Abstract
Retigeric acid B (RAB), a natural compound isolated from lichen, has been demonstrated to inhibit cell growth and promote apoptosis in prostate cancer (PCa) cells. The present study evaluated the function of RAB combined with clinical chemotherapeutic drugs in PCa cell lines by MTT assay, reverse transcription quantitative polymerase chain reaction and western blot analysis, and identified that RAB at low doses produced significant synergistic cytotoxicity in combination with cisplatin (CDDP); however, no marked synergism between RAB and the other chemotherapeutics was observed. Additional studies revealed that RAB exerted an inhibitory effect on DNA damage repair pathways, including the nucleotide excision repair and mismatch repair pathways, which are involved in the sensitivity to CDDP-based chemotherapy, as suggested by the significantly downregulated expression of certain associated repair proteins. Notably, Excision repair cross-complementing 1, a critical gene in the nucleotide excision repair pathway, exhibited the most significant decrease. When combined with CDDP, RAB-mediated impairment of DNA repair resulted in prolonged DNA damage, as demonstrated by the long-lasting appearance of phosphorylation of histone H2AX at Ser139, which potentially enhanced the chemosensitivity to CDDP. Concurrently, the proapoptotic protein death receptor 5 (DR5) was activated by RAB, which also enhanced the chemotherapeutic response of CDDP. Knockdown of DR5 partially blocked RAB-CDDP synergism, suggesting the crucial involvement of DR5 in this event. The results of the present study identified that RAB functioned synergistically with CDDP to increase the efficacy of CDDP by inhibiting DNA damage repair and activating DR5, suggesting the mechanistic basis for the antitumor effect of RAB in combination with current chemotherapeutics.
Keywords: prostate cancer, combination therapy, Retigeric acid B, cisplatin, DNA repair, death receptor 5
Introduction
Prostate cancer (PCa) is one of the most common malignant tumors in males worldwide, representing a global public health problem (1). The development of PCa in humans presents as a multistage process, proceeding from a localized, androgen-dependent disease to invasive and metastatic hormone-refractory PCa. Chemotherapy is one of the primary treatment methods used in patients with PCa (2,3). However, the therapeutic strategies for this disease are limited as chemotherapy and radiation therapy are largely ineffective due to cross-resistance and metastatic disease frequently develops, even following potentially curative surgery (2–4). Therefore, the development of novel therapeutic options is urgently required.
Cisplatin (CDDP), one of the most widely used chemotherapy drugs, also known as cisplatinum or cis-diamminedichloridoplatinum (II), is a member of the most widely-used group of platinum-containing anticancer drugs (5,6). Platinum complexes exert antitumor activities via the formation of covalent adducts with cellular DNA, resulting in DNA damage, which in turn triggers apoptosis (6,7). CDDP is one part of the treatment modalities used for a variety of solid tumors, including ovarian, testicular, esophageal, non-small cell lung, and head and neck cancer, as well as PCa (8–10). In addition, alone or in combination with other chemotherapy agents, platinum compounds have been examined in the aforementioned clinical trials in patients with advanced PCa. Their antitumor activity as monotherapy in randomly selected patients was mostly moderate, and certain combination therapies resulted in significant toxicity. For example, in cisplatin/5-fluorouracil treatment as a chemoradiotherapy regimen for the treatment of locally advanced squamous cell carcinoma of the head and neck, high doses (cisplatin 15 mg/m2 + 5-fluorouracil 750 mg/m2 per day) resulted in significantly higher level of neutropenia and a trend towards higher rate of mucositis (11). Cisplatin in combination with bleomycin and vinca alkaloids may provoke even more chest pain presentations compared with cisplatin alone, at an incidence as high as 40% (12,13). The concurrent chemotherapy of cisplatin (30 mg/m2) and docetaxel (40 mg/m2) and external radical radiotherapy for transitional cell bladder carcinoma caused severe early and late side effects including acute gastrointestinal toxicity, myelotoxicity, stomatitis, skin toxicity and nephrotoxicity (14). The combination of docetaxel and cisplatin with radiotherapy was also demonstrated to be associated with a higher incidence of side effects compared with single-agent cisplatin with radiotherapy in high-risk early-stage cervical cancer (15).
Clinical resistance to CDDP remains a major obstacle to increasing its cytotoxic effects. The capacity for DNA repair is a crucial molecular pathway implicated in resistance to platinum-based chemotherapy (16,17). As the cytotoxicity of platinum drugs is principally attributable to the formation of platinum-DNA adducts (6,16), nucleotide excision repair (NER) is the primary DNA repair mechanism for the removal of bulky DNA lesions caused by CDDP from genomic DNA. The core proteins required for NER are xeroderma pigmentosum group A (XPA), replication protein A (RPA), XPC-UV excision repair protein RAD23 homolog B (RAD23B), transcription factor II human (TFIIH), excision repair cross-complementing 1 (ERCC1)/DNA excision repair protein ERCC-4 (XPF), ERCC excision repair 5 and endonuclease (XPG) (16,18). The downregulation of ERCC1, a critical gene in the NER pathway (16,19), was identified to increase the sensitivity of cancer cells to platinum-based chemotherapy (16,19,20). It is highly conserved during evolution and constitutively expressed in all tissues at relatively high levels. The ERCC1/XPF heterodimer is a structure-specific endonuclease and its function in NER is to create the 5′-incision on the damaged strand (20). Functional ERCC1 is essential for survival; knockdown of the ERCC1 gene in mice was observed to lead to an accelerated-aging phenotype, with brain damage, liver failure and mortality occurring following weaning (21). It has also been demonstrated that the downregulation of ERCC1 sensitized PCa cells to CDDP, and excision repair of CDDP adducts in PCa cells was attenuated to a similar extent by ERCC1 downregulation (20), suggesting that ERCC1 is a potential therapeutic target to sensitize cancer cells to chemotherapy.
