Abstract
Docosahexaenoic acid (DHA) oil is an important polyunsaturated fatty acid for the human body. Evidence has demonstrated that DHA is beneficial for inhibiting mammary carcinogenesis. However, the mechanisms of DHA mediating apoptosis induction have not been fully elucidated. Thus, in the present study, the activity levels of total-superoxide dismutase (t-SOD), catalase (CAT), glutathione-peroxidase (GSH-PX) and the concentration of malondialdehyde (MDA) were determined in DHA oil-treated human malignant breast tissues. The results revealed that compared with control, DHA significantly increased the main antioxidant enzymes levels, including t-SOD, CAT, and GSH-PX, but decreased the MDA concentration in the DHA oil treated breast cancer tissues. Furthermore, DHA significantly increased the ratio of cyclic (c)AMP/cGMP levels and promoted the expression of Toll-like receptor 4 (TLR-4) and peroxisome proliferator activated receptor (PPAR)-α, thus DHA induced breast cancer cell apoptosis. We hypothesized that the levels of TLR-4 and PPAR-α are involved in the antitumorigenesis properties of DHA in breast cancer. The results of the present study hold significance for the further clinical development of DHA oil in breast cancer treatment.
Keywords: docosahexaenoic acid, apoptosis, cAMP/cGMP, antioxidant enzymes, Toll-like receptor 4, breast cancer
Introduction
Docosahexaenoic acid (DHA; 22:6 n-3) is an important member of the family of omega-3 polyunsaturated fatty acids and numerous people have added DHA oil to their daily diet (1,2). Currently, breast cancer (BC) is one of the most common types of cancer among women worldwide (3). Dietary unsaturated fatty acids, particularly DHA, are considered to serve an important role in reducing the risk of developing BC (4). Certain evidence has demonstrated that DHA oil is beneficial for inhibiting mammary gland carcinogenesis (5–10). The mechanisms of DHA oil on the inhibition of tumor proliferation and the promotion of apoptosis are complex (5–10). Among the numerous key factors, oxidative stress serves an important role in the initiation, promotion, progression and apoptosis of BC by interfering with the intracellular signal transduction pathways, and inducing DNA damage (11). The downregulation of antioxidant enzyme (AOE) expression levels or their activities have been revealed to be associated with numerous types of cancer, including breast, prostate, bladder and hepatic cancer, and multiple myeloma (12–18). Nevertheless, other studies have reported no significant changes, higher expression or higher activity levels of AOEs in certain cancer types. For example, decreased catalase (CAT), with unchanged glutathione-peroxidase (GPH-PX) and increased levels of superoxide dismutase (SOD) levels were reported in the A549 lung cancer cell line, and lung cancer tissues (19). Therefore, maintaining the appropriate activity levels of AOEs may be essential in preventing the development of specific cancer types (20). There are specfic key AOEs with essential roles in protecting cells from oxidative stress, including SOD, CAT and GPH-PX. The regulation and increases in AOE activity are associated with cancer (12). To elucidate the anticancer mechanism of DHA in BC, antioxidant activities, including that of SOD, CAT and GSH-PX were analyzed using various assays.
The anticancer mechanisms of DHA are complex (7). Glucose uptake, glycolytic metabolism, lactate production and total glucose oxidation have been demonstrated to be significantly decreased in response to DHA supplementation, thereby decreasing oxidative metabolism, and enhancing metabolic injury (7). Furthermore, the metabolic changes in DHA led to intracellular cyclic (c)AMP and cGMP levels decreasing by 50% in MDA-MB-231, and BT-474 cancer cell lines, which mediated the phosphorylation of AMP-activated protein kinase at Thr172, a metabolic stress marker (7). It effectively provides rationale for enhancement oncurrent cancer prevention models and therapies by combining with dietary sources, including DHA oil (7).
In the present study, the tumor suppressor ratio, the activities of SOD, GSH-PX and CAT, the expression of malondialdehyde (MDA), the expression of cAMP/cGMP, and the expression of Toll-like receptor 4 (TLR-4) and peroxisome proliferator activated receptor (PPAR)-α factor were detected in human malignant BC tissues following treatment with DHA oil. The results aided in explaining the apoptosis mechanism of DHA on human malignant BC. The current study has clinical significance for the further development of DHA oil use in BC.
Materials and methods
Samples and reagents
DHA oil (21) (Bohai algae DHA oil, content ~40%) was provided by the Food Science and Engineering Laboratory of Jinzhou Medical University (Jinzhou, China). Human malignant BC tissue samples were provided by the First Affiliated Hospital of Jinzhou Medical University. The approval of removed tissues for research purposes was obtained from the Ethics Committee of The First Affiliated Hospital of Jinzhou Medical University. Written informed consent was obtained from each patient.
Hydrocortison (1 gram) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Anti-TLR4 antibody produced in rabbit (100 µg) was purchased from Sigm-Aldrich (Merck KGaA). Anti-PPARα rabbit antibody (100 µg) was purchased from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated sheep anti-rabbit IgG H&L (1 mg) was purchased from Abcam. Trypsin, Hanks, RPMI-1640 medium and Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The 10% fetal bovine serum and 10% normal goat serum was purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT, USA). Tris-buffered saline (pH 7.6; cat. no. SG EC-925) was purchased from Shanghai Sango Biotechnology Co. Ltd. (Shanghai, China). Other reagents were analytical solvents and purchased from Hongda Co. (Jinzhou, China; https://0ds13046577.atobo.com.cn/).
Human malignant BC tissue culture
Human malignant BC tissues were obtained from the Breast Surgery Department of the First Affiliated Hospital of Jinzhou Medical University. The BC tissues were sterilized with 75% alcohol for 2 min and then washed three times with RPMI-1640. The BC tissues were sliced into small pieces of 1–2 mm3. Each BC tissue sample was cultured in RPMI-1640 medium with or without DHA oil. One of BC tissue samples was used as a control (control group) and the rest were treated with DHA oil (~40%) with final DHA concentrations of 100 µg/ml (first group), 150 µg/ml (second group) and 200 µg/ml (third group). Each group contained three samples of BC tissues. Each individual BC was placed in a 3.5-cm culture well. All wells were cultured for 24 h with 5% CO2 at 37°C.
After being cultured with or without DHA for 24 h, the BC tissues were embedded in paraffin to observe the effect of DHA oil on the morphology of BC. Five random sections were obtained from each sample. For research on the signal transduction pathway of DHA, BC tissues were cultured for 24 h in RPMI-1640 medium with or without DHA oil, and then the total protein was extracted from the BC tissues. The total protein content was used to detect the effect of DHA on BC antioxidant activities. Each sample was measured three times in parallel.
