Abstract
The aim of the present study was to investigate the effects of an oleanolic acid derivative, a novel antitumor drug, on the growth of SMMC-7721 human hepatocellular carcinoma cells and the underlying mechanism. An MTT assay was performed to determine the cytotoxicity of the oleanolic acid derivative. Cell membrane integrity was assessed using fluorescence microscopy to assess the uptake of annexin V-FITC/propidium iodide (PI). Western blotting was used to detect the apoptosis-associated proteins B cell lymphoma-2 (Bcl-2), Bax, caspase-9 and caspase-3. A spectrophotometer was used to analyze the intracellular adenosine triphosphate (ATP) expression level. The loss of mitochondrial membrane potential was detected by performing the JC-1 assay. ELISA was used to evaluate the content of cytochrome c (Cyt-C). The oleanolic acid derivative reduced the cell viability of SMMC-7721 cells in a dose- and time-dependent manner. The half maximal inhibitory concentration values of the oleanolic acid derivative in SMMC-7721 cells at 24, 48 and 72 h were 26.80, 11.85, and 6.66 µM, respectively. The antiapoptotic-protein Bcl-2 was downregulated, and the proapoptotic protein Bax was upregulated following treatment with the oleanolic acid derivative for 48 h. The oleanolic acid derivative induced the cleavage of caspase-9 and caspase-3 as well as promoted annexin V-FITC/PI uptake in SMMC-7721 cells. Furthermore, treatment of SMMC-7721 cells with the oleanolic acid derivative induced a reduction of the intracellular ATP expression level, loss of ΔΨm and Cyt-C release from the mitochondria. The oleanolic acid derivative induced apoptosis in SMMC-7721 human cells. Mitochondrial dysfunction was involved in the anticancer effects of this derivative on SMMC-7721 human cells.
Keywords: oleanolic acid derivative, apoptosis, mitochondrial dysfunction, human hepatocellular carcinoma, mitochondrial membrane potential
Introduction
Liver cancer is a common type of cancer with a high mortality rate worldwide. Hepatocellular carcinoma (HCC) is the most common type of liver cancer and its incidence rate is currently on the rise (1). Hepatectomy and liver transplantation remain the curative treatments for this fatal disease; however, only 30–40% of patients with HCC are eligible. For late-stage patients, non-curative strategies, including transcatheter arterial chemoembolization and radiofrequency ablation, are available; however, the survival time is prolonged by only a few months (2). Furthermore, there are no effective chemotherapeutic drugs available due to the overexpression of multidrug-resistance genes in HCC (3). Currently, a multi-targeted tyrosine kinase inhibitor, sorafenib, only slightly improves the survival of certain patients with HCC (4). Therefore, the development of novel chemotherapeutic agents and more effective therapies for the treatment of HCC are required.
Triterpenoids are compounds that are presented extensively in numerous plants and have been used in traditional medicine. Olive oil is an important source of pentacyclic triterpenoids, including oleanolic acid. Oleanolic acid exhibits potential antitumor activity against numerous types of tumors, including hepatoma cells (5), pancreatic cancer cells (6) and bladder cancer cells (7). In addition, oleanolic acid has been revealed to arrest cell cycle progression, and to induce apoptosis via reactive oxygen species-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells (6). Furthermore, an oleanolic acid derivative has been demonstrated to induce human hepatoma cell apoptosis via a reactive oxygen species/mitogen-activated protein kinase-dependent mitochondrial signaling pathway (5). Additionally, ursolic acid has been revealed to induce an endoplasmic reticulum stress response to activate apoptosis signal-regulating kinase 1-c-Jun N-terminal kinase signaling and to induce apoptosis in T24 human bladder cancer cells (7). These findings suggest that oleanolic acid and ursolic acid may be promising agents for the treatment of HCC. However, the molecular pharmacology of oleanolic acid and ursolic acid remains unknown.
The present study investigated the molecular pharmacology of an oleanolic acid derivative, a newly synthesized compound. In addition, the potential effects of this oleanolic acid derivative on the viability of SMMC-7721 human cells were evaluated and the underlying mechanism involved in oleanolic acid derivative-mediated cell death was explored.
