Abstract
Purpose:
To compare operative outcomes of patients after canaloplasty and trabeculectomy with mitomycin C (MMC) through 2-year follow-up.
Methods:
We included 31 eyes of 31 patients with primary open-angle glaucoma (POAG) who underwent canaloplasty and 37 eyes of 37 patients with POAG who underwent trabeculectomy with MMC with 24 months of postoperative follow-up. All surgeries were performed by a single surgeon between January 2005 and May 2011. Failure was defined as intraocular pressure (IOP >18 or <4 mmHg at 2 years), second operative procedure, or loss of light perception. Change in IOP, visual acuity (VA), glaucoma medications, and complication rates at 24 months were analyzed.
Results:
Caucasians made up to half of the patients included in this study (58% vs. 43%) while the rest where either African Americans (32% vs. 43%) or Hispanic (10% vs. 14%) patients between the canaloplasty and trabeculectomy group. Both groups showed significant reduction in IOP from baseline at 24 months. Trabeculectomy patients had a greater mean reduction of IOP compared to canaloplasty patients (12.2 ± 12 vs. 4.7 ± 7.5, P = 0.003) and also achieved lower IOP at 24 months (12.2 ± 4.1 vs. 14.9 ± 6.0, P = 0.03). Postoperative glaucoma medication use was less in the trabeculectomy group (n = 0, interquartile range [IQR] 0–2) compared to those in whom canaloplasty was performed (n = 2, IQR 0–3, P = 0.02). VA showed no statistical change in either group over 2 years. Overall failure rates at 2 years were comparable between the two groups: 32% for trabeculectomy and 26% for canaloplasty (P = 0.6). Subgroup analysis revealed a lower failure rate in Caucasions (15%) when compared to Blacks (42%) and Hispanics/others (50%, P = 0.03).
Conclusion:
Canaloplasty and trabeculectomy both achieved significant reduction in IOP with comparable success rates. Trabeculectomy can achieve a greater reduction in IOP while requiring fewer medications however is associated with more intensive postoperative care and frequent interventions. Pigmented populations have worse outcomes compared to Caucasians.
Keywords: Canaloplasty, multiethnic patient population, primary open-angle glaucoma, trabeculectomy
Trabeculectomy is considered the gold standard of glaucoma surgery, and although effective, it requires the presence of a filtering bleb that can be associated with problems including infection, dysesthesia, and poor cosmesis. Canaloplasty is a procedure that involves viscodilation of the Schlemm's canal with placement of an intracanalicular tension suture with the purpose of facilitating trabeculocanalicular aqueous outflow.[1] When canaloplasty is performed correctly, there is no bleb formation. Our previously published studies demonstrated significant intraocular pressure (IOP) reduction and comparable 1 year success rates between the two procedures when performed alone or with cataract surgery.[2,3] Independently, there are several studies comparing the procedures with 1 year outcome data.[4,5] Matlach et al. have produced prospective 2 year data comparing the procedures however only included Caucasian European patients.[6] Additional studies published comparing these two procedures also do not include racial demographics.[4,5,7] We present 2-year surgical outcomes of canaloplasty and trabeculectomy with mitomycin C (MMC) performed in a multiethnic group with moderate to advanced primary open-angle glaucoma (POAG).
Methods
This retrospective, nonrandomized, comparative study received institutional review board approval. A single surgeon performed all surgeries. Medical records were reviewed of patients with moderate to advanced open-angle glaucoma who underwent either trabeculectomy with MMC or canaloplasty with or without phacoemulsification and intraocular lens implant. Glaucoma severity was graded by measures that are now considered consistent with the 2010 American Academy of Ophthalmology preferred practice pattern guidelines. All were treated by the glaucoma service at a tertiary hospital from January 2005 to December 2011. The decision to perform canaloplasty or trabeculectomy was based on the prior approval of canaloplasty by the insurance companies. All patients whose insurance companies preauthorized the canaloplasty procedure underwent canaloplasty with or without cataract surgery. The remainder of the patients underwent trabeculectomy with or without cataract surgery. The decision to perform phacoemulsification with intraocular lens implant at the time of glaucoma surgery was based on the presence of a visually significant cataract.
Eyes with a minimum of 24 months of follow-up after surgery were included. If surgery was performed on both eyes, only the first eye was included. Exclusion criteria included age <18, previous incisional surgery (excluding cataract surgery), and unsuccessful cannulation at time of canaloplasty. A total of 31 canaloplasty procedures in 31 patients and 37 trabeculectomy surgeries in 37 patients were included in our analysis.
