Abstract
Background
Acute kidney injury (AKI) secondary to sepsis is a major cause of morbidity and mortality in the human intensive care unit (ICU). Kidney function and the histological findings of AKI were investigated in an experimental rat model with sepsis induced by cecal ligation and puncture (CLP) and compared with and without treatment with carnosine (beta-alanyl-L-histidine).
Material/Methods
Twenty-four Sprague-Dawley rats were randomly divided into three groups consisting eight rats in each: Group 1 – control; Group 2 – septic shock; and Group 3 – septic shock treated with carnosine. Femoral vein and artery catheterization were applied in all rats. Rats in Group 1 underwent laparotomy and catheterization. The other two groups with septic shock underwent laparotomy, CLP, catheterization, and bladder cannulation. Rats in Group 3 received an intraperitoneal (IP) injection of 250 mg/kg carnosine, 60 min following CLP. Rats were monitored for blood pressure, pulse rate, and body temperature to assess responses to postoperative sepsis, and 10 mL/kg saline replacement was administered. Twenty-four hours following CLP, rats were sacrificed, and blood and renal tissue samples were collected.
Results
Statistically significant improvements were observed in kidney function, tissue and serum malondialdehyde levels, routine blood values, biochemical indices, and in histopathological findings in rats in Group 3 who were treated with carnosine, compared with Group 2 exposed to septic shock without carnosine treatment.
Conclusions
Carnosine (beta-alanyl-L-histidine) has been shown to have beneficial effects in reducing AKI due to septic shock in a rat model of septicemia.
MeSH Keywords: Acute Kidney Injury; Carnosine; Shock, Septic
Background
Sepsis and septicemia, in response to infectious pathogens and the effects of their toxins, are leading cause of mortality for humans in the intensive care unit (ICU) [1]. In the United States, there are more than 750,000 cases of sepsis annually, with a mortality rate of nearly 30% [1]. Sepsis is found to be associated with the development of acute kidney injury (AKI) [2]. Bacterial infections, most commonly due to Gram-negative bacteria and their endotoxins, are the most common underlying cause of sepsis [1–3]. Inflammation associated with sepsis involves a dynamic response of vascularized tissue to injury [3]. In the inflamed tissues, changes in blood flow, vascular permeability, leukocyte accumulation, and edema occur, with a variety of mediators being involved in this chain of events [3].
Carnosine (beta-alanyl-L-histidine) is a dipeptide that has been shown to scavenge reactive oxygen species (ROS) as well as alpha, beta-unsaturated aldehydes, formed from the peroxidation of cell membrane fatty acids during oxidative stress [4–6]. In experimental studies, carnosine reduces the level of proinflammatory and profibrotic cytokines [4,5]. It has been suggested that carnosine is a naturally occurring anti-aging substance in humans, with beneficial effects on the cardiovascular system [4,5]. There have been no previously published studies on the effects of carnosine on renal function in sepsis and septic shock.
Because carnosine is involved in multiple metabolic pathways and has nephroprotective properties, the present study aimed to examine the effects of carnosine in a rat model of sepsis that used cecal ligation and puncture (CLP). In this study, routine blood values, biochemical indices, and histopathological findings were used to investigate the possible effects of carnosine treatment in sepsis-induced kidney damage.
Material and Methods
The present experimental study was performed in the Physiology Department of the Experimental Laboratory and Medical Research Centre, Experimental Animal Laboratory, Eskişehir Osmangazi University, Turkey. The study protocol was approved by the Eskişehir Osmangazi University Medical Faculty Ethical Committee (approval number: 19.10.2010-176/2010).
Animals studied
Twenty-four Sprague–Dawley rats (weight, 200–300 g) were purchased from Eskisehir Osmangazi University Experimental Research Centre for Medical and Surgical Investigation. No gender discrimination was made. Animals were maintained at room temperature under standard laboratory conditions during the experiment, and fed with standard rat chow and tap water. Animal protocols conformed to the principals identified in the Guide for the Care and Use of Laboratory Animals (http://www.nap.edu/catalog/5140.html).
Experimental design
Figure 1 shows the study design. Twenty-four Sprague-Dawley rats were randomly divided into three groups consisting eight rats in each: Group 1 – control; Group 2 – septic shock; and Group 3 - septic shock treated with carnosine.
Figure 1.
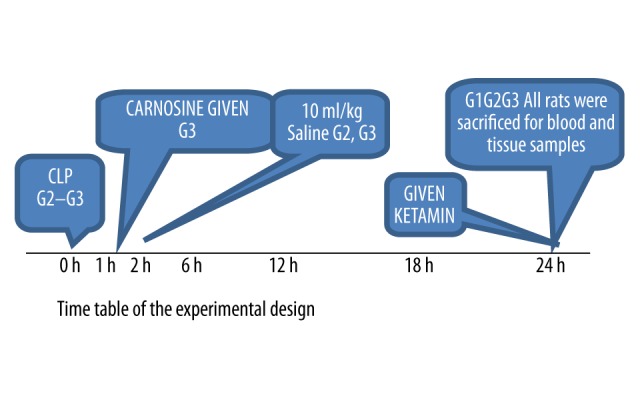
The study design and timelines.
Femoral vein and femoral artery catheterization were applied in all rats. Rats in control Group 1 underwent laparotomy and catheterization. Rats in Group 2 and Group 3, underwent laparotomy, cecal ligation and puncture (CLP), catheterization, and bladder cannulation. Animals were anesthetized with 60 mg/kg intraperitoneal (IP) ketamine hydrochloride (Parke-Davis, NJ, USA) and 5 mg/kg of xylazine (Bayer AG, Leverkusen, Germany), after fasting for 12 h. The depth of anesthesia was monitored by the lack of response to a forceps pinch on the abdominal skin at 5 min after injection of the anesthetic.
Under sterile conditions, 10% povidone iodine was swabbed on the skin, and an inguinal incision was performed. All rats were then catheterized, isolating the femoral artery and vein using pigtail 60 cm (polyethylene) catheters. The femoral vein catheter was advanced to the vena cava. Cannulas were washed with saline and 100 IU heparin before and after cannulation to prevent blockage. All surgeries were performed on a heated surgical table at 36.6°C to avoid hypothermia during surgery and follow-up.
