Abstract
The aim of our study was to correlate tumor uptake of 68Ga-DOTA-NOC positron emission tomography/computed tomography (PET/CT) with the pathological grade of neuroendocrine tumors (NETs). 68Ga-DOTA-NOC PET/CT examinations in 41 patients with histopathologically proven NETs were included in the study. Maximum standardized uptake value (SUVmax) and averaged SUV SUVmean of “main tumor lesions” were calculated for quantitative analyses after background subtraction. Uptake on main tumor lesions was compared and correlated with the tumor histological grade based on Ki-67 index and pathological differentiation. Classification was performed into three grades according to Ki-67 levels; low grade: Ki-67 <2, intermediate grade: Ki-67 3–20, and high grade: Ki-67 >20. Pathological differentiation was graded into well- and poorly differentiated groups. The values were compared and evaluated for correlation and agreement between the two parameters was performed. Our study revealed negatively fair agreement between SUVmax of tumor and Ki-67 index (r = −0.241) and negatively poor agreement between SUVmean of tumor and Ki-67 index (r = −0.094). SUVmax of low-grade, intermediate-grade, and high-grade Ki-67 index is 26.18 ± 14.56, 30.71 ± 24.44, and 6.60 ± 4.59, respectively. Meanwhile, SUVmean of low-grade, intermediate-grade, and high-grade Ki-67 is 8.92 ± 7.15, 9.09 ± 5.18, and 3.00 ± 1.38, respectively. As expected, there was statistically significant decreased SUVmax and SUVmean in high-grade tumors (poorly differentiated NETs) as compared with low- and intermediate-grade tumors (well-differentiated NETs). SUV of 68Ga-DOTA-NOC PET/CT is not correlated with histological grade of NETs. However, there was statistically significant decreased tumor uptake of 68Ga-DOTA-NOC in poorly differentiated NETs as compared with the well-differentiated group. As a result of this pilot study, we confirm that the lower tumor uptake of 68Ga-DOTA-NOC may be associated with aggressive behavior and may, therefore, result in poor prognosis.
Keywords: 68Ga-DOTA-NOC positron emission tomography/computed tomography, Ki-67, neuroendocrine tumors, standardized uptake value
Introduction
Neuroendocrine tumors (NETs) are defined as epithelial neoplasms with predominant neuroendocrine differentiation and can arise in almost any organ of the body. They involve overexpression of receptors for regulatory peptides such as somatostatin and the presence of cellular structures for amine uptake and storage. Several prognostic factors have been studied. The prognostic value of several pathological (cytology, Ki-67 index) or biological (chromogranin A) factors is known in nonmetastatic disease but is less studied in metastatic disease.[1] NETs typically have a wide range of cellular differentiation. The presence of cell surface receptors appears to depend on tumor cell differentiation, with well-differentiated tumors exhibiting a greater affinity for somatostatin.[2]
Imaging plays a key role in the evaluation of these tumors including detection, staging, response assessment, and prognostication.[3] F-18 fludeoxyglucose positron emission tomography/computed tomography PET/CT has limited value in well-differentiated NETs as these tumors often have near normal glucose turnover.[4] 68Ga DOTA-conjugated peptide-binding somatostatin receptor (SSTR) has been widely used for localizing primary tumors and to detect sites of metastatic NETs (staging) as well as to select patients with metastatic disease for peptide receptor radionuclide therapy (PRRT).[5] PRRT can be successful in controlling symptoms because of excessive hormonal secretion and has also been shown to improve overall survival in patients with progressive or symptomatic NETs.[6] Their mechanism of uptake in neuroendocrine cells is due to the increased expression of SSTR and is also the basis of imaging with SSTR scintigraphy.[7]
In our study, we aimed to analyze the correlation between tumor uptake of 68Ga DOTANOC and Ki-67 index in patients with recurrent or metastatic NETs.
