Abstract
Lymphatic and hematogenous spread are the most common ways for tumors to metastasize. Angiogenesis is essential for tumor growth and metastasis. Vascular endothelial growth factor (VEGF) particularly VEGF-A is important in the process of angiogenesis. The current research has indicated that thalidomide (THD) may be able to inhibit angiogenesis, stimulate the activity of the immune system and inhibit the adherence of cancer cells to stromal cells. These changes may lead to suppression of tumor occurrence and development. To date, to the best of our knowledge, the effects of THD on colon cancer SW480 cells have not been reported. In the present study, the effects of THD and a combination of THD and oxaliplatin (L-OHP) on the proliferation of SW480 cells have been investigated. Furthermore, the expression of VEGF-A and hypoxia-inducible factor 1 (HIF-1) was analyzed using MTT assay, quantitative polymerase chain reaction and western blot analysis. The results indicated that THD was able to inhibit SW480 cells in dose-and-time dependent manner and inhibit the expression of VEGF-A and HIF-1α. Furthermore, treatment with THD and L-OHP had synergistic inhibitory effect, which may provide a novel treatment strategy for advanced colorectal cancer.
Keywords: colon cancer, thalidomide, oxaliplatin, vascular endothelial growth factor-A, hypoxia-inducible factor 1-1α
Introduction
Colon cancer is one of the most common malignant tumors worldwide (1). In Europe, America and other western countries, colon cancer has one of the highest cancer mortality rates. In 1982, the incidence of colon cancer in China was (2). However, with a deterioration in lifestyle and eating habits, including high fat, high animal protein, high energy intake and lack of fiber (2), the incidence of colon cancer has increased year by year and has reached fifth place in rankings behind lung, liver, stomach and esophageal cancer (1).
Recurrence and metastasis are the primary causes of mortality in patients with colon cancer. The most common ways for tumors to metastasize are via the blood and/or lymph nodes, additionally, angiogenesis is essential for tumor growth and metastasis (3). Vascular endothelial growth factor (VEGF) is considered to be one of the most important angiogenic factors, which can promote the formation of new blood vessels and lymphatic vessels (4). VEGF has a number of different physiological functions. For example, VEGF is able to selectively increase the mitosis of endothelial cells, and promote proliferation of endothelia, l cells and angiogenesis. VEGF is also able to strengthen blood vessels by increasing capillary permeability and therefore promotes the extravasation of plasma proteins and other macromolecules. Furthermore, it increases deposition to the extravascular matrix and supplies nutrition for the establishment of new capillary networks (5).
Therefore, VEGF is one of the most important growth factors for angiogenesis, which has a key role in the formation of blood vessels (6). When VEGF binds with the VEGF receptor (VEGF-R), the tyrosine protein kinase pathway is activated, and the corresponding signal transduction pathways including P44/P42-mitogen-activated protein kinase and phosphoinositide 3-kinase/protein kinase B are subsequently activated (7,8). The tyrosine protein kinase pathway is involved in the normal regulation of cell proliferation and differentiation amongst other important physiological processes.
A previous study has reported that VEGF was highly expressed in numerous malignant tumor tissues including colon and esophageal cancer, and VEGF expression was closely associated with tumor metastasis (9,10). Hypoxia-inducible factor 1 (HIF-1) is an oxygen dependent transcriptional activator, which was discovered by Ratcliffe et al (11) in 1992. HIF-1 is composed of HIF-1α and HIF-1β subunits. HIF-1α is the only oxygen regulated subunit, which is mainly expressed in cells under hypoxic conditions (12). Hypoxia can increase the accumulation and activity of HIF-1α. Activated HIF-1α regulates the transcription and expression of downstream genes, and then stimulates tumor angiogenesis, inhibits the apoptosis of tumor cells and promotes metastasis (13,14). These changes enable tumor cells to adapt to hypoxic microenvironment. The tumor cells can proliferate and invade, and transcription can take place continuously (13,14). VEGF is one of the target genes of HIF-1α. HIF-1α promotes the transcription of target gene VEGF, and regulates angiogenesis in tumors under hypoxic conditions (15,16). Therefore, HIF-1α may reduce the possibility of recurrence and metastasis of tumor cells if the formation of new blood vessels in tumor tissue can be suppressed.
Thalidomide (THD), synthesized by German Chemie Gruneenthal in 1954, was used in Europe as a sedative treatment for morning sickness in pregnancy. However, the use of THD during pregnancy led to phocomelia and consequently the drug was withdrawn from the market (17). Subsequent studies have reported that THD has anti-inflammatory, immunomodulatory and anti-angiogenic effects (18–22). Due to its anti angiogenic effects, THD is able to affect fetal development in pregnant women, which leads to fetal deformity. However, at the same time this effect of THD is one of the mechanisms for its antitumor activity (18,23). THD inhibits angiogenesis, stimulates the activity of the immune system and inhibits the adherence of cancer cells to stromal cells, which suppresses the occurrence and development of tumors.
There may be several mechanisms of action for THD in the treatment of malignant tumors (24). As THD is able to mediate anti-angiogenesis effects through inhibiting the expression of VEGF and basic fibroblast growth factor (bFGF), a previous study demonstrated that 150 µg/ml THD was able to reduce tumor blood supply and inhibit tumor growth (23). THD is also able to can stimulate the proliferation of natural killer cells and induce lymphocytes to secrete interferon γ (IFN-γ) and interleukin 2 (IL-2), so as to kill tumor cells (25). Studies have also demonstrated that THD may induce tumor cell apoptosis and arrest cell growth at the G1 phase (26–28). Additionally, THD is able to inhibit the binding of NF-κB to DNA and therefore exhibits antitumor effects by blocking transcriptional activity (9). THD is also able to downregulate the expression of adhesion molecules in vascular endothelial cells and therefore inhibit tumor proliferation (29).