Materials and methods
Chemicals and reagents
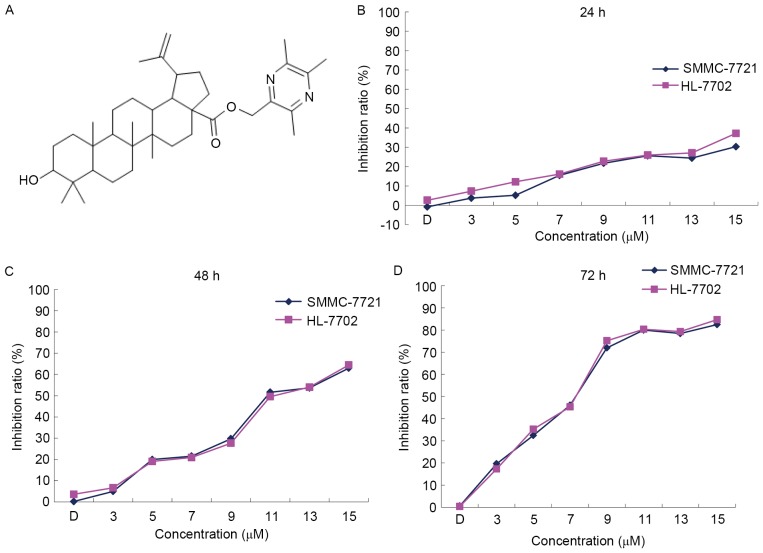
The oleanolic acid derivative was provided by Dr Lei of Beijing University of Chinese Medicine (Beijing, China) and dissolved in DMSO. The chemical structure of the oleanolic acid derivative is presented in Fig. 1A. RPMI-1640, fetal bovine serum, penicillin, streptomycin and trypsin were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). SDS, ponceaus, dithiothreitol, phenylmethylsulfonylfluoride, bovine serum albumin, MTT, the Annexin V-fluorescein isthocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit and JC-1 were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Anti-caspase-9 (catalog no., 9502), anti-caspase-3 (catalog no., 9662) were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Bcl-2 (catalog no., BS1031) and Bax (catalog no., BS1030) were obtained from Biogot Technology Co., Ltd. (Nanjing, China). Horseradish peroxidase-conjugated goat anti-rabbit antibodies (catalog no., BA1054) were obtained from Boster Biological Technology Co. Ltd (Wuhan, China). Polyvinylidene difluoride (PVDF) microporous membranes and the enhanced chemiluminescence detection system were purchased from Takara Biotechnology Co., Ltd. (Dalian, China). RIPA cell lysis buffer for western blot analysis was purchased from Beyotime Institute of Biotechnology (Haimen, China). The kit for detection of adenosine triphosphate (ATP) was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Human cytochrome c (Cyt-C) ELISA kit was purchased from Shanghai Ximei Chemical (Shanghai, China). The Cytoplasmic and Mitochondrial Protein Extraction kit was purchased from Sangon Biotech Co., Ltd. (Shanghai, China).
Figure 1.
Oleanolic acid derivative dose- and time-dependently reduced the viability of SMMC-7721 and HL-7702 cells. (A) The chemical structure of the oleanolic acid derivative used in the present study. Each value represents the mean ± standard deviation of three experiments. SMMC-7721 and HL-7702 cells were treated with 0, 3, 5, 7, 9, 11, 13 or 15 µM oleanolic acid derivative or vehicle (0.15% dimethyl sulfoxide) alone. The MTT assay was performed following incubation for (B) 24, (C) 48 or (D) 72 h.
Cell culture and treatment
SMMC-7721 human HCC cells were obtained from the American Type Culture Collection (Manassas, VA, USA). HL-7702 normal human liver cells were obtained from Obio Technology (Shanghai, China). The cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin at 37°C in a 5% CO2 incubator. Cells were treated with vehicle (0.15% DMSO) alone or with 3, 7, 9, 11, 13 or 15 µM oleanolic acid derivative for 24, 48 and 72 h.
Cell viability assay
Cell viability was determined using the MTT assay. SMMC-7721 and HL-7702 cells were seeded at a density of 1×105 cells/well in 96-well plates. Following incubation for ~24 h at 37°C in a 5% CO2 incubator, the cells were treated with various concentrations of oleanolic acid derivative (0, 3, 5, 7, 9, 11, 13 and 15 µM). The MTT assay was performed after 24, 48 and 72 h of treatment. The culture medium was discarded, then 30 µl 0.5% (w/v) MTT dissolved in 1X phosphate-buffered saline was added to each well, and the plate was incubated for 3 h at 37°C. Following incubation for 3 h, the culture medium was discarded and 120 µl DMSO was added into each well. Following incubation for 30 min and gentle agitation for 15 min at 37°C, the absorbance at 490 nm was evaluated using a microplate reader. The experiment was repeated in triplicate. Cell viability was expressed as a percentage of proliferation against the controls (untreated cells), which was set at 100%.
Detection of cell membrane integrity
The cell membrane integrity of SMMC-7721 cells was assessed using annexin V-FITC and PI double staining. Briefly, ~1×105 SMMC-7721 cells were seeded/well in 6-well plates. Following incubation for 24 h, the cells were treated with various concentrations of oleanolic acid derivative (7, 9 or 11 µM) or vehicle (0.11% DMSO) alone for 12, 24 or 36 h. Subsequently, the drug-containing medium was removed at each time point, and the SMMC-7721 cells were harvested, washed and stained with annexin V-FITC/PI, according to the manufacturer's protocol. Samples were then diluted with binding buffer and were analyzed using a fluorescence microscope (×200) within 1 h.
Western blot analysis
Following treatment with the oleanolic acid derivative (7, 9 or 11 µM) for 48 h at room temperature, the SMMC-7721 cells were harvested. Total protein was extracted using RIPA cell lysis buffer on ice. The protein concentration was determined using a BCA protein assay. Proteins were then mixed with loading buffer (Beijing ComWin Biotech Co., Ltd., Beijing, China) and boiled at 95°C for 10 min. Equal amounts of proteins (60 µg) were separated by SDS-PAGE (12% gradient gel), transferred to PVDF membranes and detected using the specific antibodies. The PVDF membranes were then incubated overnight at 4°C with anti-caspase-9 and anti-caspase-3 (dilution, 1:1,000). The immunoreactive proteins following incubation at 37°C with the horseradish peroxidase-conjugated goat anti-rabbit antibodies (dilution, 1:10,000) were detected using an enhanced chemiluminescence detection kit. The results were normalized to those of β-actin (dilution, 1:1,000).