Materials and Methods
Study design and patients
We retrospectively reviewed the medical information of 41 patients (24 men and 17 women, age range: 22–84, mean age 61.1 years) who had histologically diagnosed NETs and underwent 68Ga-DOTA-NOC PET/CT examinations in the Department of Nuclear Medicine at the Royal Liverpool University Hospital between May 2013 and April 2016. Pathological diagnoses were confirmed by total resection (n = 8) and tissue biopsy (n = 33).
Images and the nonimaging data were anonymized at the time of re-analysis for tumor uptake by a member of the clinical care team. Since the current analysis is based on anonymized data and because the implications of the study do not have any direct implications to individual patients, additional informed consent for patients to participate in this study was not deemed necessary. All patients have been informed and have consented that their anonymized data could be used in research settings without the need for further consent.
8 Ga-DOTA-NOC positron emission tomography/computed tomography imaging
68Ga-DOTA-NOC PET/CT examinations were performed at 1 h after injection 200 MBq of 68Ga-DOTA-NOC which was conducted on a discovery ST16 PET/CT scanner (GE Healthcare, Milwaukee, USA). The whole body scan was performed from vertex to mid thighs (time of flight, 3 min list mode per bed position). Low-dose CT acquisition was performed with 120 kV, 80 mA, 0.8 s per CT rotation. CT data were used for attenuation correction. Studies were interpreted on a Hermes Multimodality workstation using Hybrid Viewer software (Hermes Medical Solutions, Stockholm, Sweden).
Imaging interpretation
The 68Ga-DOTA-NOC PET/CT images were interpreted retrospectively by an experienced nuclear medicine physician. PET images were evaluated both qualitatively and semiquantitatively. At first, the maximum intensity projection images were visually examined in varying scales, and then each single transverse slice was looked over from vertex to the mid thighs in combination with the corresponding CT image and the fused image slice. Each lesion showing a focal abnormal tracer uptake was recorded by a slice number and anatomical localization, and any lesion with intensity greater than background which could not be explained by physiological activity was considered to be indicative of tumor tissue. For semiquantitative analysis of the lesions, volume of interests (VOIs) was drawn around the largest and/or the lesion with the highest pathological tracer accumulation. This was clarified as “main tumor lesion” in each patient. Maximum standardized uptake value (SUVmax) and average SUV SUVmean were calculated and tabulated.
Quantification analysis of tumor uptake on 68Ga-DOTA-NOC positron emission tomography/computed tomography
For quantification of relative tumor uptake, VOIs were drawn on all transverse consecutive PET slices along the contour of the “main tumor lesion.” The outline was based on visual assessment as per CT images. The SUVmax and SUVmean were generated by automatic software (Hermes Multimodality).
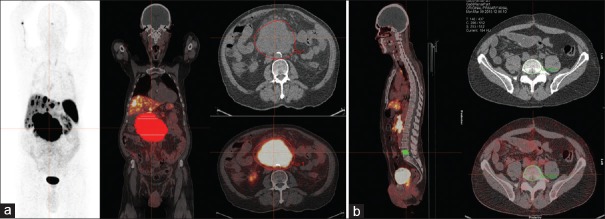
To provide some normalization and to correct for background activity, we chose a nonaffected vertebral body as a normal reference. SUVmax of tumor was derived after normalization with SUVmax of the “normal” vertebral reference, whereas SUVmean of tumor was derived after normalization with SUVmean of the “normal” vertebral reference. VOIs of main tumor lesion and vertebral reference were re-processed using Hermes Hybrid Viewer software and are shown in Figure 1. Quantitative assessment of tumor uptake on 68Ga-DOTA-NOC PET/CT was performed by an experienced nuclear medicine physician with each procedure repeated twice to reduce errors.
Figure 1.
