Abstract
Background
Melanoma is an aggressive cancer with complex etiology and poor prognosis. Surgical resection is still the primary treatment of melanoma, but shows limited efficacy in late-stage patients. Additionally, reliable prognostic markers of skin melanoma patients are still lacking. Circulating tumor cells (CTCs) have shown promise in predicting prognosis of multiple cancers.
Evaluating the prognostic value of CTC number in melanoma patients.
Material/Methods
CTCs were isolated by immunomagnetic capture from 7.5-mL samples of blood from 100 patients with cutaneous melanoma. Baseline CTC number (pre-treatment) and post-treatment CTC number were measured. Baseline CTC number and CTC number alteration were correlated with clinicopathological features and survival.
Results
Forty-three (43%) patients had more than 6 CTCs, whereas 57 (57%) had 6 cells or less. High baseline CTC count was associated with deep local invasion, lymph node metastasis, and distance metastasis, with P value of 0.003, 0.047, and 0.034, respectively. High baseline CTC count was also correlated with short overall survival time and was considered as an independent prognostic factor (P value=0.012, hazard ratio=2.262). CTC cell alteration was associated with progression-free survival and disease-specific survival (with P values of 0.012 and 0.009, respectively).
Conclusions
Baseline CTC count was correlated with adverse pathological features and was predictive of survival in melanoma patients. Alteration of CTC count before and after treatment was an indicator of therapy response and prognosis. Measuring the baseline and post-treatment CTC counts is a powerful tool in monitoring melanoma progression, drug response, and survival.
MeSH Keywords: Melanoma; Neoplastic Cells, Circulating; Prognosis; Survival
Background
Melanoma, especially the metastatic disease, is an aggressive form of cancer with complex etiology and poor prognosis [1]. In the USA, more than 76 380 patients were diagnosed with skin melanoma in 2016 alone [2]. In China, the estimated incidence of melanoma was 8000 in 2015 [3]. Cure of early-stage skin melanoma primarily relies on surgical resection [4]. However, for late-stage cases, comprehensive treatments are required [4]. Until 2010, the treatments for metastatic melanoma were very limited. In recent years, novel medications targeting the BRAF mutation and immunotherapies have shown promise in treating metastatic melanoma [5,6]. However, resistance to these novel treatment options occurs in most patients [7,8]. Reliable prognostic markers of skin melanoma patients have not yet been identified. The major cause of cancer-associated mortality is tumor metastasis [9]. During successful dissemination, tumor cells have to invade the surrounding tissue, intravasating into the blood and lymphatic vessels [9]. Thus, quantifying the number of tumor cells in peripheral blood reflects the aggressiveness of cancers. Recently, analysis of circulating tumor cells (CTCs) has shown promise in predicting prognosis of prostate cancer, breast cancer, and other solid tumors [10]. A high number of CTC was correlated with shorter overall survival (OS) and progression-free survival [10–12]. Although some previous studies have explored the prognostic value of CTC in melanoma, the conclusions remain controversial [13,14].
In the present study, we searched for prognostic markers for skin melanoma patients. As tumor development and patient survival are regulated by various factors, we included multiple parameters in our analysis, including classic pathological factors, clinically-used serum makers, and the number of CTCs. By systematically analyzing our patient cohort, we show the significance of counting CTCs in predicting skin melanoma patient outcome.
Material and Methods
Patients
We enrolled a total of 100 melanoma patients diagnosed at the People’s Hospital of Wei Fang from January 2010 to December 2014. All patients were diagnosed as melanoma based on biopsy and pathology. The primary tumors of these patients were in the skin. All patients signed informed consent and agreed to provide blood samples for evaluating the circulating tumor cells before and after they accepted surgery or systematic therapies. The first blood draw was performed right before the treatment and the second blood draw was performed 3 months after the surgery or 24 weeks after the start of non-surgery treatments (for the patients who did not accept surgery). Basic features of the patient cohort are summarized in Table 1. The TNM classification of these patients was evaluated based on the criteria of the AJCC Cancer Staging Manual, 7th Edition.
Table 1.
