Abstract
We examined the effectiveness of localized administration of fibroblast growth factor-2 containing low-molecular-weight heparin/protamine nanoparticles (FGF-2&LMWH/P NPs) on apoptosis in vivo and on healing of radiation-induced skin injury in a rat model. FGF-2 binds onto LMWH/P NPs, which can significantly enhance and stabilize FGF-2 as a local carrier. X-irradiation at a dose of 25 Gy was administered to the lower part of the back (using a lead sheet with two holes) 1 h before the administration of FGF-2&LMWH/P NPs. Cutaneous full-thickness defect wounds were then formed in X-irradiated areas to examine the time-course of wound healing, and the wound tissues were microscopically and histologically compared and examined. Wound healing was significantly delayed by X-irradiation, but FGF-2&LMWH/P NPs administration prior to irradiation led to a significantly shorter delay compared with FGF-2 alone, LMWH/P NPs alone, and controls. Furthermore, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining showed that the proportions of apoptotic dermal fibroblasts in X-irradiated skin were significantly lower in rats administered FGF-2&LMWH/P NPs than in controls. However, 8-hydroxy-2’-deoxyguanosine (8-OHdG) staining showed no differences. Thus, localized administration of FGF-2&LMWH/P NPs prior to irradiation may alleviate X-irradiation-induced healing-impaired wound repair in normal tissue.
Keywords: radioprotection, apoptosis, healing-impaired wound, FGF-2, LMWH/P NPs
INTRODUCTION
Radiation therapy has been extensively applied in the treatment of cancer and is known to be effective. However, because its mechanism of action is due to reactive oxygen species (ROS) and free radicals (FR), it also affects not only tumor tissue, but also healthy tissue, resulting in severe skin disorders and delayed wound healing [1, 2]. Acute adverse effects of radiation on the skin, such as erythema, epilation, desquamation, hyperpigmentation and erosion, collectively referred to as radiodermatitis, are common, depending on the irradiation dose [3, 4]. In addition, chronic radiation-induced skin ulcers are often observed in the region surrounding radiodermatitis [5]. These ulcers are characterized by poor healing and a high relapse rate, and they are commonly intractable. These are not only limiting factors during radiotherapy, but they are also health concerns forcing the interruption or termination of the therapeutic course [5].
Mild cases of skin damage caused by irradiation can be healed using ointments, but severe cases cannot be healed using conservative therapy. In such cases, some type of surgical procedure may be required. For example, when performing a skin graft [6], if the graft bed is irradiated, the formation of granulation tissue and blood vessels is typically decreased, and skin graft survival also decreases [6]. Moreover, a skin flap [7] that is damaged by radiation may have poor survival, i.e. if the area surrounding a lesion is irradiated, the treatment methods will be less effective because of the effects of radiation [6, 7]. Novel treatment methods and clinical trials are needed to provide efficacious therapies for radiodermatitis and chronic radiation–induced skin ulcers [1].
X-irradiation interacts with water molecules in biological systems and produces various reactive oxygen species (ROS) that can cause cell and tissue damage, together with induced excess cellular apoptosis. Several studies have shown that apoptosis plays an important role in the early stages of radiation-induced injuries on rat skin, and that X-irradiation induced apoptosis of keratinocytes, fibroblasts and dermal endothelial cells in rat skin in vivo and in vitro [8].
Intraperitoneal pre-injections of prostaglandin E1 have been shown to have a beneficial effect on radiation-induced healing-impaired skin injury [8]. However, the systemic injection of a high dose of prostaglandin E1 for radiation-induced healing-impaired skin injury may have side effects [8]. In particular, a high dose of prostaglandin E1 may induce severe changes in blood pressure or bleeding via vasodilation or platelet aggregation. On the other hand, inhalation of hydrogen-containing gas (hydrogen (1.3%) + oxygen (20.8%) + nitrogen (77.9%)) (HCG) has been shown to have a stimulatory effect on healing of radiation-induced healing-impaired skin wounds, using a rat model of radiation-induced skin injury [9, 10].
