Abstract
Lung cancers are the most commonly diagnosed malignant tumors, and are one of the leading causes of morbidity and mortality worldwide. Dexamethasone (DEX) serves an important function in the regulation of lung cancer cell proliferation; however, the mechanisms involved still remain unknown. In the present study, the effects of DEX on A549 cell proliferation and apoptosis were examined, in addition to the potential downstream regulatory mechanisms underlying these effects. A549 cells were treated with different concentrations of DEX at 12, 24 and 48 h time points, followed by the addition of SB431542, an inhibitor of the TGF-β1 receptor, to block the TGF-β1 signaling pathway. Cell proliferation was analyzed using a 3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt. The apoptosis rate was measured by Hoechst 33342 and Annexin V/propidium iodide staining and the expression of transforming growth factor (TGF)-β1, Smad family member 2 (Smad2) and caspase-3 were assessed by western blot. The results from the present study demonstrated that the proliferation of A549 cells decreased and the apoptosis rate significantly increased following DEX treatment (P<0.05). Furthermore, the expression of TGF-β1, Smad2 and caspase-3 were significantly increased following DEX stimulation (P<0.05), the effects of which were abrogated by the addition of the TGF-β1 receptor inhibitor, SB431542 (P<0.05). DEX-induced apoptosis in A549 cells, and this effect was abrogated by SB431542, an inhibitor of TGF-β1 receptor signaling, which indicated that the TGF-β1/Smad2 pathway may be associated with this process and SB431542 may function as an antitumor drug in the future.
Keywords: dexamethasone, A549 cells, SB431542, transforming growth factor-β1/Smad family member 2, caspase-3
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide, with ~1,590,000 mortalities in 2012 (1). Although there have been considerable advances in the treatment for lung cancer in previous decades, this disease still remains incurable. Dexamethasone (DEX) is widely used in the clinic, however its pharmacological effects are mainly anti-inflammatory and anti-allergic (2,3). There is a growing body of literature, which reports on the beneficial effects of DEX in tumors. DEX exerts inhibitory effects on cell migration and invasion of colon cancer (4); promotes cell proliferation via inhibiting apoptosis of bladder cancer cells (5); induces apoptosis in a leukemia cell line (6) and pre-treatment of lung cancer patients with DEX reduced hematological toxicity and enhanced efficacy of chemotherapy drugs (7). Previously, it has been reported that DEX effectively inhibits the growth of Lewis lung carcinoma, which indicated that DEX has an antitumor effect in lung cancer (8). In addition, another study reported that DEX contributed another function by inhibiting transforming growth factor (TGF)-β1 signaling by downregulating the expression and secretion of TGF-β1 (9,10).
The TGF-β family is comprised of multifunctional cytokines that function as tumor suppressors by inhibiting cell proliferation and inducing apoptosis in normal epithelial cells and precancerous tissues. However, TGF-β also accelerates the progression of established cancers by promoting cell proliferation, invasion, and metastasis (11,12) TGF-β1 is a member of the TGF-β family, and functions in the regulation of cell growth and differentiation, and contributing to the apoptotic pathway (13–16). A previous study reported that treating A549 cells with TGF-β1 may enhance the apoptosis of A549 cells but prolonged exposure to TGF-β1 inhibited the apoptosis induced by Fas/Fasl (17). SB-431542 is a small molecule inhibitor that was identified as an inhibitor of TGF-β, with the capacity to inhibit phosphorylation of Smad family member 2 (Smad2) (18). Smad2 is involved in critical role in TGF-β induced apoptosis of prostate epithelial cells, which is activated by TGF-β1 (19). The aim of the present study was to investigate the involvement of Smad2 in TGF-β1 induced apoptosis of A549 cells.
In the present study, it was observed that the proliferation of A549 cells decreased and the apoptosis rate significantly increased following exposure to DEX. Furthermore, the expression of TGF-β1 and Smad2 were significantly increased following DEX stimulation, and this effect was partially abrogated by SB431542. The results of the present study concluded that the TGF-β1/Smad2 pathway may be involved in DEX induced apoptosis of A549 cells.