Intracellular ATP evaluation
Cells (~1×105) were cultured in 96-well plates and incubated with 0, 7, 9 or 11 µM oleanolic acid derivative or vehicle (0.11% DMSO) alone for 36 h. Intracellular ATP levels were determined using an ATP determination kit, according to the manufacturer's protocol. The entire cell population, including any floating cells, were assayed. Luminescence was determined using a multimode microplate reader. The experiment was repeated in triplicate.
Evaluation of mitochondrial membrane potential (ΔΨm)
The lipophilic cationic fluorescent probe, JC-1, a mitochondrial dye that stains mitochondria in living cells in a membrane potential-dependent fashion, was used to quantify the ΔΨm. The reduction in the red/green fluorescence intensity ratio of JC-1 is directly proportional to the loss of ΔΨm. In brief, cells were treated with or without the oleanolic acid derivative (7, 9 or 11 µM) in 6-well plates for 12, 24 and 36 h. The medium was removed and replaced with 1 ml fresh culture medium (RPMI-1640 medium supplemented with 2% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin), followed by the addition of 1 ml JC-1 staining solution (final concentration of 2 mM) and incubation at 37°C for 20 min. The cells were then harvested and washed twice with JC-1 Dyeing buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Finally, 2 ml cell culture medium was added to each well, and JC-1 fluorescence was determined using a fluorescence microscope (×100).
Analysis of Cyt-C release
SMMC-7721 cells were harvested following treatment with various concentrations of the oleanolic acid derivative (7, 9 and 11 µM) for 12 h. Cytoplasmic and mitochondrial proteins were isolated using a Cytoplasmic and Mitochondrial Protein Extraction kit, according to the manufacturer's protocol. The concentrations of cytoplasmic and mitochondrial proteins were detected using the BCA assay. The concentrations of Cyt-C in the cytoplasm and mitochondria were detected according to the manufacturer's instructions using a Human Cyt-C ELISA kit, In order to ensure that the Cyt-C concentration was accurate, the cytoplasmic and mitochondrial protein were adjusted to the same level and then the sample was added to 50 µl of diluent to each well at the end. The Cyt-C content of the sample was determined by the regression equation of the standard curve of the reference standard.
Statistical analysis
All experiments were performed at least three times. All data were analyzed using SPSS version 19.0 statistical software (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation. Statistical analysis of the data for multiple groups was performed using one-way analysis of variance and Dunnett's test. GraphPad Prism version 5.02 (GraphPad Software, Inc., La Jolla, CA, USA) and Excel (2010) were used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Oleanolic acid derivative dose- and time-dependently inhibits the proliferation of SMMC-7721 and HL-7702 cells
To determine the cytotoxicity of the oleanolic acid derivative, the present study treated SMMC-7721 cells and HL-7702 normal liver cells with various concentrations of the oleanolic acid derivative (0, 3, 5, 7, 9, 11, 13 and 15 µM) for 24, 48 and 72 h, and analyzed the cell viability by performing an MTT assay. In comparison with the DMSO control, the oleanolic acid derivative inhibited the proliferation of SMMC-7721 and HL-7702 cells in a dose- and time-dependent manner (Fig. 1B). The maximum inhibition rates for the oleanolic acid derivative on SMMC-7721 cells were 30.30, 63.00 and 82.46%, and on HL-7702 cells were 37.20, 64.45 and 84.68% at 24, 48 and 72 h, respectively. The inhibition rates demonstrated that the oleanolic acid derivative had the same inhibitory effect on SMMC-7721 cells and HL-7702 normal liver cells (Fig. 1B). These results indicated that the oleanolic acid derivative dose- and time-dependently inhibited the proliferation of SMMC-7721 and HL-7702 cells.