Fibroblast growth factor (FGF)-2 has stimulatory effects on granulation tissue formation, re-epithelialization, and tissue remodeling in healing-impaired wounds, and it may alleviate the side effects of X-irradiation [11, 12]. Radioprotection by FGF-2 has been reported for damage to epithelial [13] and hematopoietic tissues [14], salivary hypofunction [15], and apoptosis of intestinal progenitor/stem cells [16] and endothelial cells in the central nervous system [17].
Diffusible FGF-2 is susceptible to heat and proteolytic enzymes such as trypsin, and its half-life in vivo simulated at 37°C and pH 7.4 is very short, ~12 h or less [12]. It is necessary to maintain a sufficient concentration of FGF-2 locally and to continue sustained release while retaining activity. Furthermore, FGF-2 is known to be a wide-spectrum survival factor, promoting cell resistance in both irradiated tumor and normal tissues [12, 15, 17].
To enhance the repetitive efficacy of FGF-2, low molecular weight heparin/protamine nanoparticles (LMWH/P NPs) were developed as polyelectrolyte complexes (PECs) [18]. LMWH/P NPs are specifically bound to FGF-2 [12, 19], hepatocyte growth factor (HGF) [20] and platelet-rich plasma (PRP) [21, 22], which are locally injected. LMWH/P NPs can be retained on the cell surfaces and matrix of various tissues in vivo, and they can protect and activate FGF-2. Moreover, localized injection of FGF-2&LMWH/P NPs showed a substantial effect in inducing vascularization and fibrous tissue formation by stabilizing, activating and gradually releasing FGF-2 from FGF-2&LMWH/P NPs [12, 19]. Furthermore, because all the components of FGF-2&LMWH/P NPs, i.e. LMWH, protamine, and FGF-2, are in clinical use, it is likely that the FGF-2&LMWH/P NPs are safe for clinical use [18, 23]. In this study, the effectiveness of LMWH/P NPs as local carriers of FGF-2 for the repair of X-irradiated–induced healing-impaired wound repair was investigated.
MATERIALS AND METHODS
Preparation of FGF-2&LMWH/P NPs
For the preparation of FGF-2&LMWH/P NPs, 0.3 ml of protamine solution (10 mg/ml; Mochida Pharmaceutical Co., Tokyo, Japan) was added to 0.7 ml of LMWH solution (Fragmin: 6.4 mg/ml; Kissei Pharmaceutical Co., Tokyo, Japan) [12, 19]. The solution was vortexed vigorously for ~1 min. To produce FGF-2&LMWH/P NPs, 0.5 ml of recombinant human FGF-2 (100 μg/ml; Fiblast; Kaken Pharmaceutical Corp., Tokyo, Japan) in saline was added to 0.5 ml of the LMWH/P NPs (12 mg/ml).
Rats and feeding conditions
All animal experiments were conducted with the approval of the Animal Ethics Committee of the National Defense Medical College (Tokorozawa, Saitama, Japan). Male Sprague-Dawley (SD) rats (SLC Japan Inc, Tokyo, Japan) weighing 300–400 g (8–12 weeks of age) were kept on a 12-h light–dark cycle at 25 ± 2°C during the experiment. They were given standard Purina chow (CREA Japan Inc., Tokyo, Japan) and water ad libitum.
