucocytes adherent in the stimulated vessels after 1 h of blood perfusion. Leukocytes are labeled white with CD45, a leukocyte-specific member of the protein tyrosine phosphatase family. Red, CD31; green, CD41a; white, CD45; and blue, nuclei. Scale bar: 50 µm. (E) Platelet adhesion on vWF fibers. Arrow, flow direction. Green, vWF; red, CD41a. Adapted from Ref. (29), copyright 2012 National Academy of Sciences.
With this perfusable vascular device, the authors were able to further study the interactions of whole blood and the endothelium. As expected, under normal conditions, the vast majority of blood cells flowed past the endothelial surfaces without noticeable adherence, and only a small amount of platelets were observed to roll along the endothelium during the blood flow (Figure 2C). On the contrary, when the vascular chip was primed with phorbol-12-myristate-13-acetate (PMA), a known secretagogue for von Willebrand factor (vWF), the platelets started to significantly aggregate and adhere to the endothelial surface (Figure 2C). After 1 h of perfusion, leukocytes were also shown to attach to the lumen walls with signs of migration through the barrier into the surrounding collagen hydrogel (Figure 2D). Mechanistic investigation revealed the secretion of vWF by the endothelial cells on surfaces, which served to bound to platelets in the blood (Figure 2E), indicating that the engineered vascular model was biologically functional and responsive to externally applied signaling molecules, capable of inducing thrombosis formation.
Thrombosis-on-a-chip model generated with bioprinting
As aforementioned, while soft lithography is convenient, it has limited flexibility in fabrication of vascular patterns due to the requirement of master molds. To this end, three-dimensional (3D) bioprinting has recently emerged as a versatile technology to fabricate volumetric tissue constructs possessing complex architectures, including those that are vascularized (30-36).
Among the various bioprinting systems such as inkjet bioprinter, microextrusion bioprinter, laser-assisted bioprinter, and stereolithography (32-34), sacrificial bioprinting strategies based on extrusion have been most widely used for generation of vascularized tissue constructs at relatively high resolutions. In a typical procedure, a microfibrous pattern is first deposited in an arbitrary shape, and cast by the hydrogel matrix that is subsequently crosslinked; the initially bioprinted microfibrous network is selectively removed from the hydrogel block to induce the formation of an interconnected microvascular network that resembles the blood vessels. The use of a bioprinting system allows significantly improved flexibility of such a method in comparison with soft lithography, as the deposition of the sacrificial template can be automated simply by altering the digital input patterns for the bioprinters. A variety of sacrificial biomaterials have been developed to enable extrusion bioprinting of vascularized constructs, ranging from carbohydrate lattices that can be removed by dissolution by perfusing medium (37), mechanical extraction of stiffened agarose microfibers (38-40), as well as thermoresponsive materials that liquefy upon temperature change, such as Pluronic F127 that transforms from the hydrogel state at room temperature to a liquid at <4 °C (41,42), or gelatin that gels at room temperature or lower but becomes a liquid 37 °C (43). Similar to soft lithography, these sacrificially bioprinted microchannels could also be functionalized with a layer of endothelial cells to introduce biological functionality.
