Abstract
Objective:
To investigate disease activity and disability progression following pregnancy-related discontinuation of natalizumab (NTZ) in patients with relapsing-remitting MS.
Methods:
A retrospective cohort study of clinical and radiologic data in patients who discontinued NTZ for pregnancy-related reasons.
Results:
Twenty-two pregnancy-related NTZ discontinuations in 17 patients were evaluated. The median time to conception was 3.4 months. Relapses were more frequent in patients in whom conception did not occur within 6 months (p = 0.022). Confirmed disability progression occurred in 27.3% and was associated with time to conception (p < 0.001).
Conclusions:
Early conception after NTZ discontinuation is associated with a reduced risk of disease activity and disability progression. Continuation of NTZ treatment until confirmed pregnancy should be considered in patients with previously active MS. However, the advantages of continuing the drug until pregnancy should be balanced against the uncertainties in postnatal outcomes.
The humanized monoclonal α-4 integrin antibody natalizumab (NTZ) (Biogen Inc., Cambridge, MA) effectively reduces relapse rate and disability progression in patients with relapsing-remitting MS (RRMS).1,2 Patients starting NTZ are likely to consider a future pregnancy because a substantial part of the patients with RRMS is women and in their childbearing years.
NTZ exposure has not been associated with a higher incidence of spontaneous abortions or large birth defects in humans, although animal studies have reported some fetal effects.3–6 However, data obtained in humans are limited by small sample sizes. Current guidelines advise women of childbearing age to use contraception and to discontinue NTZ in case of accidental pregnancy. Usually, the advice regarding family planning is to plan a washout of NTZ before conception. Unfortunately, NTZ discontinuation is associated with, potentially severe, recurrence of disease activity.7–10
Pregnancy is considered to be a protective condition in RRMS.11 However, because prior case series have reported both limited and severe disease activity in patients who discontinue NTZ because of pregnancy,12–14 it remains unclear whether disease recurrence is reduced if NTZ discontinuation is followed by a pregnancy. Because low NTZ serum concentrations have been linked to decreased drug efficacy, NTZ concentrations may play a role in (the timing of) disease recurrence after NTZ discontinuation.15
The aim of this study was to investigate MS disease activity and disability progression in pregnancy-related NTZ discontinuations in relation to time to conception and NTZ concentrations.
METHODS
Patient selection.
In 2006, an observational cohort study was initiated at the VU University Medical Center in Amsterdam to monitor different aspects of NTZ treatment. A total of 210 patients with RRMS starting NTZ have been included in this cohort and are regularly subdued to clinical testing (Expanded Disability Status Scale [EDSS]16) and annual brain MRIs. All patients of this cohort who discontinued NTZ because of (1) an accidental pregnancy while using NTZ or (2) a wish to get pregnant were included. Periods of NTZ discontinuation that ended in spontaneous abortions or no conception were also included, provided that pregnancy or the intention to become pregnant was the explicit reason to stop treatment. For patients who discontinued NTZ more than once because of pregnancy-related reasons, each period of pregnancy-related NTZ discontinuation is separately evaluated. Throughout the article, periods of pregnancy-related discontinuations will be referred to as cases. Time to conception is calculated from the date of last NTZ infusion to conception in months.
Protocol approvals and patient consent.
A waiver (reference 2016.579) was obtained from our local institutional review board stating that the requirements of the Medical Research Involving Human Subjects Act did not apply and that official institutional review board approval was not mandatory for the retrospective use of these data. Written informed consent was obtained from all participants for the use of the clinical, laboratory, and imaging data (including pregnancy outcomes) for research and teaching purposes.
Disease activity.
The 2013 criteria of Lublin et al.17 were used to define “disease activity.” According to these criteria, when referring to disease activity, the patient experiences a clinical relapse and/or the occurrence of contrast-enhancing T1 hyperintense and/or new or unequivocally enlarging T2 hyperintense lesions (active T2 lesions) on brain MRI.
Patient files of included patients were checked for relapses during the period of NTZ discontinuation and in the preceding year. A relapse was defined as a period of new neurologic deficit, existing longer than 24 hours, and not attributable to another cause than MS. For each relapse, it was noted if it was treated with steroids and when it occurred, i.e., before conception, during the first, second, or third trimester of pregnancy, or after delivery. The annualized relapse rate (ARR) was calculated by dividing the number of reported relapses by the total time of NTZ discontinuation in years.