Radiation conditions and radiation-induced healing-impaired wounds
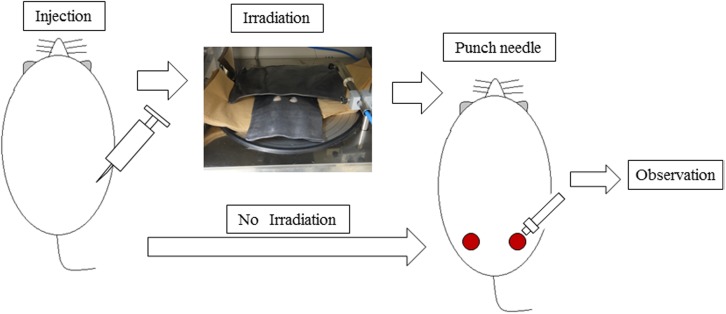
After carrying out sedation with sevoflurane (Sevoflen, Abbott Japan Inc, Osaka, Japan) in the anesthesia box, part of the dorsum hair was shaved with a clipper. Subsequently, 1 ml of FGF-2 (50 μg/ml) &LMWH/P NPs (6 mg/ml), FGF-2 alone (50 μg/ml), LMWH/P NPs (6 mg/ml) alone or saline (control) was administered by localized subcutaneous injection. Rats were anesthetized by intraperitoneal administration of 50 mg/kg pentobarbital (Somnopentyl®, 64.8 mg/ml; Kyoritsu Seiyaku, Tokyo, Japan) 1 h after administration. The distance from the radiation source to the table was 35 cm and an MBR-1505R2 X-ray irradiation apparatus (Hitachi Medical Corp., Tokyo, Japan) was used. The radiation factors were tube voltage: 150 kV, tube current: 5 mA, with a 0.5-mm added Al filter, providing a dose-rate of 1 Gy/min (total 25 Gy). The animals were protected with 3-mm-thick lead shielding that had two holes (diameter 2.3 cm) in it (Fig. 1). Lead shielding for X-irradiation is used to protect sensitive organs in animal study as well as in radiation therapy. When rats received a single dose of 15 Gy of X-rays to the snout after shielding of the remainder of the rat body with 0.5 mm-thickness lead plates in the X-ray irradiation-induced glossitis model [24]. We previously used 3 mm-thick lead shield to X-irradiation, which showed a shielding effect [8, 10]. In this study, 0.5 mm-thick showed the shielding effect, and we did not observe any gross problems in the shielded areas. After X-irradiation with 25 Gy through the two holes, cutaneous full-thickness wounds of 10 mm were made with an 8-mm biopsy dermal punch (Kai Medical, Tokyo, Japan) and a sharp blade, and the sequential effects of FGF-2&LMWH/P NPs were evaluated on the wound healing rate and on immunohistochemical analyses.
Fig. 1.
Flow chart for the X-irradiation and wound repair experiments.
Immunohistochemical analyses
Excised skin tissues in each group were fixed in 4% paraformaldehyde solution at 4°C for 12 h, embedded in paraffin, and cut into 3.5-μm-wide sections 0, 3, 7 and 14 days after X-irradiation. Sections were made perpendicular to the anteroposterior axis and perpendicular to the surface of the skin. Each section was stained with hematoxylin and eosin (H&E).
Cellular apoptosis assessed by DNA fragmentation was examined using a MEBSTAIN apoptosis TUNEL Kit II (MBL Co., Ltd, Nagano, Japan). Wound sections at 24 h after wound creation were deparaffinized and exposed to proteinase K (20 μg/ml) for 15 min at room temperature to unmask the fragment DNA 3′-OH ends. Samples were washed with distilled H2O and incubated in 0.3% hydrogen peroxide for 15 min to suppress endogenous peroxidase activity. After two rinses in phosphate-buffered saline (PBS), sections were sequentially incubated in equilibration buffer at room temperature for 10 s. After tapping off excess liquid from the samples, terminal deoxynucleotidyl transferase enzyme was applied, and the samples were incubated at 37°C for 1 h. Stop buffer was applied to the samples at room temperature for 15 min to terminate the 3′-OH labeling reaction, and peroxidase-conjugated streptavidin (Dako, Glostrup, Denmark) was applied at room temperature for 30 min. After washing the samples in PBS, DAB was added, and the samples were stained for 5 min. The sections were counterstained with Mayer’s hematoxylin (Merck, Darmstadt, Germany) to study tissue morphology.