Group 1 (control group)
Eight rats received bladder and arterial cannulation under anesthesia and underwent laparotomy only. During the 24-hour period following laparotomy, urine outflow, invasive arterial blood pressure measurements, and electrocardiogram (ECG) recordings were made, and findings were recorded every 5 min. Blood samples were taken for arterial blood gas, venous blood gas, white blood cell (WBC) count, platelet count, and biochemical measurements, including blood urea nitrogen (BUN) (mg/dL), serum creatinine (Cr) (mg/dL), and creatine kinase (CK) before catheterization and at the end of the experiment. A hemogram, or a graphic representation of the detailed blood assessment, was made. Blood samples were also obtained 24 hours postoperatively for serum malondialdehyde (MDA) measurements. Rats were sacrificed 24 hours following laparotomy using an overdose of ketamine. Kidney tissue specimens were taken for MDA measurement and histopathology examination.
Group 2 (septic shock)
Eight rats received laparotomy and cecal ligation and puncture (CLP) under anesthesia, invasive arterial blood pressure measurements, and ECG recordings were made, and findings were recorded every 5 min. The value of the mean arterial blood pressure (MAP) <60 mm/Hg were used to define septic shock, and 10 mL/kg saline replacement was administered when sepsis developed. At 24 h following CLP, blood samples were collected, as in Group 1. Rats were sacrificed 24 hours following laparotomy and the CLP procedure using an overdose of ketamine. Kidney tissue specimens were taken for MDA measurement and histopathology examination.
Group 3 (septic shock treated with carnosine)
Eight rats received laparotomy and CLP. After a further 60 min, a dose of 250 mg/kg carnosine diluted into 5 mL saline was injected. Hemodynamic responses were recorded simultaneously. Arterial pressure was measured using the Transpac® IV Disposable Pressure Transducer (ICU Medical Inc., USA). Invasive arterial blood pressure and pulse rate were monitored using an MP100 data acquisition system (Biopac, USA).
A MAP of <60 mm/Hg was used to define septic shock. The rats received an injection of 10 mL/kg saline when sepsis developed. At 24 h after CLP, blood samples were collected, as in Group 1 and Group 2. Rats were sacrificed 24 hours following laparotomy and the CLP procedure using an overdose of ketamine. Kidney tissue specimens were taken for MDA measurement and histopathology examination.
Measurements
Hemograms and biochemical analyses were performed by the standard methods used in our Hematology Department and Biochemistry Department. Renal tissue malondialdehyde (MDAT) and serum malondialdehyde (MDAS) levels were determined in our Biochemistry Department. Histopathological examination of the rat tissues was performed by our Histopathology Department. The body temperature was measured by the rectal probe, and baseline and outcome values were recorded.
Malondialdehyde measurements in serum and kidney tissue
Each tissue sample was weighed on a microbalance and homogenized in 1: 10 (0.15 M) potassium chloride (KCl) solution. Homogenates were centrifuged at 4,000 rpm at 4°C, and MDA was measured in the supernatants. Thiobarbituric acid (TBA) 1.5 mL of a 0.08% solution, pH 5.5; acetic acid, 1.5 mL of a 20% solution, pH 3.5; and sodium dodecyl sulfate (SDS) 0.2 mL of a 10% solution, pH 4.5, were added to 0.4 mL of supernatant. MDA standards were aliquoted fresh from stock solutions. Samples and standards were boiled at 100°C for 1 h. Both were then cooled in cold water, and 5 mL n-butanol was added to each. Each tube was centrifuged at 4,000 rpm for 10 min. Supernatants were used for MDA measurement. MDA measurements were also performed on plasma samples
Histological methods
Kidney tissue samples were taken from all three experimental rat groups. Specimens were fixed in 10% formalin for 48 h, and washed in tap water for 3–4 h to avoid the deposition of fixative. Washed specimens were then sequentially dehydrated in 70%, 80%, and 90% alcohol, and cleared with two 20-min incubations in xylol. After embedding in paraffin wax, tissue was sectioned on a microtome, cut sections were floated out in a water bath, and collected onto glass slides and air-dried for 1 h. After deparaffinization in two xylol baths, sections were rehydrated in sequential baths of 96%, 90%, 80%, and 70% alcohol, followed by distilled water, and stained with hematoxylin for 2 min and eosin for 10 min. After removal of excess hematoxylin and eosin (H&E) stain by washing in tap water, sections were dehydrated in an alcohol series, followed by two 30-min xylol incubations, and mounted in Entellan® rapid embedding medium (EMD Millipore, USA). H&E-stained tissue sections of rat kidney were examined by light microscopy using an Olympus BH-2 microscope and photographed with an Olympus DP-70 digital camera (Olympus Corporation, USA).
Statistical analysis
Data were analyzed using SPSS 13.0 and Sigma Stat 3.1 software. One-way analysis of variance (ANOVA) was performed for normally distributed variables, and nonparametric posthoc Tukey’s honestly significant difference (HSD) and Fisher’s least significant difference (LSD) tests were applied for multiple comparisons. Paired t-tests were used in binary (before and after) comparisons of variables where the results fit a normal distribution. Non-normally distributed variables were analyzed using Kruskal-Wallis two-way ANOVA on ranks, and binary comparison variables were analyzed, A p-value of p<0.05 was accepted as significant.
Results
The mean weight of the rats was 252.134±14 g in the control group (Group 1), 258.3734±13 g in the cecal ligation puncture (CLP) group (Group 2), and 261.165±16 g in the CLP + carnosine group (Group 3). No significant differences in weight were found between the groups (p>0.05).
Preoperative body temperature values were similar in all three groups. Postoperative body temperature was significantly lower in Group 1, compared with Groups 2 and 3. Body temperature values were increased significantly in Groups 2 and 3 after CLP.
As shown in Table 1, the mean baseline systolic arterial blood pressures were greater in Group 1 compared with Groups 2 and 3 (p<0.05). Mean systolic blood pressure values at 15, 30, and 60 min were lower in Group 2 compared with Groups 1 and 3 (p<0.05), but by 120 and 240 min, pressures were greater in Group 3 compared with Group 2 (p<0.05).
