Abstract
Previous studies have suggested that metformin, a biguanide family member widely used as an oral antidiabetic drug, may inhibit proliferation and induce apoptosis in certain types of cancer cell. However, the molecular mechanisms underlying metformin-associated anticancer effects, and in particular antimetastatic effects, remain to be fully understood. The present study assessed the efficacy of metformin in inhibiting the migration and invasion of the esophageal carcinoma cell line EC109, and evaluated the effect of metformin on the protein kinase B (AKT) signaling pathway. EC109 cells were treated with 0, 5, 10 or 20 mM metformin during the logarithmic growth phase. A Transwell assay and western blot analysis revealed that metformin inhibited the migration and invasion of EC109 cells, nuclear factor-κB activation, matrix metallopeptidase 9 and N-cadherin expression in a phosphorylated-AKT dependent manner. These results suggested that metformin inhibits the migration and invasion of human esophageal carcinoma cells by suppressing AKT phosphorylation and regulating the expression of migration- and invasion-associated genes.
Keywords: esophageal carcinoma, metformin, protein kinase B, migration, invasion
Introduction
Esophageal carcinoma, one of the most common types of mortality-inducing cancer globally, ranked ninth in cancer incidence and was the sixth leading cause of cancer-associated mortality worldwide in 2013 (1). Esophageal carcinoma is divided into two major histological subtypes: Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. In Asia and the United States, ESCC represents ~90 and ~26% of all cases of esophageal cancer, respectively (2). Current treatment options for patients with esophageal carcinoma include surgery, chemotherapy and radiotherapy. However, the 5-year survival rate for patients with ESCC remains poor (3–5). These data suggest that novel treatment approaches for advanced esophageal carcinoma are required.
Tumor metastasis is associated with complex interactions between primary tumor cells prior to invasion, intravasation, immune escape and extravasation of the circulatory system, followed by lymphangiogenesis/angiogenesis and migration towards target organs (6). The epithelial-mesenchymal transition (EMT), a key event of tumor metastasis, enables epithelial cells to cease exhibiting certain epithelial properties, obtain a mesenchymal phenotype and thereby potentially migrate and invade. This process is associated with alterations in biomarkers, including the proteins of the cell membrane, cytoskeleton, extracellular matrix (ECM), and certain transcription factors (7,8). ECM degradation provides biochemical and mechanical barriers to cell movement and is a crucial process in tumor metastasis. Matrix metallopeptidases (MMPs), a multigene family of zinc-dependent endopeptidases, degrade the majority of ECM components. MMP9 is secreted at the primary tumor site and, by degrading ECM components, may provide tumor cells access to the vasculature and thereby facilitate tumor cell migration and invasion into the target organ (9). Previous studies have suggested that MMP9 activity is increased in multiple metastatic carcinomas, including those occurring in cases of esophageal cancer (10,11).
Metformin, one of the most widely prescribed oral hypoglycemic agents, inhibits hepatic gluconeogenesis and induces the uptake and use of glucose in skeletal muscle and adipose tissue. Metformin was originally established as a glucose level-decreasing drug and became available for human consumption in the 1950s. In 2005, Evans et al (12) demonstrated that in addition to its antidiabetic function, metformin may significantly decrease tumor incidence among patients with type 2 diabetes. Previously, multiple studies have demonstrated that metformin may inhibit proliferation, induce apoptosis and arrest the cell cycle in ESCC cells (13–15). Furthermore, metformin may inhibit malignant cell migration, and the invasion of ovarian, pancreatic cancer and melanoma cells in vitro (16–19).
Materials and methods
Cell lines and culture
The human ESCC cell line EC109 was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) (both from Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) without antibiotics at 37°C in a humidified, 5% CO2 atmosphere. Cells were inoculated onto a Petri dish (106 cells/6 cm) in DMEM with 10% FBS and allowed to attach overnight prior to drug treatment. EC109 cells were treated with 100 nM insulin [a protein kinase B (AKT) activator] and 10 µM LY294002 (a PI3K inhibitor) dissolved in dimethyl sulfoxide (DMSO) for 30 min or 24 h at 37°C in a humidified, 5% CO2 atmosphere, separately or in combination. The EC109 cells treated with metformin in combination with insulin were pretreated with metformin for 23.5 h and subsequently cultured in DMEM containing 10% FBS and 100 nM insulin for 30 min at 37°C in a humidified, 5% CO2 atmosphere. DMSO and ddH2O were used as the negative controls. Metformin and LY294002, a phosphoinositide 3-kinase (PI3K) inhibitor, were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Human recombinant insulin was also from Sigma-Aldrich; Merck KGaA.