For staining with 8-hydroxy-2’-deoxyguanosine (8-OHdG), wound sections at 24 h after wound creation were deparaffinized, washed with distilled H2O, and incubated in 0.3% hydrogen peroxide for 15 min to suppress endogenous peroxidase activity. After washing with PBS, blocking solution was applied to the samples at room temperature for 10 min to adsorb non-specific proteins. The washed samples were treated with primary antibody (anti-8-OHdG mouse monoclonal antibody, Japan Institute for the Control of Aging, Shizuoka, Japan) for 12 h at 4°C. The washed samples were treated with secondary antibody (anti-mouse peroxidase-conjugated second antibody, Nichirei Biosciences, Tokyo, Japan) for 30 min at room temperature. After washing the samples, 3-3’-diaminobenzine-4HCl (DAB) (Dako) was added, and the samples were stained for 5 min. The sections were counterstained with Mayer’s hematoxylin to study tissue morphology.
Statistical analyses
Statistical testing was performed with Dunnet’s test and the Tukey–Kramer test using JMP® Pro for Windows (SAS Institute Inc., Cary, NC, USA). Values of P < 0.05 were considered significant.
RESULTS
Effects of FGF-2&LMWH/P NPs on wound healing of irradiated skin
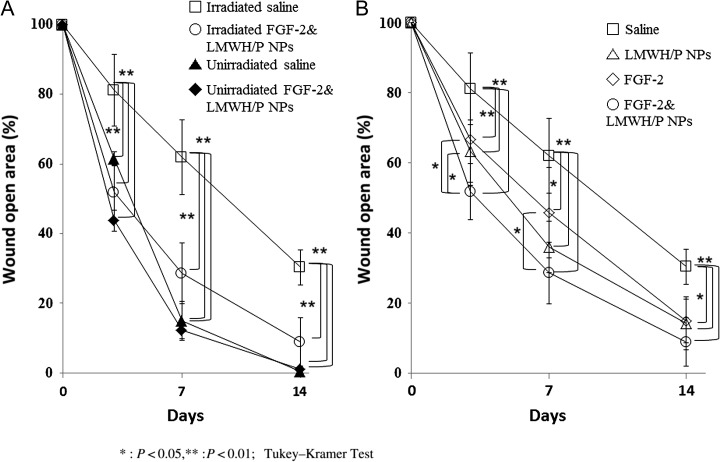
Comparison of the X-irradiated groups with the non-irradiated groups showed significantly delayed wound healing in the X-irradiated saline (control) group. In the X-irradiated control group, the wound open area was 62% and 38% after 7 and 14 days, respectively, while in the non–X-irradiated control group, the wound open area was 17% on Day 7, and all wound healing was completed by 14 days (Figs 2 and 3A). For about half of the X-irradiated control group, incomplete healing was observed on Day 14, and complete wound closure took more than 28 days. Moreover, the X-irradiated FGF-2&LMWH/P NPs-administered group had significantly higher healing rates on Days 3, 7 and 14 compared with those of the FGF-2 alone, LMWH/P NPs alone and control (saline) groups (P < 0.05) (Figs 2 and 3B).
Fig. 2.
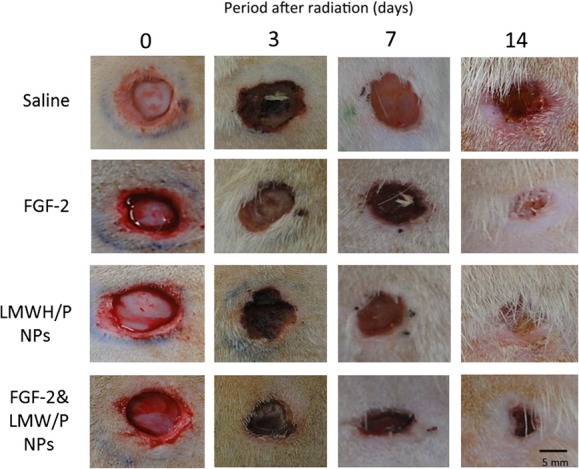
Photomicrographs of wound open areas. Wound closure was evaluated and photomicrographed 3, 7 and 14 days after wound creation in X-irradiated saline, FGF-2, LMWH/P NPs and FGF-2&LMWH/P NPs groups. Each wound on the indicated day is representative of six wounds (three rats).
Fig. 3.