Table 1.
The mean arterial systolic blood pressure values of Groups 1, 2, and 3.
| Group 1 | Group 2 | Group 3 | KW | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Baseline | 128.250 | 10.444 | 103.000 | 12.840 | 110.625 | 12.772 | 11.075 | 0.004 |
| 15th min | 119.250 | 15.267 | 98.750 | 6.135 | 112.500 | 12.906 | 10.096 | 0.006 |
| 30th min | 117.000 | 15.639 | 98.000 | 7.502 | 106.000 | 14.223 | 6.622 | 0.036 |
| 60th min | 115.750 | 10.223 | 95.500 | 4.751 | 105.500 | 8.468 | 13.326 | 0.001 |
| 120th min | 117.000 | 9.008 | 98.250 | 8.972 | 105.750 | 8.714 | 10.377 | 0.006 |
| 240th min | 114.250 | 7.285 | 100.000 | 5.237 | 107.000 | 7.559 | 10.912 | 0.004 |
G1 – control; G2 – sepsis; G3 – sepsis+carnosine.
The mean baseline diastolic arterial blood pressure values at 15, 30, 60, 120, and 240 min were greater in Groups 1 and 3 compared with Group 2 (p<0.05), as shown in Table 2. The mean arterial blood pressure (MAP) values at 15, 30, 60, 120, and 240 min were significantly lower in Group 2 compared with Group 1 and Group 3 (p<0.05), as shown in Table 3.
Table 2.
The mean diastolic blood pressure values of Groups 1, 2, and 3.
| Group 1 | Group 2 | Group 3 | KW | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Baseline | 83.500 | 8.536 | 53.625 | 4.470 | 61.750 | 8.172 | 17.725 | 0.000 |
| 15th min | 77.750 | 10.925 | 46.250 | 4.713 | 62.000 | 5.451 | 19.330 | 0.000 |
| 30th min | 86.500 | 5.732 | 45.250 | 4.528 | 63.250 | 7.005 | 20.271 | 0.000 |
| 60th min | 85.250 | 6.319 | 44.750 | 3.012 | 62.250 | 4.464 | 20.587 | 0.000 |
| 120th min | 84.750 | 5.339 | 44.750 | 5.339 | 63.500 | 5.632 | 20.498 | 0.000 |
| 240th min | 84.000 | 8.000 | 46.500 | 3.338 | 64.250 | 4.833 | 20.507 | 0.000 |
G1 – control; G2 – sepsis; G3 – sepsis+carnosine.
Table 3.
The MAP (mean arterial blood pressure) values of Groups 1, 2, and 3.
| Group 1 | Group 2 | Group 3 | KW | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Baseline | 96.200 | 10.223 | 69.300 | 10.470 | 71.750 | 12.642 | 11.050 | 0.000 |
| 15th min | 87.250 | 15.639 | 49.250 | 4.470 | 62.000 | 12.223 | 19.250 | 0.000 |
| 30th min | 96.200 | 5.736 | 47.250 | 3.012 | 52.250 | 8.468 | 13.648 | 0.000 |
| 60th min | 97.500 | 6.216 | 46.500 | 3.012 | 54.000 | 6.320 | 20.800 | 0.000 |
| 120th min | 96.250 | 5.285 | 45.500 | 5.339 | 68.500 | 5.732 | 20.500 | 0.000 |
| 240th min | 98.250 | 7.285 | 44.000 | 5.237 | 68.000 | 7.559 | 9.644 | 0.004 |
G1 – control; G2 – sepsis; G3 – sepsis+carnosine.
Baseline pulse rate was lower in Group 1 compared with Groups 2 and 3 (p<0.05). Pulse rates at 15, 30, 60, 120, and 240 min were greater in Group 2 compared with other groups (p<0.05). In Group 1, the pulse rate at 15 min was significantly greater than the baseline value (p=0.25); it then decreased significantly between 60 min and 120 min (p=0.011). In Group 2, the pulse rate at 15 min was significantly increased from the baseline (p=0.018). Other pulse rate changes in Group 2 were not significant. In Group 3, the pulse rate at 30 min was significantly increased compared with that at 15 min (p=0.017). Other pulse rate changes in this group were not significant (Table 4).
Table 4.
Pulse rate values of Groups 1, 2, and 3.
| Group 1 | Group 2 | Group 3 | KW | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Baseline | 228.250 | 26.283 | 300.000 | 24.773 | 257.250 | 28.544 | 14.033 | 0.001 |
| 15th min | 235.000 | 26.317 | 311.250 | 20.112 | 277.250 | 18.266 | 17.435 | 0.000 |
| 30th min | 251.000 | 33.226 | 311.500 | 22.110 | 283.750 | 15.135 | 12.941 | 0.002 |
| 60th min | 259.250 | 29.386 | 315.000 | 19.302 | 284.750 | 17.203 | 12.952 | 0.002 |
| 120th min | 263.250 | 28.484 | 303.250 | 20.838 | 282.250 | 14.280 | 8.517 | 0.014 |
| 240th min | 260.500 | 29.871 | 297.750 | 20.098 | 279.250 | 18.350 | 6.967 | 0.031 |
G1 – control; G2 – sepsis; G3 – sepsis+carnosine.
Preoperative blood oxygen (PaO2) values were similar in all groups and did not significantly change in Group 1 postoperatively (p=0.120). PaO2 was significantly decreased in Groups 2 and 3 following CLP (p=0.011 and p=0.021, respectively). Postoperative PaO2 values were significantly greater in Group 1 compared with Group 2 (p< 0.05), and differed significantly between Groups 2 and 3 (p=0.021).
Laboratory results
In Group 1, hemoglobin levels did not change significantly before or after surgery (p=0.091). There was a statistically significant decrease in postoperative hemoglobin level in Groups 2 and Group 3 (p=0.012 and p=0.025, respectively) compared with preoperative levels.