Oleanolic acid derivative induces membrane disintegrity of SMMC-7721 cells
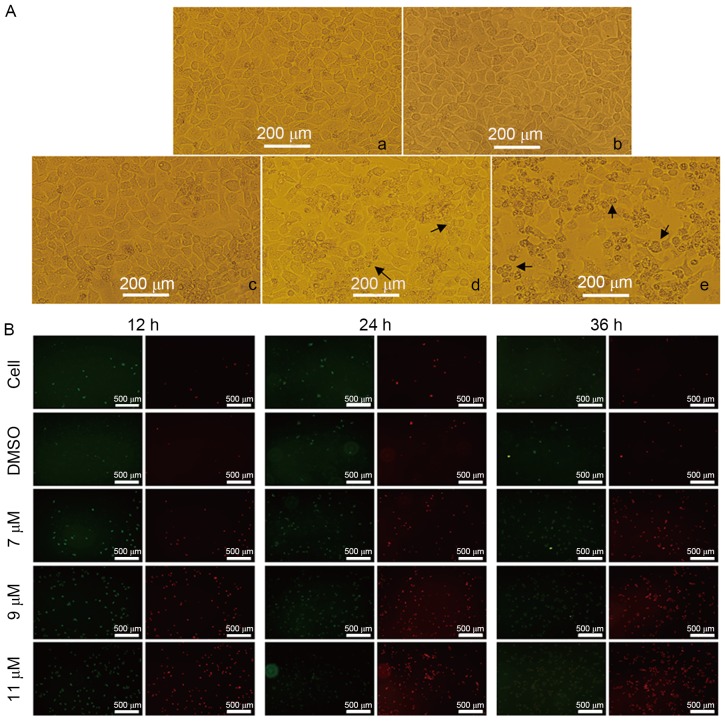
To determine whether the oleanolic acid derivative induces cell apoptosis, the present study treated SMMC-7721 cells with 0, 7, 9 or 11 µM oleanolic acid derivative for 36 h and observed cell morphology under a microscope. Compared with the DMSO-treated cells, the SMMC-7721 cells underwent the typical morphological changes of apoptosis, including cell shrinkage, membrane blebbing and accumulation of small apoptotic bodies (as indicated by the arrows in Fig. 2A). The induction of apoptosis was more evident when the oleanolic acid derivative concentration was 9 or 11 µM. These results demonstrated that the oleanolic acid derivative induced apoptosis of SMMC-7721 cells in a dose-dependent manner.
Figure 2.
Effects of the oleanolic acid derivative on the morphology and membrane integrity of SMMC-7721 cells. (A) The morphology of SMMC-7721 cells was observed using fluorescence microscopy. Following treatment with the oleanolic acid derivative for 36 h, SMMC-7721 cells were observed under a fluorescence microscope. (a) Blank control; (b) DMSO; (c) 7 µM oleanolic acid derivative; (d) 9 µM oleanolic acid derivative; (e) 11 µM oleanolic acid derivative. (B) The membrane integrities of SMMC-7721 cells were analyzed by annexin V-FITC/PI staining. Following treatment with the indicated concentration of the oleanolic acid derivative or vehicle (0.11% DMSO) alone for 12, 24, or 36 h, the SMMC-7721 cells were stained with annexin V-FITC/PI and then were observed under a fluorescence microscope. All images were captured at magnification, ×100. FITC, fluorescein isothiocyanate; PI, propidium iodide; DMSO, dimthyl sulfoxide.
Annexin V is a protein that interacts strongly and specifically with phosphatidylserine residues on the cell membrane and is widely used for the detection of apoptosis (8). PI, a nucleic acid dye, cannot cross the intact cell membrane; however, in the late-apoptotic cells and dead cells, PI is able to penetrate the cell membrane and interact with DNA, revealing red staining under a microscope. Thus, PI has been widely applied to assess cell viability. To further support the evidence that the oleanolic acid derivative induced apoptosis of SMMC-7721 cells, the present study treated SMMC-7721 cells with 0, 7, 9 or 11 µM oleanolic acid derivative for 12, 24 and 36 h and performed annexin V/PI double staining. As presented in Fig. 2B, there were markedly few red (PI-stained) and green (annexin V-stained) fluorescent cells in the control and DMSO groups, whereas the red fluorescence was increased with the increase of oleanolic acid derivative concentration and the extension of treatment time. The red fluorescence was highest at 11 µM oleanolic acid derivative treatment for 36 h. These results revealed that the oleanolic acid derivative induced membrane disintegrity of SMMC-7721 cells in a dose- and time-dependent manner.
Oleanolic acid derivative induces activation of the intrinsic apoptosis pathway in SMMC-7721 cells. To explore the mechanism underlying apoptosis induction by the oleanolic acid derivative, the present study determined the change of apoptosis-associated protein expression levels in SMMC-7721 cells following treatment with various concentrations of the oleanolic acid derivative (5, 9 and 11 µM) for 48 h by western blotting. The results revealed that anti-apoptotic protein, Bcl-2, was downregulated following 11 µM oleanolic acid derivative treatment for 48 h. Conversely, the proapoptotic protein, Bax, was upregulated following treatment of 5, 9 or 11 µM oleanolic acid derivative for 48 h (Fig. 3A). It is well known that caspase-3 is the ultimate executioner caspase of apoptosis and that activated caspase-9 cleaves downstream caspases, including caspase-3, to initiate the caspase cascade (9). Indeed, treatment of SMMC-7721 cells with the oleanolic acid derivative for 48 h induced obvious cleavage of caspase-9 and caspase-3 in a concentration-dependent manner (Fig. 3B). These results indicated that the oleanolic acid derivative induced the intrinsic pathway of apoptosis in SMMC-7721 cells.
Figure 3.
