Abstract
The aim of our retrospective study is to report a case series of ocular infections caused by a rare fungus, Scedosporium apiospermum, in a South Indian population. Thirteen cases of culture-positive S. apiospermum infections diagnosed between January 2011 and March 2016 were included in this study. The parameters evaluated were predisposing factors, treatment and final clinical outcome. The most common mode of presentation was keratitis (84.6%) followed by sclerokeratitis (15.3%). The predisposing factors involved were unspecified foreign body injury (30.7%), organic matter injury (15.3%), uncontrolled diabetes (7.6%), and recent manual small-incision cataract surgery (7.6%). Five cases (38.46%) had no predisposing factor. Of the 11 keratitis cases, nine (69.2%) responded well to combination medical therapy while one case (7.6%) required therapeutic keratoplasty. One case was lost to follow-up. Both cases which presented with sclerokeratitis showed no response to medico-surgical treatment progressing to panophthalmitis and evisceration.
Keywords: Keratitis, Scedosporium apiospermum, sclerokeratitis
Keratomycosis is commonly seen in posttraumatic cases, particularly in a rural environment. Over 60 different species of fungi have been reported to cause mycotic keratitis, Fusarium, and Aspergillus being the most common.[1]Scedosporium apiospermum, previously known as Monosporium apiospermum, is a ubiquitous dematiaceous filamentous fungus. It can be recovered from soil, decaying matter, and polluted water.[2] The histopathological morphology of this species is similar to Fusarium spp. and Aspergillus spp. Infections caused by S. apiospermum species infections can be found in immunocompetent as well as in immunocompromised individuals.[2]Pseudallescheria boydii, also known as Petriellidium boydii, is the sexual form of this fungus.[3] Here, we report a series of cases of ocular infections caused by this rare fungus, S. apiospermum, in a South Indian population.
Methods
Retrospective, observational study of medical records of patients diagnosed with culture-positive S. apiospermum-induced keratitis, scleritis, and endophthalmitis was done in the Cornea Services of Aravind Eye Hospital, Puducherry, Tamil Nadu, after obtaining the required permission from the institutional review board. Thirteen such cases diagnosed between January 2011 and March 2016 which were treated medically or surgically were included in this study. The main parameters evaluated in this study were predisposing factors, treatment modality used and outcome of various treatment modalities. Time to healing was noted as the duration from initiation of treatment at our hospital to the follow-up visit when antifungal medications were stopped upon complete scarring of infiltrate. The statistical analysis was done using SPSS software, version 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).
Results
The total number of culture-positive fungal keratitis during the study period was 1621, out of which those caused by Scedosporium were 13 (0.8%). The mean age of presentation was 54.3 years (range 30–85 years). Out of the total 13 patients, seven (53.8%) were male and six (46.15%) were female. Four (30.7%) patients had been using some ocular medications before presenting to our hospital. The average time to presentation from the onset of symptoms was 7.15 days (range 2–20 days). The different modes of presentation were keratitis 11 cases (84.6%) which was most common, followed by two cases of sclerokeratitis (15.3%). The mean size of infiltrate (longest diameter of infiltrate multiplied by its longest perpendicular diameter) was 20 mm2. Four cases (30.7%) showed hypopyon at presentation. Furthermore, one case following recent manual small-incision cataract surgery (MSICS), performed elsewhere, showed sclerocorneal wound infiltrate with superior corneal ring infiltrate. Case-wise details of every patient are elaborated in Table 1.
Table 1.
Clinical features and outcome
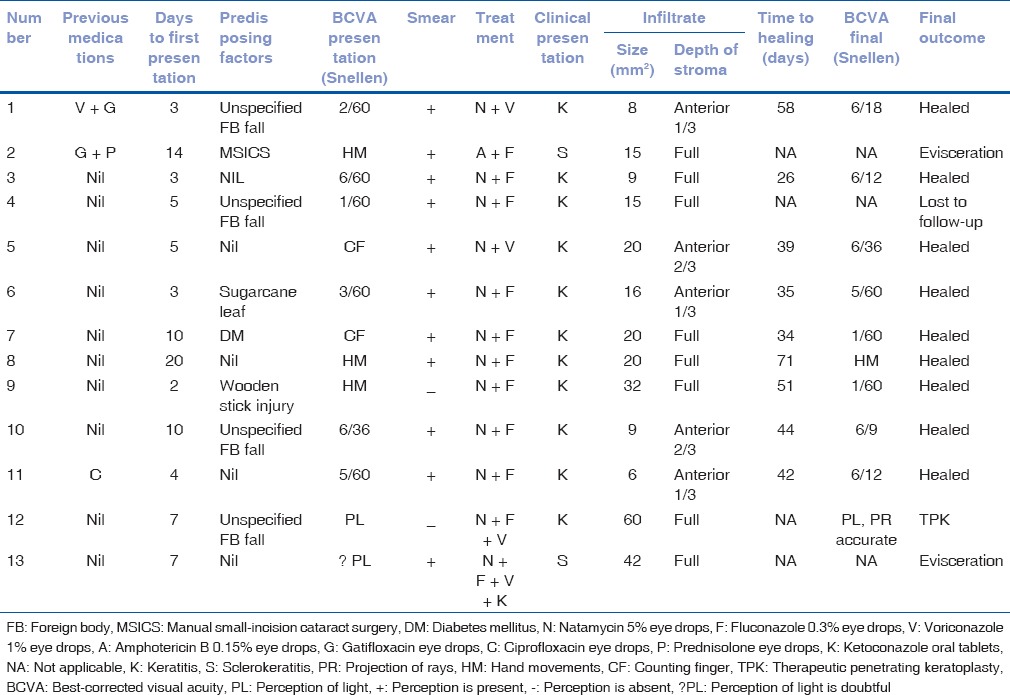
Out of the 11 keratitis cases, nine (69.2%) responded well to medical therapy with topical natamycin along with an azole (fluconazole in seven and voriconazole in two cases) while one case required therapeutic keratoplasty for progressive keratitis with perforation. Average time to healing in the nine keratitis cases that showed healing was 44.44 days (range 26–71 days). Both cases which presented with sclerokeratitis showed no response to medico-surgical treatment progressing to panophthalmitis and evisceration. One of these two sclerokeratitis cases [case no. 2 in Table 1] grew Scedosporium in the initial corneal scraping culture and Aspergillus fumigatus in the final evisceration specimen, indicating a mixed fungal infection. One case was lost to follow-up.