For each patient, we documented age, race, gender, lens status (phakic or pseudophakic), preoperative and postoperative visual acuity (VA), IOP, and number of glaucoma medications and reported these for the 1 and 2 year time points. Need for further surgical intervention and postoperative complications including choroidal effusions, suprachoroidal hemorrhage, infection, and bleb status including bleb failure or leak were reported for the 2-year duration.
Primary endpoints were IOP failure, visual failure, and operative failure, any of which was sufficient to be considered an overall failure. IOP failure was defined as an IOP >18 mmHg with or without glaucoma medications or IOP <4 mmHg at the 24-month examination. Operative failure was defined as the need for additional incisional surgery. Visual failure was defined as a loss of light perception.
Operative technique for each procedure has been described previously.[3] If the IOP was not sufficiently controlled during the follow-up of canaloplasty, the scleral flap was needled at the slit lamp. A 30-gauge needle was used to penetrate conjunctiva, loosen the scleral flap's closure, and perform micropuncture of the Descemet's window into the anterior chamber. Similarly, the bleb was needled if additional IOP control was desired during trabeculectomy follow-up. A 30-gauge needle was used to sweep any fibrous adhesions within the bleb and inject 0.2 mL of MMC (0.4 mg/mL).
All patients received the same postoperative antibiotic and steroid regimen of moxifloxacin four times daily for 1 week and prednisolone acetate 1% eight times daily initially followed by a taper of prednisolone over 2 months depending on bleb appearance. In addition, nighttime application of neomycin/polymyxin B/dexamethasone ointment was utilized for 1 week.
Statistical analysis
IOP data are summarized by means and standard deviations and VA is expressed in terms of the mean and standard error in logarithm of the minimum angle of resolution units. Gender, race, eye (left, right), and lens status were compared between groups using Fisher's exact test; the Student t-test was used to assess mean age differences. Baseline IOP, VA, and number of medications were compared between canaloplasty and trabeculectomy groups and between race and gender. IOP failure rates at 24 months, using the >18 mmHg or <4 mmHg criteria noted above, were compared between the 2 groups using the Fisher's exact test. Repeated measures analysis of variance with Bonferroni adjustment was used to compare both groups with respect to IOP and VA from preoperative baseline to 12 and 24 months.[8] Median and interquartile range (IQR) of the number of medications (preoperatively and at 12 and 24 months) were compared between groups using the nonparametric Mann–Whitney U test with changes in number of medications at 12 and 24 months relative to baseline within each group analyzed by the Wilcoxon signed-ranks test.[9] Fisher's exact test was used to compare failure rates, specific complications, and the proportion of patients requiring postoperative medications at 24 months between the groups. Freedom from reoperation was analyzed using the Kaplan–Meier product-limit method and groups compared by the log-rank test with 95% confidence intervals constructed using Greenwood's formula.[10] Statistical analysis was performed using the IBM/SPSS software package (version 21.0, IBM, Armonk, NY). Two-tailed P < 0.05 was considered significant. Power analysis indicated that a minimum sample size of 34 eyes in each group would provide 90% power to detect mean postoperative difference of 4 mmHg in IOP between the 2 groups, assuming a standard deviation of 5 mmHg (moderate effect size = 0.80) using analysis of variance (version 7.0, nQuery Advisor, Statistical Solutions, Cork, Ireland).
Results
Glaucoma management and vision
Thirty-one eyes of 31 patients who underwent canaloplasty and 37 eyes of 37 patients who underwent trabeculectomy were included in this study [Table 1]. Of the 31 patients who underwent canaloplasty, 12 (38.7%) had concurrent phacoemulsification with intraocular lens implant. Of the 37 patients who underwent trabeculectomy, 13 (35.1%) had concurrent phacoemulsification with intraocular lens implant. All 68 patients were followed for a minimum of 2 years. No differences were found in respect to age (P = 0.18), race (P = 0.48), gender P = 0.23), or right versus left eye (P = 0.76). There was a statistically significant difference in the number of individuals who were pseudophakic at the time of surgery with 7 individuals (23%) being pseudophakic before canaloplasty compared to only 1 individual (3%) in the trabeculectomy group (P = 0.02). There was no difference in preoperative VA (P = 0.45) or median number of glaucoma medications (P = 0.31). Patients undergoing trabeculectomy had a higher mean preoperative IOP (24.4 ± 12.0) compared to the canaloplasty group (19.6 ± 4.9, P = 0.03).
