Abstract
We present a 46-year-old female with pleuritic chest pain on a background of pulmonary embolism diagnosed on a single-photon emission computed tomography (SPECT) ventilation-perfusion (V/Q) imaging 3 years earlier. A SPECT V/Q scan detected a mismatched perfusion defect in the posterior basal segment of the right lower lobe, essentially unchanged from a defect identified 3 years earlier. Given the atypical finding, the patient went on to have a computed tomographic pulmonary angiogram. It revealed an intralobar bronchopulmonary sequestration as the cause of the right lower lobe mismatched perfusion defect. With growing awareness of radiation safety, the number of V/Q imaging studies being undertaken to investigate suspected pulmonary emboli, especially in young female patients, has increased. This case report serves as a timely reminder of the potential pitfalls associated with V/Q scan image interpretation.
Keywords: Bronchopulmonary sequestration, computed tomographic pulmonary angiography, lung imaging, single-photon emission computed tomography, ventilation-perfusion scan
Introduction
Increased sensitivity and specificity of single-photon emission computed tomography (SPECT) ventilation-perfusion (V/Q) imaging in detecting pulmonary emboli has been paralleled by the increased utilization of this imaging modality. This is especially the case in subsets of patients where computed tomographic pulmonary angiography (CTPA) is a relative or absolute contraindication. As SPECT V/Q studies become more commonplace, clinicians should remain mindful of the inherent limitations associated with this imaging test. We present a case of a misdiagnosed bronchopulmonary sequestration (BPS) that produced a mismatched perfusion defect on SPECT V/Q scanning, mimicking the appearance of an acute pulmonary embolism (PE).
A BPS is an aberrant region of lung tissue with no connection to the pulmonary arteries. BPS primarily affect the lower lobes; a recent study found the left and right lower lobes accounted for 71.53% and 25.97% of BPSs respectively.[1] BPS can be divided into intralobar sequestrations (ILS) and extralobar sequestrations (ELS). Both ILS and ELS receive a systemic arterial supply, usually from the aorta. ELS are usually not connected to the normal bronchial tree and are enclosed by a separate pleural covering. Conversely, ILS are closely connected to adjacent normal lung, share a common pleural lining, and drain to nearby pulmonary veins.[2] Typically seen in adults, ILS are characterized by chronic inflammation and fibrosis. ELS are seen in the fetus and are characterized by dilated terminal airways and subpleural lymphatics. ELS also drain to systemic veins.[3]
Case Report
A 46-year-old female presented with pleuritic chest pain and a history of unprovoked PE diagnosed on a SPECT V/Q scan 3 years earlier. The patient denied any other respiratory symptoms and had no other risk factors for venous thromboembolism. The patient was hemodynamically stable with normal oxygen saturation; however, an electrocardiogram suggested the right ventricular strain and the serum D-dimer assay was elevated, leading to a suspected diagnosis of pulmonary embolus.
Chest radiograph and bilateral lower limb Doppler ultrasound studies were normal. SPECT V/Q images of the lungs were acquired following inhalation of Technegas (Cyclomedica, Lucas Heights, NSW, Australia) and peripheral intravenous administration of 218 MBq of Tc-99m macroaggregated albumin. SPECT V/Q scanning demonstrated a mismatched perfusion defect within the posterior basal segment of the right lower lobe [Figure 1]. The defect was identical to that demonstrated on the previous SPECT V/Q scan 3 years earlier, which led the reporting physician to note that the SPECT V/Q scan findings would represent very unusual behavior of a pulmonary embolus. Given the atypical appearance, the patient proceeded to have CTPA.
Figure 1.
