Abstract
Purpose:
This study aimed to report the long-term outcomes of autologous Simple Limbal Epithelial Transplantation (SLET) performed for unilateral limbal stem cell deficiency (LSCD) following chemical burn at a tertiary eye center in North India.
Methods:
This was a single-center prospective interventional case series of patients who developed unilateral LSCD after suffering from ocular surface burns and who underwent SLET between October 2012 and May 2016 with a follow-up period of at least 6 months. The primary outcome measure was restoration of a completely epithelized, stable, and avascular corneal surface. The secondary outcome measure was percentage of eyes, which reported visual gain.
Results:
The study included 30 eyes of 30 patients, 18 adults and 12 children, at a median follow-up of 1.1 years (range: 6 months to 3.5 years), 21 of 30 eyes (70%; 95% confidence interval, 53.6%–86.2%) maintained successful outcome. Visual acuity gain was seen in 71.4% of successful cases. The clinical factors associated with failure were identified as acid injury, severe symblepharon at the time of presentation, and SLET combined with penetrating keratoplasty (PK).
Conclusion:
Autologous SLET is an effective limbal cell transplantation technique for the treatment of unilateral LSCD. It is especially beneficial for centers where cell cultivation laboratory is unavailable. Presence of severe symblepharon, which requires PK peroperatively, has poor outcome.
Keywords: Chemical injury, limbal stem cell deficiency, Simple Limbal Epithelial Transplantation
Corneal blindness (CB) continues to be the second most common cause of blindness in the developing world.[1] Out of all the causes for CB, ocular burns carry a poor prognosis as they may result in damage of the limbal stem cells and cause limbal stem cell deficiency (LSCD).[2] LSCD is characterized by chronic epithelial defects, neovascularization and conjunctivalization, and stromal inflammation, leading to corneal opacification and loss of vision.[3,4] Since penetrating keratoplasty (PK) carries a poor prognosis in these patients, various other treatment modalities have been described over the past few decades.[5]
In 1977, Thoft proposed autologous conjunctival transplant to treat corneas injured by ocular burn.[6] In 1989, Kenyon and Tseng described conjunctival limbal autograft (CLAU), wherein the limbal stem cells from the healthy eye were transplanted in the disease eye.[7] However, due to the fear of donor eyes developing LSCD, Pellegrini proposed a technique called cultivated limbal epithelial transplantation (CLET).[8] In this technique, smaller donor lenticule could be cultivated ex vivo into a transplantable sheet. This, however, requires a laboratory and is not feasible in developing countries.[2] To circumvent this need and at the same time strike a balance between the clinical safety and cost efficacy, Sangwan et al. in 2011 introduced Simple Limbal Epithelial Transplantation (SLET) as an alternative to CLET and CLAU.[2] This novel technique is a single-stage, affordable procedure utilizing minimal donor tissue, which achieves in vivo expansion of harvested limbal cells.[9]
Besides a study from Basu et al., limited data have been published on the long-term follow-up of SLET results.[10] After being a part of a multicentric SLET study, we are reporting our clinical outcomes of SLET performed over the last 4 years.[11] To the best of our knowledge, this study is the largest series after Basu et al., and the first of its kind from North India.[10]
Methods
Study design, patients, and approval
This is a prospective, interventional case series conducted at Cornea and Anterior Segment Services of a tertiary care eye hospital in North India. The study has been approved by the Institutional Ethics Committee. The study was conducted in adherence to the tenets of the Declaration of Helsinki. The patients or the legal guardians (in case of children) signed an informed written consent for the surgical procedure, investigations, and use of clinical photographs.
All patients who underwent SLET between October 1, 2012 and May 31, 2016 were included. Inclusion criteria were cases of unilateral LSCD with wet ocular surface for which autologous SLET was performed, with a minimum of 6 months of postoperative follow-up. LSCD was defined clinically as the absence of the limbal palisades of Vogt, dull, and irregular corneal epithelium, superficial corneal vascularization, persistent epithelial defects, and conjunctival overgrowth on the corneal surface.[2] Exclusion criteria were bilateral LSCD, LSCD secondary to mucous membrane pemphigoid, Steven–Johnson syndrome, and dry ocular surfaces.
