Abstract
Growth arrest and DNA damage-inducible-β (Gadd45β) is a stress-response protein involved in a number of processes, including cell cycle control, DNA repair, survival and death control, and stress signaling, depending on its interactions. Gadd45β expression is dysregulated in numerous types of cancer, functioning as either a tumor promoter or a tumor suppressor. However, the functions of Gadd45β in cholangiocarcinoma (CCA), particularly in metastasis, has not been studied. The immunohistochemical analysis of Gadd45β expression revealed that 75% of histological specimens from patients with CCA expressed high levels of Gadd45β, and that high Gadd45β expression was associated with metastasis. The role of Gadd45β in CCA was examined using siRNA-mediated gene knockdown in HuCCA-1, a human CCA cell line established from a Thai patient. The effects of Gadd45β downregulation upon cell viability and death, invasion, migration, matrix metalloproteinase (MMP) activity and epithelial-mesenchymal transition (EMT) marker expression were investigated. Gadd45β knockdown impaired cell viability, which was associated with the induction of apoptosis. In addition, there was a marked reduction in invasion and migration, although MMP activity was unaffected. Impairment of these metastatic properties was accompanied by the decreased expression of EMT markers, including Slug, vimentin, claudin-1 and zona occludens protein 1, whereas E-cadherin expression was increased. The present study suggests that Gadd45β is involved in regulating the viability and the metastatic potential of CCA cells, which may be mediated by the modulation of the EMT pathway.
Keywords: Gadd45β, cholangiocarcinoma, apoptosis, epithelial-mesenchymal transition, metastasis
Introduction
Cholangiocarcinoma (CCA) is a cancer of the bile duct caused by the malignant transformation of cholangiocytes, the epithelial cells lining the bile duct. CCA is the second most common type of primary hepatic malignancy (1,2). Due to late diagnosis, resistance to chemo- and radiotherapy, and the high rates of local invasion and distant metastasis, the prognosis for CCA is very poor. The median survival time of patients with CCA is <2 years (3), and the 5-year survival rate of patients receiving curative resection is 0–40% (4). The prevalence of CCA is the highest in Asia, particularly in the northeastern region of Thailand (5). Opisthorchis viverrini infection is hypothesized to be a causal factor of CCA in Thailand, as it is markedly associated with the incidence of CCA (6).
In total, >90% of cancer-associated mortality is due to the local or distant metastasis of cancer cells (7). The epithelial-mesenchymal transition (EMT) is an important process in cancer metastasis, characterized by alterations in the gene expression and morphology of cells, which leads to a reduction of intercellular adhesion, and an increase in cell motility (8–11). This process is associated with a reduction of E-cadherin expression (12,13) and an increase in the expression of vimentin, an intermediate filament protein, leading to increased cell motility and promoting tumor metastasis (14,15).
Growth arrest and DNA damage-inducible-β (Gadd45β) is a stress-response protein; its expression is induced by physiological or environmental stress. The aberrant expression of Gadd45β in various types of cancer has implicated its involvement in tumorigenesis (16). Gadd45β belongs to the Gadd45 protein family (Gadd45α, Gadd45β, Gadd45γ) (17). Gadd45β may form a homodimer or heterodimer with the other Gadd45 proteins, or interact with a variety of other proteins, including proliferating cell nuclear antigen, cyclin-dependent kinase 1, p21, mitogen-activated protein kinase kinase kinase 4, mitogen-activated protein kinase kinase 7 and p38 mitogen activated protein kinase (MAPK). The function of Gadd45β differs depending on the interacting molecules, including cell cycle control, DNA repair, survival and death control, and stress signaling (18–22).
Cancer cells must survive and propagate in a strenuous environment of hypoxia, nutrient competition and oxidative stress (23). It is essential for cells to acquire the ability to thrive in these stressful conditions. The function of Gadd45β as a stress-response protein in cancer is paradoxical; the downregulation of Gadd45β via promoter methylation in hepatocellular carcinoma suggests that Gadd45β may act as a tumor suppressor (24), whereas the upregulation of Gadd45β in colorectal cancer was associated with recurrence and mortality of patients with colorectal cancer (25), suggesting a tumor-promoting role. When the Gadd45β gene from normal adjacent tissue was over-expressed in colorectal cancer cell lines, apoptotic cell death was induced (25). In addition, Gadd45β was identified as upregulated in the metastasis of uveal melanoma to the liver (26), and the silencing of Gadd45β in human embryonic carcinoma cells decreased viability and invasiveness (27), suggesting that Gadd45β may contribute to the malignant phenotypes of cancer. However, the function of Gadd45β in metastasis and EMT is not yet fully characterized.
In the present study, it was identified that patients with CCA exhibit increased Gadd45β expression in tumor tissue, and that a high level of Gadd45β expression was associated with metastasis. Gadd45β expression in a CCA cell line, HuCCA-1, was suppressed using siRNA-mediated gene silencing, and the effects on cell viability, survival and death signaling pathways, migration, invasiveness, and the EMT pathway were studied. The data of the present study thus indicated that Gadd45β expression promoted the viability, migration and invasion of the HuCCA-1 cells, traits required for successful metastasis.
Materials and methods
Ethical approval
All procedures performed in the present study involving human participants were performed in accordance with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. The study was conducted with the approval of the Ethical Committee of Rajavithi Hospital (Bangkok, Thailand).
Immunohistochemical (IHC) staining
Paraffinized tissue samples from 28 patients with CCA who had undergone surgical treatment at Rajavithi Hospital between January 2010 and October 2010 were selected for retrospective analysis. Standard IHC technique was used for the detection of Gadd45β in paraffinized sections on glass slides. Polyclonal Gadd45β antibody (HPA029816-100UL; 1:500 dilution; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was hybridized with the sections overnight at 4°C, followed by incubation with biotinylated goat anti-rabbit IgG at RT for 1 h (E0432; dilution 1:500; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). Subsequently avidin-biotin-peroxidase conjugate was added (ABC Elite; Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature and the immunohistochemical reaction was developed with freshly prepared reagents from a Histofine SAB-PO kit (Nichirei, Inc., Tokyo, Japan). The samples were visualized at ×400 magnification using an Olympus BX53 microscope (Olympus Corporation, Tokyo, Japan) and categorized into four grades according to the intensity and percentage of positively stained cells: Negative or <5%, grade 0; weak or 5–25%, grade 1; moderate or 25–50%, grade 2; and strong or >50%, grade 3. IHC grade 0 (3 cases) and grade 1 (4 cases), which exhibited weak and/or <25% staining, were grouped as low expression, whereas grade 2 (5 cases) and grade 3 (16 cases), which exhibited moderate to strong and/or >25% staining, were grouped as high expression of Gadd45β for the purpose of statistical analysis.