Table 1.
Demographics and longitudinal outcomes for canaloplasty and trabeculectomy groups
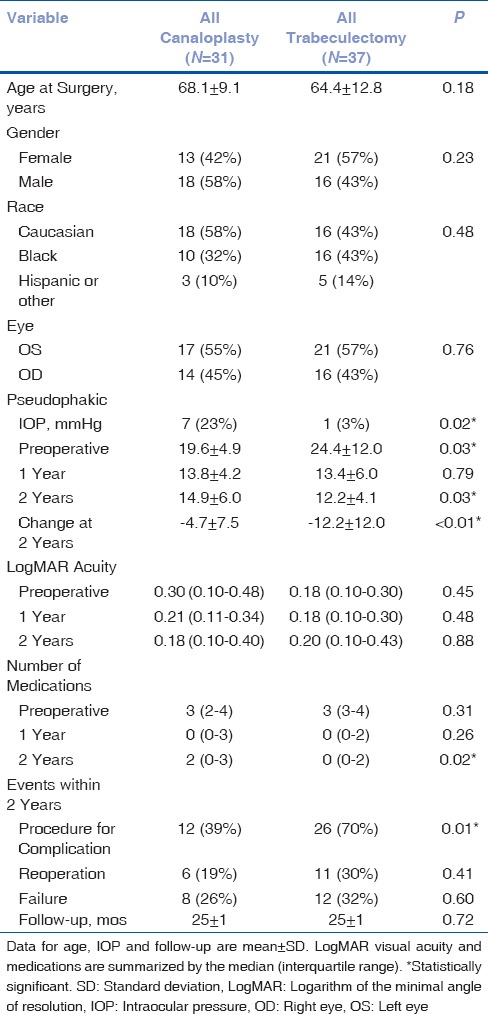
Both groups showed a statistically significant reduction in IOP at 2 years compared to baseline. This difference was greater in the trabeculectomy patients who achieved a mean reduction of 12.2 ± 12 mmHg at 2 years compared to 4.7 ± 7.5 mmHg in the canaloplasty group (P = 0.003). In addition, trabeculectomy patients achieved a lower mean IOP of 12.2 ± 4.1 compared to 14.9 ± 6.0 for canaloplasty patients (P = 0.03). Median number of glaucoma medications used at 2 years was also lower in the trabeculectomy group (n = 0, IQR 0–2) compared to those in whom canaloplasty was performed (n = 2, IQR 0–3, P = 0.02).
VA, as measured by logarithm of the minimal angle of resolution (logMAR), showed no statistical change in either group over 2 years. There was also no difference in final VA between the groups at 2 years (P = 0.88).
Failures and complications
There was no difference in 2-year failure rates [Fig. 1] for those who received canaloplasty (n = 8, 26%) versus those who received trabeculectomy (n = 12, 32%) (P = 0.6). Most failures were due to need for additional surgery. The exceptions occurred in 2 patients who had canaloplasty without cataract surgery and exceeded IOP criteria at the 2-year visit while 1 phacotrabeculectomy failed to meet IOP criteria at the 2-year examination. The reoperation rate within 2 years was not statistically different between those having canaloplasty and those having trabeculectomy (19% vs. 30%, P = 0.41). Similarly, as depicted by the Kaplan–Meier curves in Fig. 2, the time to these events is not significantly different between the surgical groups (log-rank test = 1.13, P = 0.29). Freedom from reoperation at 2 years was estimated to be 10% lower among patients treated with trabeculectomy compared to canaloplasty (71% vs. 81%); however, the difference was not statistically significant; the 95% confidence intervals provide precision as to the expected freedom from reoperation for each group at 1- and 2-year follow-up. Subgroup analysis revealed a lower failure rate in Whites (15%) when compared to African-Americans (42%) and Hispanics/others (50%, P = 0.03). Five out of the 6 surgical failures in the canaloplasty group occurred in patients who underwent canaloplasty without phacoemulsification. All surgical failures in the canaloplasty group were due to inadequately controlled IOP. As a result, 4 patients received Ahmed FP7 glaucoma valves (AGV, New World Medical, Inc.), 1 patient underwent EX-PRESS shunt (Alcon Laboratories, Inc.) with MMC, and 1 patient underwent trabeculectomy with MMC.
Figure 1.