Materials and methods
Cell culture
The A549 cells were provided by the Drug Engineering Research Center of Chongqing Medical University (Chongqing, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator containing 5% CO2 in air. The medium was changed every 2 days. A549 cells in the logarithmic growth phase treated with DEX (Tianjin Jinyao Amino Acid Co., Ltd., Tianjin, China) and SB431542 (Med Chem Express Co., Monmouth Junction, NJ, USA) were used for the following experiments. All the cells were harvested using pancreatin (Thermo Fisher Scientific, Inc.), washed with PBS and collected following centrifugation at 200 × g for 5 min at room temperature.
3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) proliferation assay
The MTS cell proliferation assay was used to quantify viable A549 cells. In brief, A549 cells were seeded onto 96-well microplates (5×104 cells/well) in 100 µl culture medium (DMEM with 10% FBS) and cultured until the cells reached 70% confluency. Following this, cells were further cultured for 0, 12, 24 or 48 h, with medium containing DEX at a range of concentrations (0, 0.1, 1.0 and 10 mmol/l). The cell proliferation assay was performed using the MTS reagent kit (MTS; Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol. In brief, 10 µl MTS was added, and cells were incubated at 37°C in a humidified incubator for 1–2 h. The absorbance values for each well were detected at 490 nM using a micro plate reader (Omega Bio-Tek Inc., Norcross, GA, USA).
Hoechst 33342 and Annexin V/propidium iodide (PI) staining
The DEX- or SB431542-treated A549 cells were fixed in 0.5 ml formalin (4%) at room temperature for 10 min, washed twice in PBS, and stained with 0.5 ml Hoechst 33342 at room temperature for 5 min. Cells were washed twice with PBS, and cell nuclei in five random fields were observed using a fluorescence microscope (Olympus Corporation, Tokyo, Japan; magnification, ×200). The apoptosis rate of A549 cells was measured using flow cytometry with Annexin V-fluorescein isothiocyanate (FITC)/PI Apoptosis Detection kit, which was purchased from the Beyotime Institute of Biotechnology (Shanghai, China). In brief, all cells were washed with PBS to remove the medium. A minimum of 1×105 cells were resuspended in 100 µl binding buffer containing Annexin V-FITC and PI (Annexin V-FITC/PI Apoptosis Detection kit; Beyotime Institute of Biotechnology). A FACScan flow cytometer was used to quantify Annexin V-FITC and PI binding using channels FL-1 (Annexin V-FITC) and FL-3 (PI). Quadrant analysis was performed using the Cellquest Pro software (version 5.1; BD Biosciences, Franklin Lanes, NJ, USA).
Western blot analysis
The pre-cooled cells from each treatment group were harvested for total protein extraction and treated with a lysis buffer containing 20 mmol/l Tris (PH 7.5), 150 mmol/l NaCl, 1% Triton X-100 and inhibitors of protease and phosphates on ice for 30 min. The cell lysis products were centrifuged for 10 min at 2,000 × g in a 4°C refrigerated centrifuge and the supernatants were collected. The final protein concentration was measured using a BCA protein kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and supernatants were boiled for 5 min. An aliquot of 40 µg of cellular protein was electrophoresed on an 8% gel using SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. The membrane was then blocked for 2 h with 5% bovine serum albumin (Beyotime Institute of Biotechnology) at room temperature, and incubated overnight with primary antibodies