Correlation between CTC levels and clinicopathological features of the melanoma patients.
| Clinicopathological features | Low CTC patients # | High CTC patients # | P value | |
|---|---|---|---|---|
| Age | <45 (%) | 25 (43.9) | 25 (58.1) | 0.157 |
| ≥45 (%) | 32 (56.1) | 18 (41.9) | ||
| Gender | Female (%) | 23 (40.4) | 15 (34.9) | 0.577 |
| Male (%) | 34 (59.6) | 28 (65.1) | ||
| Histology type | ALM (%) | 42 (73.7) | 30 (63.8) | 0.666 |
| Other (%) | 15 (26.3) | 13 (30.2) | ||
| S100B (μg/L) | <0.19 (%) | 30 (52.6) | 20 (46.5) | 0.545 |
| ≥0.19 (%) | 27 (47.3) | 23 (53.5) | ||
| Breslow thickness (mm) | <2.0 (%) | 42 (73.7) | 19 (44.2) | 0.003 |
| ≥2.0 (%) | 15 (26.3) | 24 (55.8) | ||
| Ulceration | No (%) | 42 (73.7) | 21 (48.8) | 0.011 |
| Yes (%) | 15 (26.3) | 22 (51.2) | ||
| N stage | N0/Nx (%) | 36 (63.2) | 35 (81.4) | 0.047 |
| N1+ (%) | 21 (36.8) | 8 (18.6) | ||
| M stage | No (%) | 36 (63.2) | 18 (41.8) | 0.034 |
| Yes (%) | 21 (36.8) | 25 (58.1) | ||
| TNM stage | I–II (%) | 35 (61.4) | 16 (37.2) | 0.017 |
| III–IV (%) | 22 (38.6) | 27 (62.8) | ||
| Outcome | Survived (%) | 25 (43.8) | 6 (14.0) | 0.001 |
| Died (%) | 32 (56.1) | 37 (86.0) | ||
| IFN alpha treatment | Yes (%) | 40 (70.2) | 35 (81.4) | 0.20 |
| No (%) | 17 (29.8) | 8 (18.6) | ||
ALM – acral lentiginous meloanoma.
Follow-up of these patients was performed through phone calls, home visits, or medical records. The disease-specific survival (DSS) time was defined as the interval between the date of accepting surgery or systematic treatment and the date on which the patient died of melanoma. The patients who died due to other causes were excluded from survival analysis. The average follow-up period was 27.9 months, with a median of 26 months.
CTCs counting
The CellSearch Epithelial Cell kit and CellSearch System (Veridex LLC) were used to evaluate the number of CTCs in patient blood samples, following the method described previously [15,16]. Briefly, 7.5-ml blood samples were collected from the antecubital (forearm) veins within 24 h before the scheduled surgery date or systematic treatment. Then, the blood samples were added to a CellSave tube to gently mix with 6.5 ml of dilution buffer to centrifuge for 10 min at room temperature and then were loaded to the AutoPrep system. Subsequently, the anti-Mel-CAM antibody coated with ferrofluids was added. Through the immunomagnetic isolation, the CTC cells were enriched and then stained with phycoerythrin (PE)-conjugated antibody specific to high-molecular weight melanoma-associated antigen (HMW-MAA). Allophycocyanin (APC)-conjugated anti-CD45 antibody was used to exclude the leukocytes. The cells were then transferred to Cell Track Analyzer II. The nucleated cells stained with 4’,6-diamidino-2-phenylindole, expression HMW-MAA without CD45 expression were recognized as CTCs.
Serum S100B level
Blood samples were maintained at room temperature for 30 min to clot, followed by centrifugation for 20 min to collect the serum. Then, the serum S100B level was measured using the Roche Elecsys® S100 reagent kit following the standard procedure recommended by the manufacturer.
Statistical analysis
All statistical analyses were performed using SPSS software 17.0. Receiver operating characteristic (ROC) curve was used to find the optimal cutoff point of CTC counts. The optimal cutoff point was determined by choosing the CTC count with highest sum of sensitivity and specificity regarding to the outcomes of the patients. The chi-squared test was used to analyze the relationships between CTC levels and other clinicopathological characteristics of melanoma patients. Kaplan-Meier method was used to conduct survival analysis and the log-rank test was performed to compare the difference in survival curves. The patients who withdrew from this study, were lost from follow-up, or survived at the endpoint of the follow-up were defined as censored observations. The Cox proportional hazards model was used for univariate and multivariant analysis. Two-tail P values less than 0.05 were considered as statistically significant.