Brain MRIs were based on MRI protocols according to the magnetic resonance imaging in multiple sclerosis consensus guidelines using either a 1.5-T or a 3.0-T scanner.18 A neuroradiologist scored active T2 lesions and gadolinium-enhancing T1 hyperintense lesions on the first MRI after the pregnancy-related discontinuation in comparison with the last MRI available before NTZ discontinuation.
Disability progression.
EDSS scores were assessed yearly by certified physicians in all NTZ-treated patients and postpartum at the time of restart of disease-modifying treatment (DMT). The usual definition of disability progression was applied: for a baseline EDSS score of 0, an increase of 1.5 points was required, an increase of 1 point for a baseline EDSS score of 1–5.5, and an increase of 0.5 for a baseline EDSS score of >5.5.19 A second EDSS score, at least 12 weeks after the EDSS showing progression, was used to confirm disability progression.17
NTZ serum concentration.
In the observational cohort, blood samples were obtained every 12 weeks before NTZ infusion. Sera were stored at −80°C in the biobank of the VU University Medical Center. For all patients included in this study, an NTZ trough concentration (all samples were taken right before NTZ infusion) was measured at Sanquin Laboratory in the last sample available before discontinuation of NTZ, using a cross-linking assay using polyclonal rabbit anti-NTZ F(ab)2 fragments for capture and a mouse anti-IgG4 monoclonal antibody for detection.20
Statistical analysis.
Patients were divided into 3 groups according to time to conception: (1) patients who discontinued NTZ because of an accidental pregnancy, (2) patients who conceived within 6 months after discontinuation of NTZ, and (3) patients who did not conceive within 6 months after discontinuation of NTZ. To analyze the distribution of clinical disease activity, radiologic disease activity, and disability progression across these groups, the Pearson χ2 test was used. To analyze the distribution of the ARR, the Kruskal-Wallis test was used.
Furthermore, time to conception and NTZ concentration were used as continuous variables. Both variables were not normally distributed. Therefore, nonparametric tests were used. The following tests were used to analyze the relationship between time to conception and relapses (Kruskal-Wallis test), ARR (Spearman rank test), radiologic disease activity (Mann-Whitney U test), and disability progression (Mann-Whitney U test). A possible relationship between NTZ concentration and spontaneous abortion (Mann-Whitney U test), time to conception (Spearman rank test), relapses (Kruskal-Wallis test), radiologic activity (Mann-Whitney U test), time to first relapse (Spearman rank test), and disability progression (Mann-Whitney U test) was analyzed. SPSS statistics software version 22.0 (IBM Corp., Armonk, NY) was used for statistical analyses. All reported p values are based on 2-tailed statistic tests, with a significance level set at p < 0.05.
RESULTS
Patient characteristics.
Of 210 NTZ-treated patients with RRMS, 18 women discontinued treatment for pregnancy-related reasons. One patient moved away during gestation and was lost to follow-up and excluded from analysis. Five patients restarted NTZ after the first pregnancy and later discontinued a second time for pregnancy-related reasons, resulting in a total of 22 periods of NTZ discontinuations, later referred to as cases. See table 1 for demographic and clinical baseline characteristics.
Table 1.