Discussion
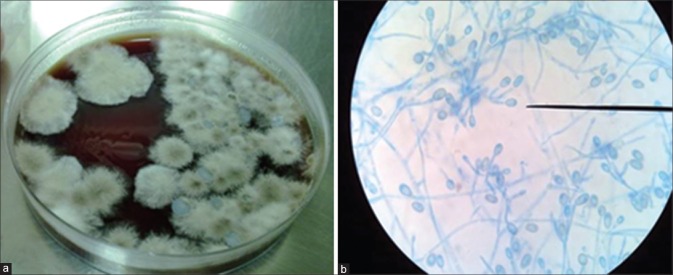
Being a rare filamentary fungus to affect the eye, reports of Scedosporium[4,5,6] ocular infections in literature are usually solitary and uncommon. The literature has attributed the low incidence of Scedosporium keratitis to difficulty in distinguishing Scedosporium from Aspergillus, Fusarium, and other hyaline hyphomycetes due to common histopathological and clinical features. However, sporulation in culture followed by staining with lactophenol cotton blue, a stain commonly used to identify fungi, helps in identifying scedosporium[1] [Fig. 1]. Even the large National Eye Institute supported studies, MUTT I[7] and MUTT II[8] that enrolled 563 patients in all had only one case of proven Scedosporium keratitis (personal communication with the authors). Only two cases series of Scedosporium ocular infections have been reported till date, one from Australia and another from southern India. The Australian study[9] has reported scleritis due to Scedosporium as the most common presenting feature, with six of nine patients with history of previous pterygium surgery. Most of our patients, on the other hand, had keratitis as the common presenting feature and even the two patients who had a scleritis component did not give any history of previous pterygium surgery. One of them had undergone a recent MSICS and was on topical steroids when she developed the sclerokeratitis, while the other patient developed Scedosporium sclerokeratitis in a previously scarred, nonseeing eye with an anterior staphyloma.
Figure 1.
(a) Chocolate agar showing dirty white cottony colonies on inoculation of corneal scraping material. Growth was seen after 3 days of incubation at 25°C. (b) Lactophenol cotton blue staining showing septate hyphae, ovoid conidia (as shown by the microscope pointer) with truncated bases suggestive of Scedosporium apiospermum as visualized using a binocular compound microscope with high power and ×45
Of special interest was the case that developed infection of the superior sclerocorneal wound along with the adjacent superior cornea, 2 weeks post-MSICS. Though culture from the initial corneal scraping revealed S. apiospermum, the final evisceration specimen grew A. fumigatus, indicating a mixed fungal infection. The concomitant use of topical steroids, delay in referral, involvement of the sclera, and a mixed fungal infection, all could have contributed to the downhill course of disease in this patient who progressed to endophthalmitis and panophthalmitis, finally necessitating evisceration. To the best of our knowledge, this is the first reported case of proven Scedosporium wound infection in the early postoperative period following cataract surgery.