Results
Basic features of the patient cohort and cutoff point determination
A total number of 100 melanoma patients were included in this study: 51 were at I–II stage (51%) and 49 were at III–IV stage (49%) when they were diagnosed; 62% were males and 38% were females. The average age was 51.3 years. The 1-, 2-, and 3-year survival rates were 86.7%, 63.7%, and 45.3%, respectively. The average CTC number was 7.9 (per 7.5ml blood).
As shown in Figure 1, ROC curve analysis was performed to determine the optimal cutoff point of CTC numbers. N=6 (6 CTCs per 7.5 ml blood) was selected as the cutoff point with the area under the curve of 0.689 (95% confidence interval (CI) was 0.584 to 0.794). N<6 (less than 6 CTCs per 7.5 ml blood) was defined as low CTC. N≥6 (more than 6 CTCs per 7.5 ml blood including 6) was defined as high CTC. A total of 57 patients had low CTCs and 43 patients had high CTCs before they accepted any treatment.
Figure 1.
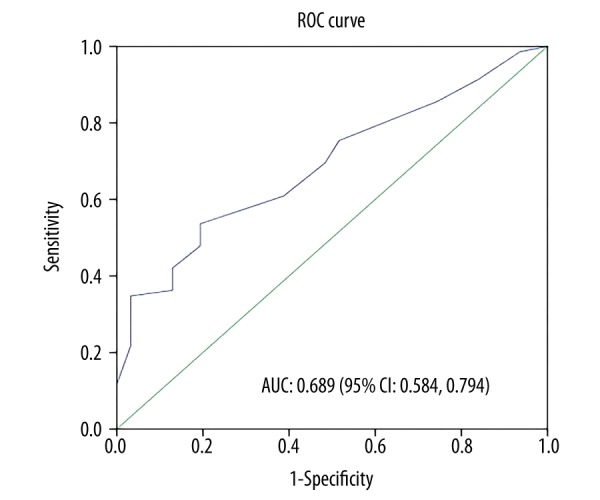
Determination of the cutoff point of CTC number by ROC curve analysis. ROC curve analysis was performed to determine the cutoff point of CTC numbers in terms of the accuracy of predicting the melanoma patient outcomes. The value with the highest sum of sensitivity and specificity was chosen as the cutoff point: CTC number >6 was determined as high CTC level and ≤6 was determined as low CTC level. The area under the curve (AUC) was 0.689 with 95% confidence interval (CI) from 0.584 to 0.794.
Relationships between baseline CTC count and the clinicopathological characteristics of melanoma patients
By analyzing the correlation between baseline CTC count and clinicopathological features of melanoma, we found that the baseline CTC number of melanoma patients was significantly associated with Breslow thickness, ulceration, regional lymph node metastasis, distant metastasis, and TNM stage, with the P values of 0.003, 0.011, 0.047, 0.034, and 0.017, respectively (Table 1). However, the number of CTCs did not show a significant association with histological type, sex, age, and S100B level. Importantly, the results indicated that CTC level was negatively associated with outcomes of these melanoma patients: higher proportions of patients who died due to melanoma during our follow-up had high CTC counts (53.6%, P value=0.001).
Baseline CTC count was associated with the DSS of melanoma patients
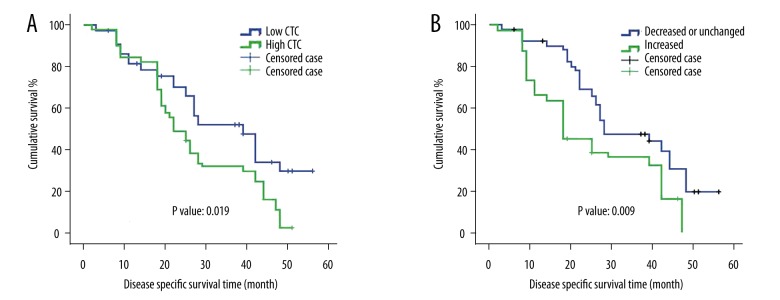
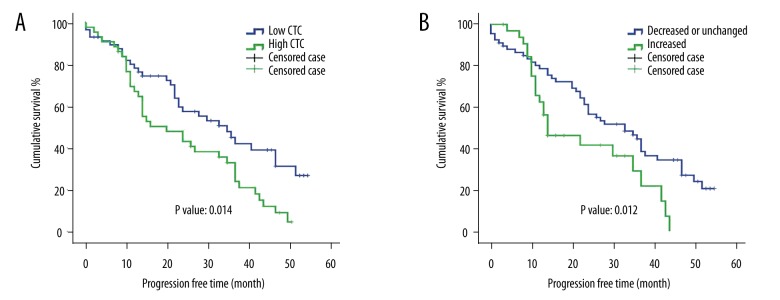
Survival analysis was performed on the melanoma patients with respect to the baseline CTC level (the CTC count before treatment) and other clinicopathological parameters. As shown in Figure 2A, the patients with high baseline CTC level had shorter cumulative survival than the patients with low baseline CTC (P value=0.019). Consistently, the patients with high baseline CTC level also had shorter progression-free survival than the patient with low baseline CTC (P value=0.014, Figure 3A). We then performed univariate and multivariant Cox proportional hazards analysis. As indicated in Tables 2 and 3, the baseline CTC count is associated with higher risk of death: hazard ratio (HR)=1.733 (1.078, 2.787) in the univariate analysis and 2.262 (1.199, 4.267) in the multivariate analysis. Other factors, such as S100B level and TNM stage, were also significant in univariant and multivariant analyses.