Volume of interest was drawn manually on all transverse consecutive positron emission tomography slices along the contour of “Main tumor lesion” (a) and normal vertebral references (b)
SUVmax (mean) of main tumor lesions = SUVmax (mean) of tumor – SUVmax (mean) of normal vertebral reference
Histopathological analysis
All NETs were graded as part of the routine workup with routine interventions including surgical resection, endoscopic biopsies, or ultrasound-guided biopsies. Pathologists were unaware of PET findings. We obtained the histological findings including the histological type and Ki-67 index of the primary tumor or metastatic sites. All NETs were classified into high, intermediate, and low-grade tumors according to the tumor histology report which were based on recent consensus statements of the European NET Society[8,9] and WHO classification,[10] using Ki-67 index or mitotic rate. Ki-67 index was expressed as the percentage of positive cells which were classified as low grade, <3%; intermediate grade, 3%–20%; and high grade, >20%. On the basis of this system, low and intermediate grades were classified as well-differentiated tumors, whereas high-grade tumors were classified as poorly differentiated tumors.
Statistical analysis
Categorical data were expressed as numbers and percentages, whereas continuous variables were expressed as the mean and range. The Spearman correlation coefficient (r) was used to evaluate correlation and the agreement between SUVmax of main tumor lesion in 68Ga-DOTA-NOC PET/CT and Ki-67 index and between SUVmean of the main tumor lesion in 68Ga-DOTA-NOC PET/CT and Ki-67 index. Scatter plots were also used to determine the correlation between the two datasets. The statistical differences of SUVs among each group of tumor grade were determined using Kruskal–Wallis test to determine the specific differences between the three groups when P < 0.05. Box and whisker plots of SUVmax and SUVmean of main tumor lesions and each group of Ki-67 index were constructed. All analyses were conducted using the SPSS software version 22 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
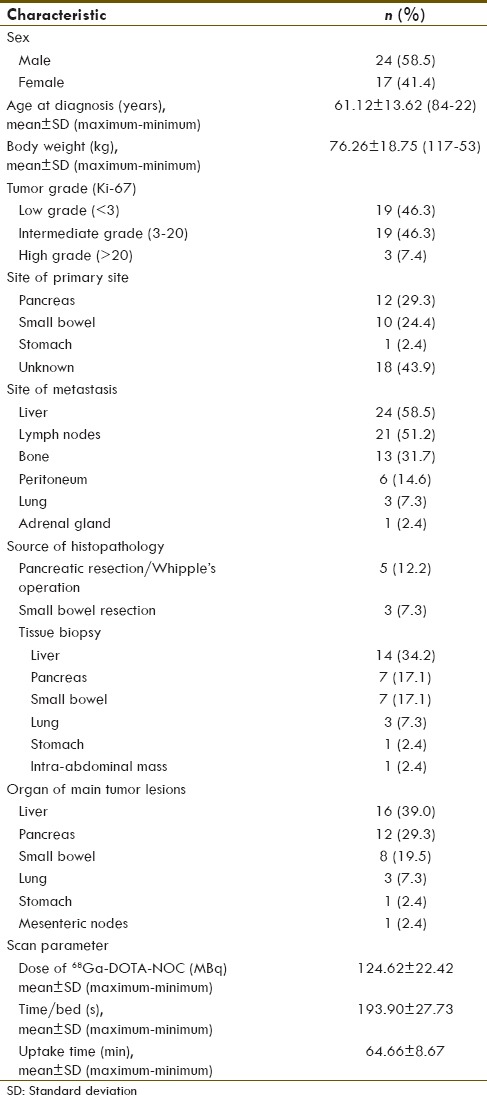
The study included 41 patients (24 males and 17 females). Primary tumor sites included pancreas (29.3%), small bowel (24.4%), and stomach (2.4%). The primary site could not be identified in 43.9%. All patients had metastatic disease, with the most common location being the liver (58.5%), followed by regional lymph nodes (51.2%) and bone (31.7%). Histologic evaluation revealed well-differentiated (n = 38) and poorly differentiated NETs (n = 3). Patient characteristics are described in more detail in Tables 1 and 2.