Western blot analysis
To extract total cell protein, the EC109 cells (5×105) were sonicated at 50 Hz twice for 3 sec, with 20 sec in between sonication, in 100 µl RIPA buffer supplemented with NaF (1 mM), sodium orthovanadate (1 mM), and phenylmethane sulfonyl fluoride (1 mM), and then homogenized for 30 min in 4°C, and then centrifuged at 4°C and 12,000 × g for 12 min. Cell nuclear protein extraction was performed as previously described (9), and protein concentration was determined using a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 30 µg protein for each sample was separated using SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). Subsequently, the membranes were then blocked with 10% non-fat milk in Tris-buffered saline with Tween-20 (TBST) for 1 h at room temperature. The membranes were incubated with the following primary antibodies: AKT (catalog no. 9272s), phosphorylated (p)-AKT (Ser473; catalog no. 4060s), nuclear factor (NF)-κB (p65; catalog no. 8242s), epithelial (E)-cadherin (catalog no. 3195s), neural (N)-cadherin (catalog no. 4061s) (dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) and MMP9 (dilution, 1:1,000; catalog no. ab38898; Abcam, Cambridge, UK) overnight at 4°C. Membranes were washed three times with TBST and incubated with a HRP-conjugated goat anti-rabbit secondary antibodies (dilution, 1:3,000; catalog no. AS006; Asbio Technology, Inc., Danvers, MA, USA) for 1 h at room temperature, then washed and developed with the ECL Plus Western Blot Detection System kit (Amersham, Piscataway, NJ USA). Antibodies against TATA-box binding protein (catalog no. 8515s) and GAPDH (catalog no. 5014s; dilution, 1:1,000; Cell Signaling Technology, Inc.) were used as loading controls.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the EC109 cells (2×105) using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and 2 µg RNA was reverse transcribed using the PrimeScript™ RT reagent kit, 20 µl volume (Takara Biotechnology Co., Ltd., Dalian, China) according to the supplier's protocol. The reactions were set up with 12.5 µl SYBR-Green PCR Master Mix (Takara Biotechnology Co., Ltd.), 1.0 µl primer mixture (10 µM) and 2 µl cDNA template, and analyzed using a Bio-Rad iCycler system (version 2.1; Bio-Rad Laboratories, Inc., Hercules, CA, USA), in accordance with the manufacturer's cycling protocol: Initial denaturation at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and at 60°C for 30 sec. Gene expression was measured using the SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.). The mRNA expression level of MMP9 for each sample was normalized to that of the housekeeping gene GAPDH by using the 2−ΔΔCq method (20). Primer sequences were as follows: MMP9 forward, 5′-CTTTGACAGCGACAAGAAGTGG-3′ and reverse, 5′-GGCACTGAGGAATGATCTAAGC-3′; GAPDH forward, 5′-GAGCCAAAAGGGTCATCATCTC-3′ and reverse, 5′-AAAGGTGGAGGAGTGGGTGTC-3′.
Migration and invasion assay
In the migration assay, EC109 cell suspensions (each with 105 cells in 200 µl serum-free DMEM) were inoculated into the upper chamber of a 24-well cell culture Transwell insert (pore size, 8 µm; Corning Incorporated, Corning, NY, USA), and the lower chamber was filled with 600 µl DMEM supplemented with 10% FBS. Following 20 h of incubation at 37°C, non-migratory cells in the upper chamber were removed from the upper surface of the filters using a PBS-soaked cotton swab, and cells that had migrated through the membrane were fixed using 92% ice methanol (10 min), stained with 0.2% hematoxylin (7 min) and 0.5% eosin (2 min) at room temperature, and then washed and dried in air. Images of ten randomly selected fields of the migrated/invaded cells were captured and counted under an upright fluorescence microscope (magnification, ×200; Ni-E; Nikon Corporation, Tokyo, Japan). Invasion assays were performed using the same protocol, except with Matrigel-coated Transwell chambers and incubating for 22 h at 37°C. Cells that were pretreated with ddH2O and the same concentration of DMSO as LY294002 were used as the negative control.