With the previous advances in the identification of the mechanisms regulating CDDP-induced apoptosis, the pleiotropic effects of CDDP on the cell may lead to the development of novel targeted therapies (22–24). Death receptor 5 (DR5) is a cell surface receptor for tumor necrosis factor-related apoptosis-inducing ligand, and triggers apoptosis via mitochondria-dependent and independent pathways (25). Previous studies have also demonstrated that DR5 upregulation sensitizes cells to the cytotoxic effects of CDDP (26–29), suggesting that combining CDDP treatment with an inducer of DR5 has the potential to sensitize cells, leading to synergy.
In light of the aforementioned results, the present study aimed to identify novel natural agents that may enhance sensitivity of PCa cells to chemotherapeutic drugs. Our previous studies demonstrated the marked antitumor activity of Retigeric acid B (RAB), a natural pentacyclic triterpenic acid isolated from the Lobaria kurokawae Yoshim, suggesting it to be a promising anticancer agent in PCa cells (30–33). In the present study, RAB was identified as an enhancer of CDDP-induced cytotoxicity. Combining RAB with CDDP resulted in a synergistic effect via the suppression of DNA repair and the activation of DR5 following the induction of DNA damage.
Materials and methods
Cell culture and treatments
Human PCa cell lines, PC3 and DU145 [American Type Culture Collection (ATCC), Manassas, VA, USA], were cultured in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences) and 100 units/ml penicillin-streptomycin (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Non-neoplastic prostate epithelial RWPE-1 cells (ATCC) were used as controls. RWPE-1 cells were maintained in keratinocyte-SFM medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with bovine pituitary extract (Gibco; Thermo Fisher Scientific, Inc.) and epidermal growth factor (Gibco, Thermo Fisher Scientific, Inc.). All the cells were maintained in a humidified incubator with 5% CO2 at 37°C.
RAB was isolated from the lichen L. kurokawae Yoshim, and its purity and structure was determined as described previously (30). RAB was prepared in dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 10 mM as stock solutions and stored at −20°C to be diluted to final concentrations of 2, 4, 5, 6, 8 and 10 µM, according to experimental requirements. Various chemotherapeutic agents including CDDP (Qilu Pharmaceutical Co., Ltd., Jinan, Shandong, China), docetaxel (DTX; Qilu Pharmaceutical Co., Ltd.), etoposide (VP-16; Qilu Pharmaceutical Co., Ltd.), doxorubicin (ADM; Shenzhen Main Luck Pharmaceuticals Inc., Shenzhen, Guangdong, China), vincristin (VCR; Shenzhen Main Luck Pharmaceuticals Inc.) were used in combination with RAB as described subsequently.
Viability assay
The effects of the indicated drugs on the viability of the human cell lines were evaluated by MTT assay (Sigma-Aldrich; Merck KGaA). PC3, DU145 and RWPE-1 cells (1×104 per well) were seeded into 96-well plates for 24 h. Different treatments were as follows: PC3 and DU145 cells were treated with different concentrations of RAB (2, 4, 6, 8 and 10 µM) for 48 h at 37°C; PC3, DU145 and RWPE-1 cells were simultaneously treated with 4 µM of RAB and chemotherapeutic agents including CDDP (2 µg/ml), ADM (300 nM), VP-16 (20 µM), DTX (10 nM) and VCR (10 nM) for 48 h at 37°C; PC3 and Du145 cells were treated with different concentrations of RAB (2, 4, 6 and 8 µM) and a fixed concentration of CDDP (2 µg/ml) for 48 h at 37°C, or treated with different concentrations of CDDP (1, 2, 3 and 4 µg/ml) and a fixed concentration of RAB (4 µM) for 48 h at 37°C; PC3 cells were treated with 2 µg/ml CDDP alone or simultaneously with 4 µM RAB for 48 h at 37°C following siRNA transfection. Then, the RPMI-1640 medium (HyClone; GE Healthcare Life Sciences) was removed and the cells were incubated with 10 µl MTT for 4 h. Subsequently, the formazan crystals were dissolved using 0.05% (v/v) DMSO. The cell growth response was detected by measuring the light absorbance at 570 nm using a Multiskan™ microplate reader (Thermo Fisher Scientific, Inc.). The viability assay was performed in triplicate.
Apoptosis assay
Following treatment with RAB (4 µM) and CDDP (2 µg/ml) alone or in combination for 48 h at 37°C, PC3 cells were digested and centrifugalized at 120 × g for 5 min at 4°C. Following 2 washes with PBS, levels of apoptosis were analyzed using an Annexin V-fluorescein isothiocyanate/propidium iodide Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer's protocol. Quantification of fluorescence was determined by flow cytometry (FACSCalibur™; BD Biosciences), and the data were analyzed by WinMDI Software 2.8 (Purdue University Cytometry Laboratories, West Lafayette, IN, USA).