Baseline characteristics
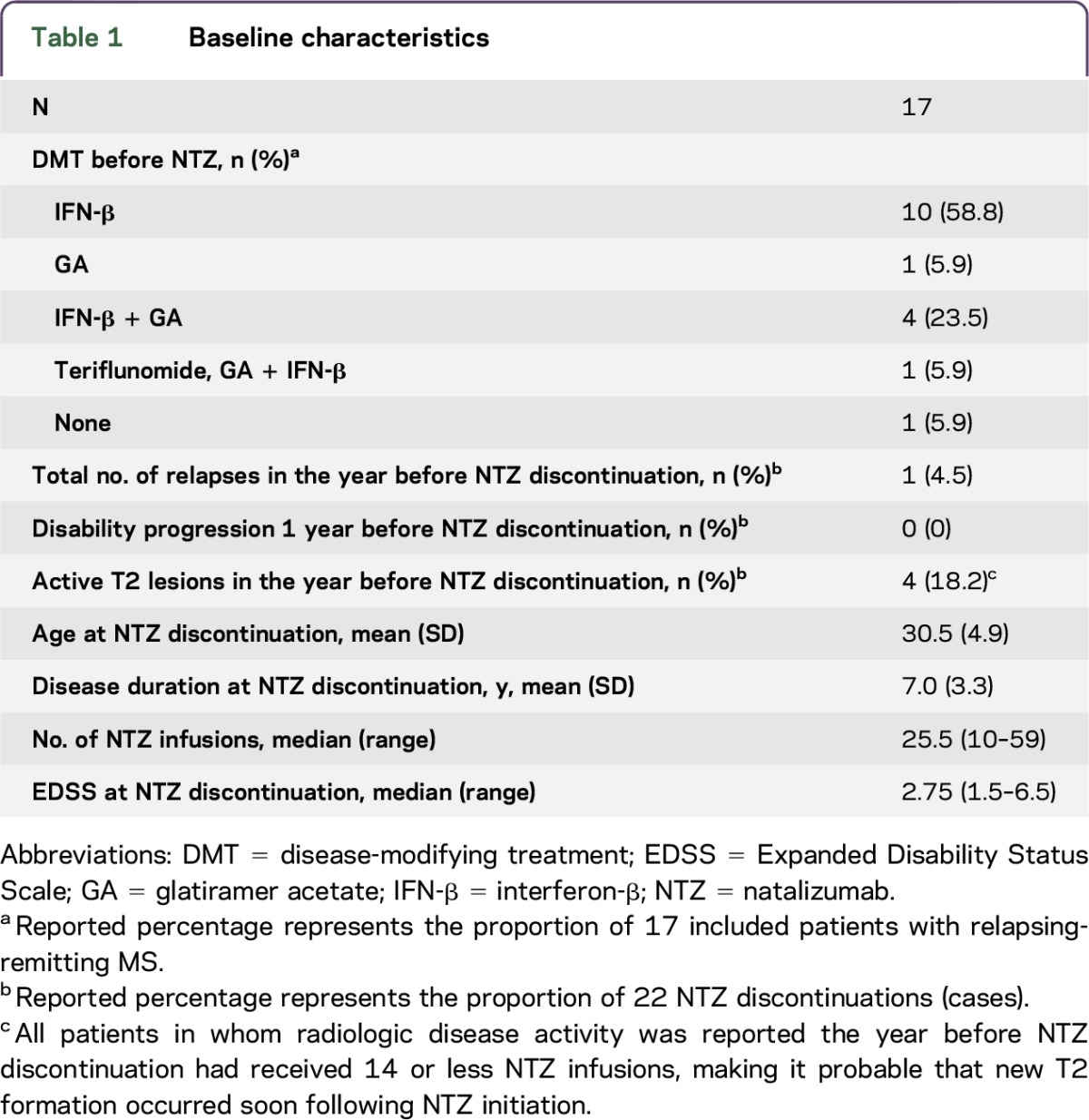
Accidental pregnancy was the reason for NTZ discontinuation in 7 cases. In all cases with an accidental pregnancy, NTZ was discontinued immediately. In 15 cases, NTZ was discontinued because of a future pregnancy wish; conception occurred within 6 months in 9 cases. The median time to conception of the 22 cases was 3.4 months. Of the patients who stopped NTZ before conception, patients conceived at a median time of 4.6 months (range 2.1–23.9 months) after discontinuation.
Because of severe clinical disease activity after NTZ discontinuation, 3 patients chose to withhold from pregnancy and restarted NTZ treatment after 10.8, 13.7, and 6.2 months of discontinuation (table 2, cases refer to patients 4, 8, and the first case of patient 11). Two of these 3 patients stopped NTZ again because of pregnancy shortly after restarting treatment (2 and 6 NTZ infusions). DMT was restarted with a median of 1.1 months (range 0.16–5.5 months) after delivery following the 19 full-term pregnancies. See table 2 for a case overview.
Table 2.
Case overview for 22 pregnancy-related NTZ discontinuations in 17 patients with RRMS
Disease activity.