Histological observation
For hematoxylin-eosin (H.E.) staining, sections were rinsed in double distilled water and were incubated with hematoxylin solution stain for 5 min at 25°C. After 5 min, sections were washed with running tap water, sequentially followed by differentiation for 30 sec in 1% acid-alcohol (hydrochloric acid and ethanol) at 25°C and then washed for 1 min again with running tap water. Subsequently, all sections were stained with eosin for 30 sec and then dehydrated with different concentrations alcohol (70, 80, 90 and 100%) for 2 min each. Sections were covered with xylene-based mounting medium, after two changes of xylene for 5 min each time at 25°C. The effect of DHA on the H.E. BC sections was observed using an inverted microscope (OLYMPUS, IX73+DP73, 12V 100W halogen lamp). Analysis of the tissue areas occupied was performed using Image Pro 5.0 Plus software (Media Cybernetics, Inc., Rockville, MD, USA). Suppressor ratio (%)=(DHA treatment group entity tissue area-the control group entity tissue area)/control group entity tissue area ×100%.
Effect of DHA on the antioxidant activities of BC tissues
Detection of the activities of antioxidant enzymes
The BC tissues were centrifuged at 1,500 × g for 15 min at 4°C to remove the debris following homogenizing with normal saline solution (0.9% sodium chloride). The supernatant was transferred into new tubes for the evaluation of the SOD, CAT and GSH-PX activities. The measurements were performed following the manufacturer's protocol of an assay kit (SOD: cat. no. A001-1; CAT: cat. no. A007-2; GSH-PX: cat. no. A005; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
SOD activities were determined (at 550 nm) using the Xanthine oxidase method and expressed as nU/mg protein (22). CAT was measured by the reaction of CAT scavenging H2O2, and ammonium molybdate was added to generate a pale yellow complex (maximum absorption peak at 405 nm). CAT activities were expressed as mU/mg protein (23). GSH-PX was reacted with dithiobis-nitrobenzoic acid to produce a yellow compound, 5-dithio-bis2-nitrobenzoic acid dithiobis-nitrobenzoic acid anion (maximum absorption peak at 420 nm). The concentrations of GSH-PX were expressed as nU/mg protein (24).
Detection of MDA content
Fatty-acid peroxidation MDA content in the homogenate (0.1 ml) was measured using the thiobarbituric acid (TBA) method according to the manufacturer's protocol (cat. no. A003-1; Nanjing KeyGen Biotech Co., Ltd.). MDA and TBA condensate to produce a red product (maximum absorption peak at 532 nm). Thus, the MDA content was calculated by measuring the 532 nm absorbance and expressed as nmol/mg protein (23,24).
cAMP and cGMP assays
The cAMP and cGMP content of BC tissues were determined following the manufacturer's protocol (cAMP: cat. no. 80204; cGMP: cat. no. 80104; Neweast Biotech Company, Wuhan, China) by ELISA. The cAMP and cGMP extracts were diluted (1:10) with sample diluent. Optical densities were measured at 450 nm (25,26).
Immunohistochemistry
The expression of TLR-4 factor was measured by immunohistochemistry. Briefly, 4-µm thick sections were deparaffinized and the endogenous peroxidase activity was blocked for 10 min with 3% hydrogen peroxide at 25°C. Subsequently, sections were treated for 30 min with 10% normal goat serum in Tris-buffered saline (pH=7.6) at 37°C. Then, sections were incubated with monoclonal anti-TLR4 rabbit antibody (1:200; cat. no. PRS3141-100UG) overnight at 4°C. After washing three times with PBS, they were incubated with the HRP-sheep anti-rabbit IgG H&L secondary antibody (1:500; cat. no. ab6721) at room temperature for 1 h, followed by incubation with the color reagent 3,3′-diaminobenzidine for 3 min at 25°C (26). Analysis of the average gray density value (GDV) of TLR-4 was performed using Image Pro 5.0 Plus software.
Western blotting
All BC tissues were stored within liquid nitrogen prior to protein extraction. Briefly, tissues were homogenized with 0.5 ml ice-cold lysis buffer (pH 7.5, 20 mM Tris-HCl, 1 mM EDTA, 1 mM DTT, 5 mM MgCl2, 20 µg/ml aprotinin, 2 mM sodium orthovanadate and 1 mM PMSF). The homogenates were centrifuged at 10,000 × g at 4°C for 20 min and the supernatant was removed. The protein concentration was determined using the bicinchoninic acid method with bovine serum albumin as the standard. Samples (30 µg/ml protein per lane) were boiled for 5 min, separated using 15% SDS-PAGE and transferred onto a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). Subsequently, membranes were blocked with 5% bovine serum albumin at room temperature for 1.5 h and incubated with the anti-PPARα rabbit antibody (1:1,000; cat. no. ab8934) at 4°C for 12 h. The membrane was then washed three times with TBS Tween 20 buffer and incubated with the secondary HRP-conjugated sheep anti-rabbit IgG H&L antibody (1:200; cat. no. ab6721) at room temperature for 1 h. The GDV of specific bands was measured with Quantity One version 4.62 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) (27).
Statistical analysis
Each experiment was performed in parallel three times. All data are expressed as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance and was analyzed further by Tukey's honest significant difference test (28). Different lowercase letters in the same column represent significant differences at P≤0.05 and different capital letters represent significant differences at P≤0.01. All data analyses were conducted using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
Results
Effects of DHA on BC morphology and the suppressor ratio of DHA in BC
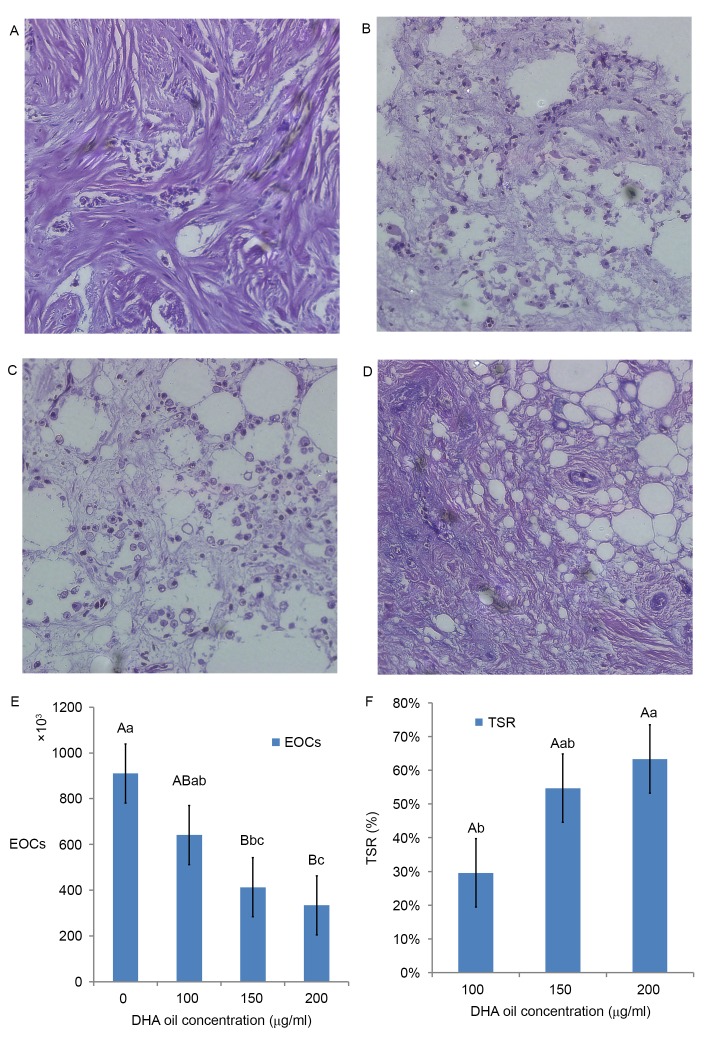
From the results of paraffin sections (H.E. staining), as DHA oil concentration increased, the morphology of BC cells became more irregular. For example, apoptosis of BC cells was more evident compared with the control group (Fig. 1A). The surrounding of tissues appeared to be shedding, phagocytized and the cell connections had disappeared (Fig. 1B). There were more vesicles observed within cells (Fig. 1C). The overall volume of BC cells had shrunk, whereas the cytoplasmic density of BC cells had increased. Chromatin agglutination occurred, and the nuclear membrane and nucleolus were broken or absent (Fig. 1D).