Statistical analysis
Data were expressed as the mean ± standard error of the mean of three independent experiments and analyzed using GraphPad Prism version 6.0 software (GraphPad, Inc., La Jolla, CA, USA). One-way ANOVA with the Student-Newman-Keuls as the post hoc test was used to determine statistical differences between parametric data. P<0.05 and P<0.01 were considered to indicate a statistically significant difference.
Results
Effects of metformin on migration and invasion in human EC109 cells
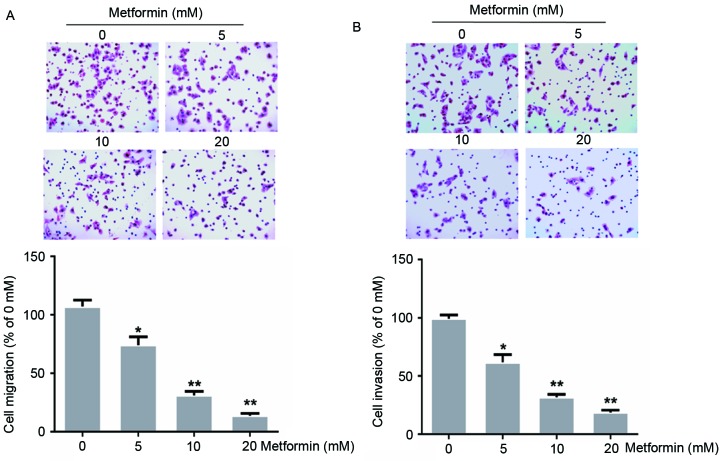
A previous study demonstrated that treatment with 20 mM metformin for 72 h suppressed EC109 cell proliferation (13). To evaluate whether metformin treatment influenced EC109 cell metastasis, the present study evaluated the ability of EC109 cells to migrate through a Transwell membrane coated with Matrigel to assess invasion, or without to assess migration, following treatment with 0, 5, 10 or 20 mM metformin for 24 h. In the present study, metformin treatment decreased the migration and invasion of EC109 cells dose-dependently (Fig. 1). Treatment with 20 mM metformin inhibited EC109 cell migration and invasion by 87 and 81%, respectively (P<0.01).
Figure 1.
Effects of metformin on the migration and invasion of the human esophageal carcinoma cell line EC109. Cells were treated with 0, 5, 10 or 20 mM metformin for 24 h. (A) Migration and (B) invasion were evaluated using a Transwell assay without and with Matrigel, respectively. Data were presented as the mean ± standard error of three independent experiments performed in triplicate (magnification, ×200). *P<0.05 and **P<0.01 vs. control cells.
Effect of metformin on the expression of p-AKT and NF-κB in human EC109 cells
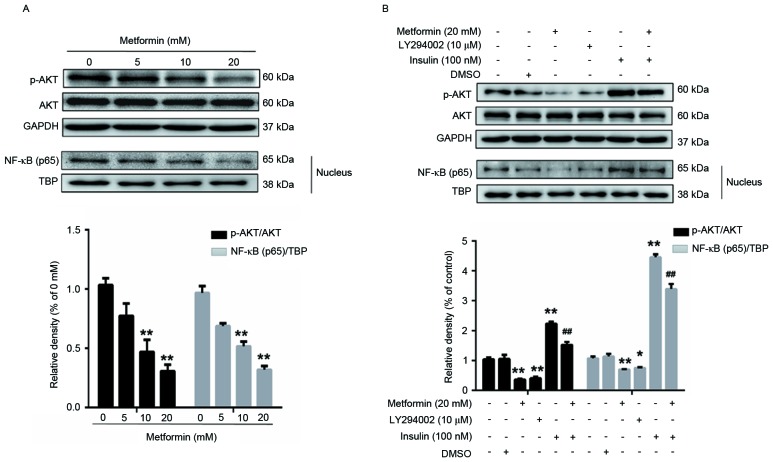
AKT, a key downstream effector of the PI3K signaling pathway, is associated with metastasis in colorectal cancer, intrahepatic cholangiocarcinoma, lung, breast cancer (21–24) and esophageal carcinoma (25). In these human epithelial malignancies, AKT is a key mediator of EMT induction. In addition, previous studies have reported that NF-κB is a target of AKT (26,27). Therefore, the present study used western blot analysis to assess the expression of p-AKT and NF-κB (p65) in EC109 cells following metformin treatment (Fig. 2). Following treatment with 0, 5, 10 or 20 mM metformin for 24 h, the expression of p-AKT and NF-κB (p65) in EC109 cells decreased dose dependently (Fig. 2A). Treatment with 20 mM metformin decreased the expression of p-AKT by 70% and that of NF-κB (p65), a p-AKT target, by 68% in EC109 cells, compared with untreated EC109 cells.