Postoperative hematocrit levels did not change significantly in Group 1 (p=0.069) or in Group 3 (p=0.093), but were significantly decreased in Group 2 (p=0.017). Preoperative WBC counts were similar in all groups. Postoperative WBC levels were significantly higher in Group 2 compared with Group 1 (p<0.05), and considerably lower in Group 3 compared with Groups 1 and 2 (p<0.05). WBC levels increased substantially in all groups after surgery for all three groups (p=0.012).
Pre-operative and postoperative blood urea nitrogen (BUN) levels did not differ significantly in Group 1 (p=0.062). There was a significant increase in preoperative BUN levels in Groups 2 and 3 (p=0.12 for both). Postoperative BUN levels were significantly greater in Group 2, relative to the other groups (Table 5).
Table 5.
Laboratory results of Groups 1, 2, and 3.
| Grup 1 | Grup 2 | Grup 3 | KW | p | ||||
|---|---|---|---|---|---|---|---|---|
| Ort | Ss | Ort | Ss | Ort | Ss | |||
| WBC 1 (preop) | 7.102 | 842 | 7.228 | 1.281 | 7.370 | 1254 | 0.184 | 0.912 |
| WBC 2 (postop) | 11.470 | 2.008 | 16.656 | 1.767 | 14.957 | 1242 | 16.293 | 0.000 |
| BUN 1 (preop) | 22.000 | 2.777 | 24.625 | 2.973 | 26.000 | 2.673 | 6.203 | 0.045 |
| BUN 2 (postop) | 35.625 | 4.596 | 93.875 | 12.005 | 62.000 | 8.435 | 20.348 | 0.000 |
| Cr 1 (preop) | 0.513 | 0.155 | 0.550 | 0.131 | 0.625 | 0.183 | 1.762 | 0.414 |
| Cr 2 (postop) | 0.625 | 0.149 | 0.950 | 0.288 | 0.775 | 0.205 | 6.156 | 0.046 |
| CLcr | 0.888 | 0.247 | 0.190 | 0.130 | 0.575 | 0.128 | 18.582 | 0.000 |
| CK | 43.375 | 2.774 | 417.250 | 2.053 | 223.875 | 2.748 | 20.401 | 0.000 |
| MDAS | 2.390 | 0.551 | 7.533 | 1.380 | 3.723 | 0.563 | 19.314 | 0.000 |
| MDAT | 11.300 | 1.225 | 19.975 | 1.676 | 12.494 | 1.430 | 16.758 | 0.000 |
p<0.05,
p<0.001 (Kruskal-Wallis test for multiple comparisons),
Mean ±SD (ANOVA used for multiple comparisons).
G1 – Control; G2 – sepsis; G3 – sepsis+carnosine. WBC – white blood cell; BUN – blood urea nitrogen; Cr – creatinine; CL cr – creatinine clearance; CK – creatine kinase; MDAS – malondialdehyde serum; MDAT – malondialdehyde tissue.
Preoperative blood creatinine levels were similar in all groups. In Groups 1 and 3, creatinine levels did not decrease significantly after surgery, while in Group 2 creatinine was significantly increased after surgery (p=0.011); postoperative creatinine levels were lower in Groups 1 and 3 compared with Group 2 (p<0.05) (Figure 2). Urine output volume was significantly greater in Group 1 compared with Groups 2 and 3, and significantly lower in Group 2 compared with Group 3 (Figure 3).
Figure 2.
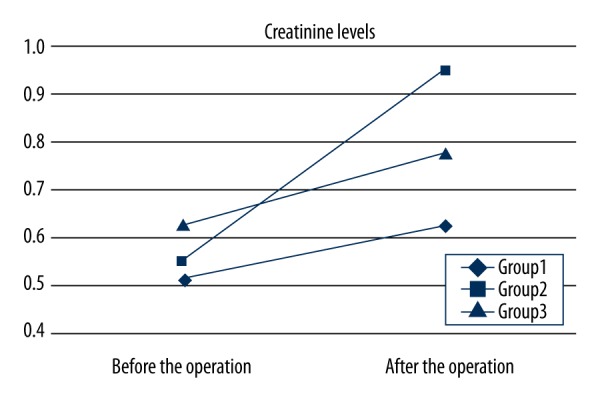
Creatinine levels in Groups 1, 2 and 3. There were significant differences in postoperative creatinine between the groups. Creatinine levels were lowest in Group 1, highest in Group 2 and 3 (p<0.05 for both).
Figure 3.
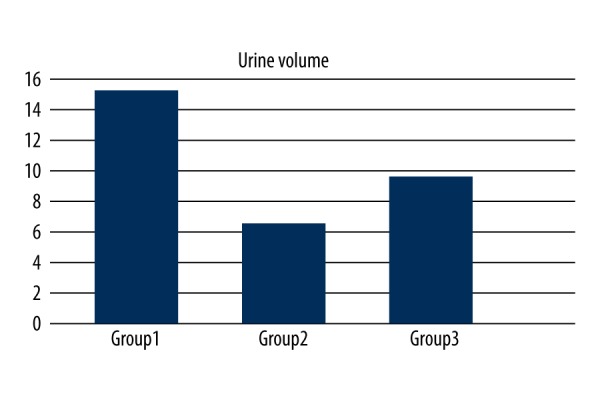
Diagram of the urine output volume of Groups 1,2, and 3. Urine output volume was significantly greater in Group 1 than in Groups 2 and 3, and significantly lower in Group 2 than in Group 3.
There were significant differences in postoperative creatinine clearance (CLcr) and CK, between the groups. Creatinine clearance levels were lowest, and creatine kinase (CK) levels were greatest in Group 2 (p<0.05 for both). Malondialdehyde in serum (MDAS) and malondialdehyde in tissue (MDAT) levels were significantly greater in Group 2 compared with Groups 1 and 3 (p<0.05 for both) (Table 5).
Histology results
Light microscopic examination of sections of rat kidneys from the control group (Group 1) showed that the renal cortex, Malpighian bodies, renal glomeruli, and Bowman’s capsule in the cortex, renal tubules, and renal medulla, all appeared histologically normal (Figure 4 and Table 6). Light microscopic examination of sections of rat kidneys from Group 2 (cecal ligation with septic shock) showed degeneration of the renal cortex and medullary tubules, as well as Malpighian bodies, and renal glomeruli, and dilatation of cortical tubules and interstitial hemorrhages were noted. Additionally, necrotic areas and tubular epithelial atrophy were seen (Figure 5 and Table 6).