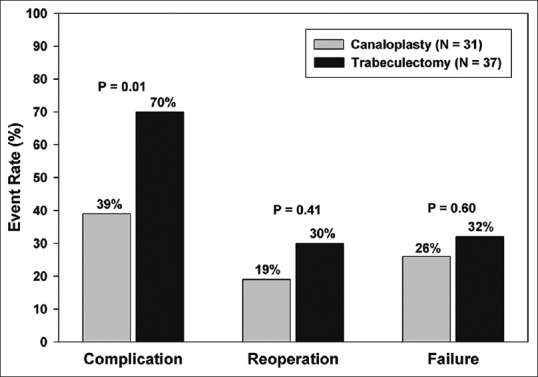
Adverse event rates at 2 years for canaloplasty and trabeculectomy
Figure 2.
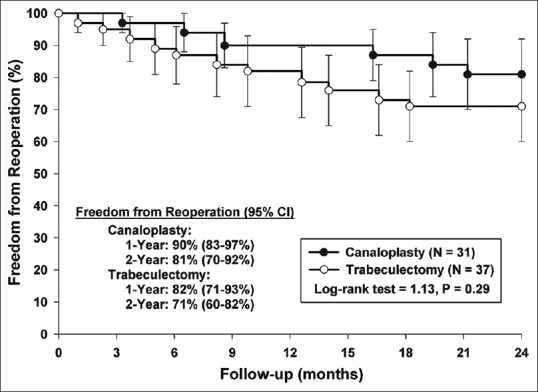
Kaplan–Meier curves depicting freedom from reoperation at 2 years
The 11 surgical failures in the trabeculectomy group were evenly distributed between individuals who did and did not receive simultaneous phacoemulsification. For the 6 failures that occurred with trabeculectomy alone, 4 patients developed a cystic leaking bleb that required surgical repair, whereas the two remaining patients underwent AGV placement. For the 5 patients who underwent combined trabeculectomy and phacoemulsification, 2 developed a cystic leaking bleb requiring surgical repair, 2 developed inadequate IOP control with subsequent placement of AGVs, and 1 developed a dislocated IOL and cystic leaking bleb, which required lens repositioning and bleb repair.
The number of individuals who had complications requiring additional surgery was much less common in those who received canaloplasty (n = 12, 39%) than those who received trabeculectomy (n = 26, 70%) (P = 0.007). In this study, 7 patients who underwent canaloplasty developed inadequate IOP control and had subsequent needling. Of those patients, 1 underwent successful needling and developed a functioning bleb at 2 years with an IOP of 10 on 2 medications. Four patients underwent needling, which were ultimately unsuccessful and went on to receive tube shunts. Two patients had unintended filtering blebs on postoperative day 1 with small leaks which were fixed with slit lamp suturing. These blebs subsequently scarred down with concurrent rise of IOP. These blebs were then needled with MMC and were successful at lowering the IOP, requiring no further interventions. Two patients had suture placement at the slit lamp to repair leaks. One of which developed hypotony with choroidal effusions that resolved after repair of the leak. Descemet's membrane detachment occurred in 4 individuals following canaloplasty. One of these was visually significant and drainage of the detachment was performed with a 30-gauge needle at the slit lamp successfully. Cataract formation was infrequent with only 1 patient requiring cataract surgery. Significant hyphema (>1 mm) occurred in 6 individuals with 1 patient developing corneal blood staining which resolved spontaneously.
Hypotony was the most common complication occurring after trabeculectomy. Fourteen individuals experienced some transient hypotony (IOP <5 mmHg or presence of choroidal effusions). Half of these individuals had hypotony related to cystic leaks requiring revision. Two patients’ hypotony resolved with medications. The remaining 5 individuals received either a transconjunctival suture for overfiltration or slit lamp repair of a wound leak.
Cataract was the second most common complication following trabeculectomy. Twenty-three of the 24 patients who had trabeculectomy without phacoemulsification were phakic at the time of surgery. Of those 23 patients, 11 (48%) developed cataracts necessitating surgery. It should be noted only 12 out of 19 individuals were phakic before primary canaloplasty. Of those 12 phakic patients, only 1 developed a cataract requiring surgery (8%).
Needling with MMC was performed in 6 individuals for bleb failure following trabeculectomy. One patient developed blebitis following needling which was medically treated but ultimately led to complete bleb fibrosis necessitating AGV placement. Finally, 1 patient developed a blood clot in the surgical ostomy resulting in an IOP of 50, which was medically managed and required no further intervention.