Surgical technique
The surgical technique was similar to the one described by Sangwan et al.[2] All surgeries were a single-stage procedure performed by a single surgeon (NG), with more than 5 years of surgical experience in corneal surgeries. Before the procedure, peribulbar anesthesia was administered in both eyes in adults and general anesthesia was administered in children. A small piece of limbal tissue (1–2 clock hours) was harvested from the unaffected eye. Subconjunctival dissection was continued until the limbus was reached followed by a shallow dissection 1 mm into the clear cornea. The limbal tissue was excised and kept in balanced salt solution; excess conjunctiva was reposited back and sealed with fibrin glue (TISSEEL Kit from Baxter AG, Vienna, Austria). Fibrovascular pannus was excised from the eye with LSCD, and human amniotic membrane (hAM) was spread over the bare surface, using fibrin glue as adhesive. The donor tissue was cut into 12–16 small pieces with either Vannas scissors (Asian Surgicals Ltd, India) or a #15 surgical blade (Asian Surgicals, India). These limbal transplants were then uniformly distributed on the hAM leaving a clear visual axis and were held in place with fibrin glue. The surgical technique was different for partial LSCD. Superficial keratectomy was done avoiding the intact limbus area. AMG was placed only in the areas of keratectomy but sutures were taken with 10-0 nylon through the episclera beyond 2 mm limbus in areas where the limbus was intact. The pieces of limbal biopsy were not placed in intact limbal area. There was only one patient with <6 clock hours of LSCD. In this patient, a banana-shaped AMG was placed with glue in the involved area, and limbal biopsy was placed only in this area 2 mm inside the limbus on cornea. At the end of the surgery, a soft bandage contact lens (Purecon™, size 18 mm) was placed over the cornea for 2–3 weeks and one drop of topical antibiotic eye drop (moxifloxacin 5 mg/mL eye drops, Vigamox®, Alcon Labs, India) was instilled into the eyes. The recipient eye was patched overnight. None of the patients underwent tarsorrhaphy during surgery. There were no cases of early BCL dislodgement, which were noted.
Postoperative follow-up schedule
All patients underwent complete eye examination of both eyes at every follow-up visit. Patients were followed up on postoperative days 1, 7, 30, 90, and at 6 months. Patients, who missed their follow-up appointment, were contacted telephonically and seen at the next earliest appointment. Patients were prescribed topical Betamethasone sodium phosphate 0.1% eye drop six times a day, which was started after surgery in the donor and the recipient eye from the following day. It was continued for 4 weeks in the donor eye and up to 3 months in the recipient eye, depending on the ocular surface inflammation. A topical antibiotic eye drop (moxifloxacin 5 mg/mL eye drops, Vigamox®, Alcon Labs, India) was used four times a day until the ocular surface epithelized. Preservative-free lubricants were used in both eyes.
Data collection and outcome measure parameters
Data collection forms were developed and were used for all patients, both pre- and post-operatively. Demographic details, etiology of LSCD, injury to presentation duration, prior surgery performed, presentation to SLET duration, and clinical details including visual acuity at presentation, extent of LSCD (in clock hours), presence or absence of eyelid abnormalities, symblepharon, simultaneous PK, and persistent epithelial defects were noted.
The primary outcome measure was success of SLET, defined clinically as a completely epithelized, avascular, stable corneal surface till the last follow-up. Secondary outcome measure included the percentage of eyes gaining vision.
Statistical analysis
Statistical analysis was carried out using SPSS statistical software (SPSS version 21. Inc., Chicago, IL, USA). All descriptive parameters were noted in the form of mean and standard deviation if the data were parametric or in the form of median if the data were nonparametric. Statistical significance was defined at a level of 5% (P < 0.05). A Cox proportional hazards analysis was done to assess the association of preoperative characteristics with risk of failure.