Figure 1.
Hematoxylin and eosin staining of human malignant breast cancer tissues following DHA oil treatment for 1 day. Representative images of the (A) control group, (B) 100 µg/ml DHA group, (C) 150 µg/ml DHA group and (D) 200 µg/ml DHA group. Magnification, ×200. Effect of DHA on the (E) area sizes and (F) the tumor suppression ratios of human malignant breast tissues. The results are expressed as the mean ± standard deviation. Different lowercase and uppercase letters indicate the significance of multiple comparisons tests of the results. Different lowercase letters in the same column represent significance differences at P≤0.05 and different capital letters represent significant differences at P≤0.01 (Table I). EOCs, entity organization areas; DHA, docosahexaenoic acid; TSR, tumor suppression ratio.
In Fig. 1E, the area sizes of human malignant breast tissues in the 150 µg/ml DHA group were significantly reduced compared with the control (0 µg/ml DHA group). The suppressor ratio of DHA in the 200 µg/ml DHA group was significantly higher compared with the ratio of the 100 µg/ml DHA group (Fig. 1F).
Effect of DHA on the activities of AOEs
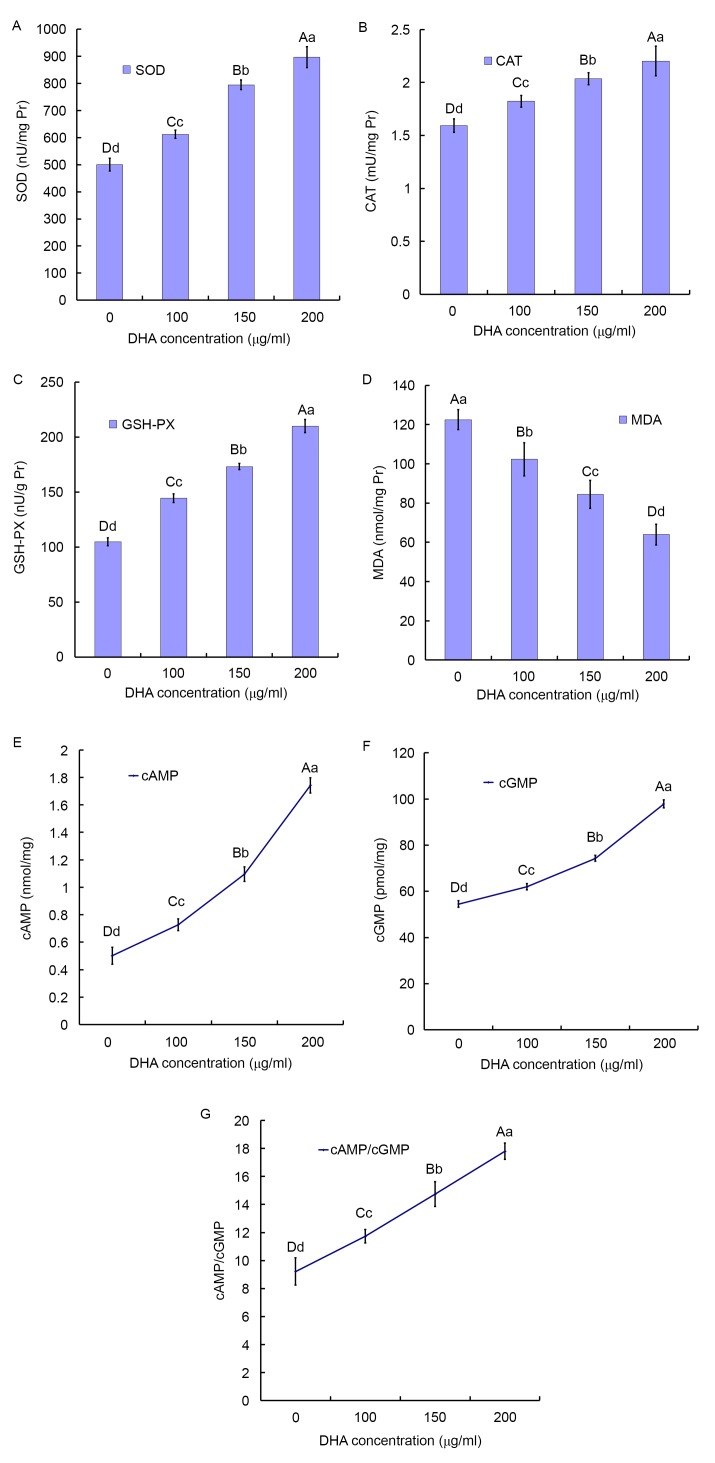
The activities of t-SOD, CAT and GSH-PX in the BC tissues increased in a DHA dose-dependent manner. Compared with the control group (500.33±23.20 nU/mg protein), the activities of SOD was significantly enhanced by 22.4% in the 100 µg/ml DHA group (612.37±14.98 nU/mg protein; P<0.001) and reached the maximum at 79.24% in the 200 µg/ml DHA group (896.78±38.87 nU/mg protein; P<0.001; Fig. 2A).
Figure 2.
Effect of DHA on the activity of antioxidant enzymes and secondary messengers. (A) SOD, (B) GSH-PX and (C) CAT activities, and (D) MDA concentration in the BCTs incubated for 24 h with increasing concentrations of DHA. The results are expressed as the mean ± standard deviation of four independent experiments, two samples per experiment performed in triplicate. The homogeneity of variance comparisons were analyzed using SPSS 19.0 software. Statistical analysis was analyzed by Tukey's honest significant difference test following one-way analysis of variance. Different lowercase letters in the same column represent significant differences at P≤0.05 and different capital letters represent significant differences at P≤0.01, but not significantly different (P>0.05) for the same letter (Table I). DHA serves as a death receptor via cancer-specific cAMP/cGMP upregulation. Effects of DHA on (E) cAMP and (F) cGMP levels in the BCTs following 24 h. (G) Effects of DHA on the ratio of cAMP/cGMP levels in the BCTs following 24 h. Different lowercase and uppercase letters indicate the significance of multiple comparisons tests of the results. Different lowercase letters in the same column represent significance differences at P≤0.05 and different capital letters represent significant differences at P≤0.01 (Table I). SOD, super oxide dismutase; DHA, docosahexaenoic acid; CAT, catalase; GSH-PX, glutathione-peroxidase; MDA, malondialdehyde; cAMP, cyclic AMP; cGMP, cyclic GMP; BCTs, breast cancer tissues.