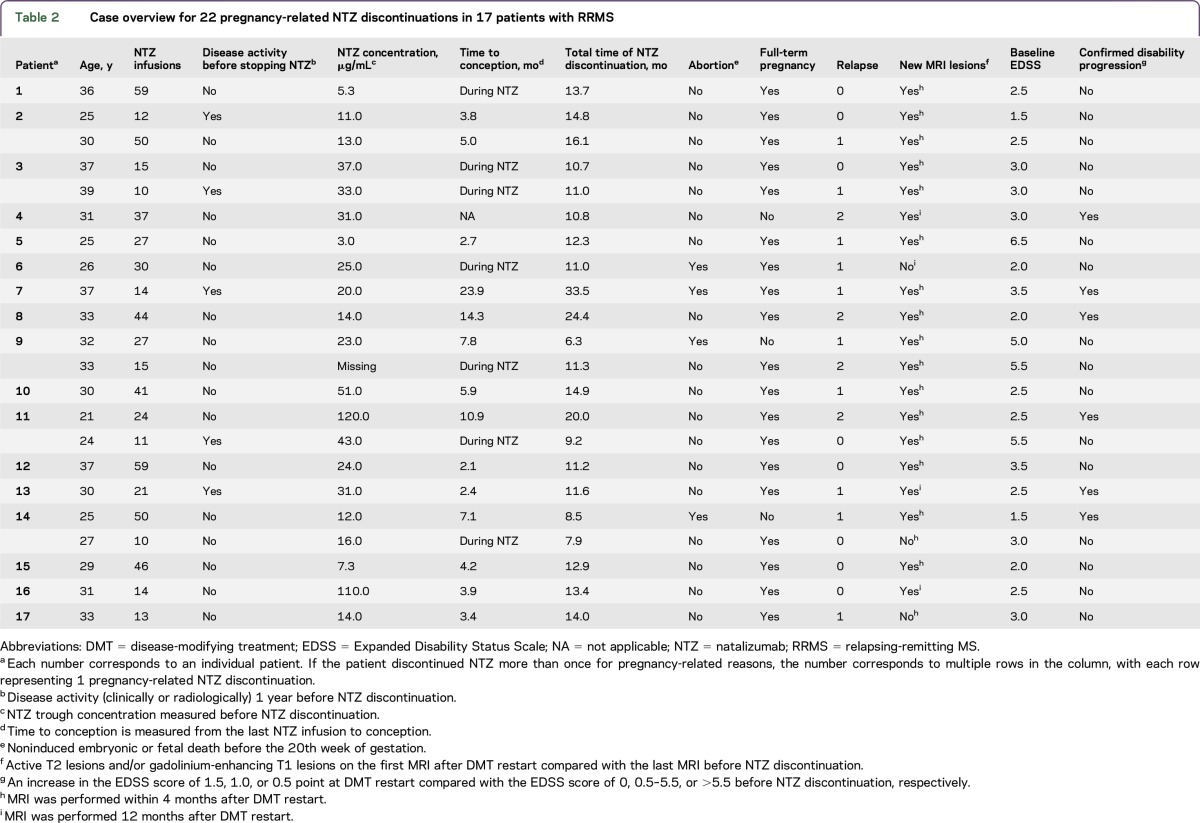
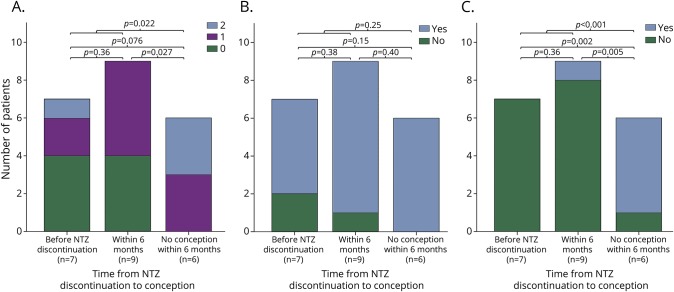
Disease activity (either clinically or radiologically) was observed after NTZ discontinuation in 21 cases (95.5%). In the case that did not show disease activity, conception occurred before NTZ discontinuation. Seventeen relapses occurred in 13 cases. Eight relapses (47.1%) were reported during pregnancy: 2 in the first trimester, 4 in the second trimester, and 2 in the third trimester. Six relapses (35.3%) occurred before conception and 3 relapses (17.6%) after delivery. All relapses before or after pregnancy were treated with steroids. Two of the 8 relapses that occurred during pregnancy were treated with steroids, 1 in the first trimester and 1 in the third trimester. If conception did not occur within 6 months of NTZ discontinuation, patients experienced significantly more relapses when comparing with patients in whom conception took place within 6 months of NTZ discontinuation (p = 0.022; figure). The ARR also increased across the groups with a median of 0 (range 0–2.1) when conception occurred under NTZ, 0.75 (range 0–1.0) if conception occurred within 6 months, and 1.3 (range 0.4–2.2) if conception did not occur within 6 months (p = 0.040). When analyzing relapses to time to conception as a continuous variable, more relapses occurred when time to conception increased, but this finding was not statistically significant (p = 0.11).