Open area of cutaneous full-thickness wounds. The X-irradiated control group shows a much greater wound open area than the non–X-irradiated control group on Days 3, 7 and 14 (A). The X-irradiated FGF-2&LMWH/P NPs group has a significantly higher healing rate on Days 3, 7 and 14 than the FGF-2, LMWH/P NPs alone, and control (saline) groups (B) (P < 0.05, n = 6).
Wound healing and angiogenic bioactivity of localized administered FGF-2&LMWH/P NPs were examined in the X-irradiated groups by photomicrographic observation of H&E-stained specimens. Figure 4 shows representative photomicrographs of saline (control)-, FGF-2-, LMWH/P NPs- and FGF-2&LMWH/P NPs-administered wounds in the X-irradiated groups on Days 1, 3 and 7. At 7 days after X-irradiation, the group with localized injection of FGF-2&LMWH/P NPs showed a substantial effect, with induction of vascularization and fibrous tissue formation, compared with the control and other groups.
Fig. 4.
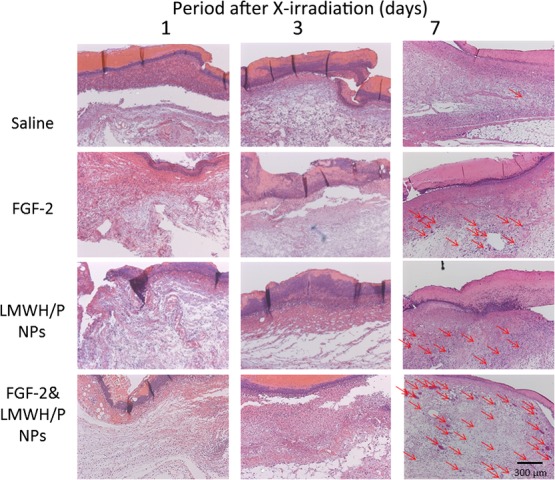
Each photomicrograph of the saline (control)-, FGF-2-, LMWH/P NPs- and FGF-2&LMWH/P NPs-administered wounds in the X-irradiated groups on Days 1, 3 and 7 is representative of six preparations. The red arrows indicate blood vessels with several erythrocytes.
TUNEL– and 8-OHdG–staining of X-irradiated skin
TUNEL-staining at 24 h after wound creation showed a large number of apoptotic dermal fibroblast cells in both the X-irradiated FGF-2&LMWH/P NPs and control groups (Fig. 5). The proportion of apoptotic cells in the wound tissue was significantly higher in the X-irradiated control group than in the X-irradiated FGF-2&LMWH/P NPs group (Fig. 5).
Fig. 5.
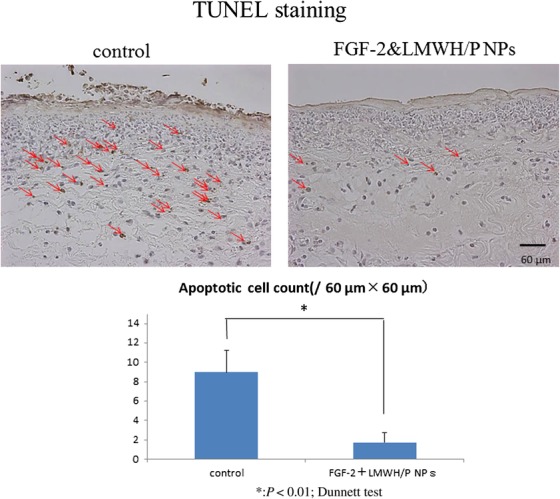
TUNEL-staining of X-irradiated skin. Control- and FGF-2&LMWH/P NPs-administered X-irradiated wounds were TUNEL-stained 24 h after wound creation. The red arrows indicate TUNEL-stained spots. Each photomicrograph is representative of eight preparations. The number of TUNEL-stained spots is significantly higher in saline (control)-administered X-irradiated wounds than in FGF-2&LMWH/P NPs-administered X-irradiated wounds. (P < 0.01, n = 8).