Cell line and culture condition
The human CCA cell line, HuCCA-1 (28), developed from a Thai patient with CCA, was used for the study. The cells were provided by Professor Satitaya Sirisinha (Mahidol University, Bangkok, Thailand). These cells were grown in HAM's F-12 medium supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in 5% CO2 humidified atmosphere.
Small interfering (si)RNA-mediated Gadd45β gene silencing
Commercial Gadd45β siRNA [GADD45β siRNA(h): sc-37416], a pool of 3 different siRNA duplexes to the Gadd45β gene (NCBI RefSeq. 12759252), was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Silencer® Negative control siRNA no. 1 (cat. no. 4404021; Ambion; Thermo Fisher Scientific, Inc.) was used as negative control siRNA. The cells were washed with PBS and detached using 0.25% Trypsin/EDTA (Gibco; Thermo Fisher Scientific, Inc.). Cells were seeded at 2×105 cells/well into a 6 well plate for 24 h, then transfected with 40 nM siRNA using Lipofectamine® RNAiMax reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The medium containing transfection reagents was changed for 10% FBS supplemented HAM's-F12 at 24 h after transfection.
Western blot analysis
At 48 h post-transfection, total cellular protein was extracted and prepared following a protocol described previously (29). Each protein sample of 40 µg was separated via SDS-PAGE (12% gel; 120 V for 2 h) followed by an electroblotting transfer of the protein to a nitrocellulose membrane at 30 V at 4°C for 15 h. The membranes were blocked in 1.5% skimmed milk at room temperature for 1 h, the membranes were incubated with the primary antibodies at 4°C overnight on a rocking platform. The next day, the membranes were washed with TBS with Tween-20 prior to incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Following 3 washes with TBST for 10 min, ECL Plus Western Blotting Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to visualize the immunoreactive bands in G:Box ChemiXL 1.4 (Syngene; Synoptics, Cambridge, UK).
Rabbit polyclonal Gadd45β antibodies were purchased from Abcam (Cambridge, UK) and goat polyclonal GAPDH antibodies from Santa Cruz Biotechnology, Inc. The rest of the antibodies used in the study were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA); the details regarding all antibodies used in western blotting are included in Table I. Densitometry analysis was performed using ImageJ software (version 1.49v; National Institutes of Health, Bethesda, MD, USA) (30). The density of the protein of interest relative to GAPDH was determined in HuCCA-1 cells transfected with Gadd45β or negative control siRNA. The activation of Akt, p38 MAPK and extracellular signal-regulated kinase (ERK)1/2 signaling pathways was examined using phospho-specific antibodies at 48 h after siRNA transfection. The densitometry measurements for total Akt, ERK1/2 and p38 MAPK were used for the normalization of protein loading. The phospho-protein/total protein density ratio was determined in HuCCA-1 cells transfected with Gadd45β siRNA or negative control siRNA. The phospho-protein/total protein density ratio of cells transfected with negative control siRNA was designated as 100%.
Table I.
The primary antibodies used for western blotting in the present study.
| Antibody | Cat. no. | Clone no. | Type of antibody | Organism | Company | Dilution |
|---|---|---|---|---|---|---|
| Gadd45β | ab105060 | Polyclonal | Rabbit | Abcam | 1:2,000 | |
| GAPDH | sc-48166 | I-19 | Polyclonal | Goat | Santa Cruz Biotechnology, Inc. | 1:2,000 |
| Cleaved PARP (Asp214) | #5625 | D64E10 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| Total PARP | #9542 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| Cleaved caspase-3 (Asp175) | #9664 | 5A1E | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| Full-length caspase 3 | #9662 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| Phospho-p38 MAPK (Thr180/Tyr182) | #9211 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| p38 MAPK | #9212 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| Phospho-Akt (Ser473) | #4058 | 193H12 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| Akt | #9272 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| Phospho-ERK1/2 (Thr202/Tyr204) | #9101 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| ERK1/2 | #9102 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 | |
| Vimentin | #5741 | D21H3 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| Claudin-1 | #13255 | D5H1D | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| β-catenin | #8480 | D10A8 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| ZO-1 | #8193 | D7D12 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| Slug | #9585 | C19G7 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
| E-cadherin | #3195 | 24E10 | Monoclonal | Rabbit | Cell Signaling Technology, Inc. | 1:1,000 |
Gadd45β, growth arrest and DNA damage-inducible-β; PARP, Poly (ADP-ribose) polymerase; p38 MAPK, p38 mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; ZO-1, zona occludens protein 1.
Cell viability assays
Live/Dead® cell viability assay
HuCCA-1 cells were grown on coverslips prior to transfection with siRNA. At 48 h post-transfection, cell viability was assessed using the Live/Dead® Viability/Cytotoxicity kit for mammalian cells (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Briefly, a working solution, containing 2 µM calcein AM and 4 µM ethidium homodimer (EthD-1), was prepared in PBS. The cells were washed with pre-warmed PBS, and 150 µl of Live/Dead solution was applied to each cover slip. Following incubation at 37°C in a 5% CO2 humidified atmosphere for 30 min, the reagents were removed and the cover slips were washed with PBS, mounted on the glass-slides, and viewed using a Nikon Eclipse TE2000-U microscope (Nikon Corporation, Tokyo, Japan) using B-2A (green, calcein) and G-2A (red, EthD-1) excitation filters. Images were recorded at ×100 total magnification using ACT-1C software (version 1.02; Nikon Corporation). Live cells were stained with calcein-AM (green), whereas dead cells were stained with ethidium homodimer (red).
WST-1 assay
A total of 5,000 HuCCA-1 cells were seeded in 96-well plates for 24 h prior to siRNA transfection. At 24, 48 and 72 h post-transfection, 10 µl of WST-1 reagent (Roche Diagnostics, Basel, Switzerland) was added into each well. The plates were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 2 h. Then the absorbance at 450 nm was measured.
Hoechst staining
At 48 h post-transfection, the culture media was carefully removed to avoid the loss of detached cells. HuCCA-1 cells were then fixed in 2% w/v paraformaldehyde solution for 30 min at room temperature prior to permeabilization using 0.03% v/v Triton X-100 for 30 min. Nuclear staining was performed with Hoechst 333258 dye at room temperature (5 µg/ml; Thermo Fisher Scientific, Inc.) for 2 h, followed by visualization at ×400 total magnification with an Olympus IX83 live cell fluorescence microscope (Olympus Corporation).
Gelatin zymography
To analyze the activity of MMPs, the gelatinase activity of the enzymes secreted into the medium was determined using gelatin zymography assay, following a previously described protocol (29). The gelatinolytic activity of MMPs was observed as clear bands on the blue background of the Coomassie-stained gel. Densitometry analysis was performed using ImageJ software (30).