against TGF-β1 (cat. no. ab92486), phosphorylated (p-)Smad2 (cat. no. ab53100), cleaved caspase-3 (cat. no. ab136812) and β-actin (cat. no. ab8226; 1:1,000; Abcam, Cambridge, MA, USA) at 4°C. Membranes were washed with Tris-buffered saline containing 0.1% Tween-20 and incubated for 2 h with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G secondary antibody (cat. no. ab97035) at room temperature (1:1,000; Abcam). The immunoreactivity of each protein was visualized using the Millipore western blot chemiluminescence horseradish peroxidase substrate ECL Chemiluminescence reagent kit (EMD Millipore, Billerica, MA, USA). The results were analyzed using Quantity One software (version 4.4.02; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All the experiments were performed in triplicate and data were expressed as the mean ± standard deviation. Statistical significance was analyzed using a one-way analysis of variance followed by Dunnett's post hoc test to analyze the difference between DEX groups. A student's t-test was performed to compare the differences among medicine groups (GraphPad Prism, version 5.01; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
DEX treatment decreased the proliferation and increased the apoptosis rate of A549 cells
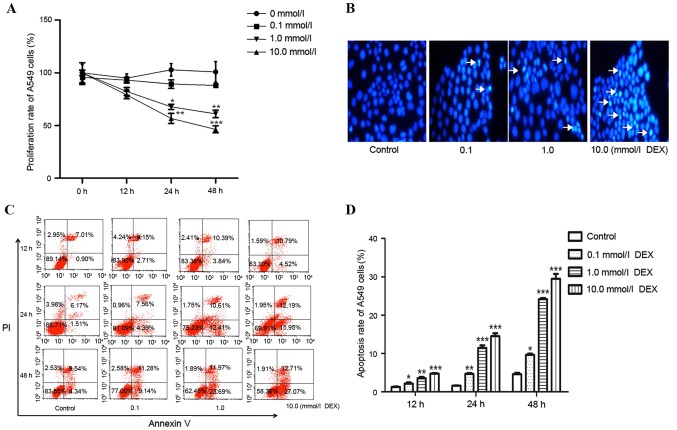
In order to investigate whether DEX decreases the proliferation of A549 cells in a dose-time-dependent manner, cells were treated with DEX at concentrations of 0.1, 1.0 and 10.0 mmol for 0, 12, 24 and 48 h (Fig. 1A). A significant time- and dose-dependent decrease of proliferation in A549 cells was observed from 1.0–10.0 mmol DEX, following 24 and 48 h of culture. Hoechst 33342 staining was performed to observe the nuclei change in A549 cells. The nuclei of the DEX-treated group emitted white blue fluorescence, whereas the control group emitted blue fluorescence, which indicated a dose-dependent increase in apoptosis in the DEX-treated group (Fig. 1B). Flow cytometry was conducted to test the apoptotic rate of cells. The results demonstrated that the rate of early apoptotic death in DEX-treated groups was significantly higher compared with that of the control. In addition, DEX induced apoptosis in a time and dose-dependent manner (Fig. 1C-D).
Figure 1.
The anticancer effect of DEX in A549 cells. (A) Cell proliferation rate following DEX treatment (0, 0.1, 1.0 or 10.0 mmol/l) for 0, 12, 24 and 48 h, as assessed by 3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt assay. (B) Nuclear morphological changes of apoptotic cells following Hoechst staining (magnification, ×200). Arrows indicate pathologic changes of apoptosis. (C) A549 cells were treated with DEX (0, 0.1, 1.0 or 10.0 mmol/l) for 12, 24 and 48 h, and the apoptosis rate was tested by flow cytometry, with (D) quantification. *P<0.05, **P<0.01 and ***P<0.001 vs. control. DEX, dexamethasone.
Protein expression levels of TGF-β1, Smad2 and caspase-3 in A549 cells were significantly increased following DEX exposure
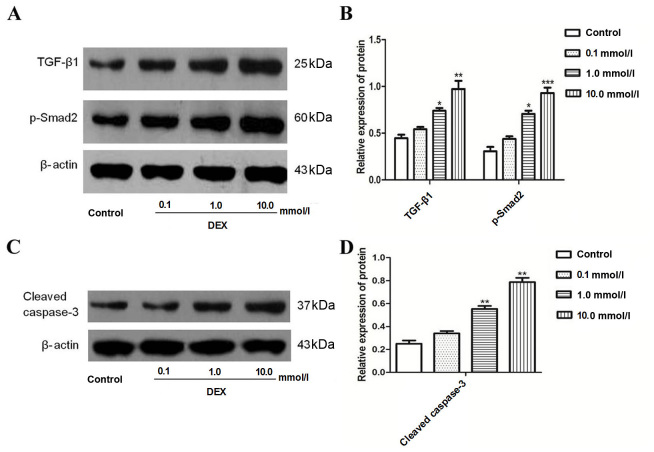
To investigate whether DEX induced the expression of TGF-β1 and Smad2 in A549 cells, cells were treated with 0.1–10.0 mmol/l DEX for 48 h. The results demonstrated that DEX significantly increased the expression of TGF-β1 and Smad2 when compared with the control (Fig. 2A and B). Cleaved caspase-3, an indicator of apoptosis, was also measured. The results from the present study revealed that DEX significantly increased the expression of cleaved caspase-3 (Fig. 2C and D).