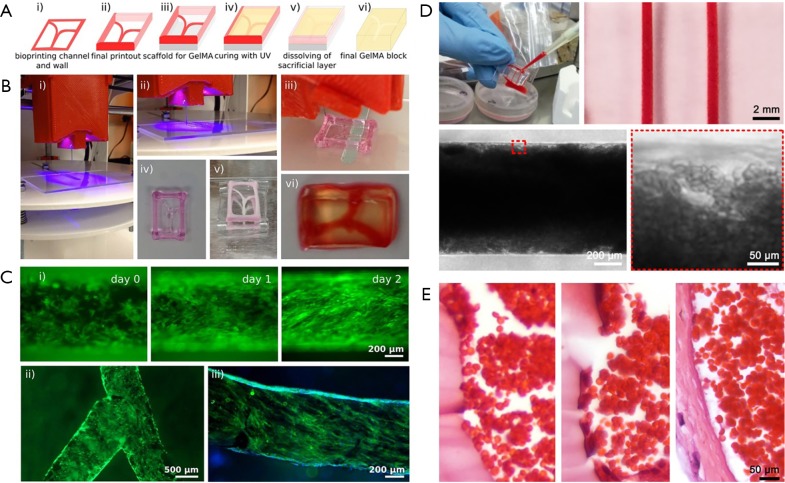
Using a modified sacrificial bioprinting strategy based on Pluronic, we and co-workers recently reported the fabrication of a thrombosis-on-a-chip model (9). A Pluronic template, containing the vascular pattern and the outside frame, was first bioprinted and then dried in air overnight; this bioprinted structure was placed on a substrate, filled with a gelatin methacryloyl (GelMA) hydrogel pre-polymer, and photocrosslinked; eventually, the entire construct was placed in a cold buffer bath to rehydrate the Pluronic and to dissolve it out (Figure 3A,B). The bioprinting of not only the vascular template but also the surrounding frame followed by desiccation served as the container to hold the hydrogel block in place, eliminating the need for additional molds. Both straight and branching microchannels could be generated using this sacrificial bioprinting method, which could be further endothelialized (Figure 3C). The obtained microchannels resembling the blood vessels were filled with human whole blood induced to clot, leading to formation of a biomimetic thrombosis model with aggregated blood cells (Figure 3D). By comparing with a human clot, the bioengineered thrombosis-on-a-chip model exhibited strong similarity when an endothelial barrier was present (Figure 3E). It was further demonstrated that, the acute clots in these bioprinted thrombosis models could be dissolved away by perfusing with tPA, while fibroblasts embedded within the surrounding GelMA hydrogel matrix were able to migrate into the thrombus in the absence of an intact endothelium, depositing collagens that resulted in the aging of the clot on the chip.
Figure 3.
Thrombosis-on-a-chip model generated using bioprinting. (A) Schematic of the bioprinting process: (i,ii) bioprinting of a Pluronic template; (iii) dried template is placed on a PDMS support; (iv) the mold is filled with GelMA followed by UV crosslinking; and (v) dissolution of the sacrificial channels and frame to produce (vi) the final construct with hollow channels. (B) Photographs showing the experimental depiction of the corresponding steps of the sacrificial bioprinting process illustrated in (A). (C) Endothelialization of the hollow microchannels inside the GelMA construct for (i) a linear and (ii) bifurcating microchannels; (iii) CD31 (green) and nuclei (blue) staining of the confluent layer of HUVECs. (D) Photographs and optical micrographs showing the infusion of human whole blood into the endothelialized microchannels and the formed thrombosis-on-a-chip model. (E) Optical micrograph showing hematoxylin & eosin-stained transvers sections of (left) a thrombus without HUVECs and (middle) a thrombus with HUVECs, both at 7 days post clotting, and (right) a venous thrombus formed in vivo at 7 days. Adapted with permission from Ref. (9), copyright 2016 Royal Society of Chemistry.
Conclusions
We have discussed recent advances in methods and techniques, primarily based on soft lithography and 3D bioprinting, for engineering biomimetic in vitro models of thrombosis. These models feature a hydrogel matrix mimicking the extracellular matrix of the biological tissue, endothelialized microchannels resembling the blood vessels, and the ability to flow human whole blood through microchannels stimulated with specific agents to induce platelet aggregation in situ or to directly infuse blood induced to coagulate within the microchannels. These thrombosis-on-a-chip models could faithfully reproduce this important vascular disorder in vitro, enabling accurate investigations into their biology and treatment. Although still preliminary, we foresee that further development of these models will eventually allow for personalized screening of intravascular interventions for treatment of thrombosis in a patient-specific manner using cells and imaging data derived from individual patients.
