Abstract
Purpose:
To evaluate the association between retinal and choroidal thickness and volume along with choroidal vessel volume in children using optical coherence tomography (OCT) images.
Methods:
113 normal eyes of children ranging from 5-17 years of age were imaged with a clinical OCT scanner (Optovue Inc., Fremont, USA). The retina, choroid and choroidal vessels were automatically segmented with algorithms. Parameters evaluated were thickness and volume. Location specific analyses of thickness were also performed at a distance of 2.5 mm from foveal center. Multivariate analyses of variance were used to analyze the effect of age and myopia. Manual segmentation of the fovea and subfoveal choroid thickness was also performed to compare with the algorithm segmentation.
Results:
There was excellent agreement between manual and automatic segmentation (intra-class correlation of 0.95). Within the same eye, total retinal and choroid thickness of nasal and temporal location were significantly lower than the superior and inferior thickness (P < 0.0001). With age (P = 0.026) and myopia (P < 0.001), foveal thickness increased. Choroid volume, vessel volume and temporal choroid thickness increased with increasing myopia (P < 0.05). There was significant positive correlation between choroid volume and retinal volume (r = 0.62, P < 0.0001), choroid volume and vessel volume (r = 0.48, P < 0.0001), and with foveal thickness (r = 0.31, P = 0.009). Choroid vessel volume also showed significant positive correlations with the other metrics (P < 0.05).
Conclusion:
Retinal and choroidal structural features were quantified simultaneously from OCT images. Magnitude of myopia had a greater effect on retino-choroid features than age in children.
Keywords: Myopia, normal pediatric retina, optical coherence tomography
Myopia is a very common ocular disorder and a potentially blinding oculopathy making it a major concern.[1] Although myopia can begin in adulthood,[2] it is most commonly known to develop and progress in childhood.[3] Previous studies have attributed changes in structure and function of retina to increase in axial length (AL) and associated stretching of retina caused by myopia.[4,5] This has been shown by a study using optical coherence tomography (OCT) which demonstrated a correlation between macular thickness and refractive error or AL.[5] Furthermore, studies on animal models and humans have shown that structure/function changes in choroid were associated with the development of refractive errors.[6] Therefore, examining retina and choroid in myopia pediatric population may provide insights into the potential role of age and refractive error in structural changes in retina and choroid.[5,6,7]
Spectral domain-OCT (SD-OCT) has proved to be very useful in the accurate detection of normal and pathologic structures, which is important for the diagnosis of ocular diseases.[8] Several techniques have been developed for automated robust segmentation of retinal layers, choroid, and choroid vessels.[9,10,11,12,13] However, studies with simultaneous assessment of retina, choroid, and choroid vessels from OCT images are still lacking. The purpose of this novel study was to examine simultaneous changes in the retina, choroid as well as choroid vessels with age and myopia in pediatric eyes and to assess their physiological implications. The study aimed at segmentation of retina choroid and choroid vessels using previously established algorithms.[11,14,15] Further, the study quantified thickness and volume of retina, choroid, and choroid vessels, adjusted for AL, in normal pediatric eyes and assessed their correlations with age and degree of myopia.
Methods
Study population
This prospective, cross-sectional study was approved by the Institutional Ethics Committee of Narayana Nethralaya Super-Specialty Hospital, Bangalore, India and adhered to the tenets of Declaration of Helsinki. One hundred and thirteen normal eyes of 68 pediatric participants of Asian-Indian origin were included in this study. As the computed parameters between both eyes of the same participant were poorly correlated, both eyes were included in the study. The subjects were between 5 and 17 years of age (under 18 years of age). Images were acquired with the RTVue Premier (Optovue, Inc., CA, USA). The default AL in the device was 23.82 mm. The refractive power of the participants was determined after cycloplegia using Retinoscope (Welch Allyn, NY, USA). All participants underwent a comprehensive ophthalmic examination including assessment of corrected distance visual acuity (CDVA), slit-lamp biomicroscopy, direct funduscopy, and intraocular pressure (IOP) measurement. AL was measured using IOL master (Carl Zeiss Meditec, Jena, Germany). Inclusion criteria were normal corneas on slit-lamp examination, no prior ocular or corneal surgery, absence of autoimmune disorders, normal IOP (<18 mmHg), and absence of ocular or corneal inflammation.