Figure 2.
Expression of TGF-β1, Smad2 and caspase-3 proteins in A549 cells following DEX exposure. (A) The expression of TGF-β1 and Smad2 as assessed by western blotting following DEX treatment (0, 0.1, 1.0 or 10.0 mmol/l) for 48 h. (B) The graph represents densitometry analysis, which demonstrates the increase in TGF-β1 and Smad2 following DEX treatment at (1.0 or 10.0 mmol/l) for 48 h. (C) The expression of cleaved caspase-3 as assessed by western blotting. (D) The graph represents densitometry analysis, which demonstrates the increase in cleaved caspase-3 following DEX treatment at (1.0 or 10.0 mmol/l) for 48 h. Densitometry analysis represents three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control. TGF-β1, transforming growth factor-β1; DEX, dexamethasone.
Cell apoptosis induced by DEX exposure is inhibited by SB431542 treatment
To explore whether TGF-β1/Smad2 signaling was involved in DEX-induced apoptosis of A549 cells, the TGF-β1 receptor was blocked with SB431542 at a concentration of 10 mmol/l, which was previously verified in a preliminary experiment (data not shown). The results of flow cytometry demonstrated that DEX increased the apoptosis rate of A549 cells, and in response to SB431542, the apoptosis of A549 cells induced by DEX was significantly inhibited (Fig. 3A and B; P<0.05). Hoechst staining revealed that SB431542 treatment also protected A549 cells from DEX induced apoptosis (Fig. 3C).
Figure 3.
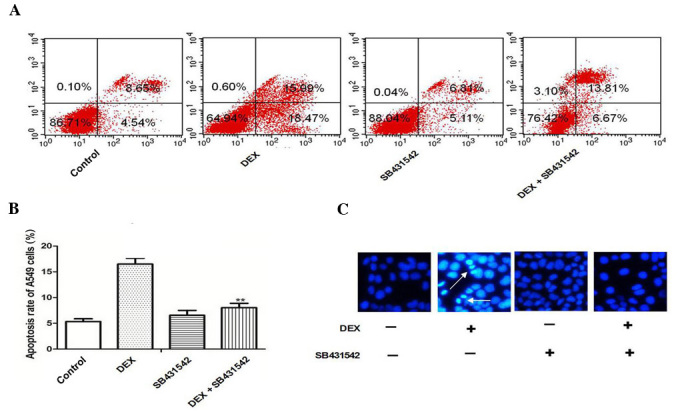
Effects of SB431542 on DEX-treated A549 cells. (A) The apoptosis rate of A549 cells following SB431542 exposure was analyzed by flow cytometry, with (B) quantification. (C) The nuclear morphological changes of apoptotic cells following Hoechst staining (magnification, ×200; arrows indicate apoptotic cells). The graph represents densitometry of three independent experiments. **P<0.01 vs. control. DEX, dexamethasone.
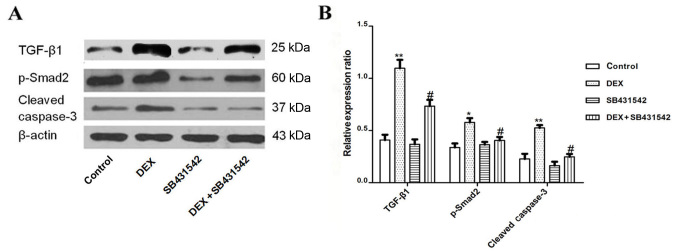
DEX-induced protein expression of TGF-β1, Smad2 and caspase-3 was significantly inhibited by SB431542
The present study demonstrated that SB431542 inhibited apoptosis of A549 cells, however the mechanism involved remains unknown. Therefore, in the present study, the TGF-β1 receptor was blocked with SB431542, and the expression of TGF-β1, Smad2 and caspase-3 were analyzed by western blot. The expression of TGF-β1, Smad2 and caspase-3 were significantly decreased in the SB431542 group when compared with the DEX group alone (Fig. 4A and B).