For further histological observation, 8-OHdG immunohistochemical staining of skin sections at 24 h after wound creation was performed to measure the 8-OHdG–positive spots. The 8-OHdG staining indicates the hyperoxidation state of the nucleic acids, allowing the anti-oxidative effect to be evaluated [10, 25]. We found 8-OHdG–positive staining at the wound surface in both the X-irradiated FGF-2&LMWH/P NPs and control groups (Fig. 6). However, there was no significant difference in the number of 8-OHdG–positive stained cells in the total wound between the X-irradiated FGF-2&LMWH/P NPs and control groups.
Fig. 6.
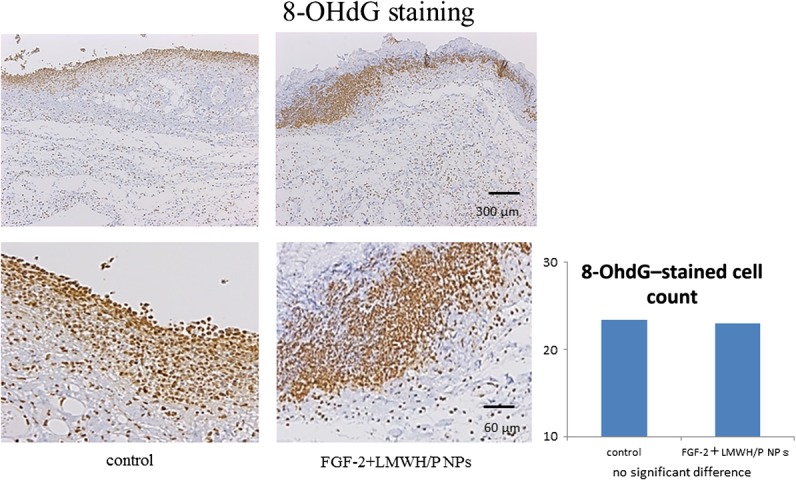
Immunostaining (8-OHdG–staining) of X-irradiated skin. Control- and FGF-2&LMWH/P NPs-administered X-irradiated wounds were 8-OHdG–stained 24 h after wound creation. The red arrows indicate 8-OHdG–stained spots. Each photomicrograph is representative of eight preparations. There are no differences in the numbers of 8-OHdG–stained spots between saline (control)-administered and FGF-2&LMWH/P NPs-administered X-irradiated wounds. (P < 0.01, n = 8).
DISCUSSION
Heparin-binding growth factors such as FGF-2 are known to bind to heparinoids with high affinity [11]. Heparinoids prolong the biological half-life of FGF-2 and protect FGF-2 from heat and proteolytic inactivation [11]. It has been reported that LMWH/P NPs are polyelectrolyte complexes that are specifically bound to FGF-2 and can be retained locally, and they significantly enhance and stabilize FGF-2 activity as a local carrier [12, 19]. In the present study, X-irradiated wound repair was found to be significantly delayed in vivo (Fig. 3A), and localized FGF-2&LMWH/P NPs administration prior to X-irradiation resulted in a significantly shorter delay (Fig. 3B), as well as inducing vascularization and fibrous tissue formation, compared with administration with FGF-2, LMWH/P NPs or controls (Fig. 4). Furthermore, TUNEL-staining showed that the proportions of apoptotic dermal fibroblasts in X-irradiated skin were significantly lower in rats administered FGF-2&LMWH/P NPs than in controls (Fig. 5). Thus, localized administration of FGF-2&LMWH/P NPs prior to irradiation may alleviate X-irradiated healing-impaired wound repair. However, a reduction in the number of 8-OHdG–stained cells was not observed in X-irradiated skin of rats administered FGF-2&LMWH/P NPs compared with controls (Fig. 6). The 8-OHdG staining indicates the hyperoxidation state of the nucleic acids, allowing the antioxidative effect to be evaluated [25]. Since the reduction in the number of 8-OHdG–stained cells is related to the direct scavenging of hydroxyl radicals [9, 25], FGF-2&LMWH/P NPs appear to have little effect on scavenging of hydroxyl radicals.