Image analyses
All the OCT scans were acquired in chorioretinal mode of RTVue Premier. This mode focuses the instrument closer to posterior eye than the standard imaging mode to enhance the visibility of choroid in the OCT scans.[16] For retina, choroid and choroid vessel segmentation and volume computation, 17 parallel raster scans in a 6 mm × 4 mm area, centered on the macula and with a spacing of 0.25 mm between the individual scans, were used. The image pixel resolution was approximately 4.9 μm/pixel.
In this study, the inner retina layer (IRL) (extending from nerve fiber layer [NFL] to outer boundary of inner nuclear layer [INL]) and outer retinal layer (ORL) (extending from inner boundary of outer plexiform layer [OPL] to retinal pigment epithelium [RPE]) were segmented using a previously described gradient-based segmentation algorithm.[14] The RPE is the most hyperreflective layer in the SD-OCT image and was detected by searching for the brightest pixel in each column of the image. The vitreous-NFL and INL-OPL boundaries are prominent due to high contrast in the intensity of the pixels in these layers. Thus, using a gradient image created by a gradient operator [−1;1], the IRL and ORL were segmented. To segment the choroid, the Bruch's membrane was detected by searching for pixels with the largest gradient below the RPE as described in a previous method.[11] Using a previously validated choroidal segmentation protocol, the choroid-sclera interface was also detected.[15] The contour of the choroid-sclera interface is a polynomial fit of an estimated boundary that is concave. The algorithms used are described in brief here as the referred papers[11,14,15] have already provided a detailed explanation of these algorithms. The retina and choroid thickness parameters were measured at fovea, 3 mm from foveal pit.
The cross-section of choroid vessels appeared as vacant, irregular spaces in the choroid. Making use of this feature, the choroid vessels were segmented using intensity-based threshold and local binary pattern.[17] The cross-sectional area of the retina, choroid, and choroid vessels was linearly interpolated between the 17 raster scans acquired, and the volume was computed using Trapezoidal rule of integration.[18] All the above methods were implemented using MATLAB version 7.10 (MathWorks, Inc., MA, USA). All the measurements were corrected for ocular magnification using the Littman formula, which included magnification factor of the OCT scanner and the eye.[19,20] Thus, the retinal and choroid thickness was adjusted by applying the magnification factor computed from the Littman formula.[19,20] Similarly, the area and volume parameters were adjusted by multiplying with the squared and cubed value of the magnification factor, respectively. Further, the foveal thickness and the thickness of the choroid at the fovea were manually segmented to compare with the magnitudes reported by the algorithms.
Statistical analyses
All continuous variables were reported as a mean ± standard error of the mean (SEM) after confirming normality of distribution with Kolmogorov–Smirnov test. The variables analyzed were age (years), spherical equivalent (SE in diopter), choroid vessel volume (mm3), choroid volume (mm3), choroid thickness (μm), foveal thickness (μm), inner retinal thickness (μm), outer retinal thickness (μm), total retinal thickness (μm), and retinal volume (mm3). The thickness measurements were taken at the foveal region and at a distance of 2.5 mm from the fovea in the temporal, superior, nasal, and inferior regions of the choroid and retina. The volumes were calculated in the 6 mm × 4 mm scan area.