Oxaliplatin (L-OHP) is a platinum anticancer drug with a broad spectrum of antitumor effects and is used to treat metastatic colorectal cancer, particularly as an adjuvant treatment for patients with stage III colon cancer with complete resections of primary tumor (30). However, if L-OHP has been used for an extended period time, incidences of resistance to the drug can increase (31). Furthermore, increasing the dose of L-OHP results in increased adverse reactions.
Previous studies have indicated that THD, when used with other chemotherapeutics may result in synergistic antitumor effects (32,33). However, to the best of our knowledge, the anti angiogenic effects of THD on SW480 cells has not been reported. In the present study, SW480 cells were cultured and treated with various concentrations of THD (12.5, 25, 50, 100 and 200 µg/ml). Optical density values were evaluated by MTT assay, and inhibition ratio was calculated for all treatment groups. The mRNA expression of VEGF-A in the treated cells was detected by quantitative polymerase chain reaction (qPCR), and the levels of HIF-1α protein were detected by western blotting. The potential mechanisms of THD on SW480 cells were investigated. Furthermore, the effect of treatment with a combination of THD and L-OHP on SW480 cells was analyzed. The results of the present study may provide experimental basis for future clinical treatment of colon cancer.
Materials and methods
Cell culture
The SW480 cell line and the HeLa cell lines were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China), and cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal calf serum (Sijiqing company, Hangzhou, China). SW480 cells and HeLa cells were cultured in an incubator containing 5% CO2 at 37°C.
Cell treatment
THD (catalog no. 14080431; Changzhou Pharmaceutical Factory, Changzhou, China) and L-OHP (catalog no. 14091515; Jiangsu Hengrui Pharmaceutical Factory, Jiangsu, China) were used. The SW480 cells were treated with 12.5, 25, 50, 100 and 200 µg/ml THD for 24, 48 or 72 h and 4, 8, 16, 32, 64 and 128 mg/l L-OHP + 5% glucose for 48 h prior to subsequent assays. The final volume was 200 µl, and the THD control group was treated with DMSO only. The L-OHP control group was treated with 5% glucose. SW480 cell proliferation was assayed subsequent to after THD/L-OHP treatment. Treatment was performed at 37°C. Cell proliferation following THD treatment and half maximal inhibitory concentration (IC50) values following L-OHP treatment were determined. SW480 cells were then treated with 25, 50, 100 µg/ml THD and 64 mg/l L-OHP and 50 µg/ml THD + 64 mg/l L-OHP for 48 h at 37°C. The control group for mRNA/VEGF detection was SW480 cells that had not been previously treated with any drugs, and the control group and positive group for HIF-1α detection were SW480 and HeLa cells, respectively. Neither group had been previously treated with any drugs. mRNA, VEGF and the expression levels of HIF-1α were detected following culture for 48 h.
Cell proliferation assay
Cells were treated as aforementioned. Experiments at each concentration were repeated 5 times. The MTT spectrophotometric dye assay (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) was used to detect cell proliferation ability following THD treatment and determine IC50 values following L-OHP treatment. SW480 cells were seeded in 96-well plates at a density of 2,500 cells/well. At 24, 48 and 72 h post-treatment, cells were incubated with 20 µl MTT for 4 h. Color was developed by incubating the cells with 150 µl DMSO; and the absorbance was detected at a wavelength of 490 nm using Microplate Reader (Elx800; BioTek Instruments, Inc., Winooski, VT, USA).
RNA extraction
The SW480 cells were treated as aforementioned. RNA extraction from the cells was undertaken using Trizol according to the manufacturer's protocols (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was eluted in RNase-free water. The RNA concentration was determined with the NanoDrop 2000/2000C spectrophotometer (cat. no. 2000/2000C; NanoDrop Technologies; Thermo Fisher Scientific, Inc.).
Reverse transcription
To obtain complementary DNA (cDNA), 1 µl Oligo dT (0.5 µg/µl) and 2.0 µg Total RNA were used according to the instructions for Promega M-MLV kit (Promega Corporation, Madison, WI, USA). Following incubation at 70°C for 10 min, the mixture was immediately placed in ice bath for 2 min. M-MLV RT 5X reaction buffer, M-MLV Reverse Transcriptase RNase Minus, and 10 mM dNTP (Promega Corporation) were added to the mixture and then incubated at 42°C for 1 h, followed by 70°C for 10 min. cDNA was stored at −80°C.
qPCR
The total RNA was extracted from the SW480 cells and reverse transcribed to template cDNA. RNA extraction from cells was performed according to the manufacturers protocol (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was eluted in RNase-free water. RNA concentrations were determined using the NanoDrop 2000/2000C spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc.). To obtain cDNA (according to the Promega M-MLV protocol), 1 µl Oligo dT (0.5 µg/µl; Axygen Scientific Inc., Union City, CA, USA) and 2.0 µg total RNA were used. Following incubation at 70°C for 10 min, the mixture was placed in an ice bath immediately for 2 min. M-MLV RT 5× reaction buffers, M-MLV Reverse Transcriptase RNase Minus and 10 mM dNTP (Promega Corporation) were added to the mixture and then incubated at 42°C for 1 h, followed by 70°C for 10 min. cDNA was stored at −80°C. qPCR was performed on the Takara PCR thermal cycler using the SYBR Green detection system (Takara Bio, Inc., Otsu, Japan). Cycling conditions consisted of a 30 sec hot start at 95°C, followed by 45 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec, extension at 95°C for 15 sec, 55°C for 30 sec, and then a final inactivation at 95°C for 15 sec. Dissociation curve analyses were performed at the end of the cycle to confirm that one specific product was measured in each reaction. This was repeated 3 times. Relative quantification was performed using the 2−ΔΔCq method (34). The specific primers for each gene are as follows: VEGF-A (product size, 89 bp) forward 5′-GCTTACTCTCACCTGCTTCTG-3′, reverse 5′-GGCTGCTTCTTCCAACAATG-3′ and GAPDH (product size, 121 bp) forward 5′-TGACTTCAACAGCGACACCCA-3′ and reverse 5′-CACCCTGTTGCTGTAGCCAAA-3′.