On the other hand, the stimulatory effects of inhalation of hydrogen-containing gas (HCG) on healing of radiation-induced healing-impaired skin wounds have been demonstrated [9, 10]. The radioprotective, antiapoptotic and antioxidant mechanisms by which pre-inhalation of HCG exerts its effects remain to be studied in vitro and in vivo. An in vivo study showed that pre-inhalation of HCG significantly reduced the severity of X-irradiation–induced oxidative injury and the apoptosis responses of X-irradiated skin areas, as evidenced by the levels of both 8-OHdG and TUNEL-staining [9, 10]. Thus, HCG is believed to have an inhibitory effect on the apoptosis of epidermal keratinocyte cells and dermal fibroblast cells that may be caused by the inactivation of cytotoxic ROS generated by X-irradiation [9, 10].
Tissues with rapid cell turnover, such as the gut, bone marrow, and hair follicles, are hypersensitive to X-irradiation–induced apoptosis [16]. Diffusible FGF-2 is known to be a wide-spectrum survival factor, promoting cell resistance in both irradiated normal and tumor tissues [26], notably through the downregulation of apoptosis [27]. It has been demonstrated that intestinal crypt radioprotection by FGF-2 is dependent on regulation of Akt/p53 signaling [16]. It has been confirmed that FGF-2 as an angiogenic factor has a protective effect against DNA damage for endothelial cells [28–30]. Furthermore, radiation-induced microvascular damage regulates the endothelial cell apoptotic response to radiation in the clinically relevant range [30]. Thus, systemic administration of FGF-2 alone may depress the radiation-induced microvascular damage associated with endothelial cell apoptosis, which may lead to cancer stem cell resistance to radiation therapy [30].
In contrast to the systemic administration of FGF-2 alone, several studies demonstrated that LMWH/P NPs strongly interact with FGF-2 and that LMWH/P NPs protect FGF-2 from inactivation by heat and degradation by proteases [12, 18], and that FGF-2&LMWH/P NPs administered locally by subcutaneous or muscular injection would remain at the injection site and sustain a high concentration of FGF-2 activity, facilitating radioprotection of normal tissues. Both chemical components of the LMWH/P NPs are in clinical use, and hence, they may possess high clinical safety. It is thus proposed that FGF-2&LMWH/P NPs may be a promising new controlled FGF-2 release system for inducing vascularization in ischemic limbs.
In conclusion, wound healing was significantly delayed by X-irradiation, but localized administration of FGF-2&LMWH/P NPs prior to irradiation led to a significantly shorter delay. Furthermore, TUNEL staining showed that the proportions of apoptotic dermal fibroblasts were significantly lower in X-irradiated skin of rats administered FGF-2&LMWH/P NPs, although 8-OHdG staining showed no significant differences. Thus, localized administration of FGF-2&LMWH/P NPs prior to irradiation may alleviate X-irradiated healing-impaired wound repair.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
FUNDING
This work was funded by the National Defense Medical College in the Defense Ministry of Japan.
REFERENCES
- 1. Spałek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol 2016;9:473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hopewell JW. The skin: its structure and response to ionizing rariation. Int J Radiat Biol 1990;57:751–73. [DOI] [PubMed] [Google Scholar]
- 3. Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg 2011;53:15S–23. [DOI] [PubMed] [Google Scholar]
- 4. Herst PM. Protecting the radiation-damaged skin from friction: a mini review. J Med Radiat Sci 2014;61:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosen EM, Day R, Singh VK. New approaches to radiation protection. Front Oncol 2015;4:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takabayashi Y, Ishihara M, Kuwabara M et al. Improved survival of full-thickness skin graft with low-molecular weight heparin-protamine micro/nanoparticles including platelet-rich plasma. Ann Plast Surg 2017;78:562–8. [DOI] [PubMed] [Google Scholar]
- 7. Takikawa M, Sumi Y Ishihara M et al. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J Surg Res 2011;170:e189–96. [DOI] [PubMed] [Google Scholar]