The participants were divided into three age groups: Group 1 = 5–11 years, Group 2 = 12–14 years, and Group 3 = 15–17 years and three refractive groups on the basis of their cycloplegic refraction: Group A = emmetropes, Group B = low myopia (−2.5D < SE <−0.5), and Group C = moderate myopia (−6D < SE <−2.5D). Two-way analysis of variance (ANOVA) was performed with the analyzed variables as the dependent variable and age group and refractive group as the two factors. For the same eye, repeated measures ANOVA was performed to compare the choroid thickness, inner retinal thickness, outer retinal thickness, and total retinal thickness among the temporal, superior, nasal, and inferior regions. Linear regression was performed among the analyzed variables as well. P < 0.05 was considered statistically significant. All statistical analyses were performed in MedCalc version 16.2.1 (MedCalc, Inc., Ostend, Belgium). Only the estimated means of variables from the ANOVA analyses were used for statistical inferences and are reported in this study.
Results
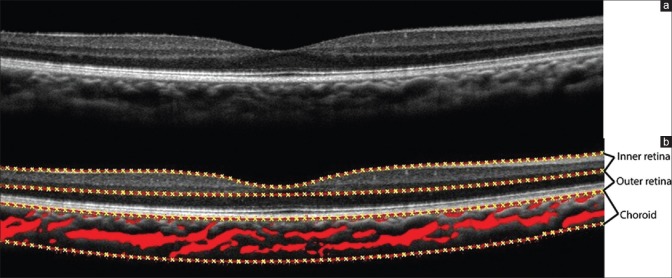
One hundred and thirteen eyes of 68 pediatric participants, 111 eyes had a corrected distance visual acuity (CDVA) of 6/6 and the remaining two eyes had a CDVA of 6/9. The number of eyes in each group was comparable with 44 in age Group 1, 36 in age group 2, and 33 in age group 3. Similarly, for each refractive group, there were 46 in Group A, 40 in Group B, and 27 in Group C. Fig. 1 (top) shows a sample image of the retina and choroid, which was segmented into inner retina, outer retina, choroid, and choroid blood vessels [Fig. 1, bottom]. The mean intraclass coefficient was 0.95 between the algorithm and manually segmented measurements of the fovea and choroid. The mean foveal thickness, subfoveal choroid thickness, retinal volume, choroid volume, and choroid vessel volume was 207 ± 1.81 μm, 316 ± 3.53 μm, 3.69 ± 0.07, 4.15 ± 0.03 mm3, and 1.85 ± 0.04 mm3 among all participants, respectively. In the same eye, the mean inner retinal thickness in the temporal, superior, nasal, and inferior regions was 135 ± 2.91 μm, 137 ± 3.23 μm, 137 ± 2.9 μm, and 144 ± 3.87 μm, respectively (P > 0.05).
Figure 1.
(a) Spectral domain-optical coherence tomography image of the retina and choroid; (b) spectral domain-optical coherence tomography image of the retina and the choroid with segmented inner retina, outer retina, choroid, and choroidal blood vessels
Similarly, the mean outer retinal thickness in the temporal, superior, nasal, and inferior regions was 120 ± 3.3 μm, 132 ± 3.36 μm, 127 ± 3.23 μm, and 140 ± 3.9 μm, respectively (P > 0.05). However [Fig. 2, top], the mean total retinal thickness was lower in the temporal (254 ± 4.03 μm) and nasal (264 ± 4.04 μm) region as compared to the superior (268 ± 2.26 μm) and inferior (287 ± 4.37 μm) region (P < 0.0001) in the same eye. Similarly [Fig. 2, bottom], the mean choroid thickness was lower in the temporal (307 ± 4.56 μm) and nasal (286 ± 3.51 μm) region as compared to the superior (319 ± 4.68 μm) and inferior (320 ± 4.74 μm) region (P < 0.0001) in the same eye.
Figure 2.