Protein extraction and western blot analysis
Hela cells and SW480 cells, which had not been previously treated with any drugs, were used as the positive and blank controls, respectively. The SW480 cells were treated as aforementioned. The cells were washed with PBS and lysed in radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Jiangsu, China). The cells were lysed with lysis buffer and centrifuged at 12,000 × g for 15 min at 4°C. Protein (20 µg) was subjected to 10% SDS-PAGE separation. The proteins were transferred to PVDF membrane (EMD Millipore, Billerica, MA, USA), blocked at 4°C overnight using TBST solution containing 5% skimmed milk. Blots were probed with HIF-1α antibody (mouse anti human; 1:500; catalog no. ab113642; Abcam, Cambridge, MA, USA). All primary antibodies were incubated 4°C overnight, and washed four times with TBST, every time 8 min, and then incubated with the secondary monoclonal antibody anti mouse immunoglobulin G (1:2,000; catalog no. sc-2005; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Blots were visualized using enhanced chemiluminescence-PLUS kit (Thermo Fisher Scientific, Inc.).
Data analysis
Statistical analysis was performed using SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA). All quantitative data are expressed as the mean ± standard deviation. One-way analysis of variance and Student-Newman-Keuls method were used for analysis. Student-Newman-Keuls analysis was used to analyze significant differences between groups, and P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of THD treatment on SW480 cells
The SW480 cells were treated with different concentrations of THD (12.5, 25, 50, 100 and 200 µg/ml) for 24, 48 and 72 h. Each concentration of THD was able to inhibit the proliferation of SW480 cells compared with the control. The growth inhibition rate of SW480 cells increased significantly (P<0.05) when the concentration of THD and duration of treatment increased (Table I).
Table I.
Effect of THD treatment on human colon cancer SW480 cells.
| Duration of treatment, h | |||
|---|---|---|---|
| Groups | 24 | 48 | 72 |
| Control (0 µg/ml) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| THD (µg/ml) | |||
| 12.5 | 24.23±1.5 | 27.5±1.37b | 33.24±1.39b |
| 25.0 | 27.73±2.05a | 31.54±1.54a,b | 35.97±1.14a,b |
| 50.0 | 31.54±1.93a | 35.12±2.18a,b | 38.35±1.38a,b |
| 100.0 | 35.24±1.4a | 37.76±1.27a,b | 40.93±0.95a,b |
| 200.0 | 37.07±0.68a | 42.11±2.11a,b | 45.13±0.97a,b |
Table demonstrates growth inhibition rate of SW480 cells following THD treatment.
P<0.05, growth inhibition rates of cells treated for the same duration for different THD concentrations were significantly different from each other.
P<0.05, growth inhibition rates of cells treated with the same concentrations of THD for different durations were significantly different from each other. Values are expressed as the mean ± standard deviation. THD, thalidomide.
Determination IC50 of L-OHP using the MTT method
According to Fig. 1, the IC50 value of L-OHP was 64 mg/l.
Figure 1.
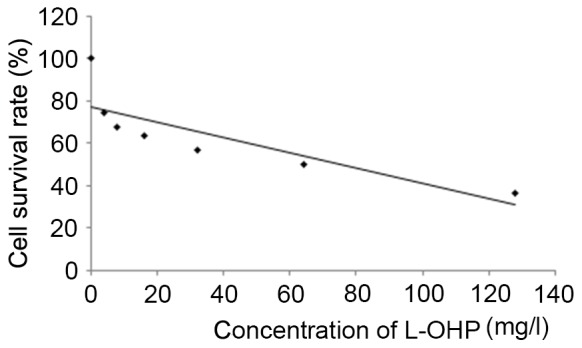
Growth curve of SW480 cells following treatment with L-OHP. L-OHP, oxaliplatin.
mRNA expression of VEGF-A in SW480 cells following treatment with various concentrations of THD, L-OHP and THD + L-OHP
The qPCR results indicated that VEGF-A mRNA expression was markedly decreased when the concentration of THD was increased (Table II). The 2−ΔΔq values for 25, 50 and 100 µg/ml THD treatment groups were 0.809±0.013, 0.685±0.035 and 0.563±0.034, respectively. The 2−ΔΔq value for the control group was 1.002±0.074. The differences between any two groups was significant (P<0.05).
Table II.
2−ΔΔq values of VEGF-A mRNA expression following THD treatment.
| Control group | 25 µg/ml THD | 50 µg/ml THD | 100 µg/ml THD |
|---|---|---|---|
| 1.002±0.074 | 0.809±0.013a | 0.685±0.035a | 0.563±0.034a |
P<0.05 front group vs. the followed group. VEGF, vascular endothelial proliferation factor; THD, thalidomide.
VEGF-A mRNA expression was also decreased following the treatment of SW480 cells with 50 µg/ml THD, 64 mg/l L-OHP and 50 µg/ml THD + 64 mg/l L-OHP for 48 h (Table III). The 2−ΔΔq values for 50 µg/ml THD, 64 mg/l L-OHP and 50 µg/ml THD + 64 mg/l L-OHP treatment groups were 0.685±0.035, 0.281±0.018, 0.167±0.007, respectively. The differences between the groups were significant P<0.05. The treatment of SW480 cells with a combination of THD and L-OHP had a synergistically decreased VEGF-A mRNA expression.
Table III.