Figure. Clinical and radiologic disease activity and confirmed disability progression across the 3 groups defined by time to conception.
(A) Relapses refer to the total number of reported relapses; no patient experienced more than 2 relapses. (B) MRI activity refers to active T2 hyperintense and/or contrast-enhanced T1 hyperintense lesions on the first MRI after DMT restart compared with the last MRI before NTZ discontinuation. (C) Confirmed disability progression defined as an increase in the EDSS score of 1.5, 1.0, or 0.5 point at DMT restart compared with the EDSS score of 0, 0.5–5.5, or >5.5 before NTZ discontinuation, respectively. DMT = disease-modifying treatment; EDSS = Expanded Disability Status Scale; NTZ = natalizumab.
The last MRI before NTZ discontinuation was obtained at a median of 1.4 months (range 0.0–9.5 months) before discontinuation. All postdiscontinuation MRIs were obtained within 4 months from DMT restart, except for 4 scans that were obtained after ±12 months (table 2). Radiologic disease activity was observed in 19 (86.4%) cases. In 14 cases, postdiscontinuation MRIs included T1 imaging after gadolinium administration. Contrast-enhancing T1 lesions were observed in 7 cases. Radiologic disease activity was not significantly different in cases in whom conception occurred before or after 6 months of NTZ discontinuation (p = 0.25; figure). Furthermore, the 3 time interval groups showed comparable occurrence of substantial T2 lesion accumulation defined as >5 new T2 lesions, i.e., 4 cases (57.1%) when conception occurred under NTZ, 5 cases (55.6%) when conception occurred within 6 months, and 3 cases (50.0%) when no conception occurred within 6 months. Radiologic disease activity was not significantly associated with time to conception as a continuous variable (p = 0.19).
Disability progression.
Median EDSS scores increased from 2.75 (range 1.5–6.5) to 3.0 (1.0–6.0) after the period of NTZ discontinuation (p = 0.084). Disability progression occurred in 8 cases and was confirmed in 6 cases (27.3%). Confirmed disability progression occurred significantly more often when conception had not occurred before 6 months (p < 0.001; figure). The incidence of confirmed disability progression also increased significantly when time to conception (as a continuous variable) increased (p = 0.006).
NTZ trough concentration.
NTZ trough concentration was measured in the latest sample available during NTZ treatment and was available in 21 cases. NTZ trough concentration ranged from 3 to 120 μg/mL, with a median concentration of 23 μg/mL. NTZ concentration was not different for patients who experienced a spontaneous abortion (p = 0.65) and was not related to time to conception (r = −0.07; p = 0.78). NTZ concentration was not related to relapses (p = 0.60) or radiologic activity (p = 0.73). The mean time to the first relapse was 7.9 ± 3.6 months. The time to the first relapse was not associated with NTZ concentration (r = 0.24; p = 0.44). The earliest relapse occurred 2.5 months after NTZ discontinuation; this patient had the lowest NTZ trough concentration of 3 μg/mL. We found no association between NTZ concentration and confirmed disability progression (p = 0.61).
Outcomes of the pregnancies.
Most patients conceived spontaneously, except for 2 patients who conceived through in vitro fertilization. Four patients experienced a spontaneous abortion; all these patients discontinued NTZ before conception. Two of these 4 patients conceived successfully again after the spontaneous abortion. In 22 cases, 19 children were born. The mean birthweight was 3,055 ± 391.4 g. No birth defects were reported. In 15 patients, the breastfeeding choice was reported; 7 patients chose breastfeeding.
DISCUSSION
This study evaluates disease activity and disability progression during 22 pregnancy-related NTZ discontinuations in 17 patients with RRMS. Recurrence of disease activity, clinically and/or radiologically, was observed in 95.5% of discontinuations, although all patients showed no or limited disease activity in the year preceding NTZ discontinuation. The extent of disease activity was partly determined by the time from NTZ discontinuation to conception. However, of the cases in whom conception occurred under NTZ treatment, still 42.9% experienced a relapse and 57.1% showed substantial T2 lesion accumulation of >5 lesions on brain MRI. Remarkably, confirmed disability progression was not observed in patients in whom conception occurred under NTZ treatment but was observed in >80% of patients who did not conceive within 6 months (p < 0.001).