Figure 2.
Survival analysis of melanoma patients regarding CTC levels and CTC alteration. (A) The patients with low CTC level had higher cumulative DSS than the patients with high CTC level (P value: 0.019). (B) The patients whose CTC levels were increased after the treatment had lower cumulative DSS than the patients whose CTC levels were not changed or decreased (P value: 0.009). (The patients who withdrew from this study, were lost from the follow-up, or survived to the endpoint of the follow-up were defined as censored cases).
Figure 3.
Progression-free time of melanoma patients with different CTC alterations after treatment. The progression-free time of each melanoma patients was used to conduct survival analysis. (A) Patients with low CTC level had longer progression-free survival than patients with high CTC level (P value: 0.014). (B) Patients with increased CTC level after the treatment showed worse survival than patients with decreased CTC level or unchanged CTC level. P value was 0.012. (The patients who withdrew from this study, were lost from the follow-up, or survived to the endpoint of the follow-up were defined as censored cases).
Table 2.
Univariate analysis of prognosticators of DSS.
| Clinicopathological features | P value | HR (95% CI) |
|---|---|---|
| Age (≥45 vs. <45) | 0.139 | 0.697 (0.432,1.125) |
| Gender (Male vs. Female) | 0.757 | 1.080 (0.664,1.775) |
| Histology type (ALM vs. other) | 0.719 | 0.904 (0.520,1.571) |
| S100B (μg/L) (≥0.19 vs. <0.19) | 0.004 | 2.063 (1.267,3.356) |
| Breslow thickness (mm) (≥2.0 vs. <2.0) | 0.153 | 1.418 (0.879,2.290) |
| Ulceration (Yes vs. No) | 0.270 | 0.750 (0.451,1.249) |
| N stage (N1+ vs. Nx/N0) | 0.803 | 1.070 (0.631,1.813) |
| M stage (Yes vs. No) | 0.825 | 0.948 (0.587,1,530) |
| TNM stage (III–IV vs. I–II) | 0.026 | 1.731 (1.067,2.808) |
| Baseline CTC (high vs. low) | 0.023 | 1.733 (1.078,2.787) |
| CTC change (increased vs. decreased or unchanged) | 0.017 | 1.884 (1.122,3.164) |
Table 3.
Multivariate analysis of prognosticators of DSS.
