Abstract
The health effects of radiation exposure from the atomic bomb fallout remain unclear. The objective of the present study is to elucidate the association between low-dose radiation exposure from the atomic bomb fallout and cancer mortality among Nagasaki atomic bomb survivors. Of 77 884 members in the Nagasaki University Atomic Bomb Survivors Cohort, 610 residents in the terrain-shielded area with fallout were selected for this analysis; 1443 residents in the terrain-shielded area without fallout were selected as a control group; and 3194 residents in the direct exposure area were also selected for study. Fifty-two deaths due to cancer in the terrain-shielded fallout area were observed during the follow-up period from 1 January 1970 to 31 December 2012. The hazard ratio for cancer mortality in the terrain-shielded fallout area was 0.90 (95% confidence interval: 0.65–1.24). No increase in the risk of cancer mortality was observed, probably because the dose of the radiation exposure was low for residents in the terrain-shielded fallout areas of the Nagasaki atomic bomb, and also because the number of study subjects was small.
Keywords: atomic bomb survivors, cancer mortality, fallout, terrain shielding, epidemiology
INTRODUCTION
On 9 August 1945, an atomic bomb was detonated 503 m above Nagasaki city [1]. After the explosion, radioactive clouds containing dust and ashes formed and passed the east area at 3 m/s. The Nishiyama region, located east of Nagasaki city, was showered by yellow-brown droplets and was contaminated more highly than the unshowered areas (Fig. 1) [2]. Mt Kompira (elevation: 366 m), which is located 2 km east of the hypocenter, shielded the Nishiyama region from direct radiation exposure. In September–November 1945, several survey groups measured the residual radioactive intensity in the Nishiyama region [2–5]. They observed readings as high as 1.08 and 1.8 mR/h using Geiger-Müller counters and 3.9 mR/h using Lauritsen electroscopes at various heights above ground (Table 1). These measurements indicate that a large portion of the Nishiyama region was contaminated with radionuclides whose radioactivity was higher than 0.8 mR/h [3]. However, in October 1948, 3 years after the bombing, no residual radioactivity was detected around the Nishiyama reservoir [5], probably because of radioactive decay and rainfall. In 1959, Shono estimated from measurements of residual radiation that the average cumulative dose until 1959 of external exposure in the Nishiyama region was 68 R [6]. Thus, although various measurements of the residual radiation were performed, the dose was not reliably estimated.
Fig. 1.
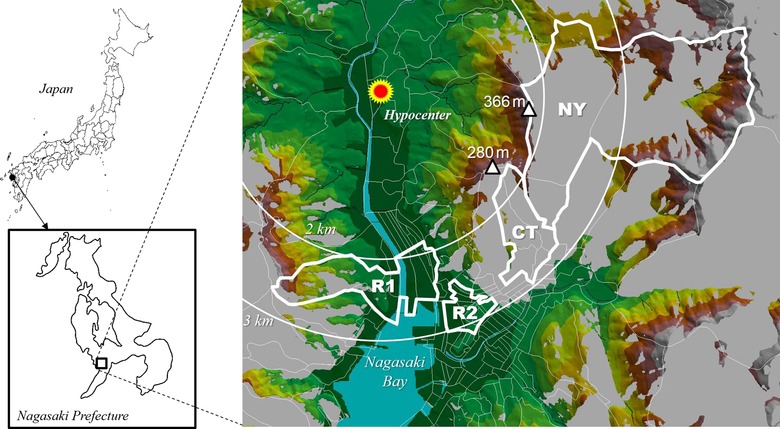
Study areas, including the terrain-shielded area. The area shielded from the detonation point of the atomic bomb 503 m above the hypocenter is shown in shades of gray. The Nishiyama region (NY) and control area (CT) were terrain shielded. Fallout was observed in the Nishiyama region, but not in the control area. The direct exposure areas on the near side (R1) and the far side (R2) of the hypocenter are also shown.
Table 1.