Western blot analysis
Different treatments were as follows: PC3 and DU145 cells were treated with different concentrations of RAB (4, 6, 8 and 10 µM) for 48 h at 37°C; PC3 and DU145 cells were treated with different concentrations of CDDP (1, 2, 3 and 4 µg/ml) and a fixed concentration of RAB (4 µM) for 48 h at 37°C; PC3 cells were treated with RAB (4 and 8 µM) for 24 and 48 h at 37°C; PC3 cells were treated with 2 µg/ml CDDP alone or simultaneously with 4 µM RAB and for 12, 24 or 48 h at 37°C; PC3 cells treated with 2 µg/ml CDDP alone or simultaneously with 4 µM RAB for 48 h at 37°C following siRNA transfection. Then PC3 and DU145 cell lysates were prepared using radioimmunoprecipitation assay lysis buffer according to the manufacturer's protocol (Beyotime Biotechnology Institute of Biotechnology, Inc., Haimen, Jiangsu, China). Proteins were quantified using the BCA protein assay (Beyotime Biotechnology Institute of Biotechnology, Inc.). Samples containing equal amounts of protein (60 µg) from the lysates were separated by 8, 10 and 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% Difco™ Skim Milk (cat. no. 232100; BD Biosciences) in TBST buffer (20 mM Tris-HCl, 137 mM NaCl, and 0.1% Tween-20, pH 8.0) for 1 h at room temperature prior to incubation with specific antibodies. Then, the membranes were incubated overnight at 4°C with the specific antibodies against poly adenosine 5′-adenosine diphosphate ribose polymerase (PARP; cat. no. sc-7150; rabbit polyclonal antibody; dilution 1:2,000), ERCC1 (cat. no. sc-10785; rabbit polyclonal antibody; dilution 1:200) from Santa Cruz Biotechnology, Inc., CA, USA, phosphorylation of histone H2AX at Ser139 (γH2AX; cat. no. 9718, mouse monoclonal antibody; dilution 1:1,000) and DR5 (cat. no. 3696; rabbit polyclonal antibody; dilution 1:1,000) from Cell Signaling Technology, Inc. Danvers, MA, USA, followed by peroxidase-conjugated appropriate secondary antibodies [anti-mouse IgG (H+L) peroxidase-labeled polyclonal antibody (cat. no. 074-1806; dilution 1:5,000); anti-rabbit IgG (H+L) peroxidase-labeled polyclonal antibody; (cat. no. 074-1506; dilution 1:5,000); both were purchased from Seracare Life Sciences Inc., Milford, MA, USA] for 1 h at room temperature. Immunocomplexes were visualized using chemiluminescence (EMD Millipore), according to the manufacturer's protocol. Membranes were stripped and re-probed with GAPDH (cat. no. sc-47724; mouse monoclonal antibody; dilution 1:2,000; Santa Cruz Biotechnology) as a protein loading control. Protein levels were quantified using densitometry of X-ray films by ImageJ 1.6 (National Institutes of Health, Bethesda, Maryland, USA).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis
PC3 and DU145 cells were treated with 0, 4 and 8 µM RAB for 24 and 48 h, or treated with 4 µM RAB and 2 µg/ml CDDP alone or simultaneously for 0, 6, 12, 18, 24 and 48 h at 37°C. Total RNA of PC3 and DU145 cells were extracted using an RNAiso plus kit (Takara Bio, Inc., Otsu, Honshu, Japan). For the RT-qPCR assays, cDNA was synthesized using a PrimeScript™ RT reagent kit (Takara Bio, Inc.). RT-qPCR was performed using the QuantiNova SYBR-Green PCR kit (Qiagen China Co., Ltd., Shanghai, China) and an ABI Prism 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR reaction conditions for all assays were as follows: 95°C for 30 sec, followed by 40 cycles of amplification (95°C for 5 sec, 58°C for 30 sec and 72°C for 30 sec). Changes in the mRNA levels of desired genes were normalized to the level of GAPDH and calculated using the 2−ΔΔCq method (34). The heat map was generated by The R Project for Statistical Computing (R version 3.4.1, The University of Auckland, Auckland, New Zealand). The sequences of primers selected are summarized in Table I.
Table I.
Primers used for reverse transcription quantitative polymerase chain reaction analysis.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| ERCC1 | GGCGACGTAATTCCCGACTA | AGTTCTTCCCCAGGCTCTGC |
| XPA | GGTCTCTTGAAGTTTGGGGTAGTC | TTCCACACGCTGCTTCTTACTG |
| XPB | CTAACTGCCTACTCCTTGTATGC | TCCATAGCTGACAGTACACAACT |
| XPC | CTTCGGAGGGCGATGAAAC | TTGAGAGGTAGTAGGTGTCCAC |
| XPD | GGAAGACAGTATCCCTGTTGGC | CAATCTCTGGCACAGTTCTTGA |
| XPF | CCTCTTTCGCCAGAAAAACAAAC | TTTACTGCTACATGGAACCTTGG |
| XPG | GACTTAGCGTCCAGTGACTCC | GGCAGTTTTGATGGCTTGTCTTT |
| RAD23B | TTCCACACCTGCATCCATCAC | TCAGTTGCTGTTGGGCTAGTA |
| TFB5 | AAGACATTGATGACACTCACGTC | GGGAAAAAGCATTTTGGTCCATT |
| TFIIH | GACCTTGTTGTGAGTCAAGTGA | CCTGCTTATGATTGGATGTGGAA |
| RPA1 | CTCGGGAATGGGTTCTACTGT | CACTTGGACTGGTAAGGAGTGA |
| MSH2 | AAGCCCAGGATGCCATTG | CATTTGACACGTGAGCAAAGC |
| MSH3 | GTGGCAAAAGGATATAAGGTGGG | AAAGGGCAGTCAATTTCCGGG |
| MSH6 | AGCTTAAAGGATCACGCCATC | AAGCACACAATAGGCTTTGCC |
| MLH1 | GCAAACCCCTGTCCAGTCAG | CTGGGAGTTCAAGCATCTCCT |
| PMS1 | CCTATTGATCGGAAGTCAGTCCA | CTACTAACTCCTTTACCGCAGTG |
| GAPDH | TGGTCACCAGGGCTGCTT | AGCTTCCCGTTCTCAGCCTT |
siRNA transfection
PC3 cells were plated into 6-well plates at 20–30% confluency, and 24 h later, knockdown of DR5 was performed by transiently transfecting small interfering RNA (siRNA) targeting DR5 (GenePharma Co., Ltd, Shanghai, China) using Invitrogen Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol (DR5 siRNA sequence, 5′-AUCAGCAUCGUGUACAAGGUGUCCC; scramble siRNA sequence, 5′-UUCUCCGAACGUGUCACGUTT). The final concentration of the siRNA was 50 nM. After 48 h of transfection, cells were treated with combinations of RAB (4 µM) and CDDP (2 µg/ml) for an additional 48 h as previously described (33,35), and the effects of different treatments on the conditioned cells were evaluated by western blot and cell viability assay, as aforementioned.
Microscopy
Morphological changes of apoptosis were determined by staining PC3 cell nuclei with DAPI. Following treatment with RAB (4 µM) and CDDP (2 µg/ml) alone or in combination for 48 h at 37°C, PC3 cells were fixed with 90% ethanol/5% acetic acid for 1 h at room temperature. Following 2 washes with PBS, cells were incubated with DAPI solution (1.5 mg/ml in PBS) for 30 min at room temperature. Images of DAPI fluorescence were captured using a fluorescence microscope (magnification, ×200; Nikon Corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard deviation of at least 3 independent experiments and analyzed by GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). The statistical significance of the mean difference between the control and treated groups was determined with two-tailed Student's t-tests. Multiple group comparisons were performed with a one-way analysis of variance, followed by Dunnett's multiple comparison test. P<0.05 was considered to indicate a statistically significant difference.