Results
During the period of this study, thirty eyes of thirty patients met the inclusion criteria. Out of the thirty patients, 18 were adults (mean age: 29.1 years, range: 20–42 years) and 12 were children (mean age: 8.1 years, range: 3–16 years) with unilateral LSCD, occurring after ocular burn. Males were more common than females (70%). Seventeen (9 adults and 8 children) patients had a minimum follow-up of at least 12 months and six (4 adults and 2 children) patients were followed up for more than 18 months (range: 6–42 months for children and 6–36 months for adults). Total LSCD was seen in 10 adults and 5 children, and the remaining 15 eyes had partial LSCD ranging from 6 to 9 clock hours of limbal involvement. Alkali burns (83.3% in children and 38.8% in adults) were by far the most common cause of LSCD, followed by acid injuries. The median duration after injury to SLET procedure was 12 months (range: 4–192 months). It was longer for children (40.5 months; range: 4–120 months) than adults (18.5 months; range: 6–192 months). Nine (75%) children and eight (44.4%) adults had symblepharon. Out of these 17 patients, 9 patients had severe symblepharon. Two of these nine patients underwent fornix formation before SLET, and the rest seven had fornix formation and SLET in the same sitting. Figs. 1–5 show a collage of slit-lamp photographs of various patients who were included in the study and underwent SLET. A PK was performed simultaneously with SLET in three (10%) cases, two adults, and one child. The etiology in two cases was chemical injury and thermal injury in the third case. One patient presented in the acute stage of injury (within 1 day of injury) and the other two in the chronic stage. The patient who presented in the acute stage had a successful outcome as opposed to the other patients who presented in the chronic stage. Table 1 summarizes demographic and baseline clinical features of the study population and compares features between adults and children.
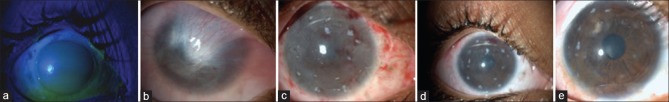
Figure 1.
(a) Grade 5 chemical (alkali) burn - first day presentation, (b) total limbal stem cell deficiency with superior symblepharon formation, 6-month postlime injury, (c) first postoperative day after Simple Limbal Epithelial Transplantation showing limbal biopsies, (d) 1-month post Simple Limbal Epithelial Transplantation, (e) 4-month post Simple Limbal Epithelial Transplantation
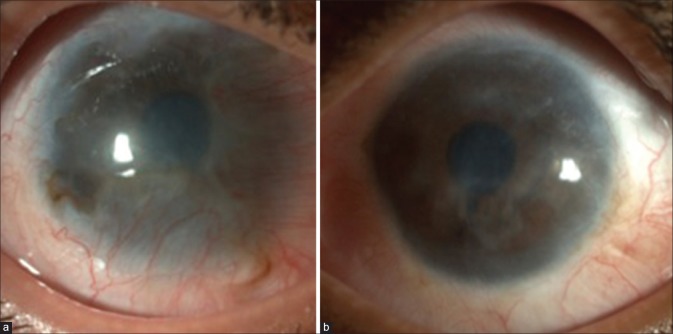
Figure 5.
(a) Two-year postfire cracker injury, the patient developed total limbal stem cell deficiency, (b) 15-month post Simple Limbal Epithelial Transplantation, the patient had focal recurrence
Table 1.