2−ΔΔq values of VEGF-A mRNA expression following THD and L-OHP treatment.
| Control group | 50 µg/ml THD | 64 mg/l L-OHP | 50 µg/ml THD+ 64 mg/l L-OHP |
|---|---|---|---|
| 1.002±0.074 | 0.685±0.035a | 0.281±0.018a | 0.167±0.007a |
P<0.05 front group vs. the followed group. VEGF, vascular endothelial proliferation factor; THD, thalidomide; L-OHP, oxaliplatin.
Expression of HIF-1α protein
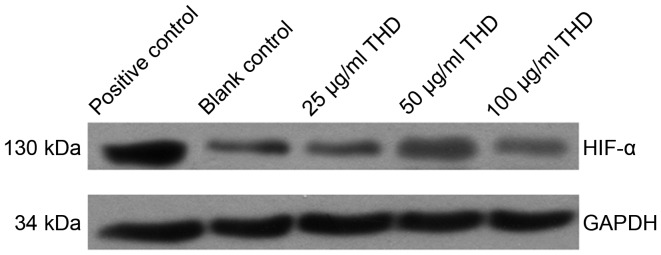
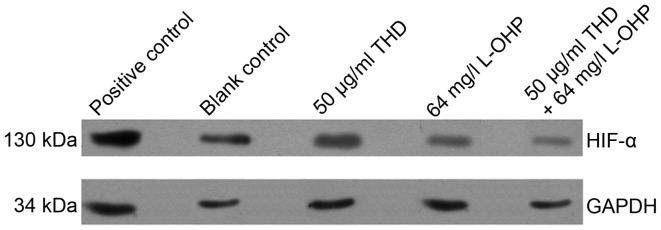
HIF-1α expression was decreased following treatment of cells with THD. In addition, there was not a significant change in expression levels as the concentration of THD increased (Fig. 2). Furthermore, HIF-1α expression was decreased following the treatment of SW480 cells with 50 µg/ml THD, 64 mg/l L-OHP and 50 µg/ml THD + 64 mg/l OHP for 48 h (Fig. 3). Results indicated that the treatment of cells with a combination of THD and L-OHP had a synergistic effect.
Figure 2.
Expression of HIF-1α following THD treatment. HIF-1α, hypoxia-inducible factor 1α; THD, thalidomide.
Figure 3.
Expression of HIF-1α following treatment with THD and L-OHP. HIF-1α, hypoxia-inducible factor 1α; L-OHP, oxaliplatin; THD, thalidomide.
Discussion
Colorectal cancer is one of the most common malignant tumors worldwide. Colorectal cancer has one of the highest rates of cancer mortality in Europe and other western countries (1). In 1971, Folkman (35) reported that angiogenesis has an important role in the process of tumor growth. Angiogenesis is necessary for tumor growth and metastasis (3). Subsequently, the study of angiogenesis attracted extra attention from researchers. VEGF is particularly important in the process of angiogenesis.
THD, a drug initially synthesized by Chemie Gruneenthal, was used to treat morning sickness during pregnancy but was later withdrawn from the market due to serious side effects of phocomelia (17). However, studies have also reported that THD has anti-inflammatory, immunomodulatory, and anti angiogenic effects (16). The effects of THD on proliferation of colon cancer cells are rarely reported. The present study investigated the effects of THD treatment on the proliferation of SW480 cells, and the effects of THD on the expression of VEGF-A and HIF-1α. Treatment with 12.5, 25, 50, 100 and 200 µg/ml THD was able to markedly decrease the proliferation of SW480 cells compared with the control. Furthermore, the growth inhibition rate increased significantly with the increase in THD concentration and duration of treatment.
Previous studies have indicated that THD was able to induce arrest at G1 phase, block DNA synthesis and inhibit the proliferation of tumor cells [KG-la human acute myelogenous leukemia cell lines, human pancreatic cancer cells (Patu-8988) and U251-MG glioma cells] (26–28). Another in vitro study on ovarian cancer also reported that THD was able to inhibit the proliferation of ovarian cancer cells SKOV3 (36), which suggested that THD may have a role in promoting anti-tumor effects by directly inhibiting proliferation. On the other hand, THD was also able to promote the apoptosis of transitional cell carcinoma cells (37).
There are three main pro-apoptotic pathways, including the mitochondrial, death receptor pathway and endoplasmic reticulum enzyme particles pathway (leading to the activation of caspase-12 and apoptosis). The B-cell lymphoma 2 (Bcl-2) family has a key function in the regulation of the mitochondrial pathway (38,39). According to the differences in function in apoptosis, the Bcl-2 family is divided into two categories: Pro-apoptotic genes and anti-apoptotic genes. The pro-apoptotic genes include BCL2-associated X (Bax), Bcl-2-like protein 7 (Bak), Bcl2-associated agonist of cell death (Bad) and BH3-interacting domain death agonist (Bid), and the anti-apoptotic genes include Bcl-2 and Bcl-xL. Among these genes, the most important are Bax and Bcl-2.
Qiao et al (40) demonstrated that the expression of Bax mRNA was upregulated, the expression of Bcl-2 gene was downregulated following the treatment of SW1990 pancreatic cancer cells with THD.
Marriott et al (41) used the THD analogue, selective cytokine inhibitory drug (SelCID-3), to treat tumor cell lines [colorectal (SW620 and LoVo), pancreatic (BxPc-3 and T3M-4), melanoma (MJT-3 and SP-1) and prostatic (PC-3 and DU-145)]. The study demonstrated that SelCID3 was able to suppress the protein expression of Bcl-2, activate caspase 3 and induce the apoptosis of cancer cells. Furthermore, THD treatment was able to reduce the proliferation of tumor cells (41).