Results
In vitro cytotoxic evaluation of RAB combined with conventional chemotherapeutic agents
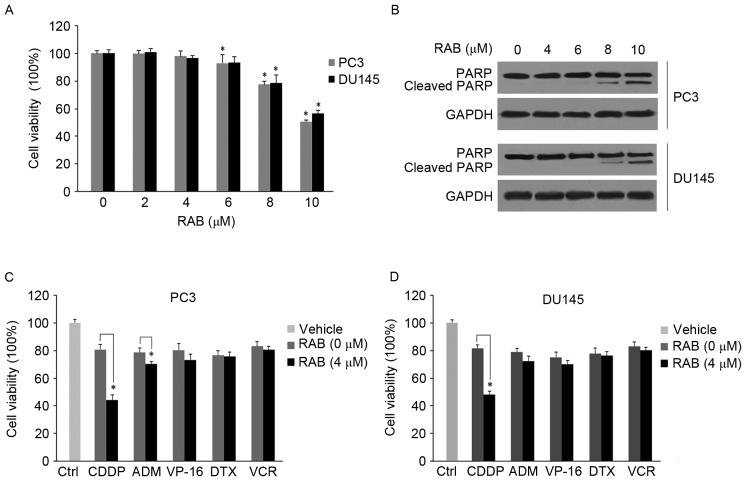
As an initial screening approach to assess the antitumor activities of RAB combined with various conventional chemotherapeutic agents against PCa cells, a cell viability assay was performed. The effect of RAB treatments (2, 4, 6, 8 and 10 µM) on cell viability and PARP cleavage was first determined using MTT and western blot analysis in 2 hormone-refractory PCa cell lines, PC3 and DU145. The results revealed that low-dose RAB did not significantly inhibit cell viability in PC3 (2–4 µM; P>0.05) or DU145 (2–6 µM, P>0.05) cells (Fig. 1A). Concurrently, apoptosis was markedly activated in the PC3 and DU145 cells upon treatment with high doses of RAB (>8 µM) for 48 h, as suggested by the levels of PARP cleavage (Fig. 1B).
Figure 1.
Combined effect of RAB and chemotherapeutic agents in PCa cells. (A) Anti-proliferative effect of RAB in PCa cell lines. PC3 and Du145 cells were treated with different concentrations of RAB and cell viability was measured by MTT assay. *P<0.05 vs. respective RAB-untreated control groups. (B) Western blot analysis of cleavage of PARP in response to RAB treatment. GAPDH served as an internal control. (C and D) Anti-proliferative activity of RAB combined with different chemotherapeutic agents; (C) PC3 and (D) Du145 cells were simultaneously treated with 4 µM of RAB and indicated chemotherapeutic agents including CDDP (2 µg/ml), ADM (300 nM), VP-16 (20 µM), DTX (10 nM) and VCR (10 nM) for 48 h at 37°C. Cell viability was measured by a MTT assay. *P<0.05 vs. single treatment with different chemotherapeutic agents. PCa, prostate cancer; CDDP, cisplatin; ADM, doxorubicin; VP-16, etoposide; DTX, docetaxel; VCR, vincristine; PARP, poly adenosine 5′-adenosine diphosphate ribose polymerase; RAB, Retigeric acid B.
Subsequently, an in vitro drug combination analysis was performed to investigate whether RAB sensitized PCa cells to chemotherapeutic agents. As demonstrated in Fig. 1C and D, PC3 and DU145 cells were simultaneously exposed to 4 µM RAB and distinct drugs, including CDDP (2 µg/ml), ADM (300 nM), VP-16 (20 µM), DTX (10 nM) and VCR (10 nM). RAB in combination with CDDP produced the greatest significant inhibitory effect on PC3 and DU145 cells compared with the other drug combinations, decreasing cell viability rate by 35–40% (P<0.05; Fig. 1C and D) compared with CDDP alone. RAB also significantly upregulated the sensitivity of PC3 cells to ADM, with a decrease in cell viability of 8–10% compared with single ADM treatment (P<0.05; Fig. 1C) in the PC3 cell line. In contrast, the cytotoxicity of all other chemotherapeutic agents was not significantly enhanced by RAB (P>0.05) in the DU145 cell line. Therefore, these results revealed the antitumor effects of low-dose RAB in combination with distinct chemotherapeutic agents, and suggested that RAB significantly increased the apoptotic ability of CDDP in PCa cells.