Baseline characteristics of patients undergoing Simple Limbal Epithelial Transplantation for limbal stem cell deficiency occurring after ocular burns
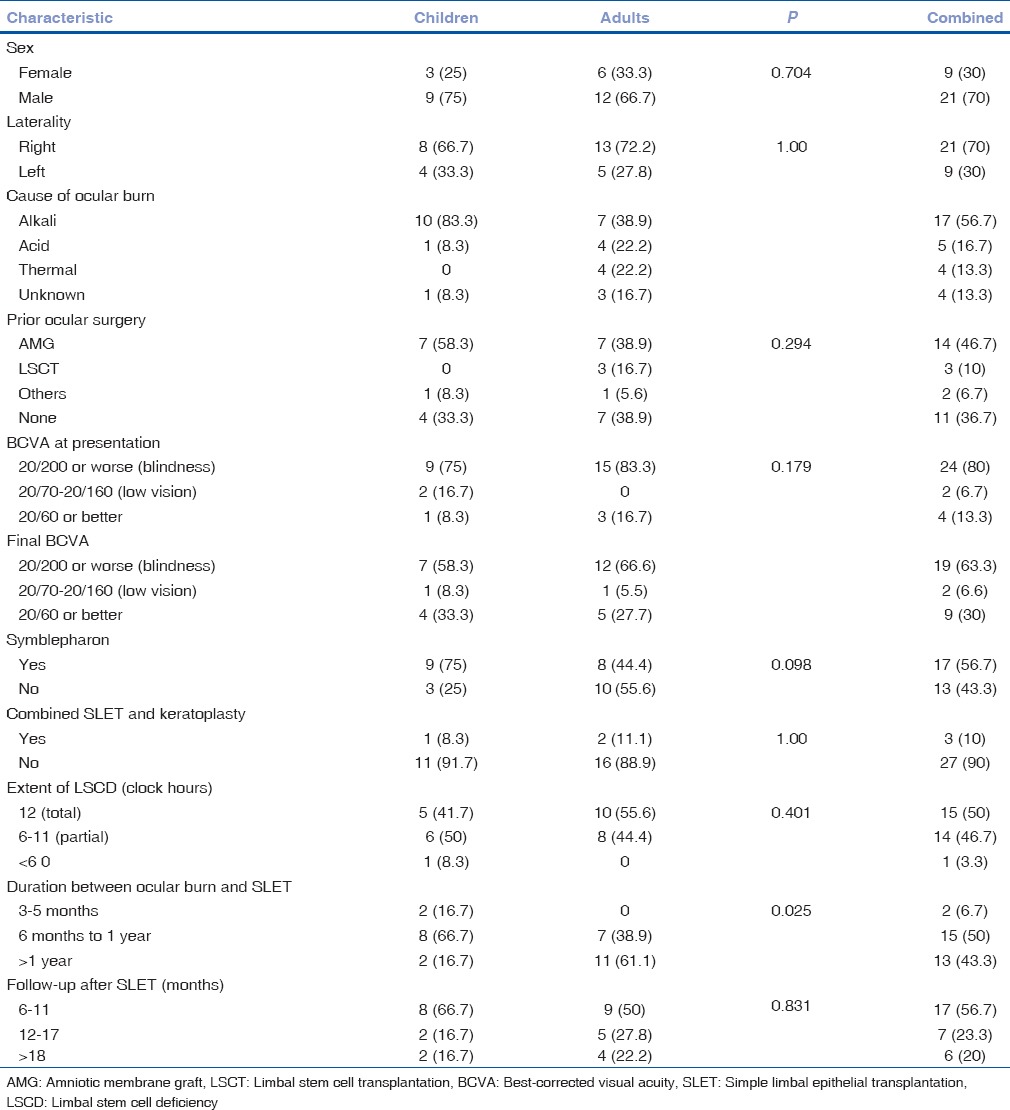
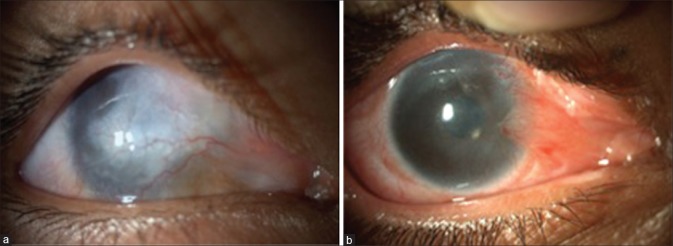
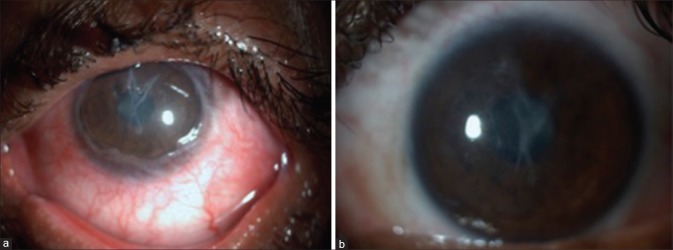
Figure 2.