Early surveys of residual radiation in the Nishiyama region
| Investigator | Instrument | Maximum exposure rate (mR/h) | Probe height above the ground (cm) | Equivalent dose rate (μSv/h) | Date measured | Survey period |
|---|---|---|---|---|---|---|
| First Technical Group of the Manhattan Engineering Districta | Geiger-Müller counter | 1.8 | 5 | 15.8 | 27 Sept 1945 | 21 Sept to 4 Oct 1945 |
| Captain Warren’s Navy Groupb | Geiger-Müller counter | 1.08 | 100 | 9.5 | Unknown (18 Oct to 17 Nov 1945) | 18 Oct to 17 Nov 1945 |
| Japanese scientistsc | Lauritsen electroscope | 3.9 | 15 | 34.1 | 1 Oct 1945 | 1 Oct 1945 to 21 Oct 1948 |
Regarding the somatic effects, temporary leukocytosis was observed in residents of the Nishiyama region; the mean leucocyte count in 25 residents increased during the period 70–80 days after the bombing, and returned to normal levels 100 days after the bombing [2]. Regarding internal exposure, Okajima [7] reported that Nishiyama residents had significantly higher concentrations of 137Cs by whole-body counting compared with sex- and age-matched controls who were not in Nagasaki city at the time of the bombing; he inferred that this was likely due to the consumption of contaminated crops. He estimated the average internal doses in males and females in the Nishiyama region to be 2.9 μSv/y and 1.9 μSv/y, respectively, and those in controls to be 1.9 μSv/y and 1.1 μSv/y, respectively. However, no abnormalities were detected in chromosome studies, and no goiter, hypothyroidism, or thyroid cancers were detected among the residents examined [8]. About 15 years after the publication of the study by Okajima [7], Nagataki et al. [9] conducted a study on thyroid disease comparing 184 Nishiyama residents and 368 controls comprising atomic bomb survivors who were exposed to <0.1 mSv of atomic bomb radiation, and observed solid thyroid nodules in nine (4.9%) and three (0.8%) of these survivors, respectively; the prevalence of solid thyroid nodules was significantly (P < 0.01) higher in Nishiyama residents than in controls. In 2014, Sakata et al. [10] reported the effects of the rain exposure in Hiroshima and Nagasaki based on a questionnaire about rain exposure completed shortly after the bombing among the Life Span Study (LSS) cohort. Of 733 subjects who reported rain exposure in Nagasaki, 394 deaths were observed during 1950–2005. In this group only, marginal association (excess relative risk = 0.08, 95% confidence interval = 0.00006–0.17, P = 0.05) was observed, but they concluded the findings may be spurious. The numbers of solid cancer deaths and leukemia deaths in the low-dose (<5 mGy) subjects and in the reference group during 1962–2005 were 43 and 1572, respectively. No association between rain exposure and cancer death was noted among subjects exposed to low-dose radiation.
The aim of the present study was to elucidate the effects of exposure to low-dose radiation on cancer mortality in the residents who were living or staying in the fallout area from the Nagasaki atomic bomb.
MATERIALS AND METHODS
The present study was reviewed and approved by the institutional ethical committee of the Nagasaki University Graduate School of Biomedical Sciences (No. 16012980). All of the data were obtained from the Nagasaki city government on the basis of the documented agreement between Nagasaki Unversity and the Nagasaki city government. Using the data was also approved in that agreement for research concerning the late effects of radiation exposure. We performed analysis with anonymized data and announced the aims and procedure to the public (http://www-sdc.med.nagasaki-u.ac.jp/abcenter/sdr/biostatistics_e.html). All methods were performed in accordance with the relevant guidelines and regulations.
Nagasaki University Atomic Bomb Survivors Cohort
Nagasaki University Atomic Bomb Survivors Cohort, which was established in 1978 and has continuously been updated, includes a large portion of atomic bomb survivors who were living in Nagasaki city as at 1 January 1970 [11]. About 60 percent of this cohort members are different from the Nagasaki members of the LSS cohort of the Radiation Effects Research Foundation.
They either currently possess, or once possessed, the Atomic Bomb Handbook as a certificate of disaster issued by the Nagasaki city government. For each cohort member, information related to the atomic bombing including the location of exposure, the distance from the hypocenter, and the shielding conditions were recorded. The individual radiation dose was estimated in those cohort members who were within ~2 km of the hypocenter at the time of the bombing [12]. Information on their death, which has been available since 2 January 1970, unless they moved out of Nagasaki city, and the date they moved out of Nagasaki city were also recorded. The underlying cause of death was selected and coded by experienced staff members of Atomic Bomb Disease Institute according to the International Statistical Classification of Diseases and Related Health Problems (ICD). We used the 9th edition (ICD-9) and the 10th edition (ICD-10) for deaths occurring from 1 January 1970 to 31 December 1994 and from 1 January 1995 onward, respectively.
Study subjects
To elucidate the health effects associated with the Nagasaki atomic bomb fallout, we first identified, the areas that were shielded from direct radiation exposure by Mt Kompira (366 m high) and a 260 m ridge to the east of the hypocenter using Geographic Information System and elevation data [13, 14]; it should be noted that the atomic bomb exploded 503 m above Nagasaki city [1]. We selected two areas from the identified areas, the Nishiyama region and the control area. According to historical records [15], no fallout was recorded in the control area adjoining the Nishiyama region. We also selected two areas from the direct exposure area: one was 2.0–2.4 km from the hypocenter (near-side direct exposure area) and the other was 2.5 km or more from the hypocenter (far-side direct exposure area) (Fig. 1).