Table 1.
Patients characteristic, tumor localizations, Ki-67 index, tumor grade and tracer uptake
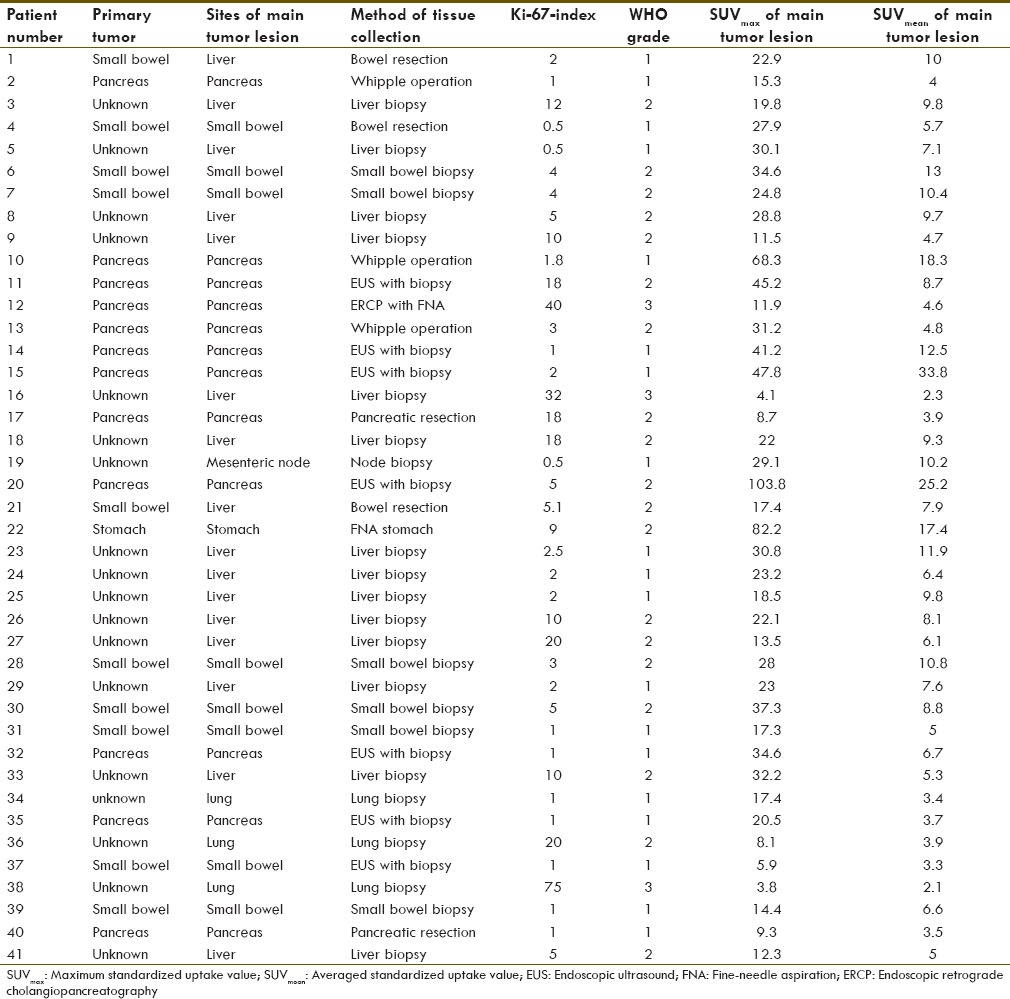
Table 2.