Cell invasion and migration assays
Cells were harvested at 48 h post-transfection for in vitro invasion and migration assays using transwell chambers, as described by Li et al (31). Briefly, HuCCA-1 cells (1×105 cells/well) transfected with Gadd45β siRNA or negative control siRNA were seeded into the upper chamber of the transwells coated with Matrigel for the invasion assay and with no coating for the migration assay, and analyzed after 6 h of incubation at 37°C. HAM's F-12 supplemented with 10% FBS, used as chemoattractant, was added to the lower chamber. Cells were seeded in the upper chamber in serum free medium. Three independent experiments were performed in duplicate transwells.
Statistical analysis
Data is presented as the mean ± standard deviation and every experiment was performed at least three times. The χ2 test was used for clinicopathological data analysis, and experimental data analysis was performed using a paired t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
High levels of Gadd45β expression are associated with metastatic incidence in CCA
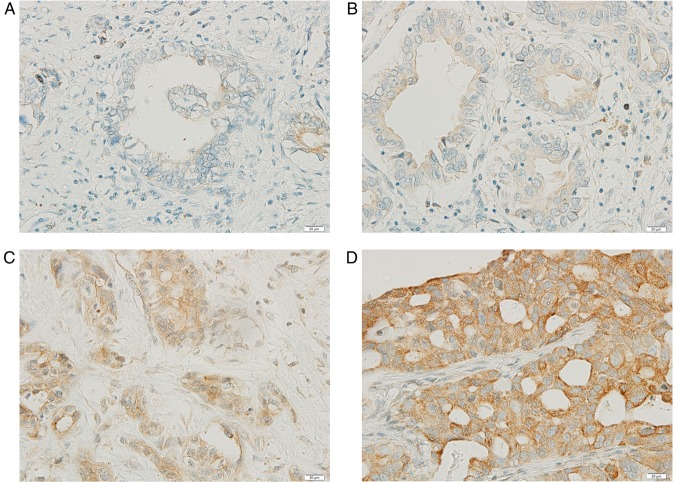
Gadd45β expression in the histological specimens of CCA tissue from 28 patients was examined with IHC staining. The expression of Gadd45β was detected in all tumor tissues (n=28), and 75% (n=21) exhibited higher Gadd45β expression than the surrounding stroma (Fig. 1A-D). Although Gadd45β expression was previously reported to be moderate in the liver, gall bladder and pancreas (32), the expression level in cholangiocytes or CCA tissue has not previously been reported, to the best of our knowledge. A high level of Gadd45β expression was associated with the incidence of metastasis in patients (P=0.035), although no statistically significant association was observed between the level of Gadd45β expression and age, sex, lymph node involvement or differentiation status (Table I).
Figure 1.
Gadd45β expression in CCA tissues, as determined by IHC. Paraffinized CCA tissue sections from patients were stained with a polyclonal anti-Gadd45β antibody. A total of 21/28 patients (75%) exhibited the high levels of Gadd45β expression. Representative images of IHC (A) grade 0 and (B) grade 1+, which were designated as low expression, and (C) grade 2+ and (D) grade 3+; which were designated as high expression, are presented. Gadd45β, growth arrest and DNA damage-inducible-β; CCA, cholangiocarcinoma; IHC, immunohistochemistry.
Gadd45β knockdown reduces the viability of HuCCA-1 cells
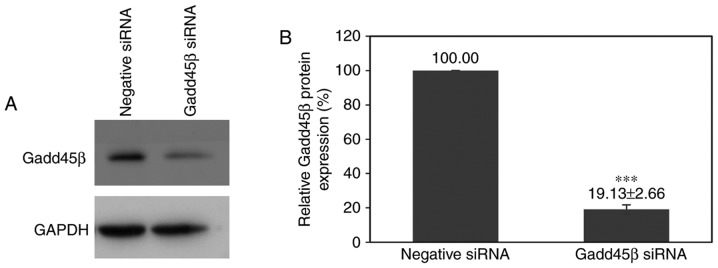
To investigate the functional importance of Gadd45β in CCA, Gadd45β expression was suppressed using siRNA-mediated silencing in HuCCA-1, a CCA cell line that expresses moderate levels of Gadd45β. Gadd45β siRNA transfection decreased the expression of Gadd45β in HuCCA-1 to 19.13±2.66% relative to negative control siRNA transfection (Fig. 2A and B).
Figure 2.
siRNA-mediated gene silencing efficiency in HuCCA-1 cells. (A) Representative images of the western blot analysis of Gadd45β silencing in HuCCA-1. (B) Densitometry analysis of the efficiency of Gadd45β silencing, using GAPDH as an internal control. Columns, mean of three independent experiments; bars, standard deviation. ***P=0.0004 compared with negative control siRNA transfection. siRNA, small interfering RNA; Gadd45β, growth arrest and DNA damage-inducible-β.
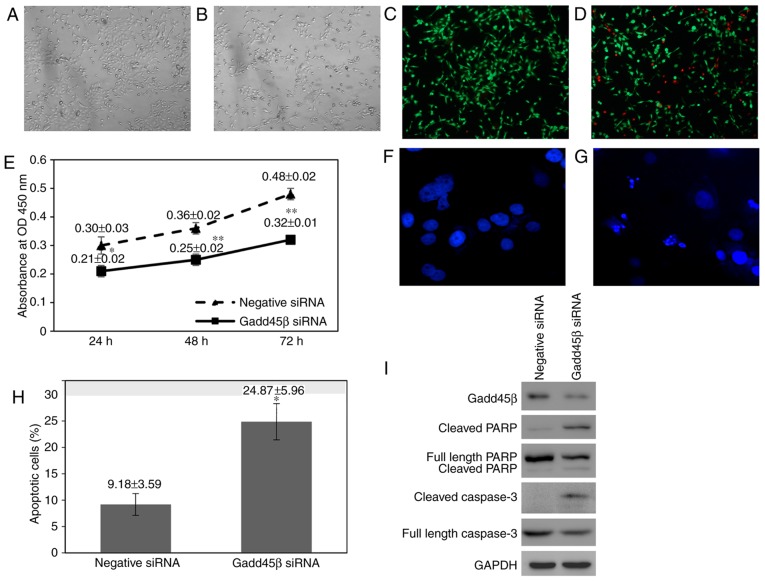
During Gadd45β silencing experiments, a decrease in the confluence of Gadd45β-silenced HuCCA-1 cells was observed compared with negative control siRNA-transfected cells (Fig. 3A and B). Furthermore, there was an increase in the number of cells floating in the medium of Gadd45β-silenced HuCCA-1 cells, indicating that silencing of Gadd45β perturbed the viability of HuCCA-1 cells (data not shown). A Live/Dead® cell viability assay confirmed that there was an increase in the number of dead cells in HuCCA-1 cells when Gadd45β was silenced (Fig. 3C and D). Cell viability was assessed using a WST-1 assay, in which the WST-1 reagent was added directly into the cells without removing the culture medium, preventing the loss of detached viable cells. Consistent with visual observation, Gadd45β silencing significantly decreased the viability of HuCCA-1 cells (Fig. 3E).