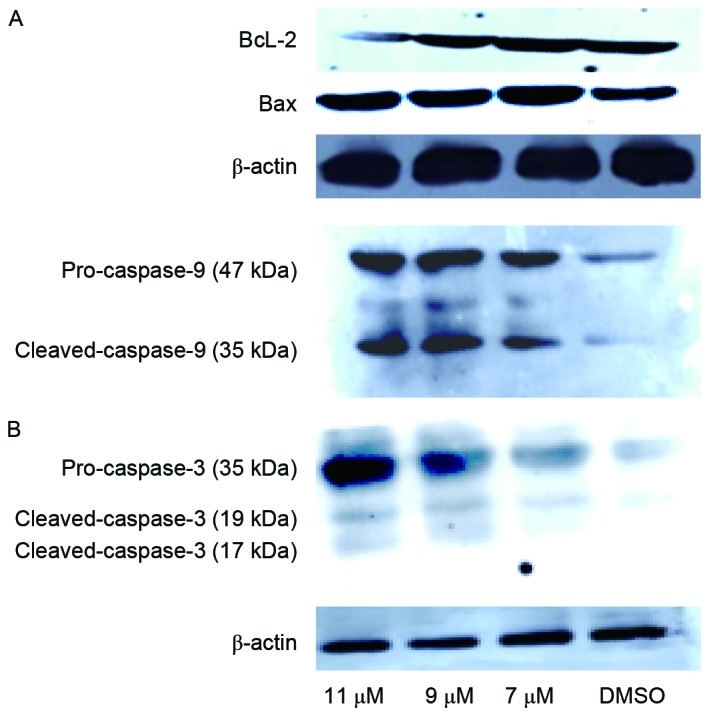
Oleanolic acid derivative altered the expression levels of apoptotic proteins in SMMC-7721 cells. Cell lysates were prepared from SMMC-7721 cells following incubation with the oleanolic acid derivative (7, 9 or 11 µM) for 48 h. Dimethyl sulfoxide-treated cell lysate was used as the control. Approximately 60 µg of protein per sample was used for immunoblotting of (A) Bcl-2 and Bax, and (B) caspase-9/caspase-3, with β-actin used as the loading control. Bcl-2, B cell lymphoma 2.
Oleanolic acid derivative reduces the intracellular ATP expression level
Mitochondria serve a key role in activation of the intrinsic cell apoptosis pathway. The activation of caspase-9 following oleanolic acid derivative treatment suggested that the oleanolic acid derivative may disrupt mitochondrial function. To investigate this hypothesis, the present study first assessed the intracellular ATP expression level, a decrease of which indicates an impairment of mitochondrial function. As presented Fig. 4, the ATP expression levels were significantly decreased in a dose-dependent manner following oleanolic acid derivative treatment. These results demonstrated that the oleanolic acid derivative impaired mitochondrial function.
Figure 4.
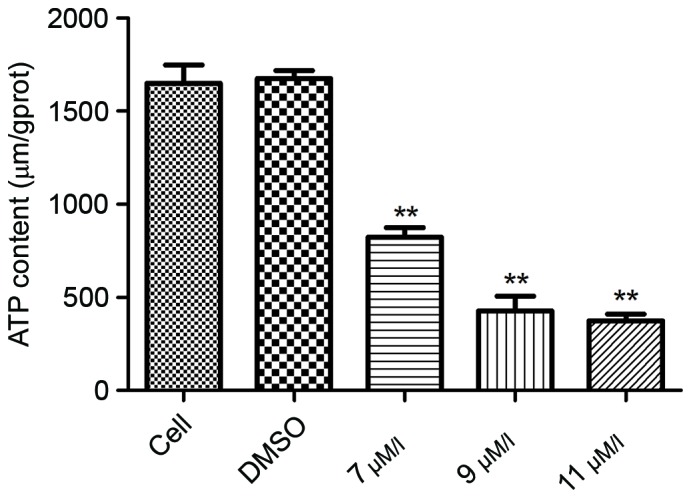
Effect of the oleanolic acid derivative on cellular ATP expression levels in SMMC-7721 cells. SMMC-7721 cells were incubated with 0, 7, 9 or 11 µM oleanolic acid derivative or vehicle (0.11% DMSO) alone for 36 h. Cells were harvested 36 h after treatment, and the cellular total ATP expression levels were analyzed using an ATP determination kit. Differences among groups were analyzed by one-way analysis of variance and Dunnett's test. **P<0.05 vs. DMSO.
Oleanolic acid derivative treatment results in loss of ΔΨm
Impairment of mitochondrial function is closely associated with the loss of ΔΨm. Subsequently, the present study treated SMMC-7721 cells with 0, 7, 9 or 11 µM oleanolic acid derivative for 12, 24 and 36 h, and determined the ΔΨm using the lipophilic cationic fluorescent probe JC-1 assay. Compared with the blank and DMSO controls, the oleanolic acid derivative significantly decreased the red/green fluorescence ratio in SMMC-7721 cells in a dose- and time-dependent manner (Fig. 5). Furthermore, the cell adherence rate declined markedly when the cells were exposed to 11 µM oleanolic acid derivative. Numerous floating cells were removed during the experiments (data not shown). These results indicated that the oleanolic acid derivative induced a decrease of ΔΨm in SMMC-7721 cells.
Figure 5.