| Clinicopathological features | P value | HR (95% CI) |
|---|---|---|
| Age (≥45 vs. <45) | 0.923 | 1.037 (0.499, 2.153) |
| Gender (Male vs. Female) | 0.646 | 0.860 (0.453, 1.633) |
| Histology type (ALM vs. other) | 0.405 | 0.682 (0.277,1.679) |
| S100B (μg/L) (≥0.19 vs. <0.19) | 0.043 | 1.778 (1.018,3.105) |
| Breslow thickness (mm) (≥2.0 vs. <2.0) | 0.370 | 1.312 (0.725,2.372) |
| Ulceration (Yes vs. No) | 0.266 | 0.680 (0.344,1.343) |
| N stage (N1+ vs. Nx/N0) | 0.752 | 1.112 (0.576,2.144) |
| M stage (Yes vs. No) | 0.607 | 0.844 (0.443,1.610) |
| TNM stage (III–IV vs. I–II) | 0.005 | 2.398 (1.296,4.436) |
| Baseline CTC (high vs. low) | 0.012 | 2.262 (1.199,4.267) |
| CTC change (increased vs. decreased or unchanged) | 0.009 | 2.913 (1.304,6.507) |
Prognostic value of CTC number alteration after treatments
For the stage I and stage II tumors and diseases without obvious metastasis at diagnosis, patients were treated by wide excision plus sentinel lymph node biopsy with/without interferon alpha (29 patients accepted interferon alpha treatment). For metastatic diseases, patients (n=46) were treated with interferon alpha and general supportive care. After the initial treatment (surgery or 24-week interferon alpha treatment), 67 (67%) patients showed decreased number of CTCs, while 33 (33%) patients showed increased number of CTCs compared to their CTC levels before surgery. The clinicopathological features of the patients with increased CTCs and decreased CTCs are summarized in Table 4. Based on the survival curve, patients with increased CTCs had worse survival than patients with decreased or unchanged CTCs (Figure 2B, log-rank test P value=0.009). Multivariate analysis showed that the increased CTC number was an adverse independent prognostic marker of melanoma (HR=2.913 with 95% CI of from 1.304 to 6.507, Table 3). We correlated the CTC alteration with disease progression survival and found that decreasing CTC numbers after treatment predicted longer progression-free time after treatments (P value=0.012, Figure 3B).
Table 4.
Correlation between CTC alterations and clinicopathological features of the melanoma patients.
| Clinicopathological features | Patient # of decreased or unchanged CTC | Patient # of increased CTC | P value | |
|---|---|---|---|---|
| Age | <45 (%) | 33 (49.3) | 17 (51.5) | 0.832 |
| ≥45 (%) | 34 (50.7) | 16 (32) | ||
| Gender | Female (%) | 24 (42.1) | 14 (32.6) | 0.330 |
| Male (%) | 33 (57.8) | 29 (67.4) | ||
| Histology type | ALM (%) | 47 (70.1) | 25 (75.8) | 0.557 |
| Other (%) | 20 (29.9) | 8 (24.2) | ||
| S100B (μg/L) | <0.19 (%) | 31 (46.3) | 19 (57.8) | 0.288 |
| ≥0.19 (%) | 36 (53.7) | 14 (42.4) | ||
| Breslow thickness (mm) | <2.0 (%) | 38 (56.7) | 23 (69.7) | 0.211 |
| ≥2.0 (%) | 29 (43.3) | 10 (30.3) | ||
| Ulceration | No (%) | 45 (67.2) | 18 (54.5) | 0.219 |
| Yes (%) | 22 (32.8) | 15 (45.5) | ||
| N stage | N0/Nx (%) | 45 (67.2) | 26 (78.8) | 0.228 |
| N1+ (%) | 22 (32.8) | 7 (21.3) | ||
| M stage | No (%) | 38 (56.7) | 16 (48.5) | 0.437 |
| Yes (%) | 29 (43.3) | 17 (51.5) | ||
| TNM stage | I–II (%) | 36 (53.7) | 17 (51.5) | 0.835 |
| III–IV (%) | 31 (46.3) | 16 (48.5) | ||
| Outcome | Survived (%) | 22 (32.8) | 9 (27.3) | 0.572 |
| Died (%) | 45 (67.2) | 24 (72.7) | ||
ALM – acral lentiginous meloanoma.
Discussion
Skin melanoma that originates in the pigment-producing melanocytes in the basal layer of the epidermis is the most dangerous skin cancer type. If melanoma is recognized and treated early, it is usually curable [4]. However, due to the aggressive biological nature of the disease and lack of appropriate monitoring, certain patients were diagnosed at late-stage with metastatic lesions. Treating advanced-stage melanoma relies on aggressive systematic treatments; however, little survival improvement was achieved until the launch of novel immunotherapies and BRAF targeted therapies [4–6]. CTC has shown unique values in diagnosing cancers, evaluating drug response, and predicting prognosis [17]. However, its prognostic value in melanoma patients has been controversial [13,14]. In the present study, we aimed to investigate the prognostic value of CTC number in melanoma patients.