(a) Eighteen-month postlime injury, (b) total limbal stem cell deficiency, (c) 3-month post Simple Limbal Epithelial Transplantation
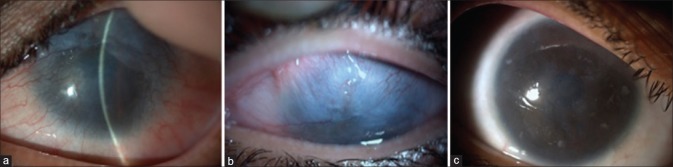
Figure 3.
(a) Ten-year postlime injury, (b) 1-year post Simple Limbal Epithelial Transplantation
Figure 4.
(a) Two-month postlime injury, the patient underwent amniotic membrane graft in the acute stage, (b) 2-year post Simple Limbal Epithelial Transplantation
Efficacy of Simple Limbal Epithelial Transplantation and outcome measures
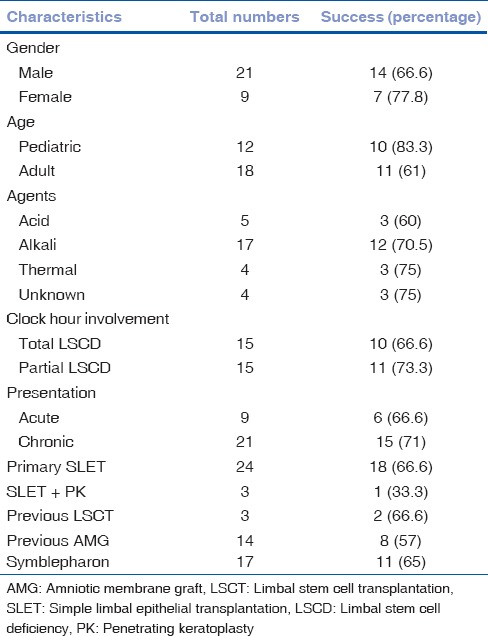
Successful outcome was achieved in 21 patients (10 children and 11 adults) and overall success rate of SLET was 70% (95% confidence interval [CI]; 53.2%–86.2). The nine patients, who did not improve after SLET, were counseled for a re-surgery. Eight of those patients declined to get another procedure done. One patient had a repeat SLET, but that went into failure as well. Improvement in visual acuity was seen in 71.4% of patients. Progressive conjunctivalization occurred in 30% of eyes. Table 2 describes the success and failure rates across different parameters. Successful regeneration of the corneal surface described as maintenance of a completely epithelized, avascular, and stable corneal surface without progressive conjunctivalization was observed in 60% eyes of adults and 80% eyes of children (P = 0.69) with total LSCD. Among patients with partial LSCD, successful outcomes were observed in 62.5% eyes of adults and 85.7% eyes of children (P = 1.0). Best-corrected visual acuity (BCVA) improved significantly after SLET both in adults and children (P = 0.00).
Table 2.
Primary outcome in various subgroups
Donor-site safety
None of our patients (adults and children) showed any donor-site complications and/or LSCD. Three patients complained of foreign body sensation, and all the patients had the epithelial defect completely healed by 1 week.