Baseline patients characteristic (n=41)
Correlation between standardized uptake value of main tumor lesions and Ki-67
SUVmax of low-, intermediate-, and high-grade tumors is 26.18 ± 14.56, 30.71 ± 24.44, and 6.60 ± 4.59, respectively. Meanwhile, SUVmean of low-, intermediate-, and high-grade tumors is 8.92 ± 7.15, 9.09 ± 5.18, and 3.00 ± 1.38, respectively as described in Table 3. Correlation of SUVmax and SUVmean and Ki-67 is shown in Figures 2 and 3, respectively. Correlation between SUVmax and SUVmean of main tumor lesion and Ki-67 is −0.241 and −0.094, respectively. Box and whisker plots of SUVmax and SUVmean of main tumor lesions and each group of Ki-67 were constructed [Figures 4 and 5]. On comparison of tumor SUV of 68Ga-DOTA-NOC and Ki-67 index, there was statistically significant decreased SUVmax in high-grade tumor (poorly differentiated NETs) as compared with low- (P = 0.014) and intermediate-grade (P = 0.010) tumor (well-differentiated NETs) but no significant difference of SUVmax between low and intermediate grades (P = 0.850). For SUVmean, there was also statistically significant decreased SUVmean in high-grade tumor (poorly differentiated NETs) as compared with low- (P = 0.025) and intermediate-grade (P = 0.010) tumor (well-differentiated NETs) but no significant difference of SUVmean between low and intermediate grades (P = 0.516).
Table 3.
Maximum standardized uptake value and averaged standardized uptake value of main tumor lesions in each grade of tumors
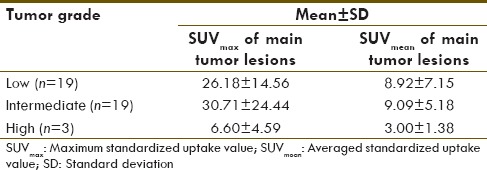
Figure 2.
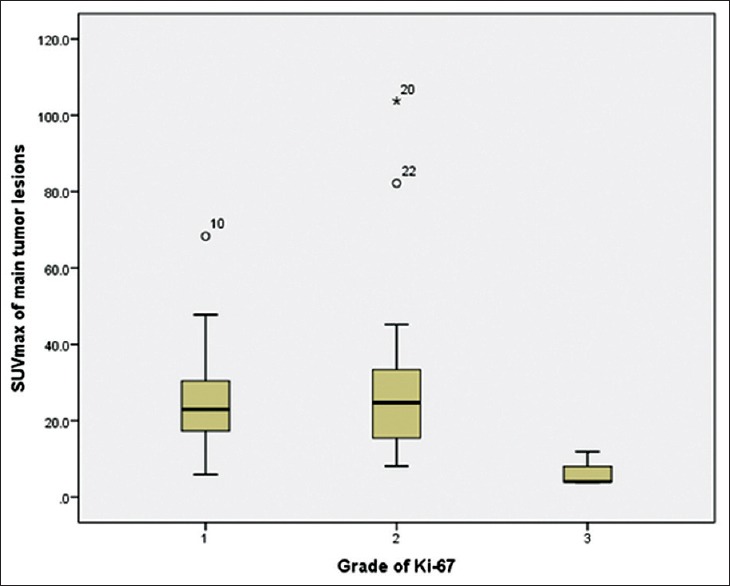
Box and whisker plot of different maximum standardized uptake value and grades of tumor
Figure 3.