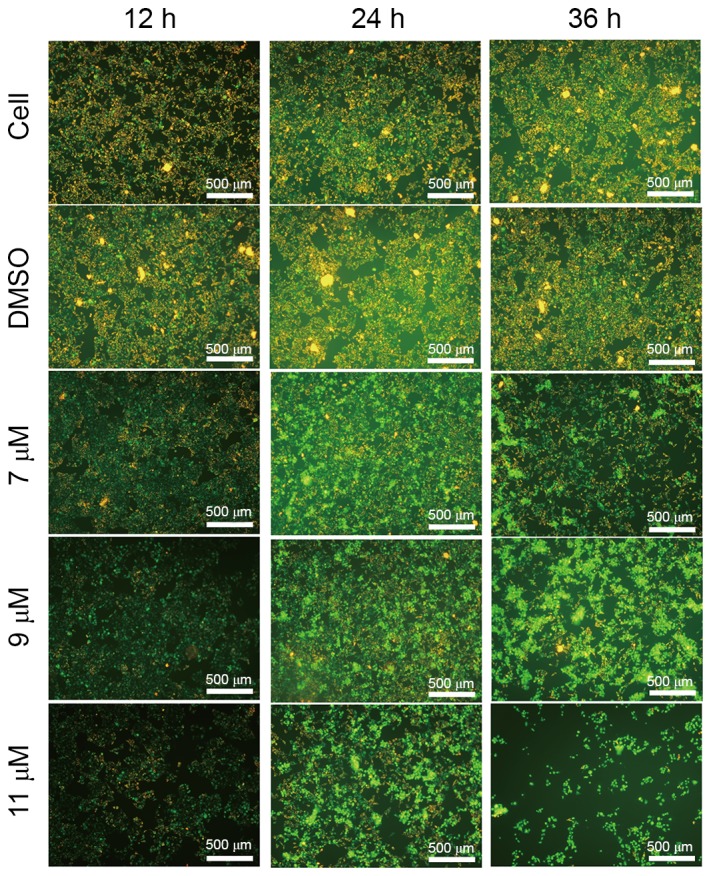
Effects of the oleanolic acid derivative on ΔΨm. SMMC-7721 cells were incubated in the absence or presence of the oleanolic acid derivative (7, 9 or 11 µM) for the indicated times (12, 24 or 36 h). Cells were incubated with JC-1 for the detection of ΔΨm using fluorescence microscopic analysis. All images were captured at magnification ×100. ΔΨm, mitochondrial membrane potential; DMSO, dimethyl sulfoxide.
Oleanolic acid derivative induces Cyt-C release from the mitochondria
It is well known that Cyt-C release from the mitochondria during apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization, indicating that a reduction in ΔΨm and caspase activation occurs considerably later than Cyt-C release (10). In the present study, depolarization of ΔΨm was revealed in SMMC-7721 cells exposed to the oleanolic acid derivative for 12 h (Fig. 5). Therefore, the present study detected the mitochondrial and cytoplasmic content of Cyt-C in SMMC-7721 cells treated with various concentrations of the oleanolic acid derivative for 12 h. The standard curve regression equation was y=0.0114×-0.0391 (R2=0.9989; Fig. 6A). The content of the sample was determined by the regression equation of the standard curve of the reference standard. The results demonstrated that when SMMC-7721 cells were treated with various concentrations of the oleanolic acid derivative for 12 h, the mitochondrial content of Cyt-C was decreased significantly in a dose-dependent manner, with a 65.48% decrease by 11 µM oleanolic acid derivative compared with the controls. In the cytoplasm, the content of Cyt-C was increased when the cells were treated with 7 µM oleanolic acid derivative and then gradually decreased with 9 and 11 µM oleanolic acid derivative (Fig. 6B). These results demonstrated that the oleanolic acid derivative induced Cyt-C release from the mitochondria in SMMC-7721 cells.
Figure 6.
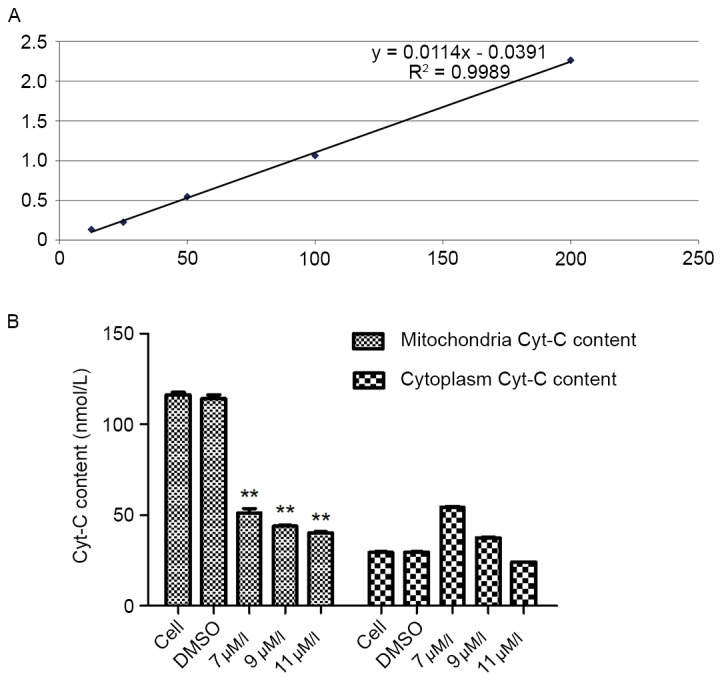
Effects of the oleanolic acid derivative on Cyt-C release from the mitochondria in SMMC-7721 cells. (A) The standard curve of Cyt-C. The standard curve regression equation was y=0.0114×-0.0391 (R2=0.9989). (B) SMMC-7721 cells were treated with the oleanolic acid derivative (0, 7, 9 or 11 µM) or vehicle (0.11% DMSO) alone. Cytoplasmic and mitochondrial fractions were obtained at 12 h after treatment, and the content of Cyt-C was analyzed using a Human Cyt-C ELISA kit. Cyt-C, cytochrome C; DMSO, dimethyl sulfoxide. **P<0.05 vs. DMSO.