VEGF is highly specific. VEGF has a number of isoforms, including VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PIGF). VEGF-A can bind with vascular endothelial growth factor receptor (VEGFR)-1 and VEGFR-2. VEGF has reported to be a key factor in promoting angiogenesis (42). A number of studies have reported that VEGF has an important role in promoting proliferation, migration and chemotactic response in bone, lung, kidney, brain, vascular endothelial cells, tumor and other tissues (43–48). Under pathological conditions, hypoxia is the main factor that promotes the synthesis of VEGF (49). A previous study has demonstrated that the capacity of VEGF synthesis in cells under hypoxic conditions would increase 3–12 times (50). Hypoxia in cells may cause the release of HIF-1 and promote the transcription of VEGF. When different concentrations of THD were incubated with human vascular endothelial cells in vitro, it was observed that the secretion of VEGF, cell adhesion ability of collagen and cell migration ability were all inhibited. Therefore, the formation of new capillaries was also reduced. This may be associated with the THD-induced downregulation of VEGF (51,52). At the same time, THD was able to block the NF-κB signaling pathway, its downstream adhesion molecules and the expression of inflammatory mediators including TNF-α (53).
It was verified in a previous study that NF-κB activates IL-6, and IL-6 is able to increase the expression of VEGF (54). Therefore, if the activation of NF-κB was inhibited, this may inhibit IL-6 activation, thereby decreasing VEGF expression. The results of q-PCR indicated that the VEGF-A mRNA expression was markedly decreased following THD treatment, and the decrease was concentration dependent. Therefore, THD may be able to reduce angiogenesis in tumor cells. VEGF is one of the target genes of HIF-1α (55). Under hypoxic conditions, signal transducer and activator of transcription 3 (STAT3) is activated. Activated STAT3 induces the transcription and expression of HIF-1α, which transcribes and expresses the target gene VEGF. VEGF participates in tumor angiogenesis, and HIF-1α can promote the expression of erythropoietin and inhibit the apoptosis of cancer cells in a hypoxic environment, ultimately result in the constant growth of tumor cells (56). Nechemia-Arbely et al (57) also identified in an in vitro study that activated STAT3 was able to induce the upregulation of HIF-1α in mouse renal cells.
The inflammatory microenvironment is an important factor in promoting tumor progression. The IL-6-mediated downstream signal STAT3 has an important role in tumor development and metastasis. The IL-6 receptor binds with soluble IL-6 (sIL-6R), and the IL-6/sIL-6R compound then activates membrane glycoprotein 130, and causes the activation of STAT3. This leads to the induction of tumor cells to proliferate, migrate and undergo angiogenesis (58). Therefore, blocking the IL-6 signal transduction pathway may inhibit the activation of STAT3, leading to downregulation of HIF-1α and reduced transcription. This consequently reduces tumor angiogenesis. In the present study, it was observed that HIF-1α expression was markedly decreased following the treatment of SW480 cells with THD for 48 h; however, as the concentration of THD increased, there was not a significant change in HIF-1α expression levels. At present, the antitumor mechanisms of THD that have been reported included changes in the levels of cytokines e.g., concentration of tumor necrosis factor (TNF)-α, IL-6 and IFN-γ. In addition, it was indicated that these changes were able to regulate the immune status of the body (59). An in vitro study indicated that treatment with 100 µM THD was able to inhibit the expression of IL-6, IL-8, TNF-α and VEGF (60). Therefore, this led to the inhibition of STAT3 activation and decreased HIF-1α expression (60). THD has been demonstrated to inhibit the expression of HIF-1α through the STAT3 signaling pathway mediated by IL-6. Furthermore, a number of studies have indicated that THD was also able to directly inhibit HIF-1α expression (52,61).
Surgery is the main treatment for colorectal cancer, and its effectiveness combined with post-surgical radiotherapy and/or chemotherapy has been widely recognized (62). Systemic chemotherapy is the primary treatment following surgery, its purpose is to eliminate micro metastases in vivo, to prolong the life of patients and improve the quality of life of the patients. At present, the National Comprehensive Cancer Network recommends FOLFOX, a combination of drugs which include 5-Fu, leucovorin and L-OHP for the treatment of colon cancer (30,63). L-OHP is the third generation platinum compound, and it is a new platinum group, which contains 1,2-diamine cyclohexane (DACH). L-OHP overcomes the toxicity of I and II generation platinum drugs. Cisplatin is a second-generation platinum drug, and cisplatin-resistant tumor cell lines exhibit varying degrees of resistance to L-OHP (64). L-OHP is a type of anticancer drug with good prospects and has attracted the attention of researchers. The mechanism for L-OHP is to induce platinum DNA adducts (Pt-GG and Pt-AG) which are intra chain complexes that block DNA transcription and replication (63). However, if L-OHP was used alone, it may cause neurotoxicity, hematological toxicity, gastrointestinal toxicity and drug-resistance, which may lead to the failure of the chemotherapy.
Studies have reported that the use of a combination of anti-angiogenic drugs is able to improve the efficacy of chemotherapy. Cremolini et al (65) discovered that progression-free survival and the median survival time of patients with metastatic colorectal cancer treated with bevacizumab plus FOLFOXIRI were improved compared with patients treated with FOLFOXIRI alone. However due to the high price of bevacizumab, this hindered the use of the drug in some patients.
Studies have reported that the use of THD in combination with other antineoplastic drugs may have a synergistic antitumor effect.
Murphy et al (66) reported that THD may increase cisplatin content in intracranial tumor and significantly increase the efficacy of cisplatin in the rat intracranial glioma model using a combination of cisplatin with THD. A phase II randomized controlled trial indicated that patients with metastatic colorectal cancer, who received L-OHP/capecitabine (XELOX) therapy plus THD as first-line treatment had good tolerance, and the disease control rate was significantly improved compared with the control group receiving XELOX alone) (32).