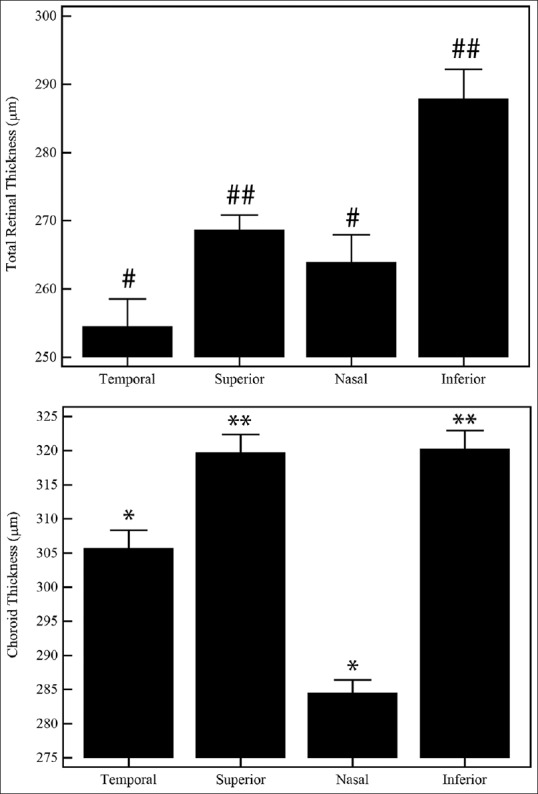
Regional variations in the total retinal and choroidal thickness within the same eye. Estimated mean ± standard error of the means was plotted. Symbols above the bar plots indicate statistical significance as mentioned in the text. P < 0.05 was considered statistically significant
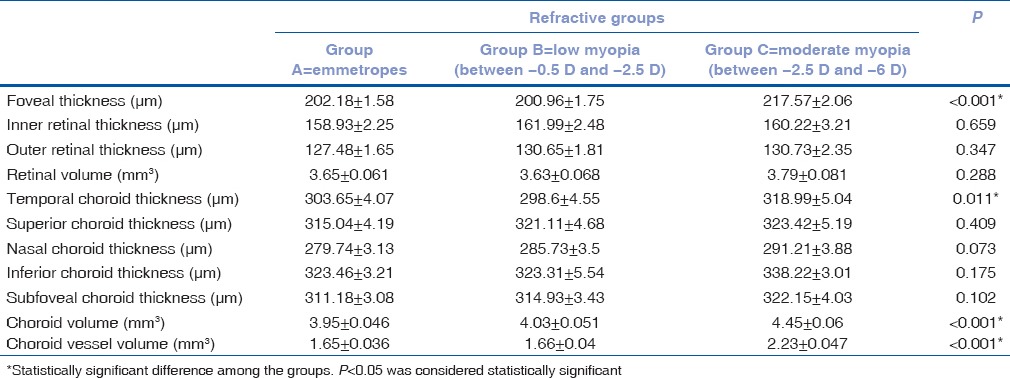
Table 1 gives the mean SEM of the variables among the three age groups. Other than foveal thickness, none of the other variables were significantly different among the age groups. Specifically, though there was a difference in the foveal thickness between age groups 1 (209.82 ± 1.69 μm) and 3 (202.95 ± 1.89 μm) (P = 0.026), this was not clinically significant. Table 2 gives the mean SEM of the variables among the three refractive groups. The foveal thickness was different between Groups A and C (P < 0.001) and between Groups B and C (P < 0.001). The choroid volume [Table 2] was significantly different between Group A (3.95 ± 0.046 mm3) and C (4.45 ± 0.06 mm3) (P < 0.001) and between Group B (4.03 ± 0.051 mm3) and C (P < 0.001). The choroid vessel volume was significantly different between Group A (1.65 ± 0.036 mm3) and C (2.23 ± 0.047 mm3) (P < 0.001) and between Group B (1.66 ± 0.04 mm3) and C (P < 0.001). Similarly, the temporal choroid thickness was different between Groups A (303.65 ± 4.07 μm) and C (318.99 ± 5.04 μm) and between Groups B (298.6 ± 4.55 μm) and C (P = 0.011).
Table 1.