Compared with the control group (1.59±0.06 mU/mg protein), the activities of CAT was enhanced by 14.44% the 100 µg/ml DHA group (1.82±0.05 mU/mg protein; P<0.001) and reached the maximum at 38.30% in the 200 µg/ml DHA group (2.20±0.14 mU/mg protein; P<0.001; Fig. 2B).
Compared with the control group (104.91±3.51 mU/mg protein), the activity of GSH-PX was enhanced by 37.75% in the 100 µg/ml DHA group (144.51±3.95 mU/mg protein; P<0.001) and reached the maximum at 100.17% in the 200 µg/ml DHA group (210.00±6.01 mU/mg protein; P<0.001; Fig. 2C).
Effect of DHA on the concentration of MDA in the BC tissues
In Fig. 2D, the MDA concentration of BC tissues was significantly reduced compared with the control. Compared with the control group (122.49±5.15 nmol/mg protein), the mean MDA concentration in the 100 µg/ml DHA group (102.31±8.49 nmol/mg protein) was significantly decreased by 16.48% (P<0.001) and in the 200 µg/ml DHA group was decreased by 52.22% (63.96±5.24 nmol/mg protein; P<0.001).
DHA serves as a death receptor via the upregulation of cancer-specific cAMP/cGMP
Intracellular cAMP and cGMP are the secondary messengers involved in the generation of BC. The levels of cAMP and cGMP of the BC tissues with DHA treatment were significantly higher compared with the control groups (Fig. 2E and F).
Compared with the control group (0.50±0.062 nmol/mg), the mean concentration of cAMP in the 100 µg/ml DHA group (0.73±0.043 nmol/mg; P<0.001) significantly increased by 44.78% and reached the maximum at 246.77% in the 200 µg/ml DHA group (1.74±0.056 nmol/mg; P<0.001). Compared with the 100 and 150 µg/ml DHA group (0.73±0.043 and 1.10±0.053 nmol/mg, respectively), the mean concentration of cAMP in the 200 µg/ml DHA group was significantly increased by 139.52, and 59.13%, respectively (Fig. 2E).
Compared with the control group (54.50±1.42 m nmol/mg), the mean concentration of cGMP in the 100 µg/ml DHA group (62.01±1.41 nmol/mg; P<0.001) significantly increased by 13.79% and reached the maximum at 79.64% in the 200 µg/ml DHA group (97.89±1.78 nmol/mg; P<0.001). Compared with the 100 and 150 µg/ml DHA group (62.01±1.41 and 74.32±1.27 nmol/mg, respectively), the mean concentration of cGMP in the 200 µg/ml DHA group significantly increased by 57.87, and 31.72% respectively (Fig. 2F).
Compared with the control group, the 200 µg/ml DHA group revealed significantly higher concentrations of cAMP/cGMP with a 2-fold increase (Fig. 2G).
Effect of DHA on the expression and localization of TLR-4 factor by immunohistochemistry
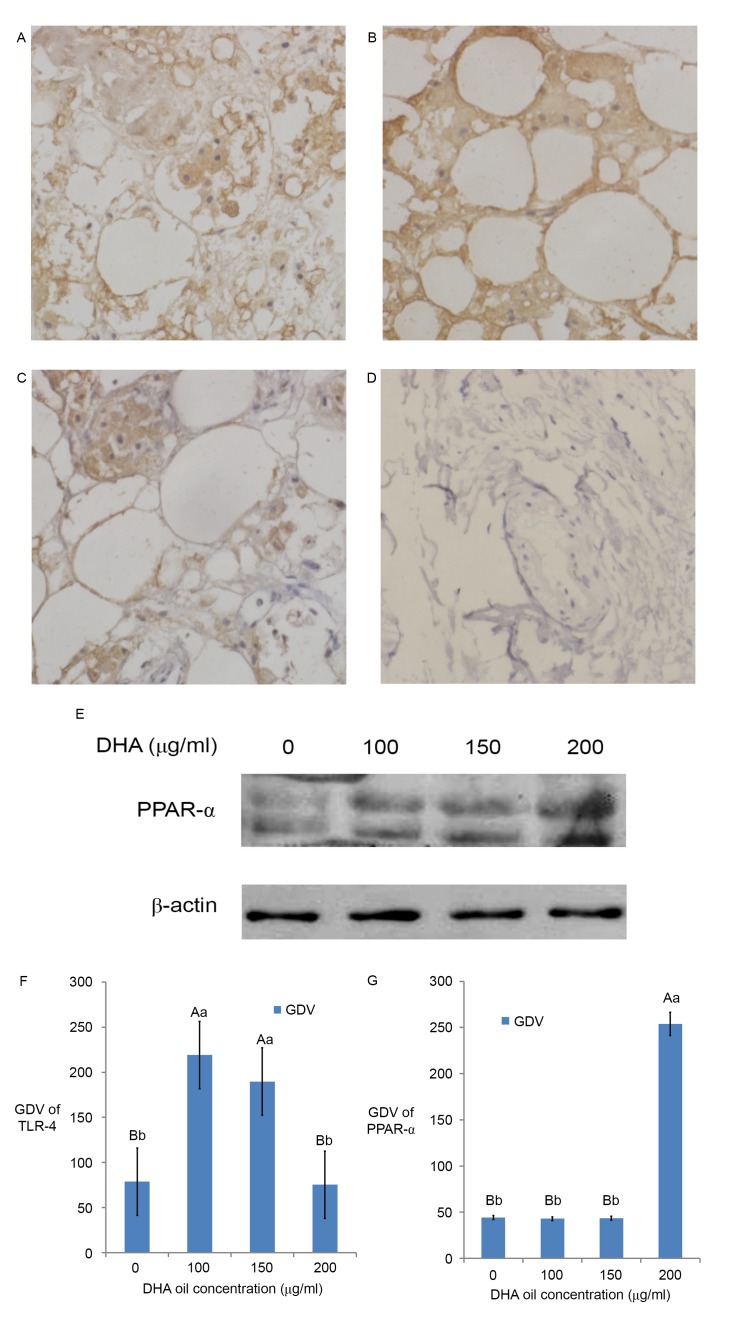
The immunohistochemical staining of the control group (Fig. 3) was brown in the cell membrane and cytoplasm. The GDV of TLR-4 showed that TLR-4 mainly existed in the cell membrane and cytoplasm (Fig. 3A-D and F). The results revealed that there was TLR-4 expression in the immunohistochemistry sections of breast cancer tissues (Fig. 3A and F), thus TLR-4 may participate in the generation of BC and localized primarily in the membrane and cytoplasm of BC cells. When DHA was added at 100 µg/ml (Fig. 3B), TLR-4 expression reached the maximum (Fig. 3F); however, the expression of TLR-4 in the 200 µg/ml (Fig. 3D) DHA group was similar to that of the control group (P>0.05, Fig. 3F). The expression of TLR-4 in the 150 µg/ml (Fig. 3C) DHA group was similar to the 100 µg/ml DHA group (Fig. 3B; P>0.05, Fig. 3F).