Prior studies report conflicting results on disease activity in patients with RRMS who discontinued NTZ for pregnancy-related reasons. In 35 women who accidentally became pregnant while using NTZ, no disease rebound was observed during pregnancy or postpartum.13 However, a recent case series described disease activity in 4 pregnant patients after discontinuing NTZ.12 In 2 of these patients, recurrence of disease activity was severe. Furthermore, the case of a young woman whose pregnancy wish was hampered by recurrent rebound disease activity after NTZ discontinuation demonstrates the impact that NTZ treatment may have on family planning.14 This is in line with the findings reported here, indicating that disease activity and disability progression are more likely to be limited when patients conceive before or shortly after the last NTZ infusion. In this study, clinical disease activity occurred more often when conception had not occurred within 6 months of NTZ discontinuation, in line with prior studies that show that recurrence of disease activity typically occurs 4–6 months after stopping NTZ.7,9 In contrast to other published reports, no increase in the relapse frequency was observed postpartum in our study.6,11 This is probably attributed to the early resumption of DMT in most cases. Radiologic disease activity exceeded clinical activity and was observed in 86.4% of pregnancy-related NTZ discontinuations. When comparing the 3 groups (0, <6, and >6 months of interval), no significant difference in radiologic disease activity was found. However, the fact that all cases without radiologic activity conceived within 6 months from NTZ discontinuation suggests at least some effect of time to conception on radiologic disease activity.
In our study, confirmed disability progression occurred in 6 cases (27.3%). Although a number of studies describe the relapse rate during and after pregnancy in women treated with NTZ,11,13 disability progression is described only in case reports.12,14,21 To our knowledge, our study is the first to describe disability progression in a cohort of patients with RRMS discontinuing NTZ for pregnancy-related reasons. Our results demonstrate a significant relation of confirmed disability progression and time to conception. Although our cohort is relatively small, this may be valuable information for women regarding decision making about a possible NTZ washout period vs continuing NTZ until pregnancy.
Intraindividual NTZ trough concentrations are shown to be stable within patients during NTZ treatment and correlate with disease activity.15,22 Desaturation of the α-4 integrin receptor occurs when the concentration falls under 2–2.5 μg/mL.23,24 We found a large variability of NTZ trough concentrations with a mean concentration of 23 μg/mL, which is in agreement with recently presented data.25 We tested NTZ trough concentrations before discontinuation of therapy, with the expectation of a high concentration as a possible protective factor for (early) recurrence of disease activity. No association between clinical disease activity and NTZ trough concentrations was observed. This finding could be influenced by the heterogeneity and small sample size of our group. Furthermore, contrary to expectations, we did not find an association between time to the first relapse and NTZ trough concentration. This might be explained by the mean time to the first relapse being 7.9 ± 3.6 months, when NTZ is expected to have long fallen under therapeutic levels.26 Also, we did not have longitudinal NTZ concentrations, and pharmacodynamics might differ between patients causing a difference in clearance of NTZ. It remains notable that the patient with the earliest relapse (2.5 months after NTZ discontinuation) showed the lowest NTZ trough concentration under treatment (3 μg/mL).
In our cohort, spontaneous abortions occurred in 18.2% of all conceptions, which is in line with reported rates in healthy populations (8%–22%).27,28 The Tysabri Pregnancy Exposure Registry reported lower rates of spontaneous abortions in NTZ-exposed women (9.0%).6 Of interest, in 102 NTZ-exposed pregnancies, the incidence of spontaneous abortions was increased in NTZ-exposed women (17.3%) as well as disease-matched controls (21.0%) compared with healthy controls (4.1%).5
NTZ, an IgG4 antibody, crosses the placenta in the second and third trimesters.29 In our study, no birth defects were reported in 19 born babies. Prior studies have not related antenatal NTZ exposure to specific large birth defects.3–6 However, sample sizes are insufficient to form conclusions, as detection of a 2-fold increase in birth defects would need a sample size of at least 200 patients and 200 controls.5 Antenatal NTZ exposure may cause mild hematologic deviations at birth, mainly thrombocytopenia and anemia.26 Additional research with larger study populations and long-term follow-up is needed to further evaluate the safety of antenatal NTZ exposure.