To study the prognostic value of CTC count in melanoma patients, we enrolled both relative early stage cases and late-stage cases. CTC can be identified from both early stage and late-stage patients. Intravasation and circulating in the peripheral blood are critical steps in establishing metastatic tumors[18]. Hematogenous spreading is particularly importantly for malignant melanoma[19]. Therefore, we expect to see higher CTC count in relatively late-stage patients. As expected, CTC count was positively correlated with invasion depth, lymph node metastasis, distance metastasis, and tumor stage. This data is particularly interesting as it highlighted the potential of predicting metastasis by measuring CTC level.
Current prognostic systems (e.g., TNM staging system, primary tumor molecular features, and serum biomarkers) are not perfect for optimal therapeutic management of melanoma patients [20]. Many radically treated early-stage patients experience disease recurrence and progression [20]. Therefore, developing a novel prognostic system that can dynamically monitor disease progression and treatment response is important. In lung cancer, it was shown that the first cycle of chemotherapy reduced CTC count in certain sensitive cases [21]. In our study, we tested baseline CTC number and post-treatment CTC number. The baseline CTC count and CTC count alteration (post-treatment CTC count minus baseline CTC count) were identified as independent prognostic factors. Interestingly, some previous studies also indicated that presence of/high baseline CTC count was associated with poor survival of melanoma patients [13,22]. We also noticed that patients with decreased CTC numbers after treatment tended to have longer disease-free time. These data suggest the significance of monitoring CTC count to predict treatment response. Nevertheless, caution is recommended when utilizing this result, since there still exists a possibility that the observed prognostic significance was enhanced by the higher ratio of early-stage patients in the low CTC group. In our study, the 1-, 2-, and 3-year disease-specific survival of all patients was 86.7%, 63.7%, and 45.3%, respectively; however, according to previously reported epidemiological trends of melanoma, the 1- and 5-year survival rate was more than 90% and 80%, respectively [23,24]. The high ratio of advanced-stage patients (49%) in our study might have contributed in part to this difference [23,24].
Conclusions
We conclude that CTC counts are useful in predicting the survival of Chinese melanoma patients. More importantly, dynamic monitoring CTC counts also had significant value in predicting treatment response and survival of melanoma patients.
Acknowledgement
We thank the grant support from The People’s Hospital of Weifang.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Perlis C, Herlyn M. Recent advances in melanoma biology. Oncologist. 2004;9:182–87. doi: 10.1634/theoncologist.9-2-182. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Collichio F, Ollila D, Moschos S. Historical review of melanoma treatment and outcomes. Clin Dermatol. 2013;31:141–47. doi: 10.1016/j.clindermatol.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Sanlorenzo M, Vujic I, Posch C, et al. Melanoma immunotherapy. Cancer Biol Ther. 2014;15:665–74. doi: 10.4161/cbt.28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res. 2017;77(4):817–22. doi: 10.1158/0008-5472.CAN-16-2379. [DOI] [PubMed] [Google Scholar]
- 8.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–9. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 11.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging – predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 12.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Mocellin S, Hoon D, Ambrosi A, et al. The prognostic value of circulating tumor cells in patients with melanoma: A systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–13. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 14.Gogas H, Kefala G, Bafaloukos D, et al. Prognostic significance of the sequential detection of circulating melanoma cells by RT-PCR in high-risk melanoma patients receiving adjuvant interferon. Br J Cancer. 2002;87:181–86. doi: 10.1038/sj.bjc.6600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch System. Clin Cancer Res. 2007;13:920–28. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 16.Terai M, Mu Z, Eschelman DJ, et al. Arterial blood, rather than venous blood, is a better source for circulating melanoma cells. EBioMedicine. 2015;2:1821–26. doi: 10.1016/j.ebiom.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maheswaran S, Haber DA. Circulating tumor cells: A window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20:96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brossart P, Keilholz U, Willhauck M, et al. Hematogenous spread of malignant melanoma cells in different stages of disease. J Invest Dermatol. 1993;101:887–89. doi: 10.1111/1523-1747.ep12371713. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 21.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–63. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 22.Schuster R, Bechrakis NE, Stroux A, et al. Circulating tumor cells as prognostic factor for distant metastases and survival in patients with primary uveal melanoma. Clin Cancer Res. 2007;13:1171–78. doi: 10.1158/1078-0432.CCR-06-2329. [DOI] [PubMed] [Google Scholar]
- 23.Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65:S78–86. doi: 10.1016/j.jaad.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–85. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]