In the present study, as THD was able to inhibit the proliferation of SW480 cells, downregulate VEGF-A expression and inhibit the expression of HIF-1α according to the preceding study, it was assumed that a combination of THD and L-OHP have a synergistic effect on SW480 cells. The IC50 of L-OHP in the SW480 cells was 64 mg/l. Additionally, 50 µg/ml THD and 64 mg/l L-OHP were selected to treat the SW480 cells. The cells were either treated with THD and L-OHP separately or together for 48 h. The expression of VEGF-A mRNA and HIF-1α protein were subsequently detected. The experiment identified that VEGF-A mRNA expression and the HIF-1α protein expression were decreased following the treatment of SW480 cells with 50 µg/ml THD, 64 mg/l L-OHP and 50 µg/ml THD + 64 mg/l L-OHP for 48 h. The combined treatment of two drugs had a synergistic effect. Research has demonstrated that IL-6 may activate STAT3 in colorectal cancer (67,68), and it has been reported that L-OHP may downregulate the expression of HIF-1α through inhibiting the STAT3 signaling pathway mediated by IL-6 (69). THD has been demonstrated to inhibit the expression of HIF-1α through the STAT3 signaling pathway mediated by IL-6 aforementioned (52,60,61). Two drugs have interacted together in this pathway, and had a synergistic effect. HIF-1α is an upstream regulatory gene of VEGF and therefore downregulation of HIF-1α resulted in downregulation of VEGF-A-mRNA.
Therefore, it was not unexpected to detect the inhibitory effects of THD on proliferation of cancer cells in the present study. Additionally, THD is also able to have an inhibitory effect on the expression of VEGF-A and its upstream gene HIF-1α. Treatment with a combination of THD and L-OHP has a synergistic inhibitory effect on the growth of the SW480 cells and this provides a new strategy for the treatment of advanced colorectal cancer.
References
- 1.International Agency for Research on Cancer, corp-author. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Colorectal cancer estimated incidence, mortality and prevalence worldwide in 2012 [EB/OL]
- 2.Zong-Guang Zhou, Lie Yang, Yuan Li, et al. The 30 years' changes of colorectal cancer and the strategies in China. Chin J Practic Surg. 2012;32:693–696. (In Chinese) [Google Scholar]
- 3.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Wieck MM, Spurrier RG, Levin DE, Mojica SG, Hiatt MJ, Reddy R, Hou X, Navarro S, Lee J, Lundin A, et al. Sequestration of vascular endothelial growth factor (VEGF) induces late restrictive lung disease. PLoS One. 2016;11:e0148323. doi: 10.1371/journal.pone.0148323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin LJ, Zheng CQ, Jin Y, Ma Y, Jiang WG, Ma T. Expression of survivin protein in human colorectal carcinogenesis. World J Gastroenterol. 2003;9:974–977. doi: 10.3748/wjg.v9.i5.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willems-Widyastuti A, Vanaudenaerde BM, Vos R, Dilisen E, Verleden SE, De Vleeschauwer SI, Vaneylen A, Mooi WJ, de Boer WI, Sharma HS, Verleden GM. Azithromycin attenuates fibroblast growth induced vascular endothelial growth factor via p38(MAPK)signaling in human airway smooth muscle cells. Cell Biochem Biophys. 2013;67:331–339. doi: 10.1007/s12013-011-9331-0. [DOI] [PubMed] [Google Scholar]
- 7.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiov Asc Res. 2001;49:568–581. doi: 10.1016/S0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 8.Zeng H, Sanyal S, Mukhopadhyay D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. Biol Chem. 2001;276:32714–32719. doi: 10.1074/jbc.M103130200. [DOI] [PubMed] [Google Scholar]
- 9.Xu XL, Ling ZQ, Chen W, Xu YP, Mao WM. The overexpression of VEGF in esophageal cancer is associated with a more advanced TMN stage: A meta-analysis. Cancer Biomark. 2013;13:105–113. doi: 10.3233/CBM-130343. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Liu X, Zhang J, Li L, Liu C. The expression and clinical significance of PI3K, pAkt and VEGF in colon cancer. Oncol Lett. 2012;4:763–766. doi: 10.3892/ol.2012.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratcliffe PJ. HIF-l and HIF-2: Working alone or together in hypoxia? J Clin Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Xiong ZW, Li HW, Hu HX, Zhang P, Su H, Huang Y, Zhang HD, Xun FH. Expression of COX-2, VEGF and E -cad in breast cancer and their clinicopathologic significance. Chin J Curr Adv Gen Surg. 2010;13:766–771. (In Chinese) [Google Scholar]
- 13.Zhang P, Dong L, Yan K, Long H, Yang TT, Dong MQ, Zhou Y, Fan QY, Ma BA. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol Rep. 2013;30:1753–1761. doi: 10.3892/or.2013.2619. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L, Zhang Y. Clinicopathologic significance of HIF-1α, CXCR4, and VEGF expression in colon cancer. Clin Dev Immunol. 2010;2010:pii:537531. doi: 10.1155/2010/537531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 16.De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer-associated fibroblasts (CAFs) Breast Cancer Res. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decourt B, Drumm-Gurnee D, Wilson J, Jacobson S, Belden C, Sirrel S, Ahmadi M, Shill H, Powell J, Walker A, et al. Poor safety and Tolerability Hamper Reaching a potentially therapeutic dose in the use of thalidomide for Alzheimer's disease: Results from a Double-Blind Placebo-Controlled trail. Curr Alzheimer Res. 2017;14:403–411. doi: 10.2174/1567205014666170117141330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raturi R, Patel AA, Carter JD. Two cases demonstrating thalidomide's efficacy in refractory lupus nephritis. Clin Rheumatol. 2017;36:725–728. doi: 10.1007/s10067-016-3511-7. [DOI] [PubMed] [Google Scholar]