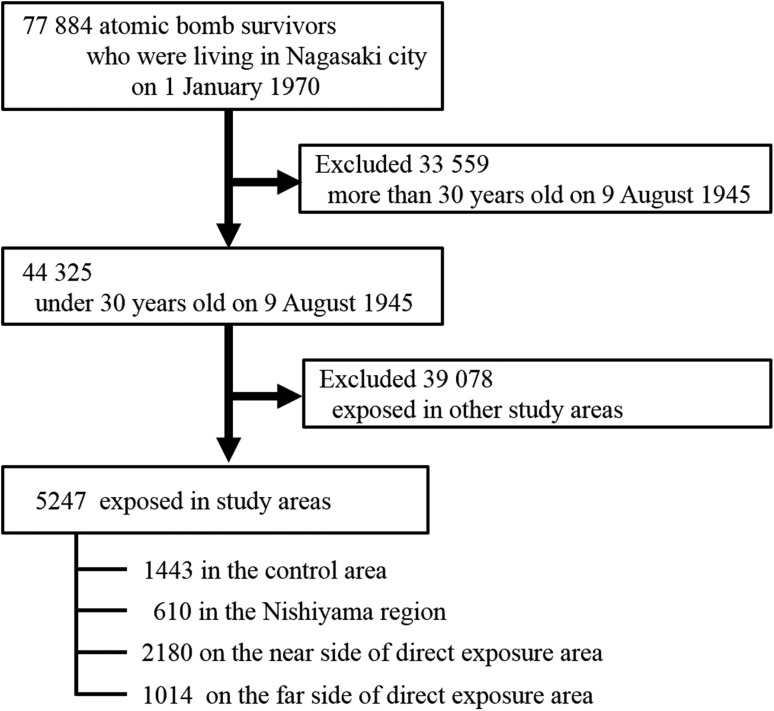
We selected subjects who were younger than 30 years of age at the time of the bombing (age ATB); survivors who were 30 years old at the time of the bombing were 55 years old at the beginning of the follow-up in 1970. Since cancer incidence usually increases at ~55 years old and after, effects of radiation exposure on cancer incidence in them will be attenuated and more difficult to detect. It would also be difficult to detect an increase in mortality in elder subjects, because the observable person-years in them should be small. Furthermore, younger people at the time of the bombing have a higher cancer risk than older people [16]. We, therefore, restricted study subjects to younger survivors. Thus, of the 77 884 survivors in the study base population, 44 325 aged under 30 years of age ATB were selected. Among these survivors, 1443, 610, 2180 and 1014 were living in the control area, the Nishiyama region, the near-side direct exposure area and the far-side direct exposure area, respectively. Finally, these 5247 subjects were included in the analysis for the present study (Fig. 2). We also investigated cancer deaths coded as ICD-9: 140–208 or ICD-10: C00-C97 during the 43 years between 1 January 1970 and 31 December 2012.
Fig. 2.
Selection of the study subjects.
The characteristics of the study subjects are shown in Table 2. The number of males aged 20–29 years at the time of the bombing was smaller than that in the other age groups. Because most of the people in this generation were soldiers, they were not in Nagasaki at that time and thus not exposed to the atomic bombing. About 55% of the study subjects were within 2.5–2.9 km of the hypocenter, and 45% were within 4.0–4.9 km; the Nishiyama region is located 2.5–5 km from the hypocenter, and the number of subjects in that area exposed to the bombing was the smallest (n = 610) among all the study areas. The proportion of subjects in direct exposure areas who were 0–9 years of age ATB was smaller than that in the shielded areas. The proportion of subjects who were exposed outside without being shielded was relatively high in the Nishiyama region, whereas this proportion was nearly identical in other areas.
Table 2.
Characteristics of the study subjects
| Characteristic | CTa | NYb | R1c | R2d | ||||
|---|---|---|---|---|---|---|---|---|
| Terrain-shielded area without fallout | Terrain-shielded area with fallout | Direct exposure area, near side | Direct exposure area, far side | |||||
| (n = 1443) | (n = 610) | (n = 2180) | (n = 1014) | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Age ATB (years)e | ||||||||
| 0–9 | 313 (54.9) | 304 (34.8) | 160 (63.2) | 144 (40.3) | 385 (42.6) | 374 (29.3) | 135 (36.7) | 145 (22.4) |
| 10–19 | 201 (35.3) | 294 (33.7) | 83 (32.8) | 115 (32.2) | 413 (45.7) | 469 (36.7) | 178 (48.4) | 242 (37.5) |
| 20–29 | 56 (9.8) | 275 (31.5) | 10 (4.0) | 98 (27.5) | 105 (11.6) | 434 (34.0) | 55 (14.9) | 259 (40.1) |
| Distance from hypocenter (km) | ||||||||
| 1.5–1.9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.6) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| 2.0–2.4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 898 (99.4) | 1275 (99.8) | 0 (0.0) | 0 (0.0) |
| 2.5–2.9 | 570 (100.0) | 873 (100.0) | 140 (55.3) | 214 (59.9) | 0 (0.0) | 0 (0.0) | 336 (91.3) | 548 (84.8) |
| 3.0–3.9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 32 (8.7) | 98 (15.2) |
| 4.0–4.9 | 0 (0.0) | 0 (0.0) | 113 (44.7) | 143 (40.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Entering near hypocenter | ||||||||
| Entered | 129 (22.6) | 193 (22.1) | 40 (15.8) | 55 (15.4) | 232 (25.7) | 335 (26.2) | 115 (31.3) | 166 (25.7) |
| Did not enter | 441 (77.4) | 680 (77.9) | 213 (84.2) | 302 (84.6) | 671 (74.3) | 942 (73.8) | 253 (68.8) | 480 (74.3) |
| Shield conditions | ||||||||
| Outside, not shielded | 110 (19.3) | 113 (12.9) | 89 (35.2) | 114 (31.9) | 158 (17.5) | 143 (11.2) | 63 (17.1) | 65 (10.1) |
| Outside, shielded | 68 (11.9) | 64 (7.3) | 24 (9.5) | 32 (9.0) | 146 (16.2) | 145 (11.4) | 33 (9.0) | 44 (6.8) |
| Inside house | 358 (62.8) | 645 (73.9) | 127 (50.2) | 197 (55.2) | 492 (54.5) | 844 (66.1) | 243 (66.0) | 499 (77.2) |
| Unknown | 34 (6.0) | 51 (5.8) | 13 (5.1) | 14 (3.9) | 107 (11.8) | 145 (11.4) | 29 (7.9) | 38 (5.9) |
| Subtotal | 570 (100.0) | 873 (100.0) | 253 (100.0) | 357 (100.0) | 903 (100.0) | 1277 (100.0) | 368 (100.0) | 646 (100.0) |
aControl area. bNishiyama region. cDirect exposure area on the near side of the hypocenter. dDirect exposure area on the far side of the hypocenter. eAge at the time of bombing.