Our relatively small sample size and multiple comparisons without correction warrant cautious interpretation of our data. A limitation of this study is the retrospective design. However, the evaluated patients were part of a well-monitored cohort that was subject to the regular EDSS and consistent MRI follow-up, resulting in very limited missing data. The composition of the pregnancy-related NTZ discontinuations was heterogeneous, with some patients not experiencing full-term gestations because of spontaneous abortions or failure to conceive, but do represent real-world clinical experience with patients with RRMS who deal with family planning.
This study shows that disease activity and disability progression during pregnancy-related NTZ discontinuations in patients with RRMS are common and that relapses and confirmed disability progression increase significantly with longer time to conception. We demonstrate a clinical advantage for patients who conceive during (or shortly after) NTZ treatment. Continuation of treatment until conception may thus be a preferred strategy to prevent relapses and disability progression in women on NTZ treatment who want to get pregnant. Therefore, future research confirming our results and ascertaining the safety of NTZ in terms of postnatal outcomes and spontaneous abortion rates is of great clinical importance.
ACKNOWLEDGMENT
The authors thank all patients included in the study for agreeing to the use of their data for research and education purposes. This research has been executed within the VUmc MS Center Amsterdam.
GLOSSARY
- ARR
annualized relapse rate
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- NTZ
natalizumab
- RRMS
relapsing-remitting MS
AUTHOR CONTRIBUTIONS
The conception and design of the study were laid out by J. Killestein and I. Kleerekooper. The acquisition of the data was done by I. Kleerekooper, C.E. Leurs, I. Dekker, T. Rispens, C.E.P. van Munster, B.A. de Jong, B.W. van Oosten, B.M.J. Uitdehaag, M.P. Wattjes, J. Killestein, and Z.L.E. van Kempen. J. Killestein, I. Kleerekooper, and Z.L.E. van Kempen interpreted the data and drafted the manuscript. Statistical analysis was conducted by I. Kleerekooper, Z.L.E. van Kempen, J. Killestein, and B.I. Lissenberg-Witte. M.P. Wattjes, C.E. Leurs, I. Dekker, B.I. Lissenberg-Witte, C.E.P. van Munster, B.A. de Jong, B.W. van Oosten, B.M.J. Uitdehaag, and T. Rispens revised the manuscript critically for intellectual content. Approval of the manuscript to be published was given by I. Kleerekooper, Z.L.E. van Kempen, C.E. Leurs, I. Dekker, M.P. Wattjes, T. Rispens, B.I. Lissenberg-Witte, C.E.P. van Munster, B.A. de Jong, B.W. van Oosten, B.M.J. Uitdehaag, and J. Killestein.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
I. Kleerekooper and Z.L.E. van Kempen report no disclosures. C.E. Leurs and I. Dekker received research support from Stichting MS Research. T. Rispens received travel funding and/or speaker honoraria from Regeneron Pfizer and received research support from the Landsteiner Foundation for Blood Transfusion Research and the Dutch Arthritis Foundation Hersenstichting. B.I. Lissendberg-Witte reports no disclosures. C.E.P. van Munster served on the scientific advisory board of Merck Serono; received speaker honoraria and consulting fees from Novartis, Biogen Idec, and Merck Serono; and received travel support from Novartis, Sanofi Genzyme, and Teva. B.A. de Jong received speaker and consulting fees from Merck Serono, Biogen, Teva, Genzyme, and Novartis. B.W. van Oosten reports no disclosures. B.M.J. Uitdehaag consulted for Novartis, Merck Serono, Biogen Idec, Teva, Sanofi Genzyme, and Roche. M.P. Wattjes received travel funding and/or speaker honoraria from Biogen, Novartis, Genzyme, and Roche; served on the editorial board of European Radiology; consulted for Biogen, Roche, and IXICO; and received speaker fees from Biogen, Novartis, Genzyme, and Novartis. J. Killestein consulted for Novartis, Merck Serono, Bioven, Genzyme, Teva, and Roche; received speaker honoraria from Biogen, Novartis, Teva, Merck Serono, and Roche; is the editor for the official scientific journal of the Dutch Society of Neurology, The Dutch Society of Neurosurgeons, and the Society of Flemish Neurologists; serves on the editorial board of MS Journal; and received research support from Schering AG, Biogen Idec, Merck Serono, Teva, Genzyme, Novartis, and Roche. The VUmc MS Center Amsterdam has received financial support for research activities from Bayer Schering Pharma, Biogen, Roche, GlaxoSmithKline, Merck Serono, Genzyme, Novartis, and Teva. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Polman CH, O'Connor PW, Havrdova E, et al. . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 2.Rudick RA, Stuart WH, Calabresi PA, et al. . Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–923. [DOI] [PubMed] [Google Scholar]