Discussion
The present study revealed that the oleanolic acid derivative dose- and time-dependently inhibited the proliferation of SMMC-7721, and HL-7702 cells. Furthermore, treatment of SMMC-7721 cells with the oleanolic acid derivative resulted in cell shrinkage, membrane blebbing and accumulation of small apoptosis bodies, accompanied with upregulation of Bax and downregulation of Bcl-2, and cleavage of caspase-9 and −3. Of note, treatment of SMMC-7721 cells with the oleanolic acid derivative induced a reduction in the intracellular ATP expression level, loss of ΔΨm and Cyt-C release from the mitochondria. The results of the present study suggested that the oleanolic acid derivative inhibited the viability of SMMC-7721 cells by inducing activation of the intrinsic cell apoptosis signaling pathway.
Firstly, the present study investigated the effect of the oleanolic acid derivative on the proliferation of human SMMC-7721 cells and revealed that the oleanolic acid derivative induced apoptosis as demonstrated by the typical signs of apoptosis, including cell shrinkage, chromatin condensation, vacuolization in the cytoplasm and apoptotic bodies. Translocation of phosphatidylserine to the outside of the cellular membrane is a key step in apoptosis (11). The annexin V-FITC/PI double-staining results revealed that oleanolic acid derivative treatment resulted in the translocation of phosphatidylserine. These results demonstrated that oleanolic acid derivative treatment resulted in characteristic morphological alterations of apoptosis.
Activation of caspases is the hallmark of apoptosis. The present study revealed that the expression levels of the proapoptotic protein, Bax, were upregulated, whereas the anti-apoptotic protein, Bcl-2, was downregulated following oleanolic acid derivative treatment. In addition, it was demonstrated that caspase-3 and caspase-9 were activated, and cleaved by the oleanolic acid derivative. Caspase-9 is one of the most representative initiators of mitochondrial apoptosis. These observations suggested that the oleanolic acid derivative triggers activation of the intrinsic pathway of apoptosis (mitochondrial apoptosis). It has been well established that mitochondria serve an important role in the regulation of programmed cell death (apoptosis) (12). There are candidate natural compounds, in various stages of drug development, that have been described to interfere with mitochondrial functions in tumor cells, leading to cell cycle arrest and/or death by apoptosis or necrosis. Therefore, the present study aimed to focus on mitochondrial function to elucidate the anticancer molecular mechanism underlying the oleanolic acid derivative.
It is well known that a decrease of the ΔΨm and redistribution of Cyt-C is associated with mitochondrial function. In addition, mitochondrial dysfunction is associated with cell apoptosis (12). It has been reported that certain anticancer compounds induce cancer cell apoptosis by reducing the ΔΨm (12). Within tumor cell subpopulations, a higher ΔΨm contributes to enhanced tumor progression and expansion (13). JC-1 exhibits ΔΨm-dependent accumulation in the normal mitochondria and emits strong red fluorescence; whereas in unhealthy mitochondria, JC-1 emits a strong green fluorescence. The present study determined whether the mitochondria were healthy or not by observing the change in the red/green fluorescence ratio. The results of the present study indicated that the oleanolic acid derivative-induced cell death was associated with a loss of ΔΨm.
Released Cyt-C from the mitochondria to the cytoplasm may initiate caspase activation (14). Previous studies have revealed the decreased content of Cyt-C in the mitochondrial fractions and that the increased content of Cyt-C in the cytosolic fractions are involved in cell death (15–18). The results of the present study indicated that the content of Cyt-C in the cytosolic fraction decreased in a dose-dependent manner at 12 h after oleanolic acid derivative treatment. In addition, it has been reported that the Cyt-C content in the cytosolic fraction increased at 2 h, but was subsequently decreased at 6 h due to heat treatment in Tobacco Bright-Yellow 2 cells (19). Similar findings have been reported in cerebellar granule cells (20). However, it has been demonstrated that there is a dynamic change in Cyt-C expression levels in the cytosolic and mitochondrial fractions. Vacca et al (19) revealed that caspase-like proteases are able to degrade Cyt-C in the cytoplasm. Therefore, it is possible that Cyt-C in the cytosolic fraction was degraded by caspase-like proteases, which were increased by the oleanolic acid derivative at 12 h after treatment.
In the presence of ATP/deoxyadenosine triphosphate, Cyt-C binding to apoptotic protease activating factor-1 triggers the activation of caspase-9 and ATP accelerates caspase-3 activation (21,22). Once activated, caspase-9 cleaves caspase-3 to induce cell death. Furthermore, the mitochondria serve an important role in the production of ATP. The results of the present study demonstrated that the ATP content decreased significantly following oleanolic acid derivative treatment in a dose-dependent manner, which is consistent with the previous observation in bladder cancer cells treated with high-dose chemotherapeutics (23). The majority of cytotoxic anticancer agents induce cell death via the mitochondrial signaling pathway (24). The decrease in ΔΨm, redistribution of Cyt-C and decrease of the intracellular ATP expression level indicate that the oleanolic acid derivative induces mitochondrial dysfunction, thereby reducing the cell viability of cancer cells.