Figure 2.
Effects of metformin, LY294002 or insulin, separately or in combination on the expression of p-AKT and NF-κB (p65) in EC109 cells. (A) Cells were treated with 0, 5, 10 or 20 mM metformin for 24 h. (B) Cells were treated with 20 mM metformin or 10 µM LY294002 for 24 h and 100 nM insulin for 30 min. Cells were lysed prior to western blot analysis using antibodies against AKT, p-AKT (Ser473) and NF-κB (p65) proteins. GAPDH was used as a loading control. Data were presented as the mean ± standard error of three independent experiments performed in triplicate. *P<0.05 and **P<0.01 vs. control cells; ##P<0.01 vs. metformin-treated cells. AKT, protein kinase B; p-, phosphorylated; NF-κB, nuclear factor-κB.
Effects of metformin on the expression of E-cadherin, N-cadherin and MMP9 in human EC109 cells
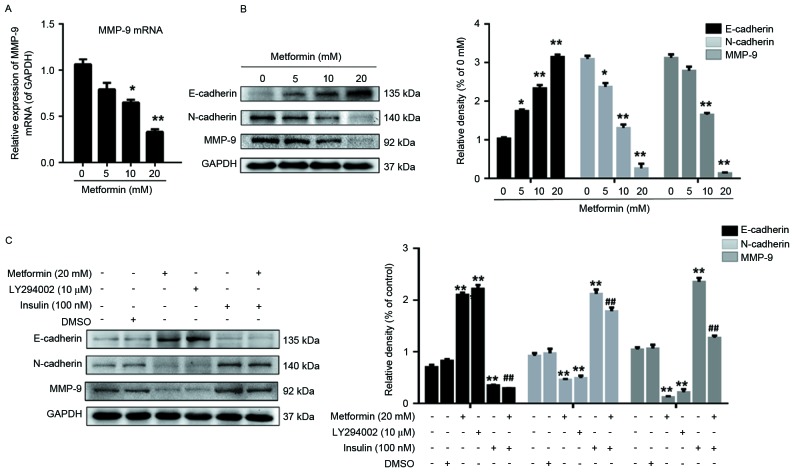
EMT results in epithelial cells acquiring fibroblast-like properties, expressing mesenchymal markers, including N-cadherin and vimentin, and decreasing the expression of epithelial markers, including E-cadherin. The present study evaluated whether metformin is associated with the regulation of EMT, a key step in tumor invasion. The results of the present study indicated that MMP9 transcription was dose-dependently decreased by metformin (Fig. 3A). In addition, the result revealed that metformin treatment upregulated and downregulated E-cadherin and N-cadherin expression, respectively, in a dose-dependent manner (Fig. 3B).
Figure 3.
Effects of metformin, LY294002 or insulin, separately or in combination on the expression of E-cadherin, N-cadherin and MMP9 in EC109 cells. (A) Cells were treated with 0, 5, 10 or 20 mM metformin for 24 h and the mRNA expression of MMP9 was subsequently measured. (B) Cells were treated with 0, 5, 10 or 20 mM metformin for 24 h, lysed prior to western blot analysis and probed with antibodies against E-cadherin, N-cadherin and MMP9 proteins. (C) Cells were treated with 20 mM metformin, 10 µM LY294002 for 24 h or 100 nM insulin for 30 min, lysed prior to western blot analysis and probed with antibodies against E-cadherin, N-cadherin and MMP9 proteins. GAPDH was used as the loading control. Data were presented as the mean ± standard error of three independent experiments performed in triplicate. *P<0.05 and **P<0.01 vs. control cells; ##P<0.01 vs. metformin. E, epithelial, N, neural, MMP, matrix metallopeptidase.