Figure 3.
Gadd45β silencing impaired proliferation and induced apoptosis in HuCCA-1 cells. Light microscopic images of (A) negative control siRNA and (B) Gadd45β siRNA transfected HuCCA-1 cells at 48 h (×40 magnification). An evident decrease in the confluence of the Gadd45β siRNA transfected cells was noted, compared with negative control siRNA-transfected cells. In a Live/Dead Viability Assay, (C) negative control HuCCA-1 cells were relatively less likely to be dead (red) compared with (D) Gadd45β-silenced cells (×40 magnification). (E) WST-1 proliferation assay. Gadd45β siRNA transfection induced a significant reduction in HuCCA-1 cell proliferation. Data points, mean of three independent experiments; bars, standard deviation. *P<0.05; **P<0.005 compared with negative control siRNA transfection. Hoechst 333258 staining of HuCCA-1 cells transfected with (F) negative control siRNA and (G) Gadd45β siRNA (×400 magnification). The nuclei of live cells were homogenously stained in blue, whereas those of apoptotic cells appeared condensed and fragmented. (H) Percentage of apoptotic cells in HuCCA-1 cells following Gadd45β silencing for 48 h. Columns, mean of three independent experiments; bars, standard deviation; *P<0.05. (I) Representative images of the western blot analysis of caspase-3 activation and cleavage of PARP in HuCCA-1 following Gadd45β silencing for 48 h. Gadd45β, growth arrest and DNA damage-inducible-β; siRNA, small interfering RNA.
Gadd45β silencing kills HuCCA-1 cells via apoptosis
The mode of cell death in HuCCA-1 cells upon Gadd45β silencing was examined by staining the nuclear DNA with Hoechst 333258. Upon silencing of Gadd45β, HuCCA-1 cells were observed to exhibit nuclear condensation and DNA fragmentation, characteristics of apoptotic cells (33). The nuclei of live cells were homogenously stained in blue, whereas those of apoptotic cells appeared condensed and fragmented. Gadd45β-silenced HuCCA-1 cells exhibited an increased rate of apoptotic cells compared with negative control cells (24.87±5.96% vs. 9.18±3.59%; Fig. 3F-H). Consistently, western blot analysis revealed that poly (ADP-ribose) polymerase (PARP) cleavage and caspase-3 cleavage were observed, indicating that HuCCA-1 cells died via apoptosis when Gadd45β was silenced (Fig. 3I) (34).
Gadd45β silencing reduces Akt activity in HuCCA-1
To determine which signaling pathways were involved, three of the principal pathways involved in the regulation of cell viability and proliferation, the Akt, ERK and p38 MAPK pathways, were studied. Akt is associated with cell proliferation, survival and apoptosis inhibition (35). p38 MAPK is a stress-activated protein kinase pathway important for cell proliferation, differentiation, survival and migration (36,37). ERK1/2 activation has been demonstrated to protect cells from apoptosis (38).
Gadd45β silencing resulted in a marked reduction of Akt phosphorylation (P=0.0237), whereas no significant alteration in p38 MAPK or ERK1/2 phosphorylation was observed (Fig. 4A-D). The decrease in Akt activity may be responsible for the reduction of viability in HuCCA-1 upon Gadd45β silencing.
Figure 4.
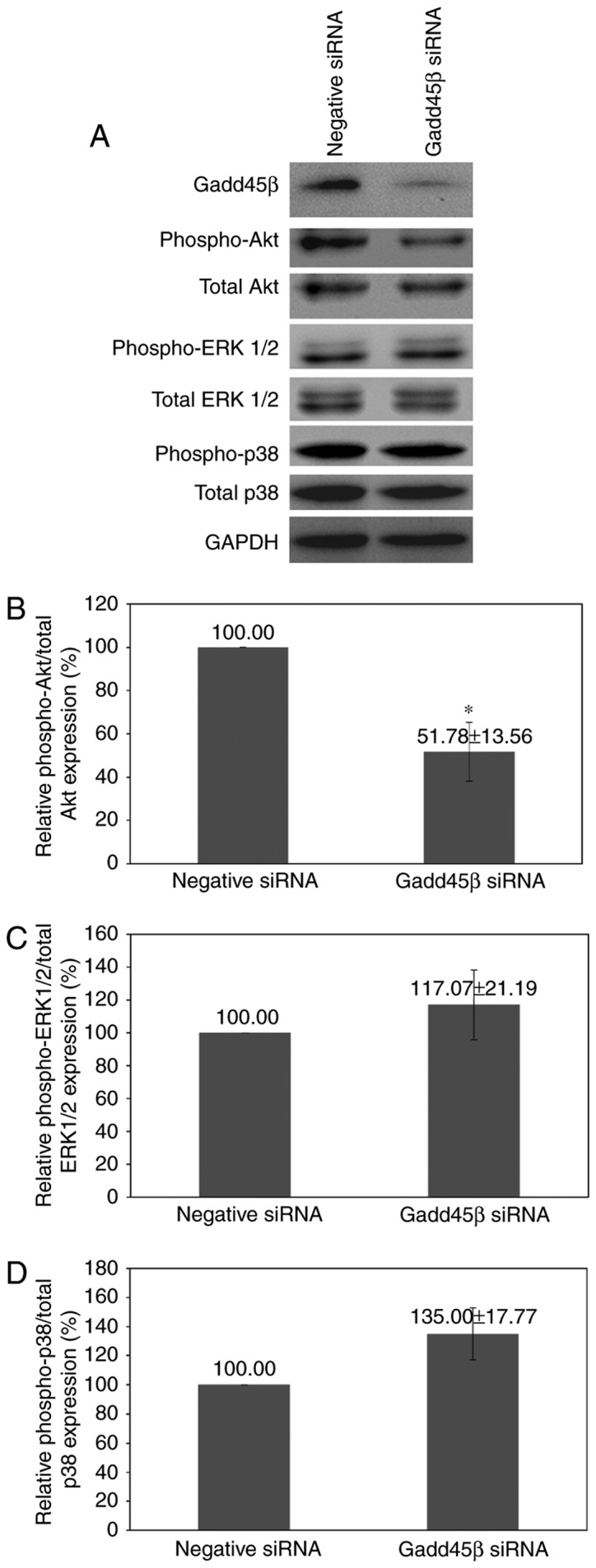
Effects of Gadd45β silencing upon major signaling pathways. (A) Representative images of the western blot analysis of p-Akt, p-p38 MAPK and p-ERK1/2 expression levels. Densitometry analysis for (B) p-Akt, (C) p-ERK1/2 and (D) p-p38 MAPK relative to the total form of each respective protein. Columns, mean of three independent experiments; bars, standard error of the mean; *P<0.05. Gadd45β, growth arrest and DNA damage-inducible-β; p38 MAPK, p38 mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase.