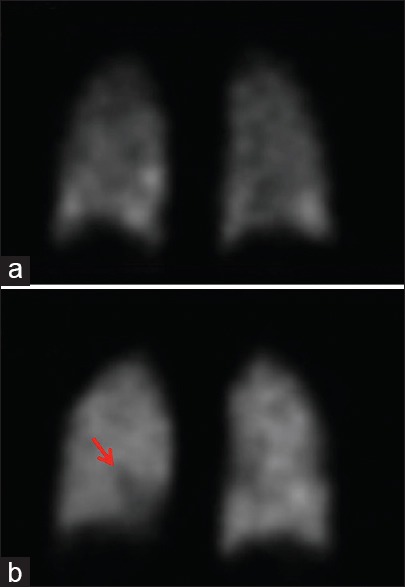
Relevant coronal sections from single-photon emission computed tomography ventilation-perfusion scan images. (a) Coronal single-photon emission computed tomography ventilation-perfusion image depicts ventilation portion of examination following inhalation of Technegas. (b) Coronal single-photon emission computed tomography ventilation-perfusion image depicts corresponding perfusion portion of examination following intravenous administration of Tc-99m macroaggregated albumin, demonstrating a mismatched perfusion defect within the right lower lobe (red arrow)
The CTPA showed no pulmonary thromboembolism. However, the region of mismatch seen within the posterior basal segment of the right lower lobe on SPECT V/Q imaging corresponded to an intralobar BPS with an arterial supply arising from the distal thoracic aorta and venous drainage through the pulmonary veins [Figure 2].
Figure 2.
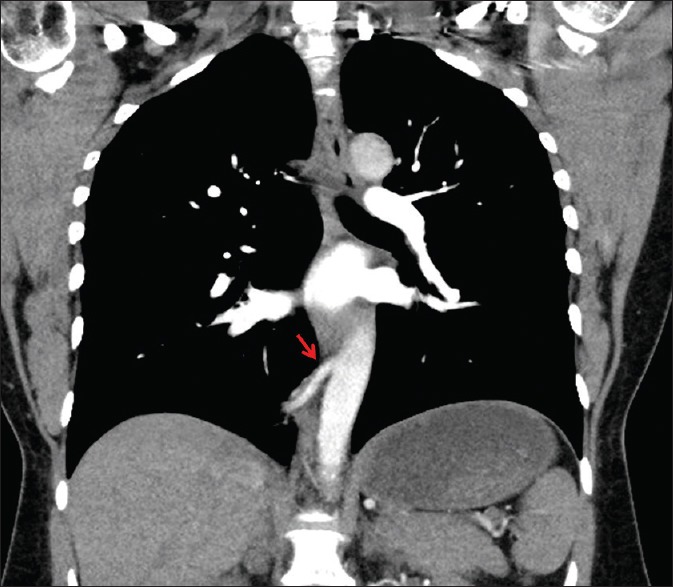
Coronal section from computed tomographic pulmonary angiogram scan. A feeding arterial vessel (red arrow) arises from the lower thoracic aorta supplying an intralobar bronchopulmonary sequestration within the right lower lobe. This intralobar bronchopulmonary sequestration correlated with the mismatched perfusion defect identified on earlier single-photon emission computed tomography ventilation-perfusion imaging
Discussion
Advances in nuclear medicine imaging technology (with SPECT) have resulted in improved accuracy of V/Q scans and a reduced number of non-diagnostic studies.[4]
V/Q scans have long been the imaging modality of choice for investigating PE in patients with an absolute contraindication to CTPA such as those with renal impairment and iodinated contrast hypersensitivity. More recently, the increased sensitivity of scintigraphy scans in younger patients, in the absence of known lung pathology, and a lower associated breast radiation dose has seen a paradigm shift in the investigation of acute PE in premenopausal females.[5] Professional bodies such as the Royal Australian and New Zealand College of Radiologists recommend V/Q scans be used as first-line imaging, where available, in cases of suspected PE in women of reproductive age unless there is significant lung pathology.[6]
In the context of increased utilization of V/Q scans, clinicians and radiologists should be aware of the limitations of this imaging modality. Some limitations are well recognized, such as the inability to detect other pathology (e.g., aortic dissection and malignancy). Less common causes of mismatched perfusion defects – which can mimic PE - include hiatus hernias and associated atelectasis[7] and other anatomical abnormalities such as BPS.