MMP9 may represent a key mediator in tumor cell migration and invasion by stimulating ECM degradation, and increased MMP9 expression is associated with tumor progression (28,29). Therefore, the present study assessed whether metformin affected MMP9 protein expression in EC109 cells, and demonstrated that MMP9 expression in EC109 cells was decreased by metformin treatment, particularly at 20 mM metformin, compared with that in untreated EC109 cells (Fig. 3B).
Metformin inhibits the migration and invasion of EC109 cells by targeting the AKT signaling pathway
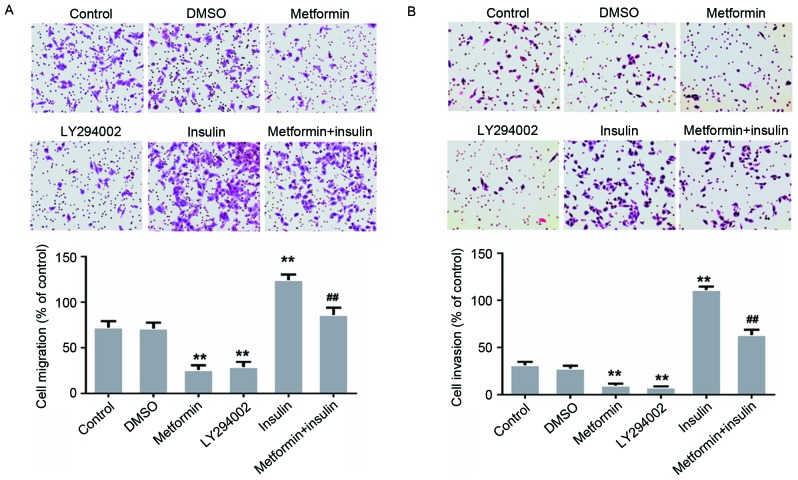
To further explore whether the AKT signaling pathway is associated with EC109 cell migration and invasion, EC109 cells were treated with 100 nM insulin (an AKT activator) and 10 µM LY294002 (a PI3K inhibitor) for 30 min or 24 h, separately or in combination. The EC109 cells treated with metformin in combination with insulin were pretreated with metformin for 23.5 h and subsequently cultured in DMEM containing 10% FBS and 100 nM insulin for 30 min (Fig. 4). The results of the present study demonstrated that LY294002 inhibited the migration and invasion of the EC109 cells (Fig. 4). However, this metformin-mediated inhibition was decreased following treatment with insulin for 30 min (Fig. 4). The results of the present study suggested that metformin inhibited the migration and invasion of EC109 cells via the AKT pathway.
Figure 4.
Effects of metformin, LY294002 or insulin, separately or in combination on the migration and invasion of the human esophageal carcinoma cell line EC109. Cells were incubated with 20 mM metformin or 10 µM LY294002 for 24 h or 100 nM insulin for 30 min. Migration and invasion were assessed using (A) Transwell migration and (B) Matrigel invasion assays, respectively. Data were presented as the mean ± standard error of three independent experiments performed in triplicate (magnification, ×200). **P<0.01 vs. control cells; ##P<0.01 vs. metformin-treated cells.
Metformin inhibits the expression of migration- and invasion-associated proteins in EC109 cells by targeting the AKT signaling pathway
EC109 cells were treated with LY294002 for 24 h or with insulin for 30 min, separately or in combination. Compared with the control cells, the expression of p-AKT/AKT and NF-κB (p65) was decreased (Fig. 2B), the protein expression of MMP9 and N-cadherin was downregulated, and the expression of E-cadherin was increased in LY294002-treated cells (Fig. 3C). Compared with the control cells, the expression of p-AKT/AKT and NF-κB (p65) in the insulin-treated EC109 cells was increased and the metformin-induced suppression of p-AKT/AKT and NF-κB (p65) was decreased (Fig. 2B). In addition, the expression of MMP9 and N-cadherin significantly increased, and the expression of E-cadherin decreased, in EC109 cells treated with insulin for 30 min compared with the control cells (Fig. 3C). The results of the present study suggested that the AKT signaling pathway may be associated with the inhibition of migration- and invasion-associated protein expression in EC109 cells by metformin.