Figure 4.
DEX-induced expression of TGF-β1, Smad2 and caspase-3 was significantly inhibited by SB431542. (A) A549 cells were co-cultured with 10.0 mmol/l DEX with or without 10.0 mmol/l SB431542 for 48 h, the expression of TGF-β1, Smad2 and caspase-3 were tested by western blot analysis and then analyzed. (B) The graph represents densitometry of the results of three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 vs. DEX group. TGF-β1, transforming growth factor-β1; DEX, dexamethasone.
Discussion
In the present study, a mechanism by which DEX induced apoptosis in A549 cells was demonstrated. The results revealed that DEX exposure significantly increased apoptotic cell accumulation, caspase-3 production and TGF-β1/Smad2 pathway activity. The results also revealed that SB431542 inhibited A549 cell apoptosis, which may act via the TGF-β1/Smad2 pathway.
Glucocorticoids are commonly used anti-inflammatory drugs in the clinic, and inhibit TGF-β1 activity (9,20). TGF-β is a multifunctional protein, which influences a variety of cellular functions including cell growth, differentiation and immune regulation. Previous studies have demonstrated that TGF-β1 is involved in the process of apoptosis (21). Smad family proteins are pivotal TGF-β signal transmission carriers and are activated in the cytoplasm prior to transferring into the nucleus, wherein they activate or inhibit the transcription of target genes (22). Miyazaki et al (23) reported that TGF-β1 stimulates or decreases cell proliferation via the down or upregulation of cyclin-dependent kinase inhibitor 1A respectively, which is a direct target of Smad proteins (24,25). Zhang et al (21) reported that inhibition of Smad2/3 gene expression partially decreased the apoptosis rate of gliomas. Yang et al (19) demonstrated that Smad2 is involved in TGF-β induced prostate epithelial cell apoptosis. Taken together, these results suggest that TGF-β1/Smad2 is involved in the regulation of the cell apoptosis process; however, this mechanism has not been previously reported in A549 cells. To the best of our knowledge, the present study was the first to demonstrate a decrease in cell proliferation and an increase in the apoptosis rate of A549 cells following DEX treatment (Fig. 1A-E). Furthermore, the expression of TGF-β1, Smad2 and caspase-3 were significantly increased following DEX exposure, which indicated that the TGF-β1/Smad2 pathway may be involved in DEX-induced apoptosis of A549 cells (Fig. 2A-D).
It has previously been reported that TGF-β1 has a dual role in apoptosis (26). Multiple types of tumor cells may at times secrete TGF-β1, which induces growth factor secretion from stromal cells, which in turn may enhance the proliferation of cancer cells (27,28). This is thought to be one mechanism by which TGF-β1 activity increases the malignancy of cancer (29). However, it was further reported that TGF-β1 enhances apoptosis in A549 cells (18,30). Thus, inhibition of growth factor induction may counteract the effects of TGF-β1 on the suppression of lung tumor growth. Therefore, in the present study, the TGF-β1 receptor was antagonized with SB431542 with or without DEX treatment, and SB431542 was observed to inhibit apoptosis and the expression of TGF-β1, Smad2 and caspase-3 in DEX treated A549 cells (Figs. 3 and 4).
To conclude, DEX-induced apoptosis of A549 cells may function via the induction of the TGF-β1/Smad2 signaling pathway, and DEX may be a potential anti-lung cancer treatment. However, it is suggested for future studies, that the aforementioned experiments in the present study be conducted in an in vivo model, to confirm these results and the therapeutic potential of dexamethasone.