RAB demonstrates synergistic antitumor effects in combination with CDDP in PCa cells
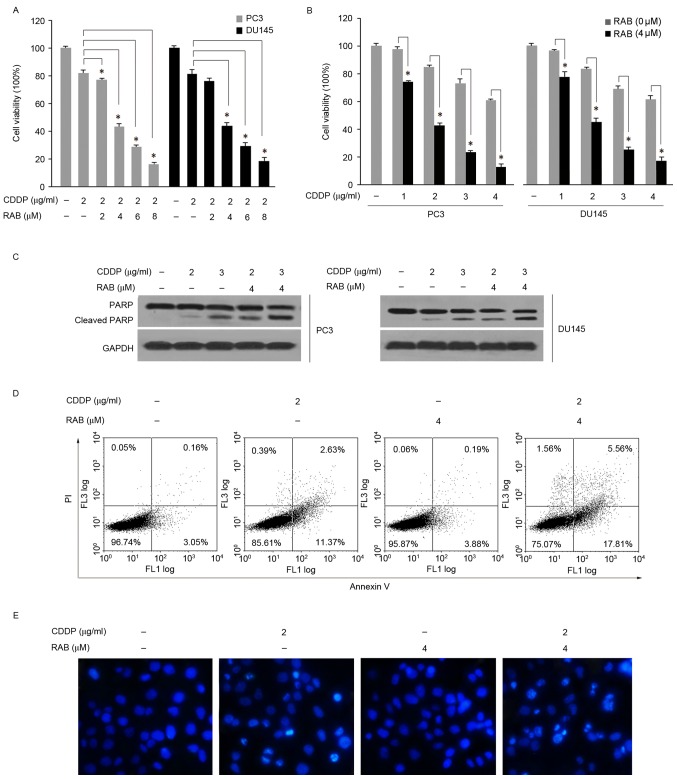
Based on the aforementioned results, it was important to additionally elucidate the potential of RAB to enhance cellular sensitivity to CDDP in PCa cells. As demonstrated in Fig. 2A, RAB at doses ranging from 2–8 µM significantly enhanced the cytotoxicity of CDDP (2 µg/ml) in a dose-dependent manner, as suggested by the more pronounced inhibition of cell viability in comparison to single CDDP treatment (Fig. 2A). As PC3 cells were exposed simultaneously to 4 µM RAB and increasing concentrations of CDDP (1–4 µg/ml), a significant decrease in cell viability was also observed, with a decreased half maximal inhibitory concentration (IC50) of CDDP from 4.5 to ~2 µg/ml (Fig. 2B). Similar results were obtained in PC3 and DU145 cells under the same experimental conditions (Fig. 2A and B). Measurement of apoptosis revealed an increased level of cleaved PARP in the combination treatments compared with the CDDP-alone group (Fig. 2C). Additionally, co-treatment with RAB (4 µM) and CDDP (2 µg/ml) caused an increased percentage of apoptotic cells, as determined by the flow cytometry assay (Fig. 2D). The sum of early and late apoptotic cell death induced by CDDP alone at 48 h was 14.0%; when used in combination with RAB the level of apoptotic cells reached 23.37% in PC3 cells. In addition, the percentage of necrotic cells increased from 0.39 to 1.56% in PC3 cells following the combined treatment. The nucleic morphological changes of apoptosis were determined by staining nuclear DNA with DAPI. Correspondingly, combined treatment of RAB with CDDP resulted in a marked increase in the number of apoptotic cells with condensed and fragmented DNA, by a more marked blue fluorescence compared with the non-apoptotic cells as observed in the microscopic images (Fig. 2E). In contrast, the non-neoplastic prostate epithelial RWPE-1 cells, were resistant to the combination treatment compared with PCa cells (data not shown). Together, these results suggested that RAB synergistically increased CDDP-mediated cell growth inhibition and apoptosis.
Figure 2.
RAB enhances the cytotoxicity of CDDP. RAB sensitized PC3 and DU145 cells to CDDP-mediated anti-proliferation and apoptosis; (A) PC3 and DU145 cells were treated with different concentrations of RAB (2–8 µM) and a fixed concentration of CDDP (2 µg/ml) for 48 h at 37°C; (B) PC3 and DU145 cells were treated with different concentrations of CDDP (1–4 µg/ml) and a fixed concentration of RAB (4 µM) for 48 h at 37°C as measured by MTT assay. *P<0.05 vs. single treatment with the corresponding concentrations of CDDP. RAB sensitized PC3 and Du145 cells to CDDP-induced apoptosis as measured by (C) PARP cleavage and (D) flow cytometry. (E) Apoptosis in PC3 cells as visualized using DAPI staining. Cells were exposed for 48 h at 37°C to the indicated treatments prior to staining with DAPI for 30 min. CDDP, cisplatin; RAB, Retigeric acid B; PI, propidium iodide; PARP, poly adenosine 5′-adenosine diphosphate ribose polymerase.
RAB-induced DNA repair inhibition enhances chemotherapy sensitization of PCa cells to CDDP
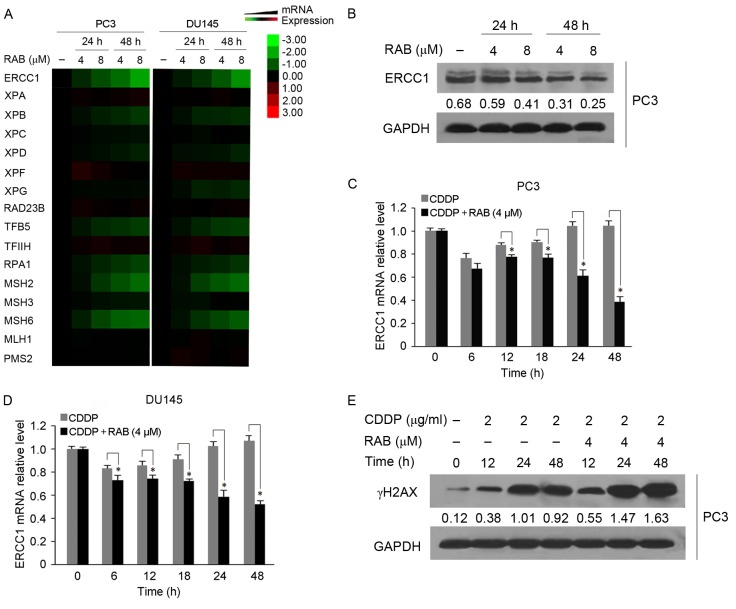
As impairment of DNA repair may cause cell death following lethal DNA damage, and the NER pathway is particularly associated with the efficacy of CDDP (16,18), the present study focused on the effect of RAB on this process to reveal the RAB-induced sensitization mechanisms of CDDP cytotoxicity. RT-qPCR analysis identified the changes in the expression of genes associated with NER in response to RAB treatment (Fig. 3A). The expression of NER-associated genes including ERCC1, TFB5 and RPA1 in RAB-treated cells markedly decreased at 24 h treatment, and their expression levels decreased further at 48 h compared with at 24 h (Fig. 3A). The levels of XPB and XPD also decreased following RAB exposure, but in a moderate manner (Fig. 3A). In contrast, XPA, XPC, XPF, RAD23B and TFIIH expression remained unchanged or increased insignificantly following RAB treatment (Fig. 3A). Levels of XPG expression remained unchanged in PC3 cells, while they decreased over time in in a moderate manner in DU145 cells. The effect of RAB on the expression of NER-associated genes demonstrated a similar pattern in PC3 and DU145 cells, as indicated in Fig. 3A. In addition, RAB demonstrated an inhibitory effect on mismatch repair (MMR), another DNA repair pathway associated with sensitivity to CDDP (16), as indicated by the marked inhibition of the expression of MMR genes MSH2 and MSH6 following RAB treatment.