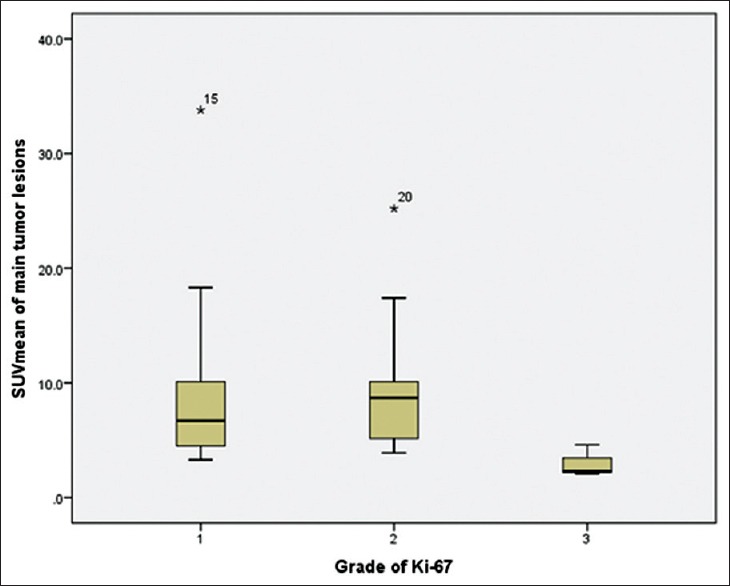
Box and whisker plot of different averaged standardized uptake value and grades of tumor
Figure 4.
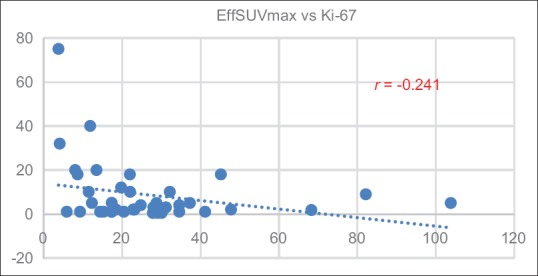
Correlation of maximum standardized uptake value and Ki-67 index
Figure 5.
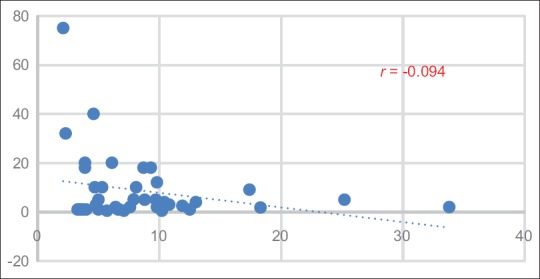
Correlation of averaged standardized uptake value and Ki-67 index
Discussion
The grade of a tumor refers to its biological aggressiveness which has been accepted as a powerful indicator for prognosis in various tumors. NETs are defined as epithelial neoplasms with predominant neuroendocrine differentiation and can arise in almost any organ of the body. The grading system of NETs is based on the rate of proliferation which is defined by the number of mitoses per ten high-power microscopic fields or 2 mm2 (mitotic rate) or as the percentage of tumor cells immunolabeled for positively for the Ki-67 antigen (Ki-67 index).[11] NETs can also be classified based on differentiation, which refers to the extent to which cancerous or neoplastic cells resemble normal cells. Patients with high-grade tumors have turnover with high aggressive nature and can progress rapidly, whereas others can remain stable for a long time. It is important to distinguish between rapidly progressive tumors and relatively stable tumors because treatment for aggressive tumors can have significant long-term toxicity and moderate efficacy.[12]
68Ga-DOTA-NOC PET/CT has been shown to have high diagnostic accuracy for the detection of primary and metastatic disease in patients with GEP-NETs.[13,14] In our study, we found no significant correlation between tumor SUVmax of 68Ga-DOTA-NOC and Ki-67 index (r = −0.241) and between tumor SUVmean of 68Ga-DOTA-NOC and Ki-67 index (r = −0.094). However, our results show significantly higher tumor SUVmax of 68Ga-DOTA-NOC in low-grade (SUVmax of 26.18) with lower tumor SUVmax in high-grade tumors (SUVmax of 6.60). In the previous study of Kayani et al.,[15] they also showed comparable results, and that there is greater uptake of 68Ga DOTATATE in low-grade NETs (median SUVmax of 29), whereas high-grade tumors had lower uptake (median SUVmax of 4.3). When we compared tumor SUVmean in our study, there is a lower uptake in high-grade tumors (SUVmean of 8.92) as compared with low-grade tumors (SUVmean of 3.00), but the difference was not so much as with tumor SUVmax. As expected, SUVmax is a more accurate estimation of the true SUV than SUVmean. In addition, SUVmax has a significantly improved reproducibility as compared to SUVmean. A concern with the use of SUVmax is that it is based on a reported value for a lesion with perhaps only one pixel. Whereas SUVmean is used in certainty boundary definition of region of interests with no evidence of resolution loss.[16] We sought to reduce the errors associated with variable injected activities, variable imaging times after injection and possible variation in body mass by “subtracting” or “normalizing” with a noninvolved body part that was reproducible between studies. We decided to choose a discrete noninvolved vertebral body confirmed on PET as well as the CT components of the PET/CT study. We sought to further reduce errors by repeating the measurements twice and ensuring conformity of results.