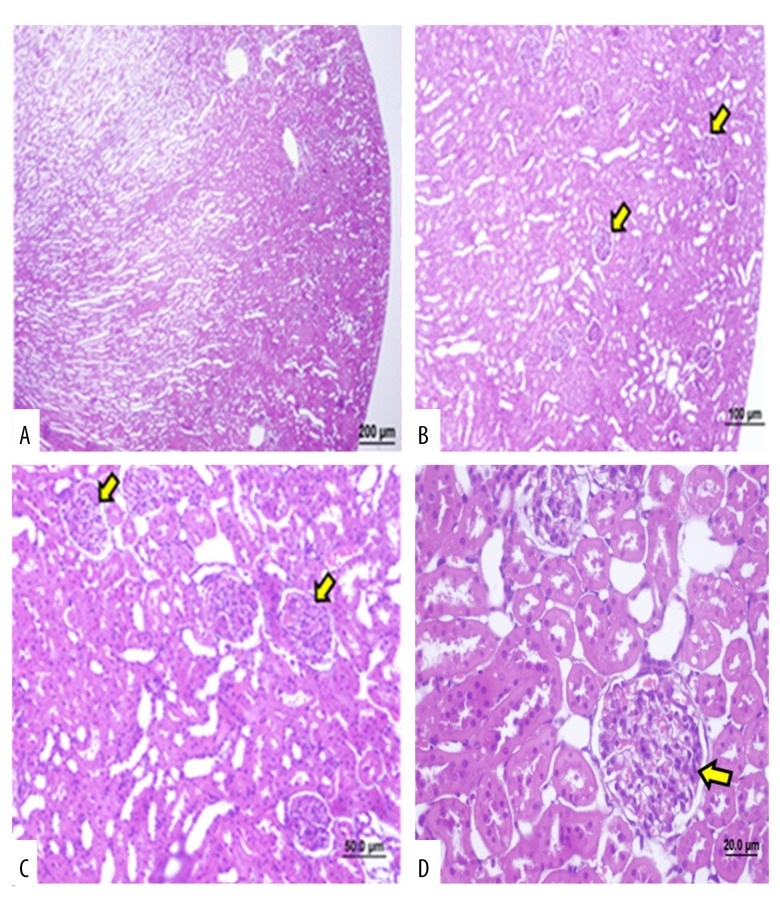
Figure 4.
Photomicrographs of the light microscopy of the kidney in Group 1 (control group). Light microscopy of the rat kidney from (Group 1). Hematoxylin and eosin (H&E). (A) Renal cortex and medullary structures. cortical tubules, and Malpighian body (arrow). (bar: 200 μm). (B–D) Rat kidney tissue sections with a normal appearance at different magnifications on light microscopic examination (bar: 100 μm, bar: 50.0 μm, bar: 20.0 μm).
Table 6.
Histology of the renal tissue of rats in of Groups 1, 2, and 3.
| Groups | Median (25th–75th) | Multiple comparison results | |||
|---|---|---|---|---|---|
| G1 | G2 | G3 | |||
| Glomerular injury | G1 | 0.00 (0.0–0.0) | * | ||
| G2 | 3.000 (1.5–3.0) | * | * | ||
| G3 | 1.500 (1.0–2.0) | * | * | ||
| H=13.506 DF=2 P=0.003 (P<0.05) | |||||
| Tubular atrophy | G1 | 0.000 (0.0–0.0) | ** | * | |
| G2 | 2.500 (1.,5–3.0) | ** | ** | ||
| G3 | 1.000 (1.0–2.0) | * | ** | ||
| H=14.82 DF=2 P<0.001 | |||||
| Necrosis | G1 | 0.000 (0.0–0.0) | ** | * | |
| G2 | 3.000 (3.0–3.0) | ** | ** | ||
| G3 | 1.500 (1,0–2,0) | * | ** | ||
| H=13,506 DF=2 P<0.001 | |||||
| Inflammation | G1 | 0.000 (0.0–0.0). | ** | * | |
| G2 | 3.000 (3.0–3.0) | ** | ** | ||
| G3 | 1.000 (0.5–1.0) | * | ** | ||
| H=13,82 DF=2 P<0.001 | |||||
| Hemorrage | G1 | 0.000 (0.0–0.0). | ** | * | |
| G2 | 3.000 (3.0–3.0) | ** | ** | ||
| G3 | 2.000 (2.0–2.0) | * | ** | ||
| H=14.82 DF=2 P<0.001 | |||||
p<0.05;
p<0.001 Kruskal-Wallis H.
G1 – Control; G2 – sepsis; G3 – sepsis+carnosine. Histological Findings: Absent (0 degree), mild (1 degree), moderate (2 degree) or severe (3 degree).
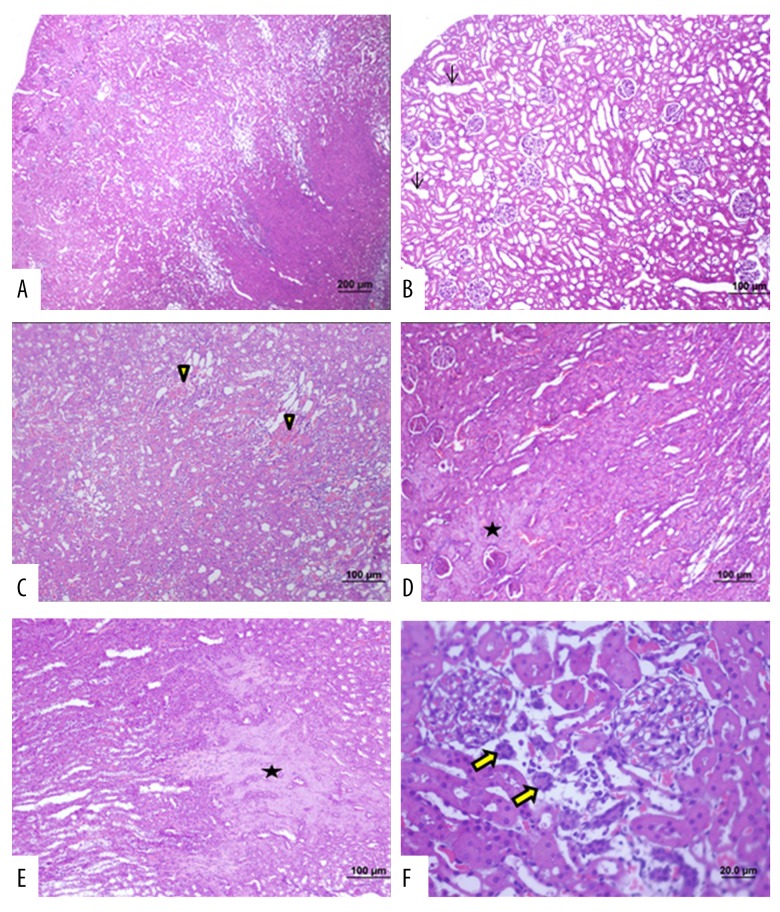
Figure 5.