Statistical analysis
First, we checked death rates based on observed person-years to obtain an overall picture of cancer deaths. For the main analysis, we performed Cox proportional hazard regression with adjustment for related factors to determine the mortality hazard ratio during the study period. We treated non-cancer disease death, survival to the end of the study period, and those who had moved out of Nagasaki city as censoring. We evaluated the fallout effects on cancer mortality by area adjusted by sex, age ATB, shielding conditions (inside the house, outside shielded, or outside unshielded), and entering into the hypocenter areas (~2 km around the hypocenter within 3 days after the bombing) on the basis of the following equation:
where t denotes the time since commencement of follow-up; λ(t) and λ0(t) denote the hazard rate and baseline hazard rate at t, respectively; S = 1(male) or 0(female) denotes sex; A denotes age ATB; Gi denotes area with G1 = 1 if the subject was exposed in the Nishiyama region and G1 = 0 otherwise; G2 = 1 if exposed in the direct exposure area on the near side of the hypocenter and G2 = 0 otherwise; G3 = 1 if exposed in the direct exposure area on the far side of the hypocenter and G3 = 0 otherwise, according to the subject’s location at the time of the bombing; Pi denotes shielding conditions, with P1 = 1 (outside) or P1 = 0 (inside house), P2 = 1 (shielded) or P2 = 0 (not shielded) and P3 = 1 (unknown shielding condition) or P3 = 0 (known shielding condition), according to the subject’s shielding from the explosion; C = 1 if the subject entered the hypocenter area within the 3 days following the bombing and C = 0 otherwise; and βs are unknown parameters to be estimated. We used the PHREG procedure in the SAS® system [17] for the calculations.
RESULTS
Among the 5247 subjects, a total of 549 cancer deaths were observed between 1 January 1970 and 31 December 2012. Table 3 presents the site classification of the observed cancer deaths among the subjects. Deaths from cancers of the lung, stomach and colon were frequently observed in both males and females, but no deaths were observed from cancers involving the thyroid, liver, melanoma, skin, ovary, prostate or bladder. To understand the overall picture of cancer deaths during the observation period, we calculated the cancer death rate by age ATB group (Table 4). In females, the cancer death rate per 100 000 population in the Nishiyama region was lower than that in the control area in every age ATB group, i.e. 43.1 vs 103.8 (rate ratio [RR] = 0.41, 95% confidence interval [95% CI] = 0.09–1.92) in those 0–9 years of age ATB, 127.4 vs 171.5 (RR = 0.74, 95% CI = 0.27–2.01) in those 10–19 years of age ATB and 530.0 vs 540.4 (RR = 0.98, 95% CI = 0.55–1.74) in those 20–29 years of age ATB. However, in males, the death rates in the Nishiyama region vs the control area in those 0–9, 10–19, and 20–29 years of age ATB were 371.1 vs 239.0 (RR = 1.55, 95% CI = 0.80–3.01), 385.8 vs 513.2 (RR = 0.75, 95% CI = 0.36–1.55) and 2105.3 vs 1256.5 (RR = 1.68, 95% CI = 0.66–4.25), respectively. In males, the death rates in those 0–9 and 20–29 years of age ATB in the Nishiyama region were higher than those in the control area. Person-years in the Nishiyama area were fairly small (285) in males 20–29 years of age ATB. On the other hand, higher death rates were observed in the near side of the direct exposure area compared with the control area. The RRs (95% CIs) of those 0–9, 10–19 and 20–29 years of age ATB were 1.20 (0.66–2.17), 1.41 (0.91–2.18) and 0.78 (0.42–1.46) in males, and 1.29 (0.55–2.97), 2.30 (1.33–3.95) and 1.07 (0.74–1.56) in females, respectively. However, this tendency weakened in the far side of the direct exposure area. The RRs of those 0–9, 10–19 and 20–29 years of age ATB were 1.04 (0.47–2.32), 1.35 (0.82–2.23) and 0.89 (0.45–1.76) in males, and 1.00 (0.31–3.23), 1.43 (0.75–2.73) and 0.58 (0.36–0.95) in females, respectively. Death rates did not show consistent results in males. We performed Cox proportional regression analysis for cancer mortality to evaluate the effects of areas with adjustment factors, i.e. sex, age ATB, shielding condition and entering the hypocenter areas within the 3 days following the bombing.