Mean±standard error of the variables among the three age groups (1, 2, and 3) ranging from 5 to 17 years
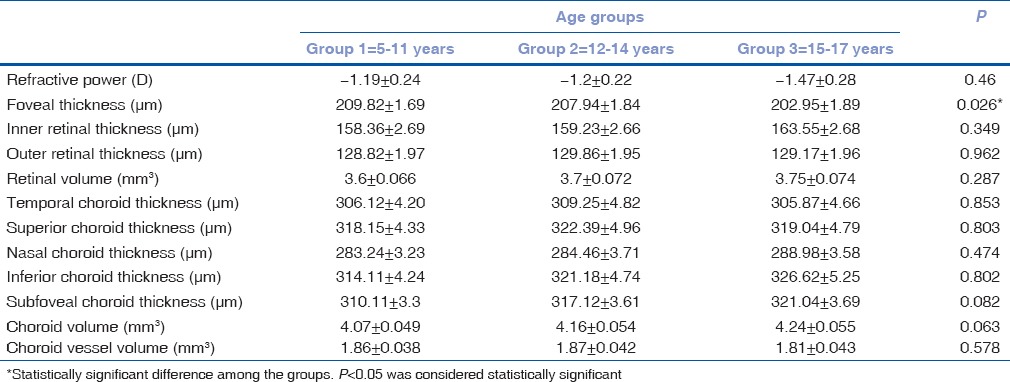
Table 2.
Mean±standard error of the variables among the three groups (A, B, and C) ranging from emmetropes to moderate myopia
However, there was no spatial (temporal, nasal, inferior, and superior) effect of age and myopia on all (total, inner, and outer) retinal thicknesses (P > 0.05). Choroid volume correlated positively with retinal volume (r = 0.62, P < 0.0001), choroid vessel volume (r = 0.48, P < 0.0001), subfoveal choroid thickness (r = 0.48, P < 0.0001), and foveal thickness (r = 0.31, P = 0.009). Choroid vessel volume also showed a positive correlation with retinal volume (r = 0.22, P = 0.018), foveal thickness (r = 0.43, P < 0.0001), and subfoveal choroid thickness (r = 0.22, P = 0.022).
Discussion
This study quantified retina and choroid thickness and volume in pediatric population of normal eyes to evaluate the effect of age and myopia on the same. The study also quantified choroid vessel volume and showed correlation with retina and choroid parameters. The results of the study demonstrated a significant relation of choroid and retina parameters with respect to age and myopia.
In this study, the mean foveal thickness was 206 μm, which was comparable to a foveal thickness of 220 μm reported in a previous study on Asian-Indian eyes.[21] Another study on myopic children reported that, with increasing degree of myopia, there was a tendency toward increase of retinal thickness in the foveal region.[22,23] These published results were consistent with the current study, which indicated an effect of myopia on foveal thickness and showed that foveal thickness increased with magnitude of myopia. As reported previously, this could be due to an increase in AL of the enlarged eyeball resulting in the mechanical stretching of the sclera which in turn causes retinal stretching at the fovea.[24] Further, a study on macular thickness measurements in a 3 mm ring around the fovea showed that the inferior and superior regions were significantly thicker than the nasal and temporal regions.[25] The study mentioned that the higher thickness of the superior and inferior regions could be because of arcuate nerve fiber bundles.[25] These results were consistent with the findings of the current study which showed the inner and outer retinal thickness to be higher in superior and inferior regions as compared to temporal and nasal. Furthermore, a recent study on Asian-Indian normal eyes demonstrated similar trends in the retinal vessel density among these zones using OCT angiography.[26] It may be speculated that there could be a physiological relationship between retinal tissue and vessel volume, which resulted in lower vessel density in the thinner retinal tissue regions. However, these trends need to be evaluated further.
In this study, retinal volume was also computed, and the mean retinal volume was 3.68 mm3 in the 6 mm × 4 mm macular area. Another study on macular measurements in myopic children reported a total retinal volume of 6.85 mm3 in 6 mm diameter central retinal region.[27] Similarly, a study on retinal thickness and volumes reported that the retinal volume in 6 mm diameter region surrounding the macula was 6.22 mm3 and that the retina in highly myopic individuals had smaller macular volume than emmetropic individuals.[24] However, the retinal volumes in low and moderate myopia were similar to emmetropic children in this study.