In conclusion, the oleanolic acid derivative promotes cell apoptosis of SMMC-7721 cells by inducing mitochondrial dysfunction. The results of the present study suggest that oleanolic acid derivatives may serve as potential therapeutic agents for human HCC.
Acknowledgements
The present study was supported by the Agricultural science and technology achievements transformation projects (grant no. 2014GB2A300003).
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng IO, Liu CL, Fan ST, Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am J Clin Pathol. 2000;113:355–363. doi: 10.1309/AC1M-4TY4-U0TN-EN7T. [DOI] [PubMed] [Google Scholar]
- 4.Vitale A, Volk ML, Pastorelli D, Lonardi S, Farinati F, Burra P, Angeli P, Cillo U. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: A cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51:165–173. doi: 10.1002/hep.23260. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Fu J, Li T, Cui R, Ling J, Yu X, Ji H, Zhang Y. NG, a novel PABA/NO-based oleanolic acid derivative, induces human hepatoma cell apoptosis via a ROS/MAPK-dependent mitochondrial pathway. Eur J Pharmacol. 2012;691:61–68. doi: 10.1016/j.ejphar.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Liu M, Liu H, Wang H, Wang F, Zhang Y, Han L, Lin X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J Appl Toxicol. 2013;33:756–765. doi: 10.1002/jat.2725. [DOI] [PubMed] [Google Scholar]
- 7.Zheng QY, Li PP, Jin FS, Yao C, Zhang GH, Zang T, Ai X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell Signal. 2013;25:206–213. doi: 10.1016/j.cellsig.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/S1534-5807(03)00090-X. [DOI] [PubMed] [Google Scholar]
- 9.Kuida K. Caspase-9. Int J Biochem Cell Biol. 2000;32:121–124. doi: 10.1016/S1357-2725(99)00024-2. [DOI] [PubMed] [Google Scholar]
- 10.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mourdjeva M, Kyurkchiev D, Mandinova A, Altankova I, Kehayov I, Kyurkchiev S. Dynamics of membrane translocation of phosphatidylserine during apoptosis detected by a monoclonal antibody. Apoptosis. 2005;10:209–217. doi: 10.1007/s10495-005-6076-5. [DOI] [PubMed] [Google Scholar]
- 12.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 13.Houston MA, Augenlicht LH, Heerdt BG. Stable differences in intrinsic mitochondrial membrane potential of tumor cell subpopulations reflect phenotypic heterogeneity. Int J Cell Biol. 2011;2011:978583. doi: 10.1155/2011/978583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Samuel S, Tumilasci VF, Oliere S, Nguyên TL, Shamy A, Bell J, Hiscott J. VSV oncolysis in combination with the BCL-2 inhibitor obatoclax overcomes apoptosis resistance in chronic lymphocytic leukemia. Mol Ther. 2010;18:2094–2103. doi: 10.1038/mt.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbu EM, Shirazi F, McGrath DM, Albert N, Sidman RL, Pasqualini R, Arap W, Kontoyiannis DP. An antimicrobial peptidomimetic induces Mucorales cell death through mitochondria-mediated apoptosis. PLoS One. 2013;8:e76981. doi: 10.1371/journal.pone.0076981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JH, Cho YC, Kim KH, Lee KS, Lee J, Kim DE, Park JS, Jang BC, Kim S, Kwon TK, Park JW. BAI, a novel Cdk inhibitor, enhances farnesyltransferase inhibitor LB42708-mediated apoptosis in renal carcinoma cells through the downregulation of Bcl-2 and c-FLIP (L) Int J Oncol. 2014;45:1680–1690. doi: 10.3892/ijo.2014.2534. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Beitler JJ, Wang H, Lee MJ, Huang W, Koenig L, Nannapaneni S, Amin AR, Bonner M, Shin HJ, et al. Honokiol enhances paclitaxel efficacy in multi-drug resistant human cancer model through the induction of apoptosis. PLoS One. 2014;9:e86369. doi: 10.1371/journal.pone.0086369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E. Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiol. 2006;141:208–219. doi: 10.1104/pp.106.078683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobba A, Atlante A, Giannattasio S, Sgaramella G, Calissano P, Marra E. Early release and subsequent caspase-mediated degradation of cytochrome c in apoptotic cerebellar granule cells. FEBS Lett. 1999;457:126–130. doi: 10.1016/S0014-5793(99)01018-2. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 22.Zou H, Li Y, Liu X, Wang X. An APAF1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Okuyama H, Nakayama M, Nishimura K, Nonomura N, Inoue M. Rapid decrease of ATP followed by necrosis-like cell death in bladder cancer cells after exposure to high-dose chemotherapeutics used in intravesical therapy. Cancer Res. 2014;74:1341. doi: 10.1158/1538-7445.AM2014-1341. [DOI] [Google Scholar]
- 24.Galluzzi L, Kroemer G. Necroptosis: A specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]