Risk factors
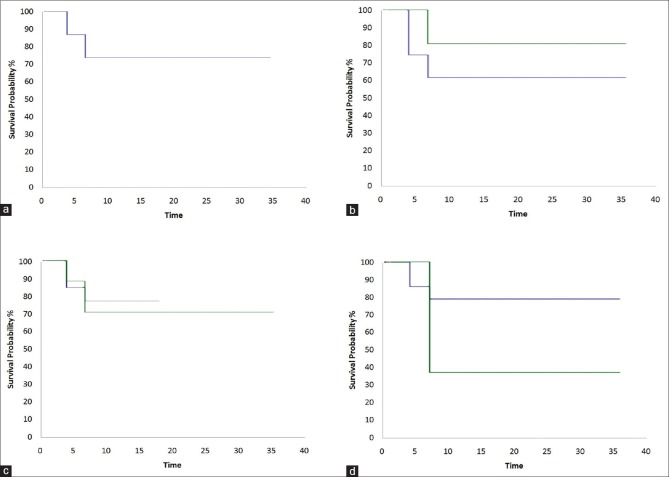
In the Cox proportional hazards analysis, presence of symblepharon (hazard ratio [HR] 1.24; 95% CI: 0.30–5.06) and simultaneous keratoplasty (HR 2.76; 95% CI: 0.29–25.82) was found to be associated with a risk of failure of SLET. Fig. 6 shows Kaplan–Meier survival curves in various subgroups and clinical settings.
Figure 6.
Kaplan–Meier survival curves comparing the success rates of Simple Limbal Epithelial Transplantation in different subgroups. (a) Overall survival probability, (b) survival probability in children and adults, (c) survival probability in symblepharon cases, and (d) Simple Limbal Epithelial Transplantation combined with keratoplasty during surgery
Discussion
The research foundation in the field of stem cells dates back to early 1800s,[12] and their self-renewal and differentiation abilities have being widely used in the medical field in the recent years. Our study is modeled over the novel technique described by Sangwan et al. and its use as an alternative to CLET and CLAU.[2]
The overall success of SLET in this study was 70% at a median follow-up of 1.1 years. This is comparable to the limited data, which is available on SLET. Other major SLET studies done in the recent years have described their success as 76% (Basu et al.), 66% (Jain et al.), and 83% (Vazirani et al.), with a mean follow-up period of 35.5 months, 6.2 months, and 12 months, respectively.[9,10,11] Holland and Baylis reviewed multiple studies on CLET.[13,14] The results have been encouraging, varying from 46.7% to 76%. The fundamental difference between the two procedures is the need for ex vivo expansion of cells in CLET, which makes it limited to few centers across the world. On the other hand, even though literature review of CLAU shows that the ocular surface was improved in 82%–94% of the patients, the success was limited to patients in whom large grafts (>120°) were used.[15,16] In addition, visual acuity Dropped to 60% when smaller grafts were used. The exact amount of tissue from the healthy eye that can be transplanted safely without jeopardizing the donor eye is still debatable.[17]
Interestingly, the success seen in children in our study was 83.3%. Not only is this much better than CLET (46.7%[18] and 37%[19] as mentioned in literature), but it is also better when compared to other studies on SLET.[9,10,18] We feel that this is a topic of further research, as some studies have attributed the failure to strong inflammatory response to inciting injury;[9] whereas, other studies have mentioned good results because of better inherent potential of individual transplants of young patients.[18]
Visual improvement was seen in 71.4% of our patients which is comparable with other studies on CLET by Baylis et al. and Zhao and Ma who reported improvement in 51% and 67%, respectively.[14,20] Our figures are even comparable with that of other SLET studies, which report two-line improvement in 65.7% and 66.6%.[9,11] Among the five patients whose vision remained same, one patient developed amblyopia, two patients had corneal scar who subsequently required PK, and three patients had partial LSCD with good vision preoperatively, two had 20/30 and third one 20/40.