Figure 3.
Effect of DHA on the signaling molecules in breast cancer. Immunohistochemical results of TLR-4 in breast cancer: (A) Control, (B) 100 µg/ml DHA group, (C) 150 µg/ml DHA group and (D) 200 µg/ml DHA group. Magnification, ×400. (E) Western blotting results of PPAR-α. The GDV of (F) TLR-4- and (G) PPAR-α-positive expression areas in different groups. The results are expressed as the mean ± standard deviation. Different lowercase and uppercase letters indicate the significance of multiple comparisons tests of the results. Different lowercase letters in the same column represent significance differences at P≤0.05 and different capital letters represent significant differences at P≤0.01 (Table I). DHA, docosahexaenoic acid; PPAR, peroxisome proliferator activated receptor; GDV, gray density value; TLR-4, Toll-like receptor 4.
Effect of DHA on the expression of PPAR-α by western blotting
The western blotting result (Fig. 3E) and the GDV (Fig. 3G) of the control group bands revealed that PPAR-α protein expressed in breast cancer tissue. The results revealed that PPAR-α participated in the generation of BC in Fig. 3E and G. The expression of PPAR-α in BC tissues increased with DHA and reached the maximum in the 200 µg/ml DHA group (Fig. 3E; P<0.01; Fig. 3G). The expression of PPAR-α in the control group, 100 µg/ml DHA group and 150 µg/ml DHA group are similar (Fig. 3E; P>0.05).
Discussion
The results of the H.E. staining in the present study demonstrated that DHA oil induced the apoptosis of BC cells. The suppressor ratio of DHA treatment revealed that DHA significantly inhibited the growth and induced the apoptosis of BC cells.
Oxidative stress is the most important cause of cell damage, leading to the occurrence and development of cardiovascular disease. AOEs protect cells from free radicals and oxidative stress, contributing to the prevention of cancer. Numerous studies have reported that the upregulated expression or higher activity of AOEs may be used as effective strategies for cancer prevention and therapy. For example, MRN-100, as an adjuvant therapy was demonstrated to be effective in the treatment of esophageal/gastric carcinoma, exerting an antioxidant effect in the stomach and blood tissues by increasing the levels of GSH-PX, SOD, CAT, GSH-PX, and the total antioxidant capacity (29). Ganoderma lucidum significantly enhanced the levels of SOD, CAT and GPH-PX in the plasma, liver, and mammary tissues, thus being an effective chemopreventive agent against BC (30). Tangeretin increased the levels of AOEs, including SOD, CAT, GST and GSH-PX significantly, indicating to be effective, and efficient for the treatment of BC (31).
SOD is a class of AOEs that catalyzes the dismutation of superoxide radicals into H2O2 and O2. Subsequently, CAT and GSH-PX catalyze H2O2 decomposition to H2O and O2 (20). H2O2 is not only a reactive oxygen species, but also a major signaling molecule (32). Multiple studies have indicated that mitochondrial H2O2 is a direct and effective apoptosis inducer (32,33). The present study demonstrated that DHA was able to simultaneously upregulate the expression levels and activities of SOD, CAT, and GSH-PX in BC tissues. Therefore, DHA may inhibit the proliferation of BC cells and the associated oxidative stress mechanism.
Oxidative stress produces fatty-acid peroxidation whose metabolites possess high toxicities and mutagenic properties (34). The main fatty-acid peroxidation is MDA (34). The results in the current study revealed that DHA significantly decreased the MDA concentration of BC tissues.
The cyclic nucleotides cAMP and cGMP have been recognized as important signaling molecules within cells. Under normal physiological conditions, cyclic nucleotides regulate a myriad of biological processes. In addition, altered cyclic nucleotide signaling has been observed in a number of pathophysiological conditions, including cancer. Several studies have demonstrated that activation of cyclic nucleotide signaling leads to the inhibition of proliferation and activation of apoptosis in numerous types of cancer cells, such as bladder (35,36), breast (37–41), colon (42–44), hepatoma (45), leukemia (46,47), lung (36,48), lymphoma (49,50), ovarian (36,51), pituitary (52), prostate (36) and skin cancer (53). In the present study, the results demonstrated that DHA produced significant increase in the ratio of cAMP/cGMP levels (P<0.001), suggesting that DHA inhibits the proliferation and induces apoptosis of BC cells by increasing the ratio of cAMP/cGMP.
Increasing evidence suggests an association between chronic inflammation and cancer development (54). Recent evidence suggests that inflammation and oxidative stress play pivotal roles in the development of clinical conditions like cancers (55) and metabolic syndrome (56). Nonetheless, the underlying molecular signaling pathways associating inflammation, oxidative stress and BC cell death are not well defined.
TLR-4 is an important member of the Toll-like receptor family that are exogenous or endogenous ligands, and activate the nuclear factor (NF)-κB signal transduction pathway and the transcription of the early inflammatory cytokine genes. Ahmed et al (57) demonstrated that lipopolysaccharide, the TLR-4 agonist, enhances 4T1 tumor growth and migration, by increasing the rate of angiogenesis, vascular invasiveness, and tumor invasion. This effect is more evident in TLR-4−/− mice, suggesting that TLR-4 on host immune cells may serve an essential role in inhibiting BC genesis and tumor metastasis (58). TLR4 exerts both a defensive role at the host level and a negative role at the cancer cell level in this murine metastatic breast tumor model (58). Other data suggested that the TLR-4 agonist may induce pro- or anti-tumorigenic effects (57–60). The results in the present study revealed that TLR-4 was highly expressed in the BC tissue of the 100 µg/ml DHA treatment group. In the BC tissue, TLR-4 was localized in the cell membrane and cytoplasm, similar to that observed by Yang et al (61). The expression of TLR-4 in the cytoplasm may be due to the presence of BC with lymph node metastasis. Thus, this suggests that TLR-4 participates in the generation of BC and DHA upregulated TLR-4 to induce the apoptosis of BC. The expression of TLR-4 was significantly reduced when DHA was at 200 µg/ml, this may be due to the fact that different concentrations of DHA use different signaling pathways in breast cancer. The low concentrations DHA (100 µg/ml) stimulated the expression of TLR-4 to induce the apoptosis of BC. However, high concentrations DHA (200 µg/ml) decreased the expression of TLR-4 signaling molecules to promote the BC tissue apoptosis thoroughly.