Discussion
Esophageal carcinoma is characterized by a decreased cure rate and the potential development of invasive properties. The increased mortality rate with which esophageal carcinoma is associated is primarily due to metastasis and the side effects and increased rate of recurrence associated with treatment. Therefore, the present study assessed the antimetastatic effect of metformin on human esophageal cancer cells.
Patients treated with metformin, a nontoxic and antihyperglycemic drug rarely report the side effect of lactic acidosis. Previous studies have evaluated the effects metformin may induce through AMP-activated protein kinase-dependent mechanisms, and have demonstrated that metformin decreases the expression of p-AKT in bladder cancer, NRAS proto-oncogene mutant melanoma cell line DO4 and squamous cell carcinoma (SCC) cell line A431 (30–32). In the present study, metformin inhibited EC109 cell migration and invasion. Previous studies have demonstrated that the mechanisms underlying these effects were associated with the downregulation of p-AKT expression (33,34). Consistent with these previous studies, the results of the present study suggested that metformin inhibited invasion in EC109 cells by inhibiting AKT activation. The present study also demonstrated that the PI3K inhibitor LY294002 inhibited EC109 cell migration and invasion, while the AKT activator insulin decreased metformin-induced metastasis inhibition in EC109 cells. These results suggested that the AKT pathway was suppressed in the metformin-treated EC109 cells, which may indicate that metformin inhibits migration and invasion in ESCC cells. To the best of our knowledge, the present study is the first to demonstrate in vitro that metformin inhibits ESCC metastasis by inhibiting AKT activation.
In tumor cells, the nuclear transcription factor NF-κB is formed as an inactive complex in the cytoplasm by p65, p50 and NFKB inhibitor α (NFKBIA). Once this complex is activated, IκB kinase (IKK) phosphorylates NFKBIA, p65 is subsequently released and NF-κB translocates to the nucleus and regulates the expression of multiple genes (35), including MMPs, resulting in tumor cell invasion, migration and metastasis (36,37). AKT, which phosphorylates IKKα, is associated with NF-κB activation (38). In the gastric cancer cell line BGC-823, the overexpression of death associated protein kinase 3 (DAPK3) induced AKT activation and increased the phosphorylation of IKKα, NFKBIA and NF-κB, while the AKT inhibitor LY294002 significantly decreased the phosphorylation of IKKα, NFKBIA, NF-κB, and prevented DAPK3-induced BGC-823 cell migration and invasion (39). In the present study, NF-κB (p65) expression was decreased in the EC109 cells treated with metformin or LY294002, but increased in insulin-treated EC109 cells pretreated with or without metformin.
MMPs participate in normal connective tissue homeostasis and remodeling, and are associated with ECM degradation, alterations to cell-cell and cell-ECM interactions, tumor formation, invasion and metastasis (40,41). Since AKT promoted invasion in the human fibrosarcoma cell line HT1080 by increasing cell motility and MMP9 expression in a previous study (42), LY294002-induced suppression of MMP9 expression may have resulted in the inhibition of EC109 cell invasion demonstrated in the present study. The present study evaluated the expression of MMP9 in the EC09 cells following metformin treatment for 24 h. The expression of MMP9 was inhibited by metformin (Fig. 3A and B) and LY294002 (Fig. 3C). Therefore, the suppression of the AKT pathway by metformin may have resulted in the decrease in MMP9 expression in the EC09 cells identified in the present study.
E-cadherin is a Ca2+-dependent cell adhesion molecule expressed by epithelial cells. The positive expression of E-cadherin is involved in establishing cell-cell connections, and maintaining epithelial polarity and structural integrity. The absence of E-cadherin in epithelial cells results in decreased cell-cell adhesion and induces tumor cell invasion and metastasis (43). N-cadherin, a transmembrane glycoprotein, is expressed by numerous types of cell, including neuroepithelial cells, neurons and mesenchymal cells. N-cadherin mediates Ca2+-dependent cell-cell adhesion through the homophilic interactions of its extracellular domains during mesenchymal condensation (11). A previous study suggested that N-cadherin is associated with increased invasion in tumor cells and may contribute to the mesenchymal-scattered phenotype associated with decreased E-cadherin and cadherin 3 expression in SCC cells (44). Consistent with this previous study, the expression of E-cadherin and N-cadherin in the present study was regulated in the metformin-treated EC109 cells.