- 19.Anargyrou K, Dimopoulos MA, Sezer O, Terpos E. Novel anti-myeloma agents and angiogenesis. Leuk Lymphoma. 2008;49:677–689. doi: 10.1080/10428190701861686. [DOI] [PubMed] [Google Scholar]
- 20.Bombini G, Canetti C, Rocha FA, Cunha FQ. Tumour necrosis factor-alpha mediates neutrophil migration to the knee synovial cavity during immune inflammation. Eur J Pharmacol. 2004;496:197–204. doi: 10.1016/j.ejphar.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Deng L, Ding W, Granstein RD. Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J Invest Dermatol. 2003;121:1060–1065. doi: 10.1046/j.1523-1747.2003.12565.x. [DOI] [PubMed] [Google Scholar]
- 22.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Liu F, Sun Z, Sun M, Sun S. The enhancement of radiosensitivity in human esophageal carcinoma cell by thalidomide and its potential mechanism. Cancer Biother Radiopharm. 2011;26:219–227. doi: 10.1089/cbr.2010.0897. [DOI] [PubMed] [Google Scholar]
- 24.Pan J, Lu GP, Yu ZJ. Thalidomide on anti-tumor research. Chin J Cancer Prev Treat. 2012;19:552–555. [Google Scholar]
- 25.Uach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girgis E, Mahoney J, Darling-Reed S, Soliman M. Arsenic trioxde enhance the cytotoxic effect of thalidomide in a KG-la human acute mylogenous leukemia cell line. Oncol Lett. 2010;1:473–479. doi: 10.3892/ol_00000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Xiong G, Li E, Zhu H, Lio H, Shen G, Wu S. Effect of thalidomide on cytodynamics and VEGF expression of human pancreatic cancer cells. Chin J Gastroenterol. 2012;9:511–513. [Google Scholar]
- 28.Gao S, Yang XJ, Zhang WG, Ji YW, Pan Q. Mechanism of thalidomide to enhance cytotoxicity of temozolomide in U251-MG glioma cells in vitro. Chin Med J (Engl) 2009;122:1260–1266. [PubMed] [Google Scholar]
- 29.Lin YC, Shun CT, Wu MS, Chen CC. A novel anticancer effect of thalidomide: Inhibition of intercellular adhesion molecule-1-mediated cell invasion and metastasis through suppression of nuclear factor-kappaB. Clin Cancer Res. 2006;12:7165–7173. doi: 10.1158/1078-0432.CCR-06-1393. [DOI] [PubMed] [Google Scholar]
- 30.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 31.Dahan L, Sadok A, Formento JL, Seitz JF, Kovacic H. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br J Pharmacol. 2009;158:610–620. doi: 10.1111/j.1476-5381.2009.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv J, Liu N, Liu KW, Ding AP, Wang H, Qiu WS. A Randomised controlled phase II trial of the combination of XELOX with thalidomide for the first-line treatment of metastatic colorectal cancer. Cancer Biol Med. 2012;9:111–114. doi: 10.3969/j.issn.2095-3941.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindarajan R. Irinotecan/thalidomide in metastatic colorectal cancer. Oncology (Williston park) 2002;16(4 Suppl 3):S23–S26. [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 36.Zhang MM, Cai L, LA DD. Study on mechanism of thalidomide in human ovarian cancer cells SKOV3 in vitro. J Practi Med. 2010;26:2693–2696. [Google Scholar]
- 37.Huang YT, Cheng CC, Chiu TH, Lai PC. Therapeutic potential of thalidomide for gemcitabine-resistant bladder cancer. Int J Oncol. 2015;47:1711–1724. doi: 10.3892/ijo.2015.3155. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu W, Zhao XG, Zhang X. Formononetin induce apoptosis of SKOV3 cells through regulating the expression of Bax and Bcl-2. Sichuan J Physiol. 2014;36:60–62. [Google Scholar]
- 40.Qiao Z, Yuan J, Shen J, Wang C, He Z, Hu Y, Zhang M, Xu C. Effect of thalidomide in combination with gemcitabine on human pancreatic carcinoma SW-1990 cell lines in vitro and in vivo. Oncol Lett. 2015;9:2353–2360. doi: 10.3892/ol.2015.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marriott JB, Clarke LA, Czajks A, Dredge K, Childs K, Man HW, Schafer P, Govinda S, Muller GW, Stirling DI, Dalgleish AG. A novel subclass of thalidomide analogue with anti-solid tumor activity in which caspase-dependent apoptosis is associated with altered expression of bcl-2 family proteins. Cancer Res. 2003;63:593–599. [PubMed] [Google Scholar]
- 42.Meller S, Bhandari V. VEGF levels in humans and animal models with RDS and BPD: Temporal relationships. Exp Lung Res. 2012;38:192–203. doi: 10.3109/01902148.2012.663454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 44.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao T, Zhao W, Meng W, Liu C, Chen Y, Gerling IC, Weber KT, Bhattacharya SK, Kumar R, Sun Y. VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infracted myocardium. Am J Transl Res. 2015;7:697–709. [PMC free article] [PubMed] [Google Scholar]
- 46.Iacovelli R, Sternberg CN, Porta C, Verzoni E, de Braud F, Escudier B, Procopio G. Inhibition of the VEGF/VEGFR pathway improves survival in advanced kidney cancer: A systematic review and meta-analysis. Curr Drug Targets. 2015;16:164–170. doi: 10.2174/1389450115666141120120145. [DOI] [PubMed] [Google Scholar]