Gadd45β silencing reduces the invasion and migration of HuCCA-1 cells
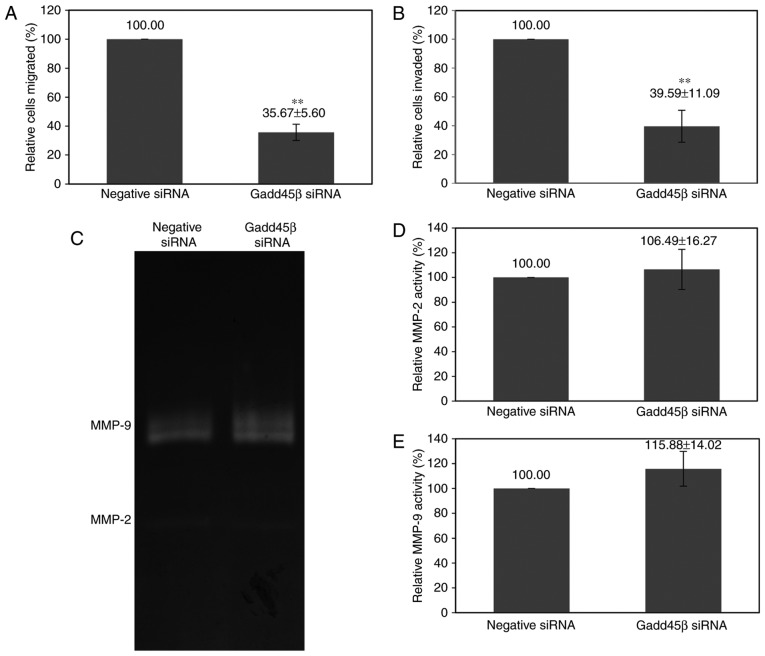
A hallmark of malignancy is the ability to invade local tissues or to spread to distant sites (39). During this process, the cancer cells must acquire the ability to invade and migrate through connective tissue barriers, including the basement membrane, surrounding matrix and existing blood vessels (40). IHC data analysis of patient samples revealed that there was an association between the high expression of Gadd45β and metastasis (Table II). Furthermore, Gadd45β was demonstrated to be important for the viability of HuCCA-1 cells in the present study; viability is required by the cancer cells for successful metastasis (41). Therefore, the effect of Gadd45β silencing on the migration and invasion abilities of HuCCA-1 cells was investigated using in vitro transwell migration and invasion assays. Gadd45β silencing decreased the migration of HuCCA-1 cells to 35.67±5.60%, and invasion to 39.59±11.09%, compared with the negative control siRNA (Fig. 5A and B).
Table II.
Association between Gadd45β expression and the clinicopathological features of patients with cholangiocarcinoma.
| Gadd45β expression | ||||
|---|---|---|---|---|
| Clinicopathological parameter | Total | Low | High | P-value |
| Total, n (%) | 28 | 7 (25.0) | 21 (75.0) | |
| Age, years | 0.378 | |||
| Mean ± standard deviation | 59.68±10.41 | |||
| <60, n (%) | 12 (42.9) | 4 (14.3) | 8 (28.6) | |
| ≥60, n (%) | 16 (57.1) | 3 (10.7) | 13 (46.4) | |
| Sex, n (%) | 0.512 | |||
| Male | 13 (46.4) | 4 (14.3) | 9 (32.1) | |
| Female | 15 (53.6) | 3 (10.7) | 12 (42.9) | |
| Tumor size, cm | 0.385a | |||
| Mean ± standard deviation | 2.90±2.4 | |||
| <5, n (%) | 25 (92.6) | 7 (25.9) | 18 (66.7) | |
| ≥5, n (%) | 2 (7.4) | 0 (0) | 2 (7.4) | |
| Lymph node metastasis, n (%) | 0.190 | |||
| Absent | 14 (50.0) | 5 (17.9) | 9 (32.1) | |
| Present | 14 (50.0) | 2 (7.1) | 12 (42.9) | |
| Distant metastasis, n (%) | 0.035c | |||
| Absent | 19 (67.9) | 7 (25.0) | 12 (42.9) | |
| Present | 9 (32.1) | 0 (0) | 9 (32.1) | |
| Differentiation status, n (%) | 0.696b | |||
| Well | 11 (52.4) | 3 (14.3) | 8 (38.1) | |
| Moderate | 10 (47.6) | 2 (9.5) | 8 (38.1) | |
| Type of surgery, n (%) | 0.076 | |||
| R0 | 14 (50.0) | 6 (21.0) | 8 (28.6) | |
| R1 | 7 (25.0) | 1 (3.6) | 6 (21.4) | |
| R2 | 7 (25.0) | 0 (0) | 7 (25.0) | |
Tumor size data was not available in one case
cell differentiation status data was not available in 7 cases
P<0.05. Gadd45β, growth arrest and DNA damage-inducible-β.
Figure 5.
Gadd45β silencing decreased the in vitro migration and invasion of HuCCA-1, whereas MMP-2 and MMP-9 activity was not affected. Quantification of Transwell (A) migration and (B) invasion assays. The silencing of Gadd45β significantly decreased the in vitro invasion and migration activity of HuCCA-1 cells. (C) Gelatin zymography assay for secreted MMP-2 and −9. Densitometry analysis for (D) MMP-2 and (E) MMP-9. Columns, mean of three independent experiments; bars, standard deviation; *P<0.05; **P<0.01. Gadd45β, growth arrest and DNA damage-inducible-β; MMP, matrix metalloproteinase; siRNA, small interfering RNA.
Cancer cells may secrete matrix metalloproteinases (MMPs) or stimulate surrounding stromal cells to secrete MMPs, consequently leading to digestion of the extracellular matrix, promoting local invasion and metastasis (42,43). However, gelatin zymography analysis revealed that Gadd45β silencing did not significantly alter the activity of MMP-2 (P=0.5609) and MMP-9 (P=0.1886) secreted by HuCCA-1 cells (Fig. 5C-E).