Discussion
Our previous work showing 1-year outcomes of trabeculectomy versus canaloplasty showed similar success rates and both surgeries were able to achieve IOPs in the low teens.[2] In that study, there was a statistically nonsignificant trend for trabeculectomy patients to achieve a lower number of IOP lowering medications with lower mean IOP. Matlach et al. published the TVC study, a 2-year prospective study investigating trabeculectomy versus canaloplasty, however they reviewed outcomes in an all Caucasian group.[6] This is an atypical demographic for most glaucoma practices in the southern United States. Additional studies published comparing these two procedures do not include racial demographics.[4,5,7] Furthermore, it has been proposed that non-Caucasions are at higher risk for bleb failure following trabeculectomy.[11] Therefore, this study contributes to the current literature 2-year data comparing vision, IOP lowering medication, and mean IOP reduction between these procedures among a multiethnic group, providing broader applications among different patient populations. Subgroup analysis in our study revealed a lower failure rate in Whites (15%) when compared to Blacks (42%) and Hispanics/others (50%, P = 0.03) emphasizing this very point.
TVC study further differs from our study in that up to 50% of the patients had secondary OAG (PXF or Pigment dispersion) with no further clarification as to the stage of glaucoma (mild, moderate, or severe). Our study population includes a multiracial patient panel with moderate to severe POAG. TVC patients were all pretreated with at least one episode of laser cyclodestructive procedure whereas our patients were only treated with topical medications before surgical intervention. In regard to the postoperative bleb management, the TVC surgeons actively injected 5 FU injections (in 29/32 patients in trabeculectomy group and up to 7 injections on average).
Mean VA remained stable in both groups. Cataract formation was frequently seen in our phakic patients who underwent trabeculectomy. Although vision was stable in both groups at 2 years, cataract surgery was performed more frequently in the trabeculectomy arm. These results may be biased by our preference to remove cataracts at the time of canaloplasty. In addition, these results were likely influenced by the fact that most patients undergoing canaloplasty were already pseudophakic at the time of surgery.
Both groups achieved significant IOP reduction at 2 years. Patients who had trabeculectomy achieved a greater mean reduction than those who had canaloplasty(−12.2 vs. −4.7, P = 0.003) although this was confounded by the fact that the trabeculectomy group started with a significantly higher mean IOP. In addition, trabeculectomy patients achieved a lower mean IOP (12.2 vs. 14.9, P = 0.03). These 2-year IOP results are similar to our 1-year outcomes in our previous 2 studies. Our first study looking at canaloplasty versus trabeculectomy showed a final mean IOP of 13.8 ± 4.9 and 11.6 ± 4.0 whereas our second study showed a final mean IOP of 14.1 ± 4.4 and 11.8 ± 5.4 when comparing phacocanaloplasty versus phacotrabeculectomy.[2,3] The TVC study found 2-year mean IOP for canaloplasty to be 14.4 ± 4.2 and trabeculectomy to be 11.5 ± 3.4 which is comparable to our results.
Before surgery, the median number of glaucoma medications used was 3 for both groups. At 2 years, however, trabeculectomy patients used a median of 0 medications compared to 2 medications for canaloplasty patients (P = 0.02). These results vary considerably from what was documented in our previous work. Both of our previous studies showed 0 median IOP lowering medications at 1 year regardless of procedure. We also observe this trend in this study. However, we see that after 1 year, the medication burden increases in our patients who received canaloplasty, indicating a decreased effect of the surgery with time.
Caucasian individuals experienced a 15% failure rate, which was significantly lower compared to African-American (42%) and Hispanic/other patients (50%, P = 0.03). The TVC study defined qualified success in a similar manner to our study. In that study, which included only Caucasians, success rate for trabeculectomy was 90.3% for trabeculectomy and 82.6% for canaloplasty. This success rate is similar to that of the Caucasian patients in this study.
One distinct advantage of canaloplasty over trabeculectomy is the absence of a filtering bleb. In patients who are showing early failure following canaloplasty, we sometimes utilize flap needling with or without antimetabolite with the purpose of creating a filtering bleb. In this study, 7 patients who had canaloplasty underwent subsequent needling. Patients who inadvertently formed a bleb after canaloplasty which subsequently scarred down were able to reform functioning filtering blebs following needling with MMC. However, in the 5 patients who never formed an initial bleb, only 1 was able to form a functioning bleb after needling. This strategy, therefore, may be of limited use for lowering IOP in most canaloplasty patients. Consideration must be given to the fact that the long-term infection risks associated with trabeculectomy are likely applicable to canaloplasty patients who form blebs following needling.