- 47.Quittet MS, Touzani O, Sindji L, Cayon J, Fillesoye F, Toutain J, Divoux D, Marteau L, Lecocq M, Roussel S, et al. Effects of mesenchymal stem cell therapy, in association with pharmacologically active microcarriers releasing VEGF, in an ischaemic stroke model in the rat. Acta Biomater. 2015;15:77–88. doi: 10.1016/j.actbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Zhu H, Ge Y, Liu J, Cai J, Qin Q, Zhan L, Zhang C, Xu L, Liu Z, et al. Melittin enhances radiosensitivity of hypoxic head and neck squamous cell carcinoma by suppressing HIF-1α. Tumour Biol. 2014;35:10443–10448. doi: 10.1007/s13277-014-2218-0. [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson-Berka JL. Vasoactive factors and diabetic retinopathy: Vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr Pharm Des. 2004;10:3331–3348. doi: 10.2174/1381612043383142. [DOI] [PubMed] [Google Scholar]
- 51.Buckstein R, Kerbel R, Cheung M, Shaked Y, Chodirker L, Lee CR, Lenis M, Davidson C, Cussen MA, Reis M, et al. Lenalidomide and metronomic melphalan for CMML and higher risk MDS: A phase 2 clinical study with biomarkers of angiogenesis. Leuk Res. 2014;38:756–763. doi: 10.1016/j.leukres.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Tan H, Chen H, Xu C, Ge Z, Gao Y, Fang J, Liu W, Xiao S. Role of vascular endothelial growth factor in angiodysplasia: An interventional study with thalidomide. J Gastroenterol Hepatol. 2012;27:1094–1101. doi: 10.1111/j.1440-1746.2011.06967.x. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez Mde O, Fulco Tde O, Pinheiro RO, Pereira Rde M, Redner P, Sarno EN, Lopes UG, Sampaio EP. Thalidomide modulates Mycobacterium leprae-induced NF-κB pathway and lower cytokine response. Eur J Pharmacol. 2011;670:272–279. doi: 10.1016/j.ejphar.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 54.Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol. 2013;(Suppl 1) doi: 10.4172/2155-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang YF, Yang CH, Huang CC, Tai MH, Hsu KS. Pharmacological and genetic accumulation of hypoxia-inducible factor-1alpha enhances excitatory synaptic transmission in hippocampal neurons through the production of vascular endothelial growth factor. J Neurosci. 2010;30:6080–6093. doi: 10.1523/JNEUROSCI.5493-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carbajo-Pescador S, Ordonez R, Benet M, Jover R, García-Palomo A, Mauriz JL, González-Gallego J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signaling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 2013;109:83–91. doi: 10.1038/bjc.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nechemia-Arbely Y, Khamaisi M, Rosenberger C, Koesters R, Shina A, Geva C, Shriki A, Klaus S, Rosen S, Rose-John S, et al. In vivo evidence suggesting reciprocal renal hypoxia-inducible factor-1 upregulation and signal transducer and activator of transcription 3 activation in response to hypoxic and non-hypoxic stimuli. Clin Exp Pharmacol Physiol. 2013;40:262–272. doi: 10.1111/1440-1681.12064. [DOI] [PubMed] [Google Scholar]
- 58.Dourlat J, Liu WQ, Sancier F, Edmonds T, Pamonsinlapatham P, Cruzalegui F, Garbay C. A novel non-phosphorylated potential antitumoral peptide inhibits STAT3 biological activity. Biochimie. 2009;91:996–1002. doi: 10.1016/j.biochi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Majumder S, Sreedhara SR, Banerjee S, Chatterjee S. TNF-α signaling beholds thalidomide saga: A review of mechanistic role of TNF-α signaling under thalidomide. Curr Top Med Chem. 2012;12:1456–1467. doi: 10.2174/156802612801784443. [DOI] [PubMed] [Google Scholar]
- 60.El-Aarag BY, Kasai T, Zahran MA, Zakhary NI, Shigehiro T, Sekhar SC, Agwa HS, Mizutani A, Murakami H, Kakuta H, Seno M. In vitro anti-proliferative and anti-angiogenic activities of thalidomide dithiocarbamate analogs. Int Immunopharmacol. 2014;21:283–292. doi: 10.1016/j.intimp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Cook KM, Figg WD. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.People's Republic of China national health and Family Planning Commission of medical affairs authority, Tumor branch of Chinese Medical Association, corp-author. Standard for diagnosis and treatment of colorectal cancer (2015) Chin J Practi Surg. 2015;35:1177–1191. [Google Scholar]
- 63.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 64.Voland C, Bord A, Péleraux A, Pénarier G, Carrière D, Galiègue S, Cvitkovic E, Jbilo O, Casellas P. Repression of cell cycle-related proteins by oxaliplatin but not cisplatin in human colon cancer cells. Mol Cancer Ther. 2006;5:2149–2157. doi: 10.1158/1535-7163.MCT-05-0212. [DOI] [PubMed] [Google Scholar]
- 65.Cremolini C, Loupakis F, Masi G, Lonardi S, Granetto C, Mancini ML, Chiara S, Moretto R, Rossini D, Vitello S, et al. FOLFOXIRI or FOLFOXIRI plus bevacizumab as first-line treatment of metastatic colorectal cancer: A propensity score-adjusted analysis from two randomized clinical trials. Ann Oncol. 2016;27:843–849. doi: 10.1093/annonc/mdw052. [DOI] [PubMed] [Google Scholar]
- 66.Murphy S, Davey RA, Gu XQ, Haywood MC, McCann LA, Mather LE, Boyle FM. Enhancement of cisplatin efficacy by thalidomide in a 9L rat gliosarcoma model. J Neuroancol. 2007;85:181–189. doi: 10.1007/s11060-007-9406-3. [DOI] [PubMed] [Google Scholar]
- 67.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 68.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cross-Knorr S, Lu S, Perez K, Guevara S, Brilliant K, Pisano C, Quesenberry PJ, Resnick MB, Chatterjee D. RKIP phosphorylation and STAT3 activation is inhibited by oxaliplatin and camptothecin and are associated with poor prognosis in stage II colon cancer patients. BMC Cancer. 2013;13:463. doi: 10.1186/1471-2407-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]