Gadd45β silencing reverses the EMT changes in HuCCA-1
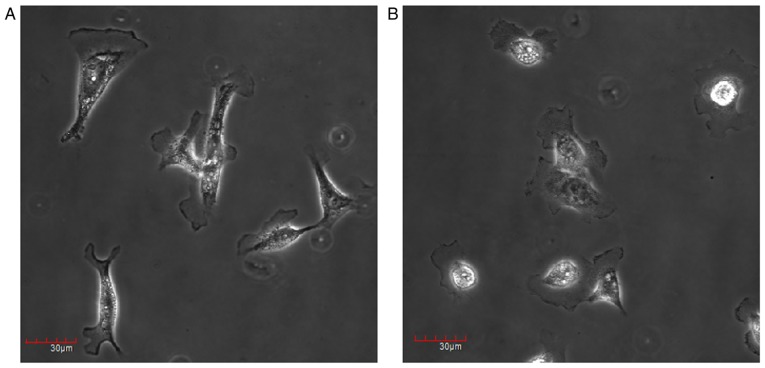
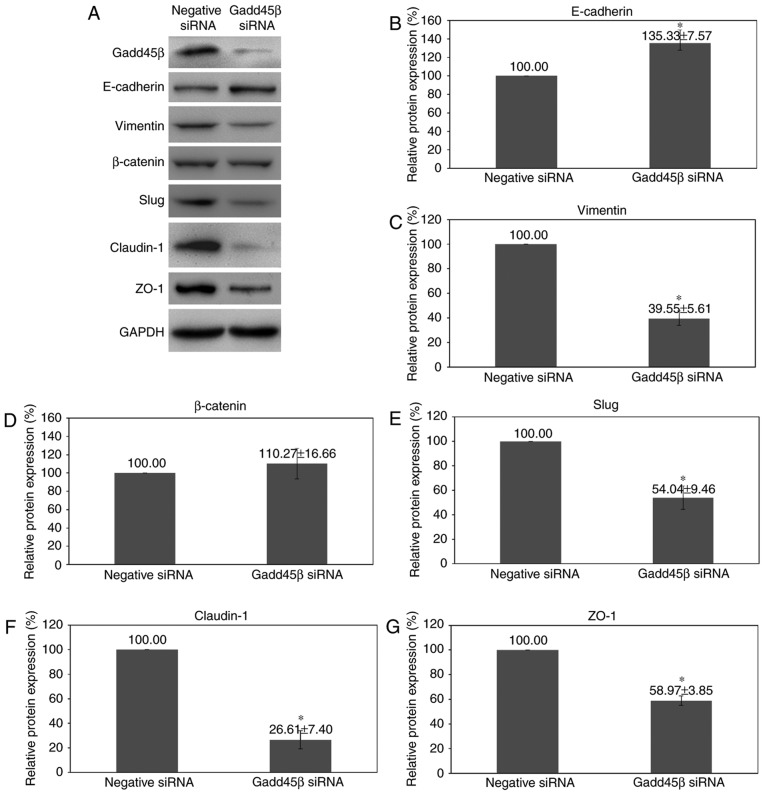
Epithelial-mesenchymal transition is an important process during cancer metastasis, denoted by acquisition of mesenchymal phenotypes, including increased motility and invasiveness (8,9,44–46). Since Gadd45β silencing decreased the invasion and migration of HuCCA-1 cells, properties associated with EMT, including morphological changes and EMT marker expression, were examined. It was demonstrated that Gadd45β silencing may have induced HuCCA-1 cells to undergo morphological changes, from spindle-shaped and fibroblast-like in appearance, to a flattened epithelial-like phenotype (Fig. 6A and B). The expression patterns of the proteins involved in the EMT pathway were further investigated with western blotting. It was identified that Gadd45β silencing affected the expression of multiple EMT markers (Fig. 7A-G). Epithelial marker E-cadherin expression was increased (P=0.0430), whereas the expression of the mesenchymal markers, Vimentin (P=0.0085) and Slug (P=0.0399), was decreased by Gadd45β silencing. However, there was no change in β-catenin expression (P=0.6006; Fig. 7A and D).
Figure 6.
Effect of Gadd45β silencing upon cell morphology. Morphology of the HuCCA-1 cells at 48 h after (A) negative control siRNA and (B) Gadd45β siRNA transfection. HuCCA-1 cells changed in morphology from a spindle-shaped and fibroblast-like appearance to a flattened epithelial-like phenotype by Gadd45β silencing. Gadd45β, growth arrest and DNA damage-inducible-β.
Figure 7.
Effect of Gadd45β silencing upon EMT marker expression. (A) Representative images of western blot analysis for the expression of E-cadherin, vimentin, β-catenin, Slug, claudin-1 and ZO-1 at 48 h after Gadd45β silencing in HuCCA-1. Densitometry analysis of (B) E-cadherin, (C) vimentin, (D) β-catenin, (E) Slug, (F) claudin-1 and (G) ZO-1. Columns, mean of three independent experiments; bars, standard error of the mean. *P<0.05. Gadd45β, growth arrest and DNA damage-inducible-β; EMT, epithelial-mesenchymal transition; ZO-1, zona occludens protein 1; siRNA, small interfering RNA.
Notably, proteins associated with tight junctions were also affected by Gadd45β silencing. Claudin-1 and zona occludens protein 1 (ZO-1) constitute the tight junctions between cells (47,48); one of the mechanisms involved in cancer invasion is collective migration, in which the expression levels of tight-junction proteins are important to allow cells to move as a cluster (49). There was a decrease in claudin-1 (P=0.0100; Fig. 7A and F) and the ZO-1 expression (P=0.0087; Fig. 7A and G) upon Gadd45β silencing.
Discussion
The IHC staining of paraffin embedded tissue samples from patients with CCA revealed that the majority of CCA tissue samples expressed increased Gadd45β relative to non-tumor tissue, and that the high level of Gadd45β in CCA was associated with an increased incidence of metastasis in patients. These findings suggest that Gadd45β may be of functional importance in CCA. As cancer cells are continuously exposed to stressful environments including hypoxia, competition for nutrients and oxidative stress during growth (23), Gadd45β appears to enable CCA cells to cope with stress and to thrive in the harsh tumor microenvironment.
In the present study, the functional importance of Gadd45β in CCA was determined using siRNA-mediated gene silencing in HuCCA-1, a cell line established from a patient with CCA. Gadd45β silencing decreased the proliferation and induced apoptosis in HuCCA-1 cells, as demonstrated by nuclear condensation and fragmentation, together with the activation of caspase-3 and PARP cleavage. The impairment of cell proliferation together with an increased rate of apoptosis in Gadd45β-silenced cells suggests that Gadd45β serves a pro-survival role in CCA. These data are in agreement with previous reports on genotoxic stress-induced apoptosis in hematopoietic cells of Gadd45β-deficient mice (50). Additionally, Gadd45β has been demonstrated to promote the survival of mouse embryo fibroblasts in response to tumor necrosis factor-α (51) and of B cells during Fas-induced apoptosis (52).
To study the underlying molecular mechanisms by which Gadd45β promotes viability in HuCCA-1, key cellular survival and death signaling pathways were studied, since the activation of death signals or the reduction of survival signals reduces the viability of cells. The results of the present study demonstrated that Gadd45β silencing significantly decreased the Akt activity, although p38 MAPK and ERK1/2 activity was unchanged. Activation of the Akt/PI3K pathway has been associated with growth and metastasis (53); therefore, the decrease in p-Akt may be responsible for the reduced growth and induction of apoptosis in HuCCA-1 cells upon Gadd45β silencing. The altered cell signaling activities appeared to shift the balance from pro-survival to pro-apoptotic following the silencing of Gadd45β.