A common problem associated with bleb-based procedures is hypotony. There was one case of hypotony in our canaloplasty group due to early bleb formation and wound leak. This is the only patient we have encountered that has experienced significant hypotony following canaloplasty. The leak was repaired at the slit lamp, the hypotony resolved, and the patient had an otherwise uneventful postoperative course. Hypotony was common in our trabeculectomy group with 38% experiencing it at some point during the 2-year study period. Of the 14 cases of hypotony, half were due to the formation of cystic leaking blebs. Our approach to this outcome is surgical repair with amniotic membrane graft and conjunctival advancement.[12] The incidence of hypotony is likely related to the high concentration of MMC used during the primary procedure. All received sponge application of MMC for 30 s at a 0.4 mg/ml concentration.
Limitations of this study include the inherent weaknesses of all retrospective studies, including selection bias, confounding, limited patient population, and lack of true randomization. It is possible that lower socioeconomic status played a role in our study, as these patients are more likely to have less permissive insurance plans. The inclusion of phakic and pseudophakic eyes as well as combination of glaucoma and cataract surgery as part of the study design may be considered as a limitation. We applied multivariate analysis to make the comparison between the groups while controlling for any possible confounders. Finally, this study analyzed the surgical results of a single surgeon (R. S. A.); thus, the results may not apply to everyone.
Conclusion
Trabeculectomy offers the potential for lower IOP and lower medication burden but at the cost of more intensive postoperative care, higher complication, and reoperation rates. Canaloplasty is a viable alternative to trabeculectomy although patient selection is important. Canaloplasty may not be the best option in patients whom necessitate very low IOP. African-American and Hispanic patients experienced greater failure rates compared with Caucasian patients between both surgeries.
Financial support and sponsorship
This research was supported in part by the Tulane Glaucoma Research Fund.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lewis RA, von Wolff K, Tetz M, Korber N, Kearney JR, Shingleton B, et al. Canaloplasty: Circumferential viscodilation and tensioning of Schlemm's canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: Interim clinical study analysis. J Cataract Refract Surg. 2007;33:1217–26. doi: 10.1016/j.jcrs.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Ayyala RS, Chaudhry AL, Okogbaa CB, Zurakowski D. Comparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months’ follow-up. Ophthalmology. 2011;118:2427–33. doi: 10.1016/j.ophtha.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Schoenberg ED, Chaudhry AL, Chod R, Zurakowski D, Ayyala RS. Comparison of surgical outcomes between phacocanoloplasty and phacotrabeculectomy at 12 months’ follow-up: A longitudinal cohort study. J Glaucoma. 2015;24:543–9. doi: 10.1097/IJG.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 4.Brüggemann A, Despouy JT, Wegent A, Müller M. Intraindividual comparison of canaloplasty versus trabeculectomy with Mitomycin C in a single-surgeon series. J Glaucoma. 2013;22:577–83. doi: 10.1097/IJG.0b013e318255bb30. [DOI] [PubMed] [Google Scholar]
- 5.Matlach J, Freiberg FJ, Leippi S, Grehn F, Klink T. Comparison of phacotrabeculectomy versus phacocanaloplasty in the treatment of patients with concomitant cataract and glaucoma. BMC Ophthalmol. 2013;13:1. doi: 10.1186/1471-2415-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matlach J, Dhillon C, Hain J, Schlunck G, Grehn F, Klink T, et al. Trabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: A prospective randomized clinical trial. Acta Ophthalmol. 2015;93:753–61. doi: 10.1111/aos.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brusini P, Caramello G, Benedetti S, Tosoni C. Canaloplasty in open-angle glaucoma: Mid-term results from a multicenter study. J Glaucoma. 2016;25:403–7. doi: 10.1097/IJG.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 8.Katz MH. Multivariable Analysis: A Practical Guide for Clinicians. 2nd ed. New York: Cambridge University Press; 2006. pp. 159–74. [Google Scholar]
- 9.Feinstein AV. Principles of Medical Statistics. Boca Raton, FL: CRC Press; 2001. pp. 269–91. [Google Scholar]
- 10.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman & Hall; 1984. pp. 48–61. [Google Scholar]
- 11.Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10:237–49. doi: 10.1097/00061198-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Sethi P, Patel RN, Goldhardt R, Ayyala RS. Conjunctival advancement with subconjunctival amniotic membrane draping technique for leaking cystic blebs. J Glaucoma. 2015;93:753–61. doi: 10.1097/IJG.0000000000000098. [DOI] [PubMed] [Google Scholar]