The other case series by Rathi et al.,[10] also from South India, showed healing with natamycin monotherapy in five of eight cases while natamycin along with voriconazole was used in two of eight cases. At our hospital, we administer natamycin monotherapy to mild ulcers (<2 mm in largest diameter, involving the anterior stromal third only and noncentral, none of the ulcers in our series fit these criteria).[11] Since polyenes and azoles act at two different levels of ergosterol biosynthesis pathway, we prefer to take advantage of this possible synergistic effect in treating moderate and severe ulcers, as in our series. Hence, we instituted a topical combination therapy of 5% natamycin, a polyene (shown to be very effective against filamentous fungi)[7] as the primary drug and an azole, either 0.3% fluconazole or the relatively newer 1% voriconazole.[12] All three drugs are commercially available and hence are easy to dispense and use. The effectiveness of topical fluconazole has been debated in literature with some studies showing poor susceptibility of filamentous fungi to fluconazole in vitro[13] and in the clinical scenario,[14] while other authors have claimed clinical benefit even with fluconazole monotherapy in filamentary fungal keratitis.[15] We found that in our series, natamycin and fluconazole combination therapy succeeded in seven of 10 cases. Of the remaining three, one was lost to follow-up, one progressed to therapeutic keratoplasty, and one had scleral involvement and progressed to evisceration.
Time to healing in our series may appear quite prolonged since it was a retrospective analysis, and we have taken the visit at which topical antifungals were stopped completely. We usually stop antifungals after 10–14 days of complete scarring as visualized on slit lamp.
Conclusion
Most Scedosporium infections of the cornea alone responded well to commercially available antifungals with natamycin as the primary drug. Cases which involved the sclera showed grave prognosis eventually leading to panophthalmitis and evisceration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kwon Chung KJ, Bennett JE. Infections due to miscellaneous molds. In: Kwon Chung KJ, Bennett JE., editors. Medical Mycology. Philadelphia: Lea & Febiger; 1992. pp. 733–67. [Google Scholar]
- 2.Guarro J, Kantarcioglu AS, Horré R, Rodriguez-Tudela JL, Cuenca Estrella M, Berenguer J, et al. Scedosporium apiospermum: Changing clinical spectrum of a therapy-refractory opportunist. Med Mycol. 2006;44:295–327. doi: 10.1080/13693780600752507. [DOI] [PubMed] [Google Scholar]
- 3.Guého E, De Hoog GS. Taxonomy of the medical species of Pseudallescheria and Scedosporium. J Mycol Med. 1991;118:3–9. [Google Scholar]
- 4.Leck A, Matheson M, Tuft S, Waheed K, Lagonowski H. Scedosporium apiospermum keratomycosis with secondary endophthalmitis. Eye (Lond) 2003;17:841–3. doi: 10.1038/sj.eye.6700477. [DOI] [PubMed] [Google Scholar]
- 5.Özkan A, Susever S, Erturan Z, Uzun M, Alparslan N, Öz Y. A case of keratitis caused by Scedosporium apiospermum. J Microbiol Infect Dis. 2013;3:45–8. [Google Scholar]
- 6.Vagefi MR, Kim ET, Alvarado RG, Duncan JL, Howes EL, Crawford JB, et al. Bilateral endogenous Scedosporium prolificans endophthalmitis after lung transplantation. Am J Ophthalmol. 2005;139:370–3. doi: 10.1016/j.ajo.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial: A randomized trial comparing natamycin vs.voriconazole. JAMA Ophthalmol. 2013;131:422–9. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prajna NV, Krishnan T, Rajaraman R, Patel S, Srinivasan M, Das M, et al. Effect of oral voriconazole on fungal keratitis in the mycotic ulcer treatment trial II (MUTT II): A Randomized clinical trial. JAMA Ophthalmol. 2016;134:1365–72. doi: 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhanji V, Yohendran J, Constantinou M, Sheorey H, Vajpayee RB. Scedosporium scleritis or keratitis or both: Case series. Eye Contact Lens. 2009;35:312–5. doi: 10.1097/ICL.0b013e3181be722e. [DOI] [PubMed] [Google Scholar]
- 10.Rathi HS, Venugopal A, Rengappa R, Ravindran M. Scedosporium keratitis: An experience from a tertiary eye hospital in South India. Cornea. 2016;35:1575–7. doi: 10.1097/ICO.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 11.Isipradit S. Efficacy of fluconazole subconjunctival injection as adjunctive therapy for severe recalcitrant fungal corneal ulcer. J Med Assoc Thai. 2008;91:309–15. [PubMed] [Google Scholar]
- 12.Lalitha P, Shapiro BL, Srinivasan M, Prajna NV, Acharya NR, Fothergill AW, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789–93. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 13.Yee RW, Cheng CJ, Meenakshi S, Ludden TM, Wallace JE, Rinaldi MG, et al. Ocular penetration and pharmacokinetics of topical fluconazole. Cornea. 1997;16:64–71. [PubMed] [Google Scholar]
- 14.Rao SK, Madhavan HN, Rao G, Padmanabhan P. Fluconazole in filamentous fungal keratitis. Cornea. 1997;16:700. [PubMed] [Google Scholar]
- 15.Sonego-Krone S, Sanchez-Di Martino D, Ayala-Lugo R, Torres-Alvariza G, Ta CN, Barbosa L, et al. Clinical results of topical fluconazole for the treatment of filamentous fungal keratitis. Graefes Arch Clin Exp Ophthalmol. 2006;244:782–7. doi: 10.1007/s00417-005-1127-8. [DOI] [PubMed] [Google Scholar]