Photomicrographs of the light microscopy of the kidney in Group 2 (septic shock group). Light microscopy of the rat kidney from (Group 2). Hematoxylin and eosin (H&E). (A) Degeneration of the tubules in the renal cortex and medulla as well as the renal glomeruli. (B) Cortical tubular dilatations are seen (arrow). (C) Interstitial hemorrhage is seen (arrowhead). (D, E) Necrotic areas are seen (*). (F) Tubular epithelial atrophy is seen (bold arrow) (bar: 200 μm; bar: 100μm; bar: 50.0 μm; bar: 20.0 μm).
Light microscopic examination of sections of rat kidneys from Group 3 (septic shock + carnosine treatment), hemorrhage and tubular damage were reduced, and renal cortex and medullary structures appeared to be normal when with the renal histology found in the kidney sections from rats in Group 1 and Group 2 (Figure 6 and Table 6).
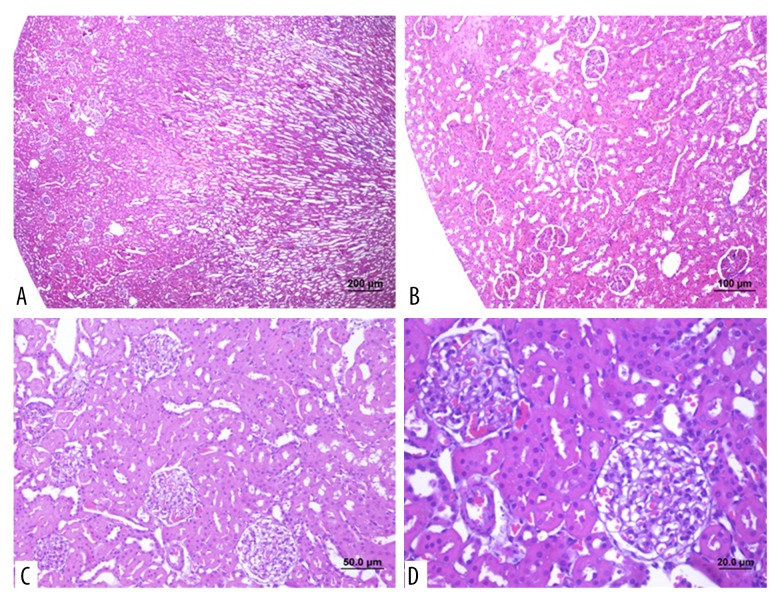
Figure 6.
Photomicrographs of the light microscopy of the kidney in Group 3 (treatment group). (A) Light microscopy of the rat kidney shows decreased hemorrhage and decreased tubular damage in the renal cortex, medullary structures, tubules and Malpighian bodies. Hematoxylin and eosin (H&E) (magnification ×4) (bar: 200 μm). (B–D) Light microscopy of the rat kidney cortical tubules and Malpighian bodies show a near-normal appearance. (H&E). (bar: 100 μm; bar: 50.0 μm; bar: 20.0 μm).
Discussion
This study used a rat model of sepsis and septicemia using cecal ligation and puncture (CLP) with acute kidney injury (AKI). The findings showed statistically significant improvements in kidney function, tissue and serum malondialdehyde (MDA) levels, routine blood values, biochemical indices, and in histopathological findings in rats in Group 3 who were treated with carnosine (beta-alanyl-L-histidine), compared with Group 2 exposed to septic shock without carnosine treatment.
Despite advances in modern medicine, sepsis and septic shock resulting in acute kidney injury (AKI) are still common clinical problems in the intensive care unit (ICU), associated with a high mortality rate, and requiring urgent treatment [7]. Antioxidants have been investigated for the treatment of septic shock treatment, although no experimental study has examined the effect of carnosine on sepsis-related AKI. In this study, we sought to investigate the histological effects of carnosine and to determine its effects on kidney damage occurring due to septic shock. We used the rat CLP model of sepsis, an easy to use method that offers the means to determine shock status, visualize the presence of any microorganisms, which reflects the changes seen in septicemia and septic shock [8–10]. The injury caused by sepsis is a result of direct action of the pathogenic microorganisms from the bowel, ischemia-reperfusion injury, and inflammation [8–10].
Carnosine (beta-alanyl-L-histidine) was isolated at the beginning of the 20th century, as a component of compounds extracted from muscle tissue [11]. This natural dipeptide exhibits antioxidative properties directed at suppression of free-radical reactions [12,13]. A previously published study that examined the antioxidative action of carnosine showed that these effects were mediated by the binding of lipid oxidation products in the course of free-radical reactions and through interaction with active oxygen species (ROS) [12,13]. Carnosine may also serve as a scavenger of peroxyl and hydroxyl radicals, singlet oxygen, and superoxide anion, and can neutralize hypochlorite anion by forming stable chloramine complexes [14]. The antioxidative properties of carnosine have led to its successful application in the healing of superficial burns of the epidermis and other wounds, in the treatment of cataracts, diabetes, neuropathy, renal dysfunction, Down syndrome, seizures, autistic spectrum disorders, ethanol intoxication, cardiomyopathy, and various inflammatory processes developing in a background of cellular membrane damage. The antioxidant, free radical and metal ion scavenging activities of carnosine cannot adequately explain these reported clinical effects [5,6,12–14]. Previous studies have shown that carnosine reacts aldehydes and ketones to protect macromolecules from cross-linking action [5,6,12–14]. Sepsis leads to the production of free oxygen radicals, and ischemic reperfusion-induced injury; carnosine is an efficient antioxidant [15].