On comparison of SUV of 68Ga-DOTA-NOC and Ki-67 index, there was statistically significant decreased SUVmax and SUVmean in high-grade tumors (poorly differentiated NETs) as compared with low- and intermediate-grade tumor (well-differentiated NETs). In patients with high-grade metastatic with low tracer uptake, there is often limited SSTR expression. Tumor grade influenced tracer uptake, and SUV values of 68Ga-labelled peptide SSTR correlate inversely with the grade of tumor.[17] The presence of cell surface receptors appears to depend on tumor cell differentiation, with well-differentiated (low grade) tumors exhibiting a greater affinity for somatostatin. As a result of this pilot study, we suggest that the lower tumor uptake may be associated with aggressive tumor behavior that has prognosis relevance. However, it is difficult to establish a correlation between tracer avidity and histopathological grade of tumors because in many patients in our study, there were a large number of tumors lesions, often multiple lesions in the same organ (such as liver metastases) and variable tracer uptake was even seen within the same lesion site. These findings suggest the wide spectrum of differentiation of NETs, heterogeneity of cellular differentiation within the same tumor mass, and also reflect the potential ability of PET to map these cellular characteristics.
Although tumor grade and proliferation appeared to be related to tumor 68Ga-DOTA-NOC uptake, there were two patients (No.37 and 40) with low-grade NETs that showed low tumor uptake. The low tumor uptake (SUVmax of 5.9) in one patient with recurrent NETs at duodenum with Ki-67 index of one from endoscopic fine-needle aspiration (FNA) of duodenum was observed [Figure 6]. It is possible that the FNA site may not fully reflect the true pathological grade of a patient with heterogeneity of cellular differentiation within the same tumor mass and this may also reflect the potential ability of 68Ga-labeled SSTR PET/CT to map these cellular characteristics. Another one patient with diagnosed recurrent NETs at the head of pancreas based on progressive imaging feature as seen on the follow-up CT scan with Ki-67 index of 1%. It also showed low tumor uptake (SUVmax of 9.3) which is possible due to small tumor volume with the main proportion of necrotic tissue which may have then resulted in a lack of detectable tracer avidity.
Figure 6.
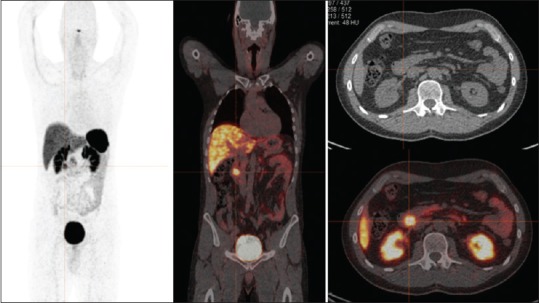
A 41-year-old male with recurrent neuroendocrine tumor at duodenum. 68Ga-DOTA-NOC positron emission tomography shows abnormal focal tracer uptake at duodenum (maximum standardized uptake value of 5.9) without other definite evidence of abnormal tracer uptake. Ki-67 index from endoscopic fine-needle aspiration of duodenum was 1%
There are some limitations to our study. First, our study population is small. The sample size in each group was not equal with the smallest number in high grade and may be insufficient to make conclusive statements on the correlation between 68Ga-DOTA-NOC uptake and pathological correlation. Second, in many patients, there were a large number of tumor lesions, often with multiple lesions in the same organ. Moreover, heterogeneous uptake within tumor lesions indicates histological findings from only one site and may not fully reflect in vivo tumor heterogeneity. Another limitation is that there was no follow-up period to confirm prognosis and survival of these patients.