References
- 1.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingawale DK, Mandlik SK, Patel SS. An emphasis on molecular mechanisms of anti-inflammatory effects and glucocorticoid resistance. J Complement Integr Med. 2015;12:1–13. doi: 10.1515/jcim-2014-0051. [DOI] [PubMed] [Google Scholar]
- 3.Choksi A, Sarojini KV, Vadnal P, Dias C, Suresh PK, Khandare J. Comparative anti-inflammatory activity of poly (amidoamine) (PAMAM) dendrimer-dexamethasone conjugates with dexamethasone-liposomes. Int J Pharm. 2013;449:28–36. doi: 10.1016/j.ijpharm.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Hwang YJ, Han SH, Lee YE, Kim S, Kim YJ, Cho JH, Kwon KA, Kim JH, Kim SH. Dexamethasone inhibits hypoxia-induced epithelial-mesenchymal transition in colon cancer. World J Gastroenterol. 2015;21:9887–9899. doi: 10.3748/wjg.v21.i34.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Izumi K, Li Y, Ishiguro H, Miyamoto H. Contrary regulation of bladder cancer cell proliferation and invasion by dexamethasone-mediated glucocorticoid receptor signals. Mol Cancer Ther. 2012;11:2621–2632. doi: 10.1158/1535-7163.MCT-12-0621. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan D, Li G, Podar K, Hideshima T, Neri P, He D, Mitsiades N, Richardson P, Chang Y, Schindler J, et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 2005;65:8350–8358. doi: 10.1158/0008-5472.CAN-05-0163. [DOI] [PubMed] [Google Scholar]
- 7.Rinehart J, Arnold S, Kloecker G, Lim A, Zaydan MA, Baeker T, Maheshwari JG, Carloss H, Slone S, Shelton B, et al. Phase II randomized trial of carboplatin and gemcitabine with or without dexamethasone pre-treatment in patients with Stage IV non-small cell lung cancer. Cancer Chemother Pharmacol. 2013;71:1375–1383. doi: 10.1007/s00280-013-2111-3. [DOI] [PubMed] [Google Scholar]
- 8.Geng Y, Wang J, Jing H, Wang HW, Bao YX. Inhibitory effect of dexamethasone on Lewis mice lung cancer cells. Genet Mol Res. 2014;13:6827–6836. doi: 10.4238/2014.August.29.4. [DOI] [PubMed] [Google Scholar]
- 9.Jang YH, Shin HS, Sun Choi H, Ryu ES, Jin Kim M, Ki Min S, Lee JH, Kook Lee H, Kim KH, Kang DH. Effects of dexamethasone on the TGF-β1-induced epithelial-to-mesenchymal transition in human peritoneal mesothelial cells. Lab Invest. 2013;93:194–206. doi: 10.1038/labinvest.2012.166. [DOI] [PubMed] [Google Scholar]
- 10.Bolkenius U, Hahn D, Gressner AM, Breitkopf K, Dooley S, Wickert L. Glucocorticoids decrease the bioavailability of TGF-beta which leads to a reduced TGF-beta signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2004;325:1264–1270. doi: 10.1016/j.bbrc.2004.10.164. [DOI] [PubMed] [Google Scholar]
- 11.Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson J, Berg T, Kurzejamska E, Pang MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P, Fuxe J. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32:5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakudo N, Kushida S, Suzuki K, Ogura T, Notodihardjo PV, Hara T, Kusumoto K. Effects of transforming growth factor-beta1 on cell motility, collagen gel contraction, myofibroblastic differentiation, and extracellular matrix expression of human adipose-derived stem cell. Hum Cell. 2012;25:87–95. doi: 10.1007/s13577-012-0049-0. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Yang S, Huang J, Ruan S, Zheng Z, Lin J. Tgf-β1 induces autophagy and promotes apoptosis in renal tubular epithelial cells. Int J Mol Med. 2012;29:781–790. doi: 10.3892/ijmm.2012.911. [DOI] [PubMed] [Google Scholar]
- 15.Miao ZF, Li WY, Wang ZN, Zhao TT, Xu YY, Song YX, Huang JY, Xu HM. Lung cancer cells induce senescence and apoptosis of pleural mesothelial cells via transforming growth factor-beta1. Tumour Biol. 2015;36:2657–2665. doi: 10.1007/s13277-014-2888-7. [DOI] [PubMed] [Google Scholar]