- 8. Takikawa M, Sumi Y, Tanaka Y et al. Protective effect of prostaglandin E1 on radiation-induced proliferative inhibition and apoptosis in keratinocytes and healing of radiation-induced skin injury in rats. J Radiat Res 2012;53:385–94. [DOI] [PubMed] [Google Scholar]
- 9. Ohsawa I, Ishikawa M, Takahashi K et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007;13:688–94. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe S, Fujita M, Ishihara M et al. Protective effect of inhalation of hydrogen gas on radiation-induced dermatitis and skin injury in rats. J Radiat Res 2014;55:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishihara M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-2 are different from that for FGF-2. Glycobiology 1994;4:817–24. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura S, Kanatani Y, Kishimoto S et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J Biomed Mater Res A 2009;91:814–23. [DOI] [PubMed] [Google Scholar]
- 13. Motomura K, Hagiwara A, Komi-Kuramochi A et al. An FGF1:FGF-2 chimeric growth factor exhibits universal FGF receptor specificity, enhanced stability and augmented activity useful for epithelial proliferation and radioprotection. Biochim Biophys Acta 2008;1780:1432–40. [DOI] [PubMed] [Google Scholar]
- 14. Ding I, Huang K, Wang X et al. Radioprotection of hematopoietic tissue by fibroblast growth factors in fractionated radiation experiments. Acta Oncol 1997;36:337–40. [DOI] [PubMed] [Google Scholar]
- 15. Cotrim AP, Soweres A, Mitchell JB et al. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 2007;15:2101–6. [DOI] [PubMed] [Google Scholar]
- 16. Liu Z, Liu H, Jiang J et al. PDGF-BB and bFGF ameliorate radiation-induced intestinal progenitor/stem cell apoptosis via Akt/p53 signaling in mice. Am J Physiol Gastrointest Liver Physiol 2014;307:G1033–43. [DOI] [PubMed] [Google Scholar]
- 17. Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 2000;60:321–7. [PubMed] [Google Scholar]
- 18. Nemeno JGE, Lee S, Yang W et al. Applications and implications of heparin and protamine in tissue engineering and regenerative medicine. Biomed Res Int 2014;2014:936196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori Y, Nakamura S, Kishimoto S et al. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int J Nanomedicine 2010;5:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kishimoto S, Ishihara M, Nakamura S et al. Fragmin/protamine microparticles to adsorb and protect HGF and to function as local HGF carrier in vivo. Acta Biomater 2013;9:4763–70. [DOI] [PubMed] [Google Scholar]
- 21. Takikawa M, Nakamura S, Nakamura S et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma containing fragmin/protamine microparticles. J Biomed Mater Res B Appl Biomater 2011;97:373–80. [DOI] [PubMed] [Google Scholar]
- 22. Takikawa M, Ishihara M, Takabayashi Y et al. Enhanced healing of mitomycin C-treated healing impaired wounds in rats with PRP-containing fragmin/protamine microparticles (PRP&F/P MPs). J Plast Surg Hand Surg 2015;49:268–74. [DOI] [PubMed] [Google Scholar]
- 23. Ishihara M, Kishimoto S, Takikawa M et al. Biomedical application of low molecular weight heparin/protamine nano/micro particles as cell- and growth factor-carriers and coating matrix. Int J Mol Sci 2015;16:11785–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakashima T, Uematsu N, Shibamori M et al. Establishment of an X-ray irradiation-induced glossitis model in rats: biphasic elevation of proinflammatory cytokines and chemokines. J Pharmacol Exp Ther 2013;347:660–8. [DOI] [PubMed] [Google Scholar]
- 25. Kinoda J, Ishihara M, Hattori H et al. Cytotoxicity of silver nanoparticle and chitin-nanofiber sheet composites caused by oxidative stress. Nanomaterials 2016;6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fuks Z, Persaud RS, Alfieri A et al. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res 1994;54:2582–90. [PubMed] [Google Scholar]
- 27. Marie M, Hafner S, Moratille S et al. FGF2 mediates DNA repair in epidermoid carcinoma cells exposed to ionizing radiation. Int J Radiat Biol 2012;88:688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389–95. [DOI] [PubMed] [Google Scholar]
- 29. Paris F, Fuks Z, Kang A et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001;293:293–7. [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Barros M, Paris F, Cordon-Cardo C et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155–9. [DOI] [PubMed] [Google Scholar]