As noted in earlier studies,[2,21] there was no loss of BCVA in healthy eyes in any case. Table 3 shows a comparison of various studies done on SLET, highlighting their important findings.
Table 3.
Comparison between various studies on Simple Limbal Epithelial Transplantation
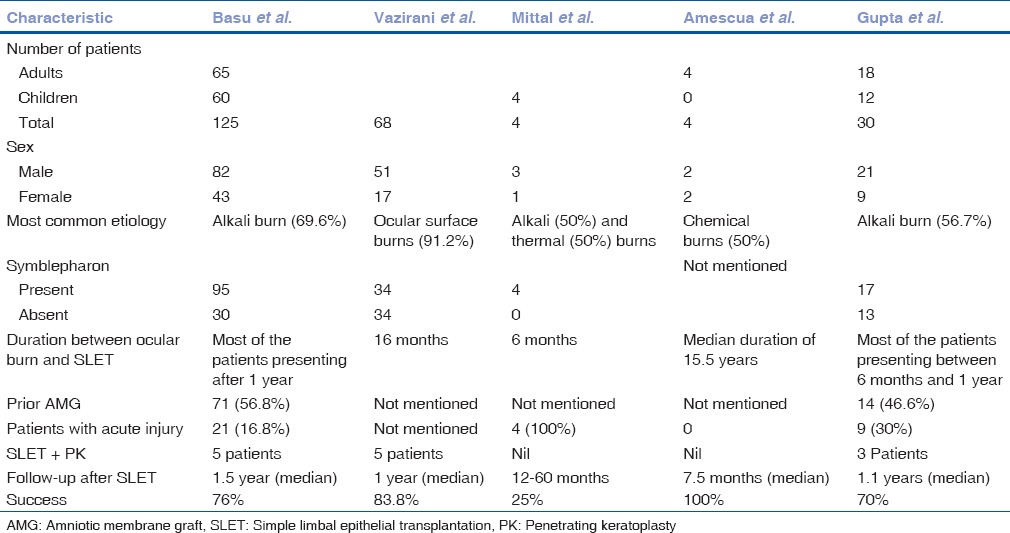
We noted that, like in other studies, the presence of symblepharon and simultaneous keratoplasty was associated with the risk of SLET failure. This has been described in almost all studies, which have described about SLET.[11,22] We recommend that such cases must be taken up with caution. Presence of symblepharon indicates toward a more severe initial insult.
We understand that the attempts for a successful stem cell transplant by any opted method (CLET/CLAU/SLET) depend on the best replicated model of corneal wound healing in the eye. Although the mechanism of stem cell transplant is unknown, it is the regeneration of dormant stem cells postinjury or reimplantation of transplanted stem cells into the niche.[12] Fate mapping studies of corneal epithelial cells have proved beyond doubt that peripherally located progenitor cells continuously divide and migrate from limbus toward central cornea to replenish corneal epithelia. In the state of a corneal wound, progenitor cells receive signals to differentiate into epithelial cells, which is regulated by specific signals from basement membrane and growth factors and cytokines.[23] The success of stem cell transplant procedure depends on the percentage of progenitor cells transplanted and presence of a favorable microenvironment.
We believe that SLET is the best possible replica of corneal wound healing since it allows the limbal biopsy to grow in the most natural favorable microenvironment and also allows the signaling pathway to reach the progenitor cells and stromal matrix to play its role. As compared to CLET, where the standard protocol for best multiplication of progenitor cells is still unavailable and variable success rate of 33%–100%, initial reports on SLET are more convincing.[23]
The uniqueness of this study is that it is the largest study from North India, with a minimum follow-up of at least 6 months. Furthermore, a single surgeon, eliminating chances of interobserver variations, did the surgery and all the follow-ups. The surgeon also observed that cases, which had AMG done in acute stage, showed easy pannus removal during SLET procedure. Finally, we noted that all the patients in the study with Grade 4 and 5 injuries (with scleral ischemia underwent tenonplasty and without scleral ischemia underwent AMG) had successful outcomes; as opposed to all the Grade 6 injuries. We acknowledge the fact that still the data are limited on SLET, and we need more patients and longer follow-ups to assess the exact outcome. However, the initial results are very encouraging, taking into consideration that these were the first few cases of SLET performed by the operating surgeon. We believe that this method should be actively taught to budding cornea surgeons, especially in developing countries, where the patients cannot afford expensive surgeries like CLET.