The antioxidative activity of carnosine and 16 related compounds, both synthetic and natural, has been determined [15–18]. Antioxidative effects were estimated by the ability of the dipeptides to prevent MDA accumulation over the course of lipid peroxidation (LPO) induced in rabbit sarcoplasmic reticulum membranes by the Fe2+ ascorbate system [18]. It was found that an antioxidative effect comparable to that of carnosine was exerted by water- soluble (cyclo-L-histidyl-L-proline) and alcohol-soluble (cyclo-L-histidyl-L-phenylalanine) dipeptides, as well as by the histidine-free cyclodipeptides (cyclo-L-tyrosyl-L-proline) [18]. However, in contrast to its synthetic analogues, carnosine not only inhibited LPO but also diminished the level of products accumulating during membrane LPO [15–18].
In a previous study that used a rat sepsis model, there was a statistically significant improvement in renal function, as assessed by increased urine output, renal blood flow, and decreased serum creatinine, in an arginine vasopressin (AVP) treatment group, when compared with a norepinephrine-treated group (p<0.05) [18]. Similarly, in our study, urine output volume and creatinine clearance rate decreased significantly, and blood urea nitrogen (BUN) and creatinine (Cr) levels increased significantly in rats exposed to sepsis. However, urine output volume, BUN, and Cr levels were significantly lower in the carnosine treatment group in our study.
In another previously reported study, Vassal et al. obtained hemodynamic improvement with amino acid infusion in an experimental porcine model of septic shock [17]. Simon et al. showed decreased mean arterial blood pressure in rats exposed to septic shock [18]. In our study, mean arterial blood pressure also decreased significantly after surgery in Group 2, compared with Groups 1 and 3. In a lipopolysaccharide-induced mouse model of acute kidney injury, Chen et al. noted decreased PaO2 values generated by septic shock [19]. These findings were supported by those in the present study, which showed that postoperative PaO2 values were significantly lower in Group 2 compared with Groups 1 and 3.
In a rat model of ischemic brain injury, Stvolinsky et al. treated rats with carnosine, and mortality was reduced from 55% to 17% after the ischemic attack; most of the monitored parameters remained at the pre-ischemic level [20]. In a previously published study by Kurata et al., a protective role for carnosine in ischemia-reperfusion oxidative organ damage was studied in rats, and their MDA results demonstrated that carnosine could be useful as a prophylactic treatment to protect against renal hypoxia-reoxygenation damage [21]. In another previous study, Sahin et al. investigated a protective role for carnosine against septic shock related oxidative damage on the liver [22]. Sabin et al. Also reported that the antioxidative properties of carnosine have led to its successful application in the healing of superficial wounds, including burns, in the treatment of cataracts, neuropathy, renal dysfunction, cataracts, seizures, ethanol intoxication, cardiomyopathy, and various inflammatory processes that develop in a background of cellular membrane damage [22].
In a previously published study, Kyoto et al. showed that l-carnosine can suppress increased renal sympathetic nerve activity during renal ischemia by its action on the central nervous system and that this suppressive effect is probably responsible for the protection against ischemia-reperfusion-induced renal injury [23]. Also, the protective effect of l-carnosine on may be induced by conversion to l-histidine mediated through the activation of histamine H3 receptors in the central nervous system [24]. Aydin et al. found that carnosine was beneficial in decreasing age-related oxidative stress, lipid peroxide (LPO), and in improving the antioxidant status of renal, heart, and brain tissues in young and aged male rats [24].
Sepsis causes organ damage and loss of function, producing oxidative stress in which LPO is a known mechanism of cellular damage [25]. Hence, LPO is used as a marker of oxidative stress in cells and tissues [25]. In our study, both serum and tissue MDA levels were significantly lower in Group 3 (the sepsis + carnosine group), compared with Group 2 (the sepsis group). As in previous studies, the findings from the present study suggested that carnosine may have an antioxidant effect in sepsis. MDA levels in plasma and tissue were significantly increased in septic rats (Group 2). Carnosine caused a significant decrease in MDA levels.
The renal histological findings from our study are supported by those of Fujii et al., who showed that the histopathological examination of rat kidneys with AKI showed renal damage, including tubular necrosis, proteinaceous casts in tubules, and medullary congestion; these changes were reduced by dietary supplementation with L-carnosine [26]. In a previous study by Soliman et al. carnosine treatment resulted in histological improvement of renal impairment generated by gentamycin (GM) [27]. The authors concluded that carnosine’s protective effect against gentamycin-induced nephrotoxicity could be attributed to its many actions: double antioxidant action, molecular protection of proteins, removal of harmfully modified proteins, activation of the immune system, preservation of membrane fluidity, and cytosolic buffering [27].
Conclusions
In a rat model of sepsis and septicemia using cecal ligation and puncture (CLP) with acute kidney injury (AKI), carnosine (beta-alanyl-L-histidine) has been shown to have beneficial effects in reducing AKI due to septic shock.
Acknowledgments
Study animals were obtained from Eskisehir Osmangazi University Faculty of Medicine, Medical and Surgical Investigation Laboratory (TICAM).