In the presented case, the patient had previously been misdiagnosed with a PE on the basis of a SPECT V/Q scan and unnecessarily treated with warfarin anticoagulation for 6 months. Along with the inconvenience and futility of this treatment is the proven morbidity and mortality associated with warfarin therapy.[8] If this patient had not undergone a CTPA scan, she would have likely been recommenced on another unnecessary course of warfarin (potentially lifelong, even) with the potential associated risks.
Three similar previous cases of BPS being mistaken for PE on scintigraphy scans have been reported in the literature from over 30 years ago.[9,10] This case presents a contemporary account of the ongoing pitfalls associated with V/Q scan interpretation despite improved imaging technology and reporting standards. A modern example of misdiagnosed BPS is relevant to clinicians and radiologists in an era of greater radiation dose awareness.
BPS should be considered in the differential for any atypical appearing lower lobe matched or mismatched perfusion defect. To completely avoid misdiagnosis of BPS when performing V/Q scans, it may be justifiable to include a low-dose CT chest in the protocol to define anatomy in patients who have no prior cross-sectional imaging. The practicality, cost–benefit ratio, and potential induction of malignancy due to the extra radiation dose associated with such a proposal are beyond the scope of this case report but genuinely warrant future consideration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wei Y, Li F. Pulmonary sequestration: A retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg. 2011;40:e39–42. doi: 10.1016/j.ejcts.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 2.Winters WD, Effmann EL. Congenital masses of the lung: Prenatal and postnatal imaging evaluation. J Thorac Imaging. 2001;16:196–206. doi: 10.1097/00005382-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Webb W, Higgins CB. Congenital bronchopulmonary lesions. In: Webb WR, editor. Thoracic Imaging: Pulmonary and Cardiovascular Radiology. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. pp. 1–29. [Google Scholar]
- 4.Reinartz P, Wildberger JE, Schaefer W, Nowak B, Mahnken AH, Buell U. Tomographic imaging in the diagnosis of pulmonary embolism: A comparison between V/Q lung scintigraphy in SPECT technique and multislice spiral CT. J Nucl Med. 2004;45:1501–8. [PubMed] [Google Scholar]
- 5.Reid JH, Coche EE, Inoue T, Kim EE, Dondi M, Watanabe N, et al. Is the lung scan alive and well? Facts and controversies in defining the role of lung scintigraphy for the diagnosis of pulmonary embolism in the era of MDCT. Eur J Nucl Med Mol Imaging. 2009;36:505–21. doi: 10.1007/s00259-008-1014-8. [DOI] [PubMed] [Google Scholar]
- 6.Goergen ST, Jong I, Zallman M. Education Modules for Appropriate Imaging Referrals. Melbourne, Australia: Royal Australian and New Zealand College of Radiologists; 2015. [Last accessed on 2017 Feb 20]. Suspected Pulmonary Embolism. Available from: http://www.ranzcr.edu.au/quality-a-safety/program/key-projects/education-modules-for-appropriate-imaging-referrals . [Google Scholar]
- 7.Wachsmann JW, Kim CK. V/Q matched defect larger than hiatal hernia itself. World J Nucl Med. 2015;14:202–4. doi: 10.4103/1450-1147.163255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro JL, Cesar JM, Fernández MA, Fontcuberta J, Reverter JC, Gol-Freixa J. Morbidity and mortality in patients treated with oral anticoagulants. Rev Esp Cardiol. 2007;60:1226–32. doi: 10.1157/13113927. [DOI] [PubMed] [Google Scholar]
- 9.Van Aman ME, Lloyd TV, Johnson JC. Focal perfusion defect caused by sequestration in two cases. AJR Am J Roentgenol. 1978;131:904–5. doi: 10.2214/ajr.131.5.904. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Abe T, Sato A, Nagai K, Nagai Y, Ibukiyama C. Radionuclide angiography in pulmonary sequestration. J Nucl Med. 1985;26:1035–8. [PubMed] [Google Scholar]