Conclusion
In our study, we found that tumor uptake of 68Ga-DOTA-NOC PET/CT is not correlated with the histological grade of NETs. However, there was statistically significant decreased tumor uptake of 68Ga-DOTA-NOC in poorly differentiated NETs which may be suggestive of aggressive tumor behavior and also reflects on the potential ability of PET/CT to map heterogeneous cellular characteristics rather than relying on histological information from only one sample.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Söreide JA, van Heerden JA, Thompson GB, Schleck C, Ilstrup -DM, Churchward M. Gastrointestinal carcinoid tumors: Long-term prognosis for surgically treated patients. World J Surg. 2000;24:1431–6. doi: 10.1007/s002680010236. [DOI] [PubMed] [Google Scholar]
- 2.Ezziddin S, Logvinski T, Yong-Hing C, Ahmadzadehfar H, Fischer HP, Palmedo H, et al. Factors predicting tracer uptake in somatostatin receptor and MIBG scintigraphy of metastatic gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2006;47:223–33. [PubMed] [Google Scholar]
- 3.Kuruva M, Bartel T, Osmany S. PET in Evaluation of Neuroendocrine Tumors. [Last cited on 2016 Nov 16]. Available from: http://www.snmmi.files.cms plus.com/ACNM/010_PET%20in%20neuroendocrine%20tumors%20for%20Spotlights%20with%20Simin%20edits%207-27-15.pdf .
- 4.Belhocine T, Foidart J, Rigo P, Najjar F, Thiry A, Quatresooz P, et al. Fluorodeoxyglucose positron emission tomography and somatostatin receptor scintigraphy for diagnosing and staging carcinoid tumours: Correlations with the pathological indexes p53 and Ki-67. Nucl Med Commun. 2002;23:727–34. doi: 10.1097/00006231-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: A review of literature. Am J Nucl Med Mol Imaging. 2014;4:426–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Vinjamuri S, Gilbert TM, Banks M, McKane G, Maltby P, Poston G, et al. Peptide receptor radionuclide therapy with (90)Y-DOTATATE/(90)Y-DOTATOC in patients with progressive metastatic neuroendocrine tumours: Assessment of response, survival and toxicity. Br J Cancer. 2013;108:1440–8. doi: 10.1038/bjc.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858–64. doi: 10.2967/jnumed.108.057505. [DOI] [PubMed] [Google Scholar]
- 8.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2007;451:757–62. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 10.Rindi G, Arnold B, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Hruban RH, Theise ND, editors. WHO Classification of Tumors of the Digestive System. Lyon, France: IARC; 2010. pp. 13–4. [Google Scholar]
- 11.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 12.Öberg K, Knigge U, Kwekkeboom D, Perren A, ESMO Guidelines Working Group Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–30. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 13.Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- 14.Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54:364–72. doi: 10.2967/jnumed.112.111724. [DOI] [PubMed] [Google Scholar]
- 15.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–55. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 16.Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR. 2010;31:496–505. doi: 10.1053/j.sult.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naswa N, Sharma P, Gupta SK, Karunanithi S, Reddy RM, Patnecha M, et al. Dual tracer functional imaging of gastroenteropancreatic neuroendocrine tumors using 68Ga-DOTA-NOC PET-CT and 18F-FDG PET-CT: Competitive or complimentary? Clin Nucl Med. 2014;39:e27–34. doi: 10.1097/RLU.0b013e31827a216b. [DOI] [PubMed] [Google Scholar]