- 3.Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs 2015;29:207–220. [DOI] [PubMed] [Google Scholar]
- 4.Duquette P, Prat A. How safe is natalizumab during pregnancy? Mult Scler 2015;21:121–122. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimi N, Herbstritt S, Gold R, Amezcua L, Koren G, Hellwig K. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy: a prospective, controlled observational study. Mult Scler 2015;21:198–205. [DOI] [PubMed] [Google Scholar]
- 6.Friend S, Richman S, Bloomgren G, Cristiano LM, Wenten M. Evaluation of pregnancy outcomes from the Tysabri (natalizumab) pregnancy exposure registry: a global, observational, follow-up study. BMC Neurol 2016;16:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox RJ, Cree BA, De Seze J, et al. . MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology 2014;82:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killestein J, Vennegoor A, Strijbis EM, et al. . Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 2010;68:392–395. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor PW, Goodman A, Kappos L, et al. . Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011;76:1858–1865. [DOI] [PubMed] [Google Scholar]
- 10.Rigau V, Mania A, Befort P, et al. . Lethal multiple sclerosis relapse after natalizumab withdrawal. Neurology 2012;79:2214–2216. [DOI] [PubMed] [Google Scholar]
- 11.Finkelsztejn A, Brooks JB, Paschoal FM Jr, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG 2011;118:790–797. [DOI] [PubMed] [Google Scholar]
- 12.De Giglio L, Gasperini C, Tortorella C, Trojano M, Pozzilli C. Natalizumab discontinuation and disease restart in pregnancy: a case series. Acta Neurol Scand 2015;131:336–340. [DOI] [PubMed] [Google Scholar]
- 13.Hellwig K, Haghikia A, Gold R. Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult Scler 2011;17:958–963. [DOI] [PubMed] [Google Scholar]
- 14.Martinelli V, Colombo B, Dalla Costa G, et al. . Recurrent disease-activity rebound in a patient with multiple sclerosis after natalizumab discontinuations for pregnancy planning. Mult Scler 2016;22:1506–1508. [DOI] [PubMed] [Google Scholar]
- 15.Vennegoor A, Rispens T, Strijbis EM, et al. . Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler 2013;19:593–600. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 17.Lublin FD, Reingold SC, Cohen JA, et al. . Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wattjes MP, Rovira A, Miller D, et al. . Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis: establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015;11:597–606. [DOI] [PubMed] [Google Scholar]
- 19.Kalincik T, Cutter G, Spelman T, et al. . Defining reliable disability outcomes in multiple sclerosis. Brain 2015;138:3287–3298. [DOI] [PubMed] [Google Scholar]
- 20.Rispens T, Leeuwen A, Vennegoor A, et al. . Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem 2011;411:271–276. [DOI] [PubMed] [Google Scholar]
- 21.Verhaeghe A, Deryck OM, Vanopdenbosch LJ. Pseudotumoral rebound of multiple sclerosis in a pregnant patient after stopping natalizumab. Mult Scler Relat Disord 2014;3:279–281. [DOI] [PubMed] [Google Scholar]
- 22.van Kempen ZL, Leurs CE, Witte BI, et al. . The majority of natalizumab-treated MS patients have high natalizumab concentrations at time of re-dosing. Mult Scler 2017:1352458517708464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri BO, Man S, Giovannoni G, et al. . Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009;72:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muralidharan KK, Kuesters G, Plavina T, et al. . Population pharmacokinetics and target engagement of natalizumab in patients with multiple sclerosis. J Clin Pharmacol 2017;57:1017–1030. [DOI] [PubMed] [Google Scholar]
- 25.Foley JF, Hoyt T, Christensen A, et al. . Natalizumab extended interval dosing reduces serum trough natalizumab concentrations and a4 integrin receptor occupancy. Mult Scler 2016;22(suppl 3):P1251. [Google Scholar]
- 26.Rispens T, Vennegoor A, Wolbink GJ, Polman CH, Killestein J. Natalizumab remains detectable in patients with multiple sclerosis long after treatment is stopped. Mult Scler 2012;18:899–901. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 2003;79:577–584. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox AJ, Weinberg CR, O'Connor JF, et al. . Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–194. [DOI] [PubMed] [Google Scholar]
- 29.Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol 2009;104:228–233. [DOI] [PubMed] [Google Scholar]