Figure 3.
NER pathway is involved in the synergistic antitumor activity of combined CDDP and RAB treatment, and the effect of RAB on activities of DNA damage repair. (A) Heat map for mRNA levels of NER and MMR genes in RAB-treated cells that were determined by RT-qPCR. Red represents overexpression, green represents under-expression and black represents unchanged expression. (B) Western blot analysis of ERCC1 in PC3 cells following RAB treatment for different time intervals. Protein levels were normalized to GAPDH, and was quantified using densitometry of X-ray films. Changes in mRNA levels of ERCC1 in response to CDDP combined with RAB; (C) PC3 and (D) DU145 cells were treated with 4 µM RAB and 2 µg/ml CDDP simultaneously for the indicated times, and ERCC1 mRNA levels were detected by RT-qPCR assay. *P<0.05 vs. respective RAB-untreated groups. (E) Effect of co-treatment with RAB and CDDP on the γH2AX expression in PC3 cells, as determined by western blot analysis. GAPDH served as a loading control. Protein levels were quantified using densitometry of X-ray films. NER, nucleotide excision repair; MMR, mismatch repair; ERCC1, Excision repair cross-complementing 1; RT-qPCR, reverse transcription quantitative polymerase chain reaction; γH2AX, phosphorylation of histone H2AX at Ser139; RAB, RAB, Retigeric acid B; CDDP, cisplatin.
Notably, the expression of ERCC1 mRNA, a critical gene in the NER pathway, presented the most significant decrease among all the genes examined following treatment with RAB (Fig. 3A). RAB markedly downregulated the protein level of ERCC1 in a dose- and time-dependent manner (Fig. 3B); therefore, it was selected for additional combination study. As indicated in Fig. 3C and D, the expression of ERCC1 in response to co-treatment with RAB and CDDP was decreased in a time-dependent manner, particularly at the later time points (12–48 h, P<0.05) compared with CDDP treatment alone. Correspondingly, RAB markedly increased the level of γH2AX, an indicator of DNA damage elicited by CDDP (Fig. 3E). It was noted that the aggravated DNA damage caused by RAB became marked following 12 h co-treatment with the two drugs and persisted up to 48 h, suggesting that the RAB-evoked DNA repair blockade may serve a pivotal function in this event. Therefore, these data demonstrated that RAB was able to impair DNA repair gene expression, which may function together with the induction of lethal DNA damage to cause cell death when combined with CDDP.
DR5 overexpression mediated by RAB accelerates CDDP-induced apoptosis
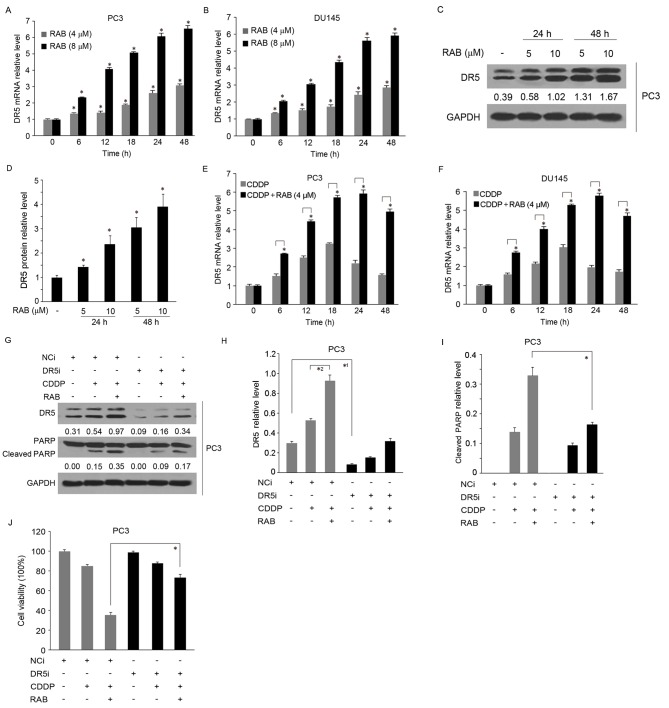
Following the observation that RAB may also increase the mRNA level of DR5, according to the microarray data obtained from our previous study (24), together with the aforementioned data suggesting that DR5 possesses the potential to promote the sensitivity of PCa cells to CDDP through DNA repair inhibition (26–29), the present study assessed whether RAB-mediated DR5 overexpression was involved in the synergistic mechanism of the increased cytotoxicity of CDDP. The upregulation of DR5 mRNA induced by RAB was validated by RT-qPCR assay (Fig. 4A and B). The results demonstrated that DR5 was elevated in a dose- and time-dependent manner in PC3 and DU145 cells. In these cell lines, the expression of DR5 mRNA significantly increased to between 1.3–1.5- and between 2 and 3-folds following 6 h treatment by 4 and 8 µM of RAB, respectively, and became more pronounced with increasing treatment durations, up to 48 h (between 2.5–3.0- and between 6–7-fold, respectively). Subsequently, the protein levels of DR5 in response to RAB were detected and revealed to be markedly increased after 24 and 48 h treatment, by ~3.0- and ~4.0-fold, respectively (Fig. 4C,D). Next, the changes in DR5 expression in response to combination application of CDDP and RAB were investigated. As indicated in Fig. 4E and F, CDDP increased the levels of DR5 at 6 h (~1.5-fold), which was maintained up to 18 h (between 3–3.5-fold), then gradually declined following 48 h treatment (~1.8-fold). In contrast, combined use with RAB enhanced CDDP-induced DR5 expression, particularly following prolonged RAB treatment (between 5–6-fold increase for 24–48 h). This effect was more marked in PC3 cells compared with DU145 cells.
Figure 4.