The results of the present study suggested that metformin inhibited EC109 cell migration and invasion by downregulating the AKT pathway and that p-AKT suppression may have induced the mesenchymal-to-epithelial reverting transition in the EC109 cells by inhibiting the NF-κB signaling pathway and decreasing MMP9 expression. Therefore, metformin potentially represents a useful therapeutic tool for controlling metastasis in patients with esophageal carcinoma.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81172263).
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Ekman S, Dreilich M, Lennartsson J, Wallner B, Brattström D, Sundbom M, Bergqvist M. Esophageal cancer: Current and emerging therapy modalities. Expert Rev Anticancer Ther. 2008;8:1433–1448. doi: 10.1586/14737140.8.9.1433. [DOI] [PubMed] [Google Scholar]
- 4.Li LY, Jiang H, Xie YM, Liao LD, Cao HH, Xu XE, Chen B, Zeng FM, Zhang YL, Du ZP, et al. Macrolide analog F806 suppresses esophageal squamous cell carcinoma (ESCC) by blocking β1 integrin activation. Oncotarget. 2015;6:15940–15952. doi: 10.18632/oncotarget.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Gu X. Silencing of insulin-like growth factor-1 receptor enhances the radiation sensitivity of human esophageal squamous cell carcinoma in vitro and in vivo. World J Surg Oncol. 2014;12:325. doi: 10.1186/1477-7819-12-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miovic M, Block S. Psychiatric disorders in advanced cancer. Cancer. 2007;110:1665–1676. doi: 10.1002/cncr.22980. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Wang Y, Li H, Li Q, Yu Y, Xu X, Xu B, Liu T. Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget. 2016;7:34356–34370. doi: 10.18632/oncotarget.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Shen KH, Chiang TA, Shih YW. Acacetin inhibits TPA-induced MMP-2 and u-PA expressions of human lung cancer cells through inactivating JNK signaling pathway and reducing binding activities of NF-kappaB and AP-1. J Food Sci. 2010;75:H30–H38. doi: 10.1111/j.1750-3841.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 10.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alizadeh AM, Shiri S, Farsinejad S. Metastasis review: From bench to bedside. Tumour Biol. 2014;35:8483–8523. doi: 10.1007/s13277-014-2421-z. [DOI] [PubMed] [Google Scholar]
- 12.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen X, Guan Y, Chen C, Jing X. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal squamous cell carcinomas in vitro and in vivo. PLoS One. 2015;10:e0133349. doi: 10.1371/journal.pone.0133349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi M, Kato K, Iwama H, Fujihara S, Nishiyama N, Mimura S, Toyota Y, Nomura T, Nomura K, Tani J, et al. Antitumor effect of metformin in esophageal cancer: In vitro study. Int J Oncol. 2013;42:517–524. doi: 10.3892/ijo.2012.1722. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu B, Li S, Sheng L, Zhu J, Gu L, Shen H, La D, Hambly BD, Bao S, Di W. Metformin inhibits the development and metastasis of ovarian cancer. Oncol Rep. 2012;28:903–908. doi: 10.3892/or.2012.1890. [DOI] [PubMed] [Google Scholar]
- 17.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483–491. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, Sarkar FH. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila) 2012;5:355–364. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, Allegra M, Giacchero D, Bahadoran P, Bertolotto C, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12:1605–1615. doi: 10.1158/1535-7163.MCT-12-1226-T. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Rui X, Yan XI, Zhang K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol Lett. 2016;11:685–688. doi: 10.3892/ol.2015.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie K, Nian J, Zhu X, Geng X, Liu F. Modulatory role of garlicin in migration and invasion of intrahepatic cholangiocarcinoma via PI3K/AKT pathway. Int J Clin Exp Pathol. 2015;8:14028–14033. [PMC free article] [PubMed] [Google Scholar]