PPAR-α, a nuclear transcription receptor, belongs to the PPAR family, and regulates the expression of numerous genes and proteins (62). Activation of PPAR-α has been reported to serve an important role in glucose homeostasis, fatty acid oxidation, lipid metabolism and the inflammatory process (63). The activation of PPAR-α was demonstrated to block the transcription of NF-κB and activator protein-1 signaling pathways (62–64). Previously, PPAR-α-specific agonists have been reported to inhibit the proliferation of various cancer cells in cultured cell lines and in engraft nude mouse models (65–70). Yessoufou et al (71) revealed that TLR-4 mutant mice exhibited significantly higher PPAR-α expression levels following a methionine/choline-deficient diet, while levels in wild types did not change. This suggests that high expression levels of TLR-4 increase the expression of PPAR-α. However, little is known regarding the molecular and cellular mechanisms of PPAR-α-mediated growth inhibition of cancer cells. In the present study, it was demonstrated that DHA promoted the expression of PPAR-α and we hypothesized that DHA regulates the glycolipid metabolic pathways in BC tissues, thus leading to the apoptosis of BC cells.
In conclusion, DHA induced the activities of SOD, CAT and GSH-PX, and decreased the concentration of MDA in the BC tissues. Furthermore, DHA significantly increased the ratio of cAMP/cGMP levels, and promoted the expression of TLR-4 and PPAR-α, in order to induce the apoptosis of BC cells (Fig. 4). DHA may be used as a dietary treatment or for prevention of BC in the future.
Figure 4.
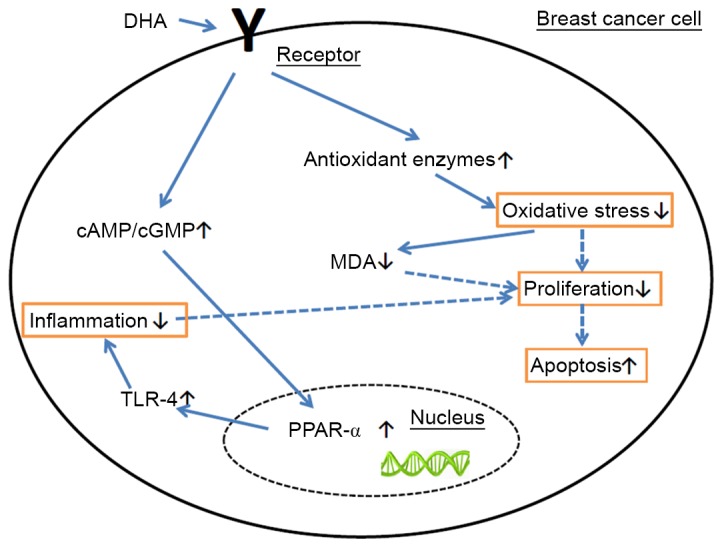
Apoptosis mechanism of DHA. DHA induced the levels of SOD, CAT, and GSH-PX and decreased the concentration of MDA in the BC tissues. DHA increased the ratio of cAMP/cGMP levels and promoted the expression of TLR-4 and PPAR-α, in order to induce the apoptosis of BC cells. DHA, docosahexaenoic acid; PPAR, peroxisome proliferator activated receptor; TLR-4, Toll-like receptor 4; CAT, catalase; MDA, malondialdehyde; cAMP, cyclic AMP; cGMP, cyclic GMP.
Table I.
Description of all experimental multiple comparison results.
| Multiple comparison | |||
|---|---|---|---|
| Figure | Group (DHA oil concentration, µg/ml) | α=0.05 | α=0.01 |
| Fig. 1E | 0 | a | A |
| 100 | ab | AB | |
| 150 | bc | B | |
| 200 | c | B | |
| Fig. 1F | 100 | b | A |
| 150 | ab | A | |
| 200 | a | A | |
| Fig. 2A | 0 | d | D |
| 100 | c | C | |
| 150 | b | B | |
| 200 | a | A | |
| Fig. 2B | 0 | d | D |
| 100 | c | C | |
| 150 | b | B | |
| 200 | a | A | |
| Fig. 2C | 0 | d | D |
| 100 | c | C | |
| 150 | b | B | |
| 200 | a | A | |
| Fig. 2D | 0 | a | A |
| 100 | b | B | |
| 150 | c | C | |
| 200 | d | D | |
| Fig. 2E | 0 | d | D |
| 100 | c | C | |
| 150 | b | B | |
| 200 | a | A | |
| Fig. 2F | 0 | d | D |
| 100 | c | C | |
| 150 | b | B | |
| 200 | a | A | |
| Fig. 3F | 0 | b | B |
| 100 | a | A | |
| 150 | a | A | |
| 200 | b | B | |
| Fig. 3G | 0 | b | B |
| 100 | b | B | |
| 150 | b | B | |
| 200 | a | A | |
Acknowledgements
The present study was supported by The National Natural Science Fund of China (grant no. 81401189), the Chancellor Hong Boze Fund of Jinzhou Medical College (grant no. XZJJ20130101-03) and The Natural Science Foundation of Liaoning Province (grant no. 20170540385).