Table 3.
Site classification of observed cancer deaths from 1970 to 2012
| Sites (ICD-10) | CTa | NYb | R1c | R2d | Total (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Terrain-shielded area without fallout | Terrain-shielded area with fallout | Direct exposure area, near side | Direct exposure area, far side | |||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Stomach (C16) | 11 | 9 | 4 | 1 | 20 | 17 | 11 | 7 | 46 (16.6) | 34 (12.5) |
| Colon (C18) | 4 | 12 | 1 | 2 | 7 | 13 | 5 | 5 | 17 (6.1) | 32 (11.8) |
| Rectal (C19–C20) | 4 | 4 | 2 | 3 | 6 | 7 | 1 | 3 | 13 (4.7) | 17 (6.3) |
| Lung (C34) | 5 | 5 | 6 | 1 | 25 | 20 | 19 | 7 | 55 (19.9) | 33 (12.1) |
| Breast (C50) | 0 | 3 | 0 | 0 | 0 | 9 | 0 | 2 | 0 (0.0) | 14 (5.1) |
| Uterus (C53–C55) | 2 | 0 | 6 | 2 | 10 (3.7) | |||||
| Myeloma (C90.0) | 1 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 4 (1.4) | 2 (0.7) |
| Leukemia (C91–C93) | 2 | 3 | 1 | 2 | 3 | 7 | 0 | 1 | 6 (2.2) | 13 (4.8) |
| Others | 35 | 32 | 18 | 14 | 69 | 64 | 24 | 22 | 146 (52.7) | 132 (48.5) |
| Total | 59 | 67 | 31 | 21 | 127 | 136 | 60 | 48 | 277 (100.0) | 272 (100.0) |
Numbers of thyroid (C73), liver (C22), melanoma (C43), skin (C44), ovary (C56), prostate (C61) and bladder (C67) cancers were zero (0) in ‘Others’. Original data were coded according to the ICD-9 or ICD-10; the ICD-10 codes are shown in this table. aControl area. bNishiyama region. cDirect exposure area on the near side of the hypocenter. dDirect exposure area on the far side of the hypocenter.
Table 4.
Area-specific and age ATB–specific cancer death rates per 100 000 from 1970 to 2012
| Age ATBe (years) | CTa | NYb | R1c | R2d | ||||
|---|---|---|---|---|---|---|---|---|
| Terrain-shielded area without fallout | Terrain-shielded area with fallout | Direct exposure area, near side | Direct exposure area, far side | |||||
| (n = 1443) | (n = 610) | (n = 2180) | (n = 1014) | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 0–9 | 239.0 | 103.8 | 371.1 | 43.1 | 285.8 | 133.5 | 248.8 | 103.3 |
| (18/7530) | (9/8673) | (17/4581) | (2/4645) | (27/9447) | (14/10 483) | (9/3617) | (4/3872) | |
| 10–19 | 513.2 | 171.5 | 385.8 | 127.4 | 723.7 | 393.8 | 691.2 | 245.2 |
| (27/5261) | (17/9913) | (10/2592) | (5/3924) | (81/11 193) | (56/14 222) | (35/5064) | (20/8157) | |
| 20–29 | 1256.5 | 540.4 | 2105.3 | 530.0 | 985.6 | 580.8 | 1118.9 | 315.0 |
| (17/1353) | (44/8142) | (6/285) | (16/3019) | (24/2435) | (75/12 914) | (16/1430) | (25/7937) | |
| Total | 438.3 | 261.9 | 442.5 | 198.5 | 572.0 | 385.4 | 593.4 | 245.4 |
| (62/14 144) | (70/26 728) | (33/7458) | (23/11 588) | (132/23 075) | (145/37 619) | (60/10 111) | (49/19 966) | |
Data in parentheses are the number of deaths and observed person years. aControl area. bNishiyama region. cDirect exposure area on the near side of the hypocenter. dDirect exposure area on the far side of the hypocenter. eAge at the time of the bombing.