Abbreviations
- AKI
acute kidney injury
- BUN
blood urea nitrogen
- CLP
cecal ligation puncture
- Cr
creatinine
- CrCL
creatinine clearance
- CK
creatine kinase
- MAP
mean arterial blood pressure
- MDA
malondialdehyde
- MDAS
malondialdehyde serum
- MDAT
malondialdehyde tissue
- WBC
white blood count
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dai X, Zeng Z, Fu C, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19:223. doi: 10.1186/s13054-015-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X-R, Xu J, Wang Y-M, et al. The effects of paeoniflorin injection on soluble triggering receptor expressed on myeloid-1 (sTREM-1) levels in severe septic rats. Korean J Physiol Pharmacol. 2016;20:565–71. doi: 10.4196/kjpp.2016.20.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guliaeva NV, Dupin AM, Levshina IP, et al. Carnosine prevents the activation of free-radical lipid oxidation during stress. Biull Eksp Biol Med. 1989;107:144–47. [PubMed] [Google Scholar]
- 5.Boldyrev AA, Stvolinsky SL, Fedorova TN, Suslina ZA. Carnosine as a natural antioxidant and geroprotector: From molecular mechanisms to clinical trials. Rejuvenation Res. 2010;13:156–58. doi: 10.1089/rej.2009.0923. [DOI] [PubMed] [Google Scholar]
- 6.Kiliś-Pstrusińska K. Carnosine, carnosinase and kidney diseases. Postepy Hig Med Dosw (Online) 2012;66:215–21. doi: 10.5604/17322693.991600. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho MAD, Freitas FGR, Silva HT, Junior, et al. Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One. 2014;9(11):e111610. doi: 10.1371/journal.pone.0111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alici O, Kavakli HS, Koca C, Altintas ND. Treatment of Nigella sativa in experimental sepsis model in rats. Pak J Pharm Sci. 2011;24:227–31. [PubMed] [Google Scholar]
- 9.Sener G, Toklu H, Ercan F, Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int Immunopharmacol. 2005;5:1387–96. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Portolés MT, Ainaga MJ, Pagani R. The induction of lipid peroxidation by E. coli lipopolysaccharide on rat hepatocytes as an important factor in the etiology of endotoxic renal damage. Biochim Biophys Acta. 1993;1158:287–92. doi: 10.1016/0304-4165(93)90027-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim MY, Kim EJ, Kim YN, et al. Effects of α-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr Res Pract. 2011;5:421–28. doi: 10.4162/nrp.2011.5.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldyrev AA, Stvolinsky SL, Fedorova TN, Suslina ZA. Carnosine as a natural antioxidant and geroprotector: From molecular mechanisms to clinical trials. Rejuvenation Res. 2010;13:156–58. doi: 10.1089/rej.2009.0923. [DOI] [PubMed] [Google Scholar]
- 13.Nikolic J, Stojanovic I, Pavlovic R, et al. The role of L-arginine in toxic renal failure: interrelation of arginase, polyamine catabolic enzymes and nitric oxide synthase. Amino Acids. 2007;32:127–31. doi: 10.1007/s00726-006-0309-y. [DOI] [PubMed] [Google Scholar]
- 14.Severina IS, Bussygina OG, Pyatakova NV. Carnosine as a regulator of soluble guanylate cyclase. Biochemistry. 2000;65:783–88. [PubMed] [Google Scholar]
- 15.Zaloga GP, Roberts PR, Black KW, et al. Carnosine is a novel peptide modulator of intracellular calcium and contractility in cardiac cells. Am J Physiol. 1997;272:462–68. doi: 10.1152/ajpheart.1997.272.1.H462. [DOI] [PubMed] [Google Scholar]
- 16.Ji MH, Yang JJ, Wu J, et al. Experimental sepsis in pigs-effects of vasopressin on renal, hepatic, and intestinal dysfunction. Ups J Med Sci. 2012;117:257–63. doi: 10.3109/03009734.2011.650796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassal O, Bonnet JM, Barthelemy A, et al. Renal haemodynamic response to amino acids infusion in an experimental porcine model of septic shock. Cta Anaesthesiol Scand. 2015;59:598–608. doi: 10.1111/aas.12507. [DOI] [PubMed] [Google Scholar]
- 18.Simon F, Giudici R, Scheuerle A, et al. Comparison of cardiac, hepatic, and renal effects of arginine vasopressin and noradrenaline during porcine fecal peritonitis: A randomized controlled trial. Crit Care. 2009;13:113. doi: 10.1186/cc7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Du Y, Li Y, et al. Panaxadiol saponin and dexamethasone improve renal function in lipopolysaccharide-induced mouse model of acute kidney injury. PLoS One. 2015;10:e0134653. doi: 10.1371/journal.pone.0134653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stvolinsky SL, Kukley ML, Dobrota D, et al. Carnosine: An endogenous neuroprotector in the ischemic brain. Cell Mol Neurobiol. 1999;19:45–56. doi: 10.1023/A:1006960407008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurata H, Fujii T, Tsutsui H, et al. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther. 2006;319:640–47. doi: 10.1124/jpet.106.110122. [DOI] [PubMed] [Google Scholar]
- 22.Sahin S, Oter S, Burukoğlu D, Sutken E. The effects of carnosine in an experimental rat model of septic shock. Med Sci Monit Basic Res. 2013;19:54–61. doi: 10.12659/MSMBR.883758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyoko U, Fumio T, Tomoko H, et al. Preventıve effect of trpv1 agonists capsaicin and resiniferatoxin on ischemia/reperfusion-induced renal injury in rats. J Cardiovasc Pharmacol. 2008;51:513–20. doi: 10.1097/FJC.0b013e31816f6884. [DOI] [PubMed] [Google Scholar]
- 24.Aydin AF, Küçükgergin C, Ozdemirler Erata G, et al. The effect of carnosine treatment on prooxidant-antioxidant balance in renal, heart and brain tissues of male aged rats. Biogerontology. 2010;11:103–9. doi: 10.1007/s10522-009-9232-4. [DOI] [PubMed] [Google Scholar]
- 25.Otero Antón E, González Quintela A, López Soto A, et al. Cecal ligation and puncture as amodel of sepsis in the rat: influence of the puncture size onmortality, bacteremia, endotoxemia and tumor necrosis factoralpha levels. Eur Surg Res. 2001;33:77–79. doi: 10.1159/000049698. [DOI] [PubMed] [Google Scholar]
- 26.Fujii T, Takaoka M, Tsuruoka N, et al. Dietary supplementation of L-carnosine prevents ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol. 2003;474:261–67. [Google Scholar]
- 27.Soliman KM, Abdul Hamid M, Othman AI. Effect of carnosine on gentamicin-induced nephrotoxicity. Med Sci Monit. 2007;13(3):73–83. [PubMed] [Google Scholar]