Acknowledgements
Funding: The authors acknowledge funding from the National Institutes of Health (K99CA201603, R21EB021148, R01HL137193, and R01EB024403). YS Zhang further acknowledges support from the Lush Prize and the Science and Technology Commission of Shanghai Municipality (STCSM) 17JC 1400200.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. New Engl J Med 1984;310:1137-40. 10.1056/NEJM198405033101801 [DOI] [PubMed] [Google Scholar]
- 2.Falk E, Fernández-Ortiz A. Role of thrombosis in atherosclerosis and its complications. Am J Cardiol 1995;75:3B-11B. 10.1016/0002-9149(95)80003-B [DOI] [PubMed] [Google Scholar]
- 3.Mai C, Hunt D. Upper-extremity deep venous thrombosis: a review. Am J Med 2011;124:402-7. 10.1016/j.amjmed.2010.11.022 [DOI] [PubMed] [Google Scholar]
- 4.Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001;86:452-63. [PubMed] [Google Scholar]
- 5.Owens AP, 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost 2010;104:432-9. 10.1160/TH09-11-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furie B, Furie BC. Mechanisms of thrombus formation. New Engl J Med 2008;359:938-49. 10.1056/NEJMra0801082 [DOI] [PubMed] [Google Scholar]
- 7.Breitenstein A, Tanner FC, Luscher TF. Tissue Factor and Cardiovascular Disease: Quo Vadis? Circ J 2010;74:3-12. 10.1253/circj.CJ-09-0818 [DOI] [PubMed] [Google Scholar]
- 8.Versteeg HH, Heemskerk JW, Levi M, et al. New fundamentals in hemostasis. Physiol Rev 2013;93:327-58. 10.1152/physrev.00016.2011 [DOI] [PubMed] [Google Scholar]
- 9.Zhang YS, Davoudi F, Walch P, et al. Bioprinted Thrombosis-on-a-Chip. Lab Chip 2016;16:4097-105. 10.1039/C6LC00380J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oklu R, Albadawi H, Watkins MT, et al. Detection of extracellular genomic DNA scaffold in human thrombus: implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol 2012;23:712-8. 10.1016/j.jvir.2012.01.072 [DOI] [PubMed] [Google Scholar]
- 11.Jaff MR, McMurtry MS, Archer SL, et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension. Circulation 2011;123:1788-830. 10.1161/CIR.0b013e318214914f [DOI] [PubMed] [Google Scholar]
- 12.Kunadian V, Gibson CM. Thrombolytics and Myocardial Infarction. Cardiovasc Ther 2012;30:e81-8. 10.1111/j.1755-5922.2010.00239.x [DOI] [PubMed] [Google Scholar]
- 13.American College of Emergency Physicians , Society for Cardiovascular Angiography and Interventions, O'Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012;379:2364-72. 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deatrick KB, Eliason JL, Lynch EM, et al. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg 2005;42:140-8. 10.1016/j.jvs.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 16.Fineschi V, Turillazzi E, Neri M, et al. Histological age determination of venous thrombosis: A neglected forensic task in fatal pulmonary thrombo-embolism. Forensic Sci Int 2009;186:22-8. 10.1016/j.forsciint.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 17.Hara T, Truelove J, Tawakol A, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography enables the detection of recurrent same-site deep vein thrombosis by illuminating recently formed, neutrophil-rich thrombus. Circulation 2014;130:1044-52. 10.1161/CIRCULATIONAHA.114.008902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosaka M, Ishida Y, Kimura A, et al. Time-dependent appearance of intrathrombus neutrophils and macrophages in a stasis-induced deep vein thrombosis model and its application to thrombus age determination. Int J Legal Med 2009;123:235-40. 10.1007/s00414-009-0324-0 [DOI] [PubMed] [Google Scholar]
- 19.Saha P, Humphries J, Modarai B, et al. Leukocytes and the Natural History of Deep Vein Thrombosis Current Concepts and Future Directions. Arterioscler Thromb Vasc Biol 2011;31:506-12. 10.1161/ATVBAHA.110.213405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008;28:387-91. 10.1161/ATVBAHA.108.162289 [DOI] [PubMed] [Google Scholar]