References
- 1.Meadus WJ, Turner TD, Dugan ME, Aalhus JL, Duff P, Rolland D, Uttaro B, Gibson LL. Fortification of pork loins with docosahexaenoic acid (DHA) and its effect on flavour. J Anim Sci Biotechnol. 2013;4:46. doi: 10.1186/2049-1891-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opperman M, Marais de W, Spinnler Benade AJ. Analysis of omega-3 fatty acid content of South African fish oil supplements. Cardiovasc J Afr. 2011;22:324–329. doi: 10.5830/CVJA-2010-080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 4.Murff HJ, Shu XO, Li H, Yang G, Wu X, Cai H, Wen W, Gao YT, Zheng W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: A prospective cohort study. Int J Cancer. 2011;128:1434–1441. doi: 10.1002/ijc.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanckaert V, Kerviel V, Lépinay A, Joubert-Durigneux V, Hondermarck H, Chénais B. Docosahexaenoic acid inhibits the invasion of MDA-MB-231 breast cancer cells through upregulation of cytokeratin-1. Int J Oncol. 2015;46:2649–2955. doi: 10.3892/ijo.2015.2936. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Ma DW. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014;6:5184–5223. doi: 10.3390/nu6115184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouradian M, Kikawa KD, Dranka BP, Komas SM, Kalyanaraman B, Pardini RS. Docosahexaenoic acid attenuates breast cancer cell metabolism and the Warburg phenotype by targeting bioenergetic function. Mol Carcinog. 2015;54:810–820. doi: 10.1002/mc.22151. [DOI] [PubMed] [Google Scholar]
- 8.Xue M, Wang Q, Zhao J, Dong L, Ge Y, Hou L, Liu Y, Zheng Z. Docosahexaenoic acid inhibited the Wnt/β-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J Nutr Biochem. 2014;25:104–110. doi: 10.1016/j.jnutbio.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MM, Veigas JM, Williams PJ, Fernandes G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res Treat. 2013;141:341–352. doi: 10.1007/s10549-013-2703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lijing G, Xingyuan Q, Zhuping S, Jialing G, Bing L, et al. Research progress in the tumor suppression mechanisms of marine polyunsaturated fatty acids DHA. Sci Technol Food Industry. 2013;22:385–391. [Google Scholar]
- 11.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 12.Khan MA, Tania M, Zhang DZ, Chen HC. Antioxidant enzymes and cancer. Chin J Cancer Res. 2010;22:87–92. doi: 10.1007/s11670-010-0087-7. [DOI] [Google Scholar]
- 13.Arsova-Sarafinovska Z, Eken A, Matevska N, Erdem O, Sayal A, Savaser A, Banev S, Petrovski D, Dzikova S, Georgiev V, et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin Biochem. 2009;42:1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 14.De Craemer D, Pauwels M, Hautekeete M, Roels F. Alterations of hepatocellular peroxisomes in patients with cancer. Catalase cytochemistry and morphometry. Cancer. 1993;71:3851–3858. doi: 10.1002/1097-0142(19930615)71:12<3851::AID-CNCR2820711210>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 16.Jeon SH, Park JH, Chang SG. Expression of antioxidant enzymes (Catalase, superoxide dismutase, and glutathione peroxidase) in human bladder cancer. Korean J Urol. 2007;48:921–926. doi: 10.4111/kju.2007.48.9.921. [DOI] [Google Scholar]
- 17.Kasapović J, Pejić S, Todorović A, Stojiljković V, Pajović SB. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages. Cell Biochem Funct. 2008;26:723–730. doi: 10.1002/cbf.1499. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Tripathi M, Satyam A, Kumar L. Study of antioxidant levels in patients with multiple myeloma. Leuk Lymphoma. 2009;50:809–815. doi: 10.1080/10428190902802323. [DOI] [PubMed] [Google Scholar]
- 19.Chung-man Ho J, Zheng S, Comhair SA, Farver C, Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61:8578–8585. [PubMed] [Google Scholar]
- 20.Khan MA, Chen HC, Wan XX, Tania M, Xu AH, Chen FZ, Zhang DZ. Regulatory effects of resveratrol on antioxidant enzymes: A mechanism of growth inhibition and apoptosis induction in cancer cells. Mol Cells. 2013;35:219–225. doi: 10.1007/s10059-013-2259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xingyuan Q, Lijing G, Weijie C, Mengyi S, Bing L, et al. Optimized detection methods of fatty acids in the Bohai seaweed by response surface method. Feed Res. 2015;1:65–70. [Google Scholar]
- 22.Ge M, Chi X, Zhang A, Luo G, Sun G, Xie H, Hei Z. Intestinal NF-E2-related factor-2 expression and antioxidant activity changes in rats undergoing orthotopic liver autotransplantation. Oncol Lett. 2013;6:1307–1312. doi: 10.3892/ol.2013.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez-Salinas J, García-Ortíz L, Morales González JA, Hernández-Rodríguez S, Ramírez-García S, Núñez-Ramos NR, Madrigal-Santillán E. In vitro effect of sodium fluoride on malondialdehyde concentration and on superoxide dismutase, catalase, and glutathione peroxidase in human erythrocytes. ScientificWorldJournal. 2013;2013:864718. doi: 10.1155/2013/864718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson K, Bruder ED, Raff H. Adrenocortical control in the neonatal rat: ACTH- and cAMP-independent corticosterone production during hypoxia. Physiol Rep. 2013;1:e00054. doi: 10.1002/phy2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thumova M, Pech V, Froehlich O, Agazatian D, Wang X, Verlander JW, Kim YH, Wall SM. Pendrin protein abundance in the kidney is regulated by nitric oxide and cAMP. Am J Physiol Renal Physiol. 2012;303:F812–F820. doi: 10.1152/ajprenal.00577.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng L, Li Q. Expression and function of heregulin-alpha and its receptors in mammary gland of mouse. Sci China Life Sci. 2010;53:1015–1024. doi: 10.1007/s11427-010-4042-0. [DOI] [PubMed] [Google Scholar]
- 28.Geng L, Zhou W, Qu X, Chen W, Li Y, Liu C, Sun J, Yu X, Wang H, Zhang Z, et al. Optimization of the preparation of pectin from aloe using a box-behnken design. Carbohydrate Polymers. 2014;105:193–199. doi: 10.1016/j.carbpol.2014.01.069. [DOI] [PubMed] [Google Scholar]
- 29.Ghoneum MH, Badr El-Din NK, Abdel Fattah SM, Pan D, Tolentino L. Hydroferrate fluid, MRN-100, provides protection against chemical-induced gastric and esophageal cancer in Wistar rats. Int J Biol Sci. 2015;11:295–303. doi: 10.7150/ijbs.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deepalakshmi K, Mirunalini S, Krishnaveni M, Arulmozhi V. In vitro and in vivo antioxidant potentials of an ethanolic extract of Ganodermalucidum in rat mammary carcinogenesis. Chin J Nat Med. 2013;11:621–627. doi: 10.1016/S1875-5364(13)60072-2. [DOI] [PubMed] [Google Scholar]
- 31.Periyasamy K, Baskaran K, Ilakkia A, Vanitha K, Selvaraj S, Sakthisekaran D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz (a) anthracene-induced proliferative breast cancer in Sprague-Dawley rats. Cancer Chemother Pharmacol. 2015;75:263–272. doi: 10.1007/s00280-014-2629-z. [DOI] [PubMed] [Google Scholar]
- 32.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 33.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/S0014-5793(01)02316-X. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: Molecular features of adipose tissue. J Transl Med. 2016;14:21. doi: 10.1186/s12967-016-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 36.Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 37.Drees M, Zimmermann R, Eisenbrand G. 3′,5′-Cyclic nucleotide phosphodiesterase in tumor cells as potential target for tumor growth inhibition. Cancer Res. 1993;53:3058–3061. [PubMed] [Google Scholar]