Conclusion
SLET seems to be a very promising treatment option for LSCD. With affordability and minimal dependence on laboratory, being its key feature, this technique will soon become the treatment of choice for LSCD of nonimmune etiology in the coming years.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pineda R. Corneal transplantation in the developing world: Lessons learned and meeting the challenge. Cornea. 2015;34(Suppl 10):S35–40. doi: 10.1097/ICO.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 2.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–4. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 3.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3(Pt 2):141–57. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 4.Kruse FE. Stem cells and corneal epithelial regeneration. Eye (Lond) 1994;8(Pt 2):170–83. doi: 10.1038/eye.1994.42. [DOI] [PubMed] [Google Scholar]
- 5.Brown SI, Bloomfield SE, Pearce DB. A follow-up report on transplantation of the alkali-burned cornea. Am J Ophthalmol. 1974;77:538–42. doi: 10.1016/0002-9394(74)90468-1. [DOI] [PubMed] [Google Scholar]
- 6.Thoft RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95:1425–7. doi: 10.1001/archopht.1977.04450080135017. [DOI] [PubMed] [Google Scholar]
- 7.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 9.Jain R, Kanaujia V, Sahu S, Das S. Management of unilateral limbal stem cell deficiency by Simple Limbal Epithelial Transplantation-our experience. MOJ Surg. 2014;1:00002. [Google Scholar]
- 10.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123:1000–10. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Vazirani J, Ali MH, Sharma N, Gupta N, Mittal V, Atallah M, et al. Autologous Simple Limbal Epithelial Transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br J Ophthalmol. 2016;100:1416–20. doi: 10.1136/bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Munozledo F. Review: Corneal epithelial stem cells, their niche and wound healing. Mol Vis. 2013;19:1600–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Holland EJ. Management of limbal stem cell deficiency: A historical perspective, past, present, and future. Cornea. 2015;34(Suppl 10):S9–15. doi: 10.1097/ICO.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 14.Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: A review of the outcomes. J Cell Biochem. 2011;112:993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 15.Atallah MR, Palioura S, Perez VL, Amescua G. Limbal stem cell transplantation: Current perspectives. Clin Ophthalmol. 2016;10:593–602. doi: 10.2147/OPTH.S83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology. 2010;117:1207–13. doi: 10.1016/j.ophtha.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Liang L, Sheha H, Li J, Tseng SC. Limbal stem cell transplantation: New progresses and challenges. Eye (Lond) 2009;23:1946–53. doi: 10.1038/eye.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittal V, Jain R, Mittal R, Vashist U, Narang P. Successful management of severe unilateral chemical burns in children using Simple Limbal Epithelial Transplantation (SLET) Br J Ophthalmol. 2016;100:1102–8. doi: 10.1136/bjophthalmol-2015-307179. [DOI] [PubMed] [Google Scholar]
- 19.Sejpal K, Ali MH, Maddileti S, Basu S, Ramappa M, Kekunnaya R, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131:731–6. doi: 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34:592–600. doi: 10.1097/ICO.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 21.Amescua G, Atallah M, Nikpoor N, Galor A, Perez VL. Modified Simple Limbal Epithelial Transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158:469–75.e2. doi: 10.1016/j.ajo.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–9. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 23.Jones RR, Hamley IW, Connon CJ. Ex vivo expansion of limbal stem cells is affected by substrate properties. Stem Cell Res. 2012;8:403–9. doi: 10.1016/j.scr.2012.01.001. [DOI] [PubMed] [Google Scholar]