- 16.Zheng RP, Bai T, Zhou XG, Xu CG, Wang W, Xu MW, Zhang J. Lefty A protein inhibits TGF-β1-mediated apoptosis in human renal tubular epithelial cells. Mol Med Rep. 2013;8:621–625. doi: 10.3892/mmr.2013.1556. [DOI] [PubMed] [Google Scholar]
- 17.Bai L, Yu Z, Wang C, Qian G, Wang G. Dual role of TGF-β1 on Fas-induced apoptosis in lung epithelial cells. Respiratory Physiol Neurobiol. 2011;177:241–246. doi: 10.1016/j.resp.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Xue L, Liu Z, Huang J, Wen J, Hu J, Bo L, Yang R. Tumor necrosis factor-like weak inducer of apoptosis accelerates the progression of renal fibrosis in lupus nephritis by activating SMAD and p38 MAPK in TGF-β1 signaling pathway. Mediators Inflamm. 2016;2016:8986451. doi: 10.1155/2016/8986451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Wahdan-Alaswad R, Danielpour D. Critical role of Smad2 in tumor suppression and transforming growth factor-beta-induced apoptosis of prostate epithelial cells. Cancer Res. 2009;69:2185–2190. doi: 10.1158/0008-5472.CAN-08-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Lei W, Wang X, Tang Y, Song J. Glucocorticoid induces mesenchymal-to-epithelial transition and inhibits TGF-β1-induced epithelial-to-mesenchymal transition and cell migration. FEBS Lett. 2010;584:4646–4654. doi: 10.1016/j.febslet.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Wu L, Wang J, Li G, Feng D, Zhang B, Li L, Yang J, Ma L, Qin H. Opposing effects of PI3K/Akt and Smad-dependent signaling pathways in NAG-1-induced glioblastoma cell apoptosis. PLoS One. 2014;9:e96283. doi: 10.1371/journal.pone.0096283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koćwin M, Jonakowski M, Przemęcka M, Zioło J, Panek M, Kuna P. The role of the TGF-SMAD signalling pathway in the etiopathogenesis of severe asthma. Pneumonol Alergol Pol. 2016;84:290–301. doi: 10.5603/PiAP.2016.0037. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki M, Ohashi R, Tsuji T, Mihara K, Gohda E, Namba M. Transforming growth factor-beta 1 stimulates or inhibits cell growth via down- or up-regulation of p21/Waf1. Biochem Biophys Res Commun. 1998;246:873–880. doi: 10.1006/bbrc.1998.8712. [DOI] [PubMed] [Google Scholar]
- 24.Yang N, Zhao B, Rasul A, Qin H, Li J, Li X. PIAS1-modulated Smad2/4 complex activation is involved in zinc-induced cancer cell apoptosis. Cell Death Dis. 2013;4:e811. doi: 10.1038/cddis.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd M, Schimmack S, Lawrence B, Alaimo D, Modlin IM. EGFR/TGFα and TGFβ/CTGF signaling in neuroendocrine neoplasia: Theoretical therapeutic targets. Neuroendocrinology. 2013;97:35–44. doi: 10.1159/000334891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Penafuerte C, Galipeau J. TGF beta secreted by B16 melanoma antagonizes cancer gene immunotherapy bystander effect. Cancer Immunol Immunother. 2008;57:1197–1206. doi: 10.1007/s00262-008-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Yu Z, Muranski P, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. Inhibition of TGF-β signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 2013;20:575–580. doi: 10.1038/gt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glick AB. TGFbeta1, back to the future: Revisiting its role as a transforming growth factor. Cancer Biol Ther. 2004;3:276–283. doi: 10.4161/cbt.3.3.849. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Gao W, Zhang D. Effects of cigarette smoke extract on A549 cells and human lung fibroblasts treated with transforming growth factor-beta1 in a coculture system. Clin Exp Med. 2010;10:159–167. doi: 10.1007/s10238-009-0081-x. [DOI] [PubMed] [Google Scholar]