- 38.Ciardiello F, Pepe S, Bianco C, Baldassarre G, Ruggiero A, et al. Down-regulation of RI alpha subunit of cAMP-dependent protein kinase induces growth inhibition of human mammary epithelial cells transformed by c-Ha-ras and c-erbB-2 proto-oncogenes. Int. J. Cancer. 1993;53:438–443. doi: 10.1002/ijc.2910530315. [DOI] [PubMed] [Google Scholar]
- 39.Singer AL, Sherwin RP, Dunn AS, Appleman M.M. Cyclic nucleotide phosphodiesterases in neoplastic and nonneoplastic human mammary tissues. Cancer Res. 1976;36:60–66. [PubMed] [Google Scholar]
- 40.Cohen LA, Straka D, Chan PC. Cyclic nucleotide phosphodiesterase activity in normal and neoplastic rat mammary cells grown in monolayer culture. Cancer Res. 1976;36:2007–2012. [PubMed] [Google Scholar]
- 41.Tinsley H.N., Gary B.D., Keeton A.B., Zhang W., Abadi A.H., Reynolds R.C., Piazza G.A. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol. Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deguchi A, Thompson WJ. Weinstein IB Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res. 2004;64:3966–3973. doi: 10.1158/0008-5472.CAN-03-3740. [DOI] [PubMed] [Google Scholar]
- 43.Carlson CC, Smithers SL, Yeh KA, Burnham LL, Dransfield DT. Protein kinase A regulatory subunits in colon cancer. Neoplasia. 1999;1:373–378. doi: 10.1038/sj.neo.7900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinsley H.N., Gary B.D., Keeton A.B., Zhang W., Abadi A.H., Reynolds R.C., Piazza G.A. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol. Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeRubertis FR, Craven PA. Sequential alterations in the hepatic content and metabolism of cyclic AMP and cyclic GMP induced by DL-ethionine: Evidence for malignant transformation of liver with a sustained increase in cyclic AMP. Metabolism. 1976;25:1611–1625. doi: 10.1016/0026-0495(76)90114-1. [DOI] [PubMed] [Google Scholar]
- 46.Lerner A, Epstein PM. Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies. Biochem. J. 2006;393:21–41. doi: 10.1042/BJ20051368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Murray F, Zahno A, Kanter JR, Chou D, et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia; Proc. Natl. Acad. Sci. USA.; 2008; pp. 19532–19537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marko D, Pahlke G, Merz KH, Eisenbrand G. Cyclic 3′,5′-nucleotide phosphodiesterases: Potential targets for anticancer therapy. Chem. Res. Toxicol. 2000;13:944–948. doi: 10.1021/tx000090l. [DOI] [PubMed] [Google Scholar]
- 49.Aleksijevic A, Lang JM, Giron C, Stoclet JC, Mayer S, et al. Alterations of peripheral blood lymphocyte cyclic AMP and cyclic GMP in untreated patients with hodgkin's disease. Clin. Immunol. Immunopathol. 1983;26:398–405. doi: 10.1016/0090-1229(83)90124-1. [DOI] [PubMed] [Google Scholar]
- 50.Aleksijevic A, Lugnier C, Giron C, Mayer S, Stoclet JC, et al. Cyclic AMP and cyclic GMP phosphodiesterase activities in Hodgkin's disease lymphocytes. Int. J. Immunopharmacol. 1987;9:525–531. doi: 10.1016/0192-0561(87)90119-6. [DOI] [PubMed] [Google Scholar]
- 51.Heinonen PK, Metsa-Ketela T. Prostanoids and cyclic nucleotides in malignant and benign ovarian tumors. Med. Oncol. Tumor Pharmacother. 1988;5:11–15. doi: 10.1007/BF03003177. [DOI] [PubMed] [Google Scholar]
- 52.Pertuit M, Barlier A, Enjalbert A, Gérard C. Signalling pathway alterations in pituitary adenomas: Involvement of Gsalpha, cAMP and mitogen-activated protein kinases. J. Neuroendocrinol. 2009;21:869–877. doi: 10.1111/j.1365-2826.2009.01910.x. [DOI] [PubMed] [Google Scholar]
- 53.Michalides R., Griekspoor A., Balkenende A., Verwoerd D., Janssen L., Jalink K., Floore A., Velds A., van't Veer L., Neefjes J. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–26, 229–232. [PubMed] [Google Scholar]
- 55.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 56.Toyoshi I, Noriyuki S, Yasutaka M. Bilirubin as an important physiological modulator of oxidative stress and chronic inflammation in metabolic syndrome and diabetes: A new aspect on old molecule. Diabetology International. 2016;7:338–341. doi: 10.1007/s13340-016-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed A, Redmond HP, Wang JH. Links between Toll-like receptor 4 and breast cancer. Oncoimmunology. 2013;2:e22945. doi: 10.4161/onci.22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed A, Wang JH, Redmond HP. Silencing of TLR4 increases tumor progression and lung metastasis in a murine model of breast cancer. Ann Surg Oncol. 2013;20(Suppl 3):S389–S396. doi: 10.1245/s10434-012-2595-9. [DOI] [PubMed] [Google Scholar]
- 59.Mai CW, Kang YB, Pichika MR. Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: Its expression and effects in the ten most common cancers. Onco Targets Ther. 2013;6:1573–1587. doi: 10.2147/OTT.S50838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghochikyan A, Pichugin A, Bagaev A, Davtyan A, Hovakimyan A, Tukhvatulin A, Davtyan H, Shcheblyakov D, Logunov D, Chulkina M, et al. Targeting TLR-4 with a novel pharmaceutical grade plant derived agonist, Immunomax®, as a therapeutic strategy for metastatic breast cancer. J Transl Med. 2014;12:322. doi: 10.1186/s12967-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H, Wang B, Wang T, Xu L, He C, Wen H, Yan J, Su H, Zhu X. Toll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis. PLoS One. 2014;9:e109980. doi: 10.1371/journal.pone.0109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delerive P, De Bosscher K, Besnard S, VandenBerghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor a negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 63.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda S, Ikeda E, Su S, Harada M, Okazaki H, Yoshioka Y, Nishimura H, Ishii H, Kakizoe K, Taniguchi A, et al. Δ (9)-THC modulation of fatty acid 2-hydroxylase (FA2H) gene expression: Possible involvement of induced levels of PPARα in MDA-MB-231 breast cancer cells. Toxicology. 2014;326:18–24. doi: 10.1016/j.tox.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 66.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/er.20.5.649. [DOI] [PubMed] [Google Scholar]
- 67.Shigeto T, Yokoyama Y, Xin B, Mizunuma H. Peroxisome proliferator-activated receptor alpha and gamma ligands inhibit the growth of human ovarian cancer. Oncol Rep. 2007;18:833–840. [PubMed] [Google Scholar]
- 68.Drukala J, Urbanska K, Wilk A, Grabacka M, Wybieralska E, Del Valle L, Madeja Z, Reiss K. ROS accumulation and IGF-IR inhibition contribute to fenofibrate/PPARalpha-mediated inhibition of glioma cell motility in vitro. Mol Cancer. 2010;9:159. doi: 10.1186/1476-4598-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptor alpha activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clin Cancer Res. 2006;12:3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- 70.Zhou J, Zhang S, Xue J, Avery J, Wu J, Lind SE, Ding WQ. Activation of peroxisome proliferator-activated receptor α (PPARα) suppresses hypoxia-inducible factor-1α (HIF-1α) signaling in cancer cells. J Biol Chem. 2012;287:35161–35169. doi: 10.1074/jbc.M112.367367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yessoufou A1, Atègbo JM, Attakpa E, Hichami A, Moutairou K, Dramane KL, Khan NA. Peroxisome proliferator-activated receptor-alpha modulates insulin gene transcription factors and inflammation in adipose tissues in mice. Mol Cell Biochem. 2009;323:101–111. doi: 10.1007/s11010-008-9968-1. [DOI] [PubMed] [Google Scholar]