In this study, mean subfoveal choroid thickness was 314 μm among all the eyes. Previous studies examining in vivo subfoveal choroidal thickness in children reported a mean value of 330 μm in 194 children of age between 4 and 12 years,[28] 337 μm in 23 Japanese children aged below 20 years of age,[29] 343 μm in 30 Korean children of age between 4 and 10 years,[30] and 313 μm in 43 children of age between 3 and 18 years,[31] which are in close agreement with the results of this study. In a study on choroidal thickness in healthy children, the nasal thickness was reported to be significantly lower than the temporal, superior, and inferior choroid thickness measured at a distance of 3 mm from the fovea.[30] Choroidal thickness measurements in healthy Japanese participants using the swept source with a longer wavelength that has the advantage of shorter imaging time and better choroidal segmentation showed that the choroid thickness in nasal and temporal regions was significantly lower than superior, inferior, and subfoveal choroid thickness.[31] However, these published results were consistent with the results obtained from the Asian-Indian pediatric normal eyes in this study [Fig. 2]. A previous study reported a choroid volume of 2.32 mm3 in a 3.45 mm macular zone[32] as compared to a 6 mm macular zone used in this study. Increasing the macular zone from 3.45 mm to 6 mm gives a proportional volume increase from 2.32 to 3.71 mm3, which was again comparable to the volume (3.68 mm3) calculated in this study.
In a study on healthy adult eyes, the choroid volume computed in the Early Treatment Diabetic Retinopathy Study (ETDRS) layout was 7.72 mm3 in 6 mm diameter macular zone.[33] Furthermore, in healthy Korean children, the mean choroid volume computed in the ETDRS layout was found to be 6.56 mm3.[34] A study on choroid volume variations during childhood reported that choroid volume increased with age during childhood.[35] In this study, the choroid volume was found to increase with both age and myopia. The mean choroid vessel volume was 1.81 mm3 in all the participants. The mean choroid vessel volume was calculated as 1.12 mm3 in a previous study.[36] The volume was calculated in 6 mm × 6 mm macula-centered region[36] compared to 6 mm × 4 mm region used in this study.
The study has a few limitations. In this study, uniformly spaced two-dimensional scans were used to approximate the volumes, unlike true (voxel) volumetric imaging. However, most commercial OCT scanners do not perform voxel imaging. Despite this limitation, volumes calculated from the uniformly spaced images used in this study were comparable to retinal and choroid volumes derived from other complex imaging protocols.[24,32,33,34,35,36] Further, the study used previously well-established and validated algorithms.[11,14,15] Thus, the automated measurements were validated with manual measurements only at the fovea and not the entire image. The segmentation protocol for choroid vessels was validated by comparing the computed volume with a previous study.[36] However, manual validation was not performed. Furthermore, the three age groups had unequal age range so as to have a similar sample size in each group. The current study compared retinal and choroid measurements with the results of other similar studies performed only in the past 5 years. However, the references have been cited based on the availability of published data. In some cases, the compared data were not of the same age range, refractive power, or ethnicity as the current study. In this study, choroid thickness was almost similar or slightly higher in moderate myopia as compared to emmetropes and low myopia [Table 2]. A previous study has reported thinning of choroid in highly myopic eyes.[6] However, similar changes in retina and choroid in highly myopic pediatric eyes were not assessed in the current study due to insufficient sample size. Furthermore, a scan length >6 mm might have provided more insight into the peripheral changes in the retina and choroid with age and myopia. Correlation analysis performed between the choroid and retinal features indicated that the choroid vessel volume increased with increase in choroid volume. Further, choroid volume increased with increase in retinal volume in pediatric eyes. These trends have not been reported previously, and further investigation is required to understand the physiological implications.