Table 5 presents the hazard ratios (HRs) for cancer mortality in the Nishiyama region and the near and far sides of the direct exposure area compared with in the control area. The HR for cancer mortality in the Nishiyama region compared with that in the control area was 0.90 with a 95% confidence interval (CI) of 0.65–1.24. No statistically significant difference was observed in cancer mortality between the Nishiyama region and the control area (P = 0.51). As for covariates, the hazard of cancer mortality for males was significantly higher (2.66-fold; 95% CI: 2.23–3.17) than that for females. The hazard of cancer mortality increased 1.08-fold (95% CI: 1.07–1.10) for each year of age ATB. This result reflects simple age-specific mortality in the Cox proportional hazard model; however, those at a younger age ATB had a higher mortality risk [16]. The hazard of cancer mortality for those entering the hypocenter area was 1.11-fold (95% CI: 0.91–1.36) higher than that for those not entering, but this difference was not significant. On the other hand, the hazard of cancer mortality in R1 was significantly (P = 0.02) higher than that in the control area (HR = 1.28; 95% CI = 1.04–1.58). The hazard of cancer mortality in R2, however, was not significantly different from that in the control area (HR = 0.96; 95% CI = 0.74–1.23).
Table 5.
Hazard ratios for cancer mortality
| Areas compared | Hazard ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Nishiyama region vs control area | 0.90 | 0.65–1.24 | 0.51 |
| Direct exposure area on the near side of the hypocenter vs control area | 1.28 | 1.04–1.58 | 0.02 |
| Direct exposure area on the far side of the hypocenter vs control area | 0.96 | 0.74–1.23 | 0.73 |
Cancer mortality was evaluated by area adjusted for sex, age at the time of bombing, the shielding condition, and entering into the hypocenter areas within the 3 days following the bombing.
DISCUSSION
No evidence of increased cancer mortality in the terrain-shielded area with radioactive fallout from the atomic bomb was found in the present study. To confirm the validity of the analysis, we simultaneously evaluated the effects of radiation exposure on cancer mortality in the direct radiation area without terrain shielding. A significant increase in cancer mortality was observed in the direct exposure area on the near side of the hypocenter (HR = 1.28; 95% CI = 1.04–1.58), but no increase was observed on the far side (HR = 0.96; 95% CI = 0.74–1.23). Subjects who were on the near side of the direct exposure area were irradiated at a distance of 2.0–2.4 km from the hypocenter, with an estimated radiation dose of 3.8–273.1 mSv. On the far side of the direct exposure area, subjects were irradiated at 2.5–3.9 km from the hypocenter, with an estimated dose of 1.9–20.9 mSv [12]. The significant increase in cancer mortality on the near side of the direct exposure area seen in the present study is consistent with the results of a previous study [16]. In contrast, no increase in cancer mortality was observed on the far side of the direct exposure area, probably because of the comparatively lower dose of radiation in that area. Similarly, no increase in cancer mortality was observed in the Nishiyama region (HR = 0.90; 95% CI = 0.65–1.24).
Although a higher prevalence of solid thyroid nodules was observed in Nishiyama residents in a previous study [9], no deaths caused by thyroid cancer were observed in the present study, probably because thyroid cancer is not typically fatal. Immediately after the bombing, a widespread residual radioactivity of ~7 μSv/h (0.8 mR/h) or higher was reported in the Nishiyama region, and radioactive hot spots of ~10–30 μSv/h around a reservoir were confirmed in previous surveys (Table 1); however, the radioactivity in the Nishiyama region decreased in the months after the bombing. The maximum cumulative external dose was estimated to be 68 R (~600 mSv) [6] in several surveys, while the majority of the estimates in the Nishiyama region was in the range of 20–40 R (~170–350 mSv)[18]. We note, however, that the estimates were based on the assumption that people were exposed to radiation outside for a lifetime. The amount of radiation they actually received would be considerably smaller than the estimates.
Although we restricted the study subjects to those aged <30 years at the time of the bombing (reasons provided in ‘Study subjects’ in Materials and Methods), we conducted a similar analysis for those aged 30 years or over at the time of the bombing. The results presented in Tables 6 and 7 indicate no effects of radiation, even between the direct exposure area near the hypocenter and the control area (i.e. the terrain-shielded area without fallout). We consider these results support our decision to restrict the study subjects to atomic bomb survivors aged <30 years at the time of the atomic bombing. The proportion of children in the direct exposure area was smaller than that in the Nishiyama region or the control area. Since the direct exposure areas were downtown and adjacent to the dockyards, which had previously been bombed in air raids, parents living downtown may have evacuated their children to keep them safe during the air raids. On the other hand, the shielded areas consisted of suburbs that had not been damaged in previous air raids. Fewer children aged 0–9 years old were thus in the direct exposure areas than in the shielded areas. Furthermore, children could not easily approach the hypocenter area immediately after the bombing. These factors, together with aspects related to the geography of the town, meant that the proportion of people entering the hypocenter area in the Nishiyama region and the control area was lower than in the direct exposure areas.
Table 6.