EMT is an important biological process that allows malignant tumor cells to acquire migratory and invasive phenotypes; prerequisites for cancer invasion and metastasis. It is indicated by the acquisition of fibroblast-like morphology, with a reduction in intercellular adhesion and increased cell motility (11–18). The IHC data from the present study indicated that Gadd45β may be involved in the metastasis of CCA. Gadd45β silencing of HuCCA-1 cells induced a marked reduction in invasiveness and migration, although the activity of MMPs was not affected. A number of reports have implicated Gadd45β in the cell migration ability, an important characteristic of metastatic cancer cells. For example Salerno et al (54) demonstrated that the granulocytes of Gadd45β-deficient mice displayed the impairment of lipopolysacchiride-stimulated chemotactic migration. Furthermore, Kodama and Negishi (55) demonstrated that the ectopic expression of Gadd45β increased, whereas siRNA-mediated silencing of Gadd45β decreased, pregnane X receptor-induced cell migration in hepatocellular carcinoma cells.
Examination of the expression of EMT markers revealed that Gadd45β silencing resulted in reduction in Slug expression, with a consequent increase in E-cadherin and decrease in vimentin expression. High Slug expression has been demonstrated to induce EMT in cancer cells (56) and the effects are orchestrated by an increase in vimentin expression (57). As Slug expression is negatively regulated by GS3Kβ (58), which in turn is inactivated by pAkt (59), the decrease in Akt activity following Gadd45β silencing may have led to the downregulation of Slug expression, with the consequent suppression of vimentin expression and an increase in the invasiveness and migration of HuCCA-1 cells.
Furthermore, a decrease in the expression of the tight junction proteins, claudin-1 and ZO-1, was induced by Gadd45β silencing. Claudins and ZO-1 are integral proteins of the tight junctions that seal adjacent epithelial cells (47,48). Although the conventional roles of claudins and ZO-1 are to control paracellular ion flux and maintain cell polarity, accumulating evidence has demonstrated that these proteins also exhibit non-junctional functions, including in cell motility, and may function in the EMT, apoptosis resistance, invasion and metastasis of cancer cells (60–62). Claudins have been demonstrated to promote collective migration, where groups of cells move in a coordinated manner and remain connected via cell-cell junctions. Additionally, claudin expression has been associated with increased MMP activity and cell survival (62). Claudin-1 expression has been demonstrated to be associated with invasive and metastatic phenotypes in a range of types of cancer including colon, liver, oral squamous cell carcinoma and melanoma (63). ZO-1 is a component of the ‘junctional plaque’ at the cytoplasmic surface of the tight junctions, which links integral membrane proteins with the cytoskeleton (64). It was identified in a previous study that ZO-1 can translocate from the cell membrane to the nucleus and may be involved in cell signaling (65). The upregulation of ZO-1 has been reported in melanoma, and the silencing of ZO-1 led to a marked reduction in the rate of cell invasion (66). Thus, the identified association between a decrease in claudin-1 and ZO-1 expression with impaired cell invasion and migration in the present study appears to be consistent with these studies.
In conclusion, the silencing of Gadd45β significantly decreased the viability, metastatic phenotypes and EMT markers of HuCCA-1 cells, suggesting that Gadd45β serves a function in the metastatic process in CCA. This is in accord with the IHC data of patients with CCA, in which Gadd45β expression was associated with metastasis. However, a limitation of this study was that the experiments were performed in only one CCA cell line. Further studies should be performed in more CCA cell lines with more stable knock down systems, as transient siRNA transfection has a limited gene silencing duration. The study of Gadd45β expression, function and Gadd45β-mediated signaling pathways in CCA will be important in understanding cancer biology, in which the cancer cells are dynamically evolving with accumulated mutations. Since the cancer cells must thrive in a competitive environment, a stress response protein like Gadd45β may be critical. The understanding or manipulation of Gadd45β-dependent survival signaling and EMT pathways may be beneficial as a potential therapeutic option, adjuvant to the conventional therapies for CCA.
Acknowledgements
The authors thank Professor Satitaya Sirisinha (Mahidol University, Bangkok, Thailand) for providing the HuCCA-1 cell line and the Central Instrument Facility, Center of Nanoimaging, Faculty of Science of Mahidol University. The authors express their thanks to Professor Tavan Janvilisri and Ms. Phorutai Pearngam for assistance in the study of EMT pathways. The present study was supported by Mahidol University (grant no. 18/2555).
Glossary
Abbreviations
- CCA
cholangiocarcinoma
- ZO-1
zona occludens protein 1
- MMP
matrix metalloproteinase
- EMT
epithelial-mesenchymal transition
- siRNA
small interfering RNA
References
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 3.Farley DR, Weaver AL, Nagorney DM. ‘Natural history’ of unresected cholangiocarcinoma: Patient outcome after noncurative intervention; Mayo Clin Proc; 1995; pp. 425–429. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9:43–57. doi: 10.1634/theoncologist.9-1-43. [DOI] [PubMed] [Google Scholar]
- 5.Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini-multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: Markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 8.Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, Willbanks A, Sarkar S. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 11.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 12.Adhikary A, Chakraborty S, Mazumdar M, Ghosh S, Mukherjee S, Manna A, Mohanty S, Nakka KK, Joshi S, De A, et al. Inhibition of epithelial to mesenchymal transition by E-cadherin up-regulation via repression of slug transcription and inhibition of E-cadherin degradation: Dual role of scaffold/matrix attachment region-binding protein 1 (SMAR1) in breast cancer cells. J Biol Chem. 2014;289:25431–25444. doi: 10.1074/jbc.M113.527267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Techasen A, Loilome W, Namwat N, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H, Yongvanit P. Loss of E-cadherin promotes migration and invasion of cholangiocarcinoma cells and serves as a potential marker of metastasis. Tumour Biol. 2014;35:8645–8652. doi: 10.1007/s13277-014-2087-6. [DOI] [PubMed] [Google Scholar]
- 14.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Qiu W, David D, Zhou B, Chu PG, Zhang B, Wu M, Xiao J, Han T, Zhu Z, Wang T, et al. Down-regulation of growth arrest DNA damage-inducible gene 45beta expression is associated with human hepatocellular carcinoma. Am J Pathol. 2003;162:1961–1974. doi: 10.1016/S0002-9440(10)64329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zumbrun SD, Hoffman B, Liebermann DA. Distinct mechanisms are utilized to induce stress sensor gadd45b by different stress stimuli. J Cell Biochem. 2009;108:1220–1231. doi: 10.1002/jcb.22354. [DOI] [PubMed] [Google Scholar]