Conclusion
Simultaneous assessment of retina and choroid thickness and volume in clinically acquired OCT images of pediatric eyes was presented in this study. Overall, myopia had a greater effect on the retinochoroid features than age in pediatric participants. This population demographic of Indian myopic children can serve as a reference for future studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Saxena R, Vashist P, Menon V. Is myopia a public health problem in india? Indian J Community Med. 2013;38:83–5. doi: 10.4103/0970-0218.112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBrien NA, Adams DW. A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group. Refractive and biometric findings. Invest Ophthalmol Vis Sci. 1997;38:321–33. [PubMed] [Google Scholar]
- 3.Zadnik K. The glenn A. Fry award lecture (1995). Myopia development in childhood. Optom Vis Sci. 1997;74:603–8. [PubMed] [Google Scholar]
- 4.Wolsley CJ, Saunders KJ, Silvestri G, Anderson RS. Investigation of changes in the myopic retina using multifocal electroretinograms, optical coherence tomography and peripheral resolution acuity. Vision Res. 2008;48:1554–61. doi: 10.1016/j.visres.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Wang B, Dong N, Ren X, Zhang T, Xiao L, et al. Macular measurements using spectral-domain optical coherence tomography in Chinese myopic children. Invest Ophthalmol Vis Sci. 2014;55:7410–6. doi: 10.1167/iovs.14-13894. [DOI] [PubMed] [Google Scholar]
- 6.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:7578–86. doi: 10.1167/iovs.13-12772. [DOI] [PubMed] [Google Scholar]
- 7.Fan DS, Lam DS, Lam RF, Lau JT, Chong KS, Cheung EY, et al. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004;45:1071–5. doi: 10.1167/iovs.03-1151. [DOI] [PubMed] [Google Scholar]
- 8.Badaró E, Novais E, Prodocimo LM, Sallum JM. Spectral-domain optical coherence tomography for macular edema. ScientificWorldJournal. 2014;2014:191847. doi: 10.1155/2014/191847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera Fernández D, Salinas HM, Puliafito CA. Automated detection of retinal layer structures on optical coherence tomography images. Opt Express. 2005;13:10200–16. doi: 10.1364/opex.13.010200. [DOI] [PubMed] [Google Scholar]
- 10.Fabritius T, Makita S, Miura M, Myllylä R, Yasuno Y. Automated segmentation of the macula by optical coherence tomography. Opt Express. 2009;17:15659–69. doi: 10.1364/OE.17.015659. [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Marziliano P, Baskaran M, Tun TA, Aung T. Automatic segmentation of the choroid in enhanced depth imaging optical coherence tomography images. Biomed Opt Express. 2013;4:397–411. doi: 10.1364/BOE.4.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danesh H, Kafieh R, Rabbani H, Hajizadeh F. Segmentation of choroidal boundary in enhanced depth imaging OCTs using a multiresolution texture based modeling in graph cuts. Comput Math Methods Med. 2014;2014:479268. doi: 10.1155/2014/479268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajić V, Esmaeelpour M, Glittenberg C, Kraus MF, Honegger J, Othara R, et al. Automated three-dimensional choroidal vessel segmentation of 3D 1060 nm OCT retinal data. Biomed Opt Express. 2013;4:134–50. doi: 10.1364/BOE.4.000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S, et al. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18:19413–28. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Fan W, Niu S, Shi J, Shen H, Yuan S, et al. Automated choroid segmentation based on gradual intensity distance in HD-OCT images. Opt Express. 2015;23:8974–94. doi: 10.1364/OE.23.008974. [DOI] [PubMed] [Google Scholar]
- 16.Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS, et al. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119:119–23. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Zhang L, Zhang D. A completed modeling of local binary pattern operator for texture classification. IEEE Trans Image Process. 2010;19:1657–63. doi: 10.1109/TIP.2010.2044957. [DOI] [PubMed] [Google Scholar]