- 23.Yoneyama R, Aoshiba K, Furukawa K, Saito M, Kataba H, Nakamura H, Ikeda N. Nicotine enhances hepatocyte growth factor-mediated lung cancer cell migration by activating the α7 nicotine acetylcholine receptor and phosphoinositide kinase-3-dependent pathway. Oncol Lett. 2016;11:673–677. doi: 10.3892/ol.2015.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Song Z, Wang Y, Yin Y, Liu Y, Yuan R, Nan X. Overexpression of SphK1 enhances cell proliferation and invasion in triple-negative breast cancer via the PI3K/AKT signaling pathway. Tumour Biol. 2016;37:10587–10593. doi: 10.1007/s13277-016-4954-9. [DOI] [PubMed] [Google Scholar]
- 25.Tantai JC, Zhang Y, Zhao H. Heterophyllin B inhibits the adhesion and invasion of ECA-109 human esophageal carcinoma cells by targeting PI3K/AKT/β-catenin signaling. Mol Med Rep. 2016;13:1097–1104. doi: 10.3892/mmr.2015.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Yang Z, Passaniti A, Lapidus RG, Liu X, Cullen KJ, Dan HC. A positive feedback loop involving EGFR/Akt/mTORC1 and IKK/NF-κB regulates head and neck squamous cell carcinoma proliferation. Oncotarget. 2016;7:31892–31906. doi: 10.18632/oncotarget.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh SC, Tsai JP, Yang SF, Tang MJ, Hsieh YH. Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a downregulation of the ERK/JNK-mediated NF-κB-dependent pathway that reduces uPA and MMP-9 expression. Amino Acids. 2014;46:2809–2822. doi: 10.1007/s00726-014-1838-4. [DOI] [PubMed] [Google Scholar]
- 30.Peng M, Su Q, Zeng Q, Li L, Liu Z, Xue L, Cheng Y, Huang Y, Tao T, Lv H, et al. High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model. Oncotarget. 2016;7:9102–9117. doi: 10.18632/oncotarget.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vujic I, Sanlorenzo M, Posch C, Esteve-Puig R, Yen AJ, Kwong A, Tsumura A, Murphy R, Rappersberger K, Ortiz-Urda S. Metformin and trametinib have synergistic effects on cell viability and tumor growth in NRAS mutant cancer. Oncotarget. 2015;6:969–978. doi: 10.18632/oncotarget.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Zhang Y, Jia K, Dong Y, Ma W. Metformin inhibits the proliferation of A431 cells by modulating the PI3K/Akt signaling pathway. Exp Ther Med. 2015;9:1401–1406. doi: 10.3892/etm.2015.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao H, Zhou Q, Gu Y, Duan T, Feng Y. Luteinizing hormone facilitates angiogenesis in ovarian epithelial tumor cells and metformin inhibits the effect through the mTOR signaling pathway. Oncol Rep. 2012;27:1873–1878. doi: 10.3892/or.2012.1745. [DOI] [PubMed] [Google Scholar]
- 34.Tan BK, Adya R, Chen J, Lehnert H, Sant Cassia LJ, Randeva HS. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2011;96:808–816. doi: 10.1210/jc.2010-1803. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Wu P, Zhao L, Huang L, Zhang Z, Zhao S, Huang J. NF-κB expression and outcomes in solid tumors: A systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1687. doi: 10.1097/MD.0000000000001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Li Z, Chang Y, Ma L, Xu W, Li M, Li J, Zhang W, Sun Q, An X, Li Z. Relationship between NF-κB, MMP9, and MICA expression in pituitary adenomas reveals a new mechanism of pituitary adenomas immune escape. Neurosci Lett. 2015;597:77–83. doi: 10.1016/j.neulet.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295:214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Deng Z, Wang Z, Wang D, Zhang L, Su Q, Lai Y, Li B, Luo Z, Chen X, et al. Zipper-interacting protein kinase promotes epithelial-mesenchymal transition, invasion and metastasis through AKT and NF-κB signaling and is associated with metastasis and poor prognosis in gastric cancer patients. Oncotarget. 2015;6:8323–8338. doi: 10.18632/oncotarget.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martano M, Corteggio A, Restucci B, De Biase ME, Borzacchiello G, Maiolino P. Extracellular matrix remodeling in equine sarcoid: An immunohistochemical and molecular study. BMC Vet Res. 2016;12:24. doi: 10.1186/s12917-016-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stallings-Mann M, Radisky D. Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs. 2007;185:104–110. doi: 10.1159/000101310. [DOI] [PubMed] [Google Scholar]
- 42.Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 43.Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. doi: 10.1016/S0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 44.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]