Area-specific and age ATB-specific cancer death rates per 100 000 from 1970 to 2012 among subjects who were 30 years and over of age ATB
| Age ATBe (years) | CTa | NYb | R1c | R2d | ||||
|---|---|---|---|---|---|---|---|---|
| Terrain-shielded area without fallout | Terrain-shielded area with fallout | Direct exposure area, near side | Direct exposure area, far side | |||||
| (n = 685) | (n = 243) | (n = 1061) | (n = 643) | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 30–39 | 1043.3 | 724.5 | 772.2 | 578.0 | 1296.5 | 930.3 | 1718.8 | 946.2 |
| (13/1246) | (41/5659) | (2/259) | (12/2076) | (39/3008) | (53/5697) | (28/1629) | (35/3699) | |
| 40–49 | 1826.0 | 1171.1 | 2427.2 | 1018.9 | 1473.4 | 1277.4 | 1584.2 | 831.2 |
| (17/931) | (31/2647) | (5/206) | (7/687) | (31/2104) | (42/3288) | (24/1515) | (13/1564) | |
| 50– | 3973.5 | 1466.3 | 1156.1 | 1162.8 | 1025.6 | 463.7 | 1333.3 | 992.1 |
| (6/151) | (10/682) | (2/173) | (3/258) | (4/390) | (3/647) | (4/300) | (5/504) | |
| Total | 1546.4 | 912.3 | 1410.7 | 728.2 | 1345.0 | 1017.4 | 1626.0 | 919.0 |
| (36/2328) | (82/8988) | (9/638) | (22/3021) | (74/5502) | (98/9632) | (56/3444) | (53/5767) | |
Data in parentheses are the number of deaths and observed person years. aControl area. bNishiyama region. cDirect exposure area on the near side of the hypocenter. dDirect exposure area on the far side of the hypocenter. eAge at the time of the bombing.
Table 7.
Hazard ratios for cancer mortality among subjects who were 30 years old and over of age at the time of bombing
| Areas compared | Hazard ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Nishiyama region vs control area | 0.82 | 0.55–1.23 | 0.34 |
| Direct exposure area on the near side of the hypocenter vs control area | 1.07 | 0.84–1.36 | 0.58 |
| Direct exposure area on the far side of the hypocenter vs control area | 1.08 | 0.85–1.41 | 0.57 |
Cancer mortality was evaluated by area adjusted for sex, age at the time of bombing, the shielding condition, and entering into the hypocenter areas within 3 days after the bombing.
The accident at the Chernobyl nuclear power plant in 1986 released a huge amount of radioactive nuclides into the environment. Although the general population was exposed to the radiation, there has been no persuasive evidence of any somatic effects due to radiation exposure, except for significant increases in thyroid cancer or solid thyroid nodules among those who took milk contaminated with 131I. Among more than 6000 children and adolescents diagnosed with thyroid cancers, only 15 deaths were observed by 2005 [19], and no increases in other cancer deaths have been observed in the areas affected by the Chernobyl accident.
Therefore, the results of the present study, suggesting no difference in the risk of cancer deaths between those exposed to radiation in the Nishiyama region and those in the control region would likely not be caused by the small number of study subjects; the quantity of the radioactive materials generated by the atomic bomb explosion was several hundred times smaller than that released to the environment due to the Chernobyl accident.
Following the accident at the Fukushima Daiichi Nuclear Power Station on 11 March 2011, the spatial radiation dose rate on 30 April 2011 at a point ~32 km north-west from the Fukushima Daiichi Nuclear Power Station was 11.57 μSv/h; this decreased to 5.69 μSv/h by 30 April 2012 [20]. On 22 April 2012, the Japanese government decided to establish an evacuation area for those areas where the ambient dose rates still exceeded 3.80 μSv/h, which is equivalent to 20 mSv/y. Areas that had similar spatial radiation dose rates of 7 μSv/h or over in the Nishiyama region after the atomic bombing were included in the evacuation area. Many residents of Fukushima were uneasy about possible future health effects resulting from exposure to the widespread radioactivity caused by the disaster. We hope that the results of the present study help to decrease anxiety among both Fukushima residents and atomic bomb survivors regarding the health effects associated with low-dose and temporary radiation exposure.
Limitations
The present study had two limitations. First, since the follow-up of Nagasaki University Atomic Bomb Survivors Cohort members started on 1 January 1970 [11], information on deaths was not available for those who had died or had moved out of Nagasaki city. Although the present analysis was restricted to those who were younger than 30 years of age ATB, the results may have been biased if the contribution to cancer mortality of such individuals was not negligible.
Second, our study was ecologic due to that no individual dose estimate was available for Nishiyama residents; the dose rates presented in Table 1 are unique information available for us. The Dosimetry System 2002 (DS02) [21] provided us with the direct radiation doses under the terrain-shielding conditions; the control area described in the present study as being shielded by a 288 m ridge was estimated to have received 3–20 mSv (Gy). Since the height of the ridge that shielded the Nishiyama region from direct exposure to the atomic bomb radiation is higher (366 m) than the ridge that shielded the control area, direct radiation doses in the Nishiyama region would have been lower than those in the control area. Further studies to estimate the individual dose in Nishiyama residents using the available information such as the location of their residence at the time of the bombing and the measurements of residual radiation presented in Table 1 are necessary.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest associated with this manuscript.
FUNDING
This work was supported by routine budget of the Atomic Bomb Disease Institute, Nagasaki University.