- 18.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–4. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 19.Littmann H. Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd. 1982;180:286–9. doi: 10.1055/s-2008-1055068. [DOI] [PubMed] [Google Scholar]
- 20.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232:361–7. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- 21.Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:3876–80. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, He X, Zhu J, Jiang K, Zheng W, Ke B, et al. Macular measurements using optical coherence tomography in healthy Chinese school age children. Invest Ophthalmol Vis Sci. 2011;52:6377–83. doi: 10.1167/iovs.11-7477. [DOI] [PubMed] [Google Scholar]
- 23.Cheng SC, Lam CS, Yap MK. Retinal thickness in myopic and non-myopic eyes. Ophthalmic Physiol Opt. 2010;30:776–84. doi: 10.1111/j.1475-1313.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu PC, Chen YJ, Chen CH, Chen YH, Shin SJ, Yang HJ, et al. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye (Lond) 2008;22:551–5. doi: 10.1038/sj.eye.6702789. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Liu X, Wu Z, Xiao H, Dustin L, Sadda S, et al. Macular thickness measurements in normal eyes with time-domain and Fourier-domain optical coherence tomography. Retina. 2009;29:980–7. doi: 10.1097/IAE.0b013e3181a2c1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadde SG, Anegondi N, Bhanushali D, Chidambara L, Yadav NK, Khurana A, et al. Quantification of vessel density in retinal optical coherence tomography angiography images using local fractal dimension. Invest Ophthalmol Vis Sci. 2016;57:246–52. doi: 10.1167/iovs.15-18287. [DOI] [PubMed] [Google Scholar]
- 27.Sheth SS, Rush RB, Natarajan S. Inner and outer retinal volumetric and morphologic analysis of the macula with spectral domain optical coherence tomography in retinitis pigmentosa. Middle East Afr J Ophthalmol. 2012;19:227–30. doi: 10.4103/0974-9233.95258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in childhood. Invest Ophthalmol Vis Sci. 2013;54:3586–93. doi: 10.1167/iovs.13-11732. [DOI] [PubMed] [Google Scholar]
- 29.Fujiwara A, Shiragami C, Shirakata Y, Manabe S, Izumibata S, Shiraga F, et al. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol. 2012;56:230–5. doi: 10.1007/s10384-012-0128-5. [DOI] [PubMed] [Google Scholar]
- 30.Park KA, Oh SY. Analysis of spectral-domain optical coherence tomography in preterm children: Retinal layer thickness and choroidal thickness profiles. Invest Ophthalmol Vis Sci. 2012;53:7201–7. doi: 10.1167/iovs.12-10599. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Moreno JM, Flores-Moreno I, Lugo F, Ruiz-Medrano J, Montero JA, Akiba M, et al. Macular choroidal thickness in normal pediatric population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:353–9. doi: 10.1167/iovs.12-10863. [DOI] [PubMed] [Google Scholar]
- 32.Noori J, Riazi Esfahani M, Hajizadeh F, Zaferani MM. Choroidal mapping; a novel approach for evaluating choroidal thickness and volume. J Ophthalmic Vis Res. 2012;7:180–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Shin JW, Shin YU, Lee BR. Choroidal thickness and volume mapping by a six radial scan protocol on spectral-domain optical coherence tomography. Ophthalmology. 2012;119:1017–23. doi: 10.1016/j.ophtha.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Lee JW, Song IS, Lee JH, Shin YU, Lim HW, Lee WJ, et al. Macular choroidal thickness and volume measured by swept-source optical coherence tomography in healthy Korean children. Korean J Ophthalmol. 2016;30:32–9. doi: 10.3341/kjo.2016.30.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mapelli C, Dell’Arti L, Barteselli G, Osnaghi S, Tabacchi E, Clerici M, et al. Choroidal volume variations during childhood. Invest Ophthalmol Vis Sci. 2013;54:6841–5. doi: 10.1167/iovs.13-12761. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Lee K, Niemeijer M, Mullins RF, Sonka M, Abràmoff MD, et al. Automated segmentation of the choroid from clinical SD-OCT. Invest Ophthalmol Vis Sci. 2012;53:7510–9. doi: 10.1167/iovs.12-10311. [DOI] [PMC free article] [PubMed] [Google Scholar]