RAB-induced DR5 overexpression enhances chemotherapy sensitivity to CDDP. RT-qPCR analysis of DR5 in RAB-treated (A) PC3 and (B) DU145 cells. *P<0.05 vs. respective RAB-untreated groups in PC3 and DU145 cells. (C and D) Western blot analysis of protein expression of DR5 in PC3 cells. *P<0.05 vs. RAB-untreated group. (D and E) Combined effect of CDDP and RAB on the expression of DR5 as determined by RT-qPCR. (E) PC3 and (F) DU145 cells were treated with 4 µM RAB and 2 µg/ml CDDP simultaneously for the indicated times. *P<0.05 vs. respective RAB-untreated groups. Silencing of DR5 expression by siRNA attenuated the cytotoxicity induced by the combined use of CDDP and RAB; the silencing efficiency and expression of cleaved PARP were examined using (G-I) western blot analysis. Equal protein loading was evaluated by GAPDH and was quantified using densitometry analysis. *1P<0.05 vs. the control in the NCi group, *2P<0.05 vs. single CDDP treatment in the NCi group. *P<0.05 vs. the combined treatment of CDDP and RAB in the NCi group. (J) Cell viability was detected by MTT assay. *P<0.05 vs. the NCi group. RT-qPCR, reverse transcription quantitative polymerase chain reaction; RAB, Retigeric acid B; CDDP, cisplatin; si, small interfering; NCi, negative control siRNA; DR5i, Death receptor 5 siRNA; PARP, poly adenosine 5′-adenosine diphosphate ribose polymerase.
To additionally examine the functional involvement of DR5 in RAB-mediated increased sensitivity of cells to CDDP, PC3 cells were transfected with DR5-targeting siRNA. As demonstrated in Fig. 4G and H, successful knockdown of DR5 expression was confirmed by western blot analysis. Cells transfected with non-target siRNA and treated with RAB exhibited DR5 expression, and the cell growth was synergistically inhibited by RAB and CDDP, while the synergistic effect was markedly decreased in PC3 and DU145 cells transfected with DR5-targeting siRNA, as evidenced by the attenuation of the levels of cleaved PARP (Fig. 4G and I). Concurrently, depletion of DR5 partially reversed the inhibitory effect of the combination treatment (RAB+CDDP) on cell proliferation by 30% (P<0.05), which additionally confirmed the role of DR5 in the mechanism of action of the RAB-CDDP treatment complex (Fig. 4J). Together, these data suggest that RAB-induced DR5 expression promoted CDDP cytotoxicity, leading to a contribution to the synergistic antitumor effect of co-treatment with RAB and CDDP.
Discussion
RAB is a natural pentacyclic triterpenic acid that exhibits potential antitumor activity in PCa cells in vitro and in vivo (31,33). The present study aimed to investigate the potential of combination treatment of RAB with chemotherapeutic drugs to promote their anticancer effects. To the best of our knowledge, it was identified here for the first time that RAB exerted potent chemotherapy sensitization to CDDP in PCa cells. To additionally elucidate the mode of action of this synergistic cytotoxicity, the function of DNA repair in the enhanced antitumor activity of CDDP by RAB-treatment was assessed. It was revealed that RAB blocked the NER pathway by inhibiting the expression of multiple NER-associated genes following prolonged treatment, which may inhibit the removal of CDDP-DNA adducts and accelerate the rate of cell death. Concurrently, treatment of tumor cells with RAB markedly promoted the expression of DR5. Regarded as a proapoptotic protein in cancer (25,27), DR5 overexpression also contributed to the cell death mediated by the combination treatment.
The development of resistance to chemotherapy remains one of the major challenges of curing advanced and metastatic PCa. Combined use of different chemotherapeutic drugs without cross resistance, with distinct mechanisms of action may decrease the risk of drug-resistant cell clone formation, and may also increase the tumor remission rate and the possibility of a cure (2,3). The hypothesis of the present study was that novel natural compounds that are able to induce cancer cell death through various signaling pathways may potentially improve the effectiveness of chemotherapy. RAB has gained attention for its potential antitumor activity in PCa. In the present study, among different treatment combinations, the combined use of CDDP and RAB exhibited the highest efficacy. As PCa cells were exposed simultaneously to low-dose RAB and increasing doses of CDDP, a significant decrease in cell viability and an induction of apoptosis were observed, with a decreased IC50 of CDDP. This treatment method may decrease the effective dose of CDDP, therapeutically, which may also contribute to decreased adverse effects of CCDP. For example, administration of epigallocatechin-3-gallate (50 mg/kg) together with CDDP (10 mg/kg) was identified to prevent CDDP-induced nephrotoxicity, ototoxicity and their consequences, including mortality (36). It was also suggested previously that reduced dosages of these two drugs achieved maximal cytotoxic effects by combining topotecan with a Checkpoint kinase 1 inhibitor (PF477736HEK1), which may potentially minimize side effects of the drugs (37).
It has been established that the formation of platinum-DNA adducts blocks replication and transcription and ultimately leads to G2 phase cell cycle arrest or cell death (5,6). However, previous studies have indicated that DNA-damaging agents only offer modest benefit for the majority of patients due to the proficient DNA repair processes available in cancer cells (6–10). Therefore, the inhibition of DNA repair remains an effective approach to improve the sensitivity to CDDP. Of the 4 major DNA repair pathways: NER, base excision repair, MMR and double-strand-break repair, NER is the major pathway regarded to remove CDDP lesions from DNA (16,18). The data from the present study revealed that γH2AX persisted at the later treatment time point (24–48 h) of the combination treatment of CDDP and RAB, while decreased accumulation of γH2AX was observed in samples treated with CDDP alone compared with the combined treatment of CDDP and RAB. The results may be due to significant NER impairment at the later time points by RAB, as supported by the decreased expression of multiple NER-associated genes. Therefore, the inhibitory effect of RAB on the NER pathway potentiated the cytotoxic activity of CDDP. Additionally, decreased sensitivity to CDDP-induced DNA damage may also occur through a loss of function of the MMR pathway (16). During MMR, CDDP-induced DNA adducts are recognized by the MMR proteins MSH2, MSH3 and MSH6, which are homologues of the bacterial protein MutS (38). Loss of MMR with respect to CDDP-DNA adducts may result in decreased apoptosis and drug resistance (16). RAB impaired the MMR pathway, as evidenced by the downregulation of MSH2 and MSH6, which function together with NER deficiency to accelerate DNA damage and cell death.