- 18.Liebermann DA, Hoffman B. Gadd45 in stress signaling. J Mol Signal. 2008;3:15. doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vairapandi M, Balliet AG, Fornace AJ, Jr, Hoffman B, Liebermann DA. The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene. 1996;12:2579–2594. [PubMed] [Google Scholar]
- 20.Smith ML, Ford JM, Hollander MC, Bortnick RA, Amundson SA, Seo YR, Deng CX, Hanawalt PC, Fornace AJ., Jr p53-mediated DNA repair responses to UV radiation: Studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol. 2000;20:3705–3714. doi: 10.1128/MCB.20.10.3705-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jónsson ZO, Hübscher U. Proliferating cell nuclear antigen: More than a clamp for DNA polymerases. Bioessays. 1997;19:967–975. doi: 10.1002/bies.950191106. [DOI] [PubMed] [Google Scholar]
- 22.Kelman Z, Hurwitz J. Protein-PCNA interactions: A DNA-scanning mechanism? Trends Biochem Sci. 1998;23:236–238. doi: 10.1016/S0968-0004(98)01223-7. [DOI] [PubMed] [Google Scholar]
- 23.Leprivier G, Rotblat B, Khan D, Jan E, Sorensen PH. Stress-mediated translational control in cancer cells. Biochim Biophys Acta. 2015;1849:845–860. doi: 10.1016/j.bbagrm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Qiu W, Zhou B, Zou H, Liu X, Chu PG, Lopez R, Shih J, Chung C, Yen Y. Hypermethylation of growth arrest DNA damage-inducible gene 45 beta promoter in human hepatocellular carcinoma. Am J Pathol. 2004;165:1689–1699. doi: 10.1016/S0002-9440(10)63425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Xiao X, Li D, Chi Y, Wei P, Wang Y, Ni S, Tan C, Zhou X, Du X. Abnormal expression of GADD45B in human colorectal carcinoma. J Transl Med. 2012;10:215. doi: 10.1186/1479-5876-10-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meir T, Dror R, Yu X, Qian J, Simon I, Pe'er J, Chowers I. Molecular characteristics of liver metastases from uveal melanoma. Invest Ophthalmol Vis Sci. 2007;48:4890–4896. doi: 10.1167/iovs.07-0215. [DOI] [PubMed] [Google Scholar]
- 27.Inowa T, Hishikawa K, Matsuzaki Y, Isagawa T, Takeuchi T, Aburatani H, Kitamura T, Fujita T. GADD45β determines chemoresistance and invasive growth of side population cells of human embryonic carcinoma. Stem Cells Int. 2010;2010:782967. doi: 10.4061/2010/782967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153–157. [PubMed] [Google Scholar]
- 29.Pongcharoen P, Jinawath A, Tohtong R. Silencing of CD44 by siRNA suppressed invasion, migration and adhesion to matrix, but not secretion of MMPs, of cholangiocarcinoma cells. Clin Exp Metastasis. 2011;28:827–839. doi: 10.1007/s10585-011-9414-8. [DOI] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YH, Zhu C. A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Clin Exp Metastasis. 1999;17:423–429. doi: 10.1023/A:1006614232388. [DOI] [PubMed] [Google Scholar]
- 32.Uhlen M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 33.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Rajendran V, Sethumadhavan R, Purohit R. Akt kinase pathway: A leading target in cancer research. ScientificWorldJournal. 2013;2013:756134. doi: 10.1155/2013/756134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebreda AR, Porras A. p38 MAP kinases: Beyond the stress response. Trends Biochem Sci. 2000;25:257–260. doi: 10.1016/S0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 37.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 38.Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/MCB.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Talmadge JE, Fidler IJ. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehlen P, Puisieux A. Metastasis: A question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 42.Davies KJ. The complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue Stroma. Int J Breast Cancer. 2014;2014:839094. doi: 10.1155/2014/839094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009;21:1323–1333. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]
- 44.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell DR, Blasky AJ, Britt SG, Artinger KB. Riding the crest of the wave: Parallels between the neural crest and cancer in epithelial-to-mesenchymal transition and migration. Wiley Interdiscip Rev Syst Biol Med. 2013;5:511–522. doi: 10.1002/wsbm.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallerand H, Cai Y, Wainberg ZA, Garraway I, Lascombe I, Nicolle G, Thiery JP, Bittard H, Radvanyi F, Reiter RR. Phospho-Akt pathway activation and inhibition depends on N-cadherin or phospho-EGFR expression in invasive human bladder cancer cell lines. Urol Oncol. 2010;28:180–188. doi: 10.1016/j.urolonc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 47.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and −2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 50.Gupta M, Gupta SK, Balliet AG, Hollander MC, Fornace AJ, Hoffman B, Liebermann DA. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene. 2005;24:7170–7179. doi: 10.1038/sj.onc.1208847. [DOI] [PubMed] [Google Scholar]
- 51.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 52.Zazzeroni F, Papa S, Algeciras-Schimnich A, Alvarez K, Melis T, Bubici C, Majewski N, Hay N, De Smaele E, Peter ME, Franzoso G. Gadd45 beta mediates the protective effects of CD40 costimulation against Fas-induced apoptosis. Blood. 2003;102:3270–3279. doi: 10.1182/blood-2003-03-0689. [DOI] [PubMed] [Google Scholar]
- 53.Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ, Loilome W. Increased activation of PI3K/Akt signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34:3637–3648. doi: 10.1007/s13277-013-0945-2. [DOI] [PubMed] [Google Scholar]
- 54.Salerno DM, Tront JS, Hoffman B, Liebermann DA. Gadd45a and Gadd45b modulate innate immune functions of granulocytes and macrophages by differential regulation of p38 and JNK signaling. J Cell Physiol. 2012;227:3613–3620. doi: 10.1002/jcp.24067. [DOI] [PubMed] [Google Scholar]
- 55.Kodama S, Negishi M. Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem. 2011;286:3570–3578. doi: 10.1074/jbc.M110.179812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 58.Kim JY, Kim YM, Yang CH, Cho SK, Lee JW, Cho M. Functional regulation of Slug/Snail2 is dependent on GSK-3β-mediated phosphorylation. FEBS J. 2012;279:2929–2939. doi: 10.1111/j.1742-4658.2012.08674.x. [DOI] [PubMed] [Google Scholar]
- 59.Cross DA, Alessi DR, Vandenheede JR, McDowell HE, Hundal HS, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: Evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303:21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bezdekova M, Brychtova S, Sedlakova E, Langova K, Brychta T, Belej K. Analysis of Snail-1, E-cadherin and claudin-1 expression in colorectal adenomas and carcinomas. Int J Mol Sci. 2012;13:1632–1643. doi: 10.3390/ijms13021632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stebbing J, Filipovic A, Giamas G. Claudin-1 as a promoter of EMT in hepatocellular carcinoma. Oncogene. 2013;32:4871–4872. doi: 10.1038/onc.2012.591. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira SS, Morgado-Diaz JA. Claudins: Multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwon MJ. Emerging roles of claudins in human cancer. Int J Mol Sci. 2013;14:18148–18180. doi: 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. doi: 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts; Proc Natl Acad Sci USA; 1996; pp. 10779–10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541–1554. doi: 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]