- 21.Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg 2011;53:500-9. 10.1016/j.jvs.2010.08.050 [DOI] [PubMed] [Google Scholar]
- 22.Kahn SR, Comerota AJ, Cushman M, et al. The Postthrombotic Syndrome: Evidence-Based Prevention, Diagnosis, and Treatment Strategies A Scientific Statement From the American Heart Association. Circulation 2014;130:1636-61. 10.1161/CIR.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 23.Fahed R, Raymond J, Ducroux C, et al. Testing flow diversion in animal models: a systematic review. Neuroradiology 2016;58:375-82. 10.1007/s00234-015-1635-0 [DOI] [PubMed] [Google Scholar]
- 24.Raj JA, Stoodley M. Experimental Animal Models of Arteriovenous Malformation: A Review. Vet Sci 2015;2:97-110. 10.3390/vetsci2020097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lysgaard Poulsen J, Stubbe J, Lindholt JS. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur J Vasc Endovasc Surg 2016;52:487-99. 10.1016/j.ejvs.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 26.Qin D, Xia Y, Whitesides GM. Soft lithography for micro-and nanoscale patterning. Nat Protoc 2010;5:491. 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 27.Khademhosseini A, Suh KY, Jon S, et al. A soft lithographic approach to fabricate patterned microfluidic channels. Anal Chem 2004;76:3675-81. 10.1021/ac035415s [DOI] [PubMed] [Google Scholar]
- 28.Ling Y, Rubin J, Deng Y, et al. A cell-laden microfluidic hydrogel. Lab Chip 2007;7:756-62. 10.1039/b615486g [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 2012;109:9342-7. 10.1073/pnas.1201240109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheth R, Balesh ER, Zhang YS, et al. Three-Dimensional Printing: An Enabling Technology for IR. J Vasc Interv Radiol 2016;27:859-65. 10.1016/j.jvir.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 31.Zhang YS, Duchamp M, Oklu R, et al. Bioprinting the Cancer Microenvironment. ACS Biomater Sci Eng 2016;2:1710-21. 10.1021/acsbiomaterials.6b00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YS, Yue K, Aleman J, et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng 2017;45:148-63. 10.1007/s10439-016-1612-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malda J, Visser J, Melchels FP, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 2013;25:5011-28. 10.1002/adma.201302042 [DOI] [PubMed] [Google Scholar]
- 34.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773-85. 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Zhang YS, Akpek A, et al. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016;9:012001. 10.1088/1758-5090/9/1/012001 [DOI] [PubMed] [Google Scholar]
- 36.Zhang YS, Pi Q, van Genderen AM. Microfluidic Bioprinting for Engineering Vascularized Tissues and Organoids. J Vis Exp 2017;(126). [DOI] [PMC free article] [PubMed]
- 37.Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 2012;11:768-74. 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014;14:2202-11. 10.1039/C4LC00030G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massa S, Sakr MA, Seo J, et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017;11:044109. 10.1063/1.4994708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YS, Duchamp M, Ellisen LW, et al. Recapitulating Mammary Ductal Carcinoma Microenvironment in vitro Using Sacrificial Bioprinting. Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington DC, USA. Philadelphia: AACR; Cancer Res 2017;77:Abstract nr 4828. [Google Scholar]
- 41.Kolesky DB, Truby RL, Gladman AS, et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv Mater 2014;26:3124-30. 10.1002/adma.201305506 [DOI] [PubMed] [Google Scholar]
- 42.Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016;113:3179-84. 10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee VK, Kim DY, Ngo H, et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014;35:8092-102. 10.1016/j.biomaterials.2014.05.083 [DOI] [PMC free article] [PubMed] [Google Scholar]