ACKNOWLEDGEMENTS
Administrative and medical data on atomic bomb survivors were obtained with the cooperation of the Nagasaki city and Nagasaki Prefectural governments. We thank the technical staff members of the Division of Scientific Data Registry, Atomic Bomb Disease Institute, Nagasaki University, for daily maintenance of the database and the data preparation for this study.
REFERENCES
- 1. Kerr GD, Solomon DL. The Epicenter of the Nagasaki Weapon: a Reanalysis of Available Data with Recommended Values. Oak Ridge, TN: Oak Ridge National Lab, 1976. ORNL-TM-5139. [Google Scholar]
- 2. Pace N, Smith RE Measurement of the residual radiation intensity at the Hiroshima and Nagasaki Atomic bomb sites. Technical Report. Atomic Bomb Casualty Commission (ABCC) TR 26–59A, 1959.
- 3. Manhattan Engineer District Final Report of Findings of the Manhattan District Atomic Bomb Investigating Groups at Hiroshima and Nagasaki. Oak Ridge, TN: Manhattan Engineer District, 1946. [Google Scholar]
- 4. Arakawa ET. Radiation dosimetry in Hiroshima–Nagasaki atomic bomb survivors. New Eng J Med 1960;263:488–93. [DOI] [PubMed] [Google Scholar]
- 5. Shinohara K, Morita Y, Kora K et al. Radiation of Ground in Nagasaki and Vicinity. II. Radioactivity near Nishiyama Reservoir. Collection of the Reports on the Investigation of the Atomic Bomb Casualties, Vol. 1 Tokyo: Japan Society for the Promotion of Science; 1953, 45–53 (in Japanese). [Google Scholar]
- 6. Shono N. Physical effects of the A-Bomb in Hiroshima and Nagasaki: amount of radiation received by A-bomb victims. J Hiroshima Med Assoc 1959;12:1041–51 (in Japanese). [Google Scholar]
- 7. Okajima S. Dose estimation from residual and fallout radioactivity. Fallout in the Nagasaki–Nishiyama district. J Radiat Res 1975;16:35–41. [DOI] [PubMed] [Google Scholar]
- 8. Okajima S, Takeshita K, Antoku S et al. Radioactive fallout effects of the Nagasaki atomic bomb. Health Phys 1978;34:621–33. [DOI] [PubMed] [Google Scholar]
- 9. Nagataki S, Hirayu H, Izumi M et al. High prevalence of thyroid nodule in area of radioactive fallout. Lancet 1989;8659:385–6. [DOI] [PubMed] [Google Scholar]
- 10. Sakata R, Grant EJ, Furukawa K et al. Long-term effects of the rain exposure shortly after the atomic bombings in Hiroshima and Nagasaki. Radiat Res 2014;182:599–606. [DOI] [PubMed] [Google Scholar]
- 11. Okajima S, Mine M, Nakamura T. Mortality of registered A-bomb survivors in Nagasaki, Japan, 1970–1984. Radiat Res 1985;103:419–31. [PubMed] [Google Scholar]
- 12. Honda S, Mine M, Okumura Y et al. Estimation of radiation doses for Nagasaki atomic bomb survivors based on ABS93D. Nagasaki Med J 1997;72:30–46 (in Japanese). [Google Scholar]
- 13. ESRI ArcGIS Desktop: Release 10.2.2 Redlands, CA: Environmental Systems Research Institute; 2014. http://www.esri.com/software/arcgis/arcgis-for-desktop/ (28 April 2017, date last accessed).
- 14. GSI The Base Map Information, 10 m Mesh Numerical Altitude Model Tokyo: Geographical Survey Institute, 2009. http://fgd.gsi.go.jp/download/ (28 April 2017, date last accessed).
- 15. Nagasaki City The Nagasaki Atomic Bomb Damage Records, General Analysis Version Vol. 1. Nagasaki: Nagasaki city, 2016, 126–31. [Google Scholar]
- 16. Ozasa K, Shimizu Y, Suyama A et al. Studies of the mortality of Atomic Bomb Survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- 17. SAS Institute Inc SAS User’s Guide: Statistics Version 9, 4th edn Cary, NC: SAS Institute Inc, 2014. [Google Scholar]
- 18. Okajima S, Fujita S, Harley JH. Radiation doses from residual radioactivity In: Roesh WC. (ed). US–Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki, Vol. 1 Hiroshima: Radiation Effects Research Foundation, 1987, 205–26. [Google Scholar]
- 19. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Health effects due to radiation from the Chernobyl accident. Report to the general assembly scientific annex D. UNSCEAR 2008 Report Volume II UNSCEAR, 2011.
- 20. Fukushima Prefecture Fukushima Prefecture Radioactivity Measurement Map.http://fukushima-radioactivity.jp/ (28 April 2017, date last accessed).
- 21. Stephen DE, Dean CK, James AR et al. Survivor shielding Part C. Improvements in terrain shielding In: Young RW, Kerr GD (eds). Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki-Dosimetry System 2002. Vol. 2 Hiroshima: Radiation Effects Research Foundation, 2005, 821. [Google Scholar]