DNA damage-mediated apoptotic signals, however, may be attenuated (4,5). Alternative therapies with potential antitumor activity that are mediated by various mechanisms have been considered. Previous studies have suggested that targeting death receptors and their respective signaling pathways to trigger apoptosis promotes the sensitivity of tumor cells (29,39,40). A variety of agents such as delphinidin (39), ursolic acid (40), carnitine (41), salirasib (42), monensin (43) and 2-tellurium-bridged β-cyclodextrin (44), have been demonstrated to sensitize tumors to apoptosis by inducing DR5. In the present study, it was identified that RAB increased the mRNA and protein levels of DR5 in PCa cells. Depletion of DR5 effectively decreased the rate of cell death in the presence of CDDP in combination with RAB, confirming the functional significance of DR5 upregulation in the enhanced cytotoxicity of CDDP.
In summary, the present study proposed a mechanistic basis for the antitumor effect of RAB in combination with CDDP, a front-line treatment for a variety of neoplasms, used to enhance the efficacy of CDDP therapy. RAB sensitized PCa cells to CDDP at low IC50 values by a combination of mechanisms, including the impairment of DNA repair and the activation of DR5, which suggested the combined use of CDDP and RAB as a potential chemotherapeutic strategy.
Acknowledgements
The present study was supported by Shandong Natural Science Foundation (grant no. 2016ZRE27236) and the National Natural Science Foundation of China (grant no. 81603140), and China Postdoctoral Science Foundation Grant (grant no. 2017M612296).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Parekh A, Graham PL, Nguyen PL. Cancer control and complications of salvage local therapy after failure of radiotherapy for prostate cancer: A systematic review. Semin Radiat Oncol. 2013;23:222–234. doi: 10.1016/j.semradonc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol. 2011;29:3686–3694. doi: 10.1200/JCO.2010.34.3996. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP. Chemotherapy for advanced hormone refractory prostate cancer. Urology. 1999;54(6A Suppl):S30–S35. doi: 10.1016/S0090-4295(99)00452-5. [DOI] [PubMed] [Google Scholar]
- 5.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo; Proc Natl Acad Sci USA; 2011; pp. 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hager S, Ackermann CJ, Joerger M, Gillessen S, Omlin A. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol. 2016;27:975–984. doi: 10.1093/annonc/mdw156. [DOI] [PubMed] [Google Scholar]
- 7.Basourakos SP, Li L, Aparicio AM, Corn PG, Kim J, Thompson TC. Combination platinum-based and DNA damage response-targeting cancer therapy: Evolution and future directions. Curr Med Chem. 2017;24:1586–1606. doi: 10.2174/0929867323666161214114948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanski M. Recent developments in the field of anticancer platinum complexes. Recent Pat Anticancer Drug Discov. 2006;1:285–295. doi: 10.2174/157489206777442287. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA, Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 10.Harzstark AL, Ryan CJ. Novel therapeutic strategies in development for prostate cancer. Expert Opin Investig Drugs. 2008;17:13–22. doi: 10.1517/13543784.17.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Su NW, Leu YS, Lee JC, Chen YJ, Chen HW, Liu CJ, Chang YF. Comparison of the efficacy and toxicity of two dose levels of cisplatin/5-fluorouracil as the chemoradiotherapy regimen for the treatment of locally advanced squamous cell carcinoma of the head and neck. Acta Otolaryngol. 2011;131:1333–1340. doi: 10.3109/00016489.2011.616226. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Grines CL, Marmagkiolis K. Vascular toxicities of cancer therapies: The old and the new-an evolving avenue. Circulation. 2016;133:1272–1289. doi: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J. 1988;9:552–556. doi: 10.1093/oxfordjournals.eurheartj.a062542. [DOI] [PubMed] [Google Scholar]
- 14.Varveris H, Delakas D, Anezinis P, Haldeopoulos D, Mazonakis M, Damilakis J, Metaxaris G, Chondros N, Mavromanolakis E, Daskalopoulos G, et al. Concurrent platinum and docetaxel chemotherapy and external radical radiotherapy in patients with invasive transitional cell bladder carcinoma. A preliminary report of tolerance and local control. Anticancer Res. 1997;17:4771–4780. [PubMed] [Google Scholar]
- 15.Pu J, Qin SS, Ding JX, Zhang Y, Zhu WG, Yu CH, Li T, Tao GZ, Ji FZ, Zhou XL, et al. A randomized controlled study of single-agent cisplatin and radiotherapy versus docetaxel/cisplatin and radiotherapy in high-risk early-stage cervical cancer after radical surgery. J Cancer Res Clin Oncol. 2013;139:703–708. doi: 10.1007/s00432-013-1373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 17.Wei Q, Frazier ML, Levin B. DNA repair: A double-edged sword. J Natl Cancer Inst. 2000;92:440–441. doi: 10.1093/jnci/92.6.440. [DOI] [PubMed] [Google Scholar]
- 18.Wood RD. Nucleotide excision repair in mammalian cells. J Biol Chem. 1997;272:23465–23468. doi: 10.1074/jbc.272.38.23465. [DOI] [PubMed] [Google Scholar]
- 19.Reed E. ERCC1 and clinical resistance to platinum-based therapy. Clin Cancer Res. 2005;11:6100–6102. doi: 10.1158/1078-0432.CCR-05-1083. [DOI] [PubMed] [Google Scholar]
- 20.Cummings M, Higginbottom K, McGurk CJ, Wong OG, Köberle B, Oliver RT, Masters JR. XPA versus ERCC1 as chemosensitising agents to cisplatin and mitomycin C in prostate cancer cells: Role of ERCC1 in homologous recombination repair. Biochem Pharmacol. 2006;72:166–175. doi: 10.1016/j.bcp.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Núñez F, Chipchase MD, Clarke AR, Melton DW. Nucleotide excision repair gene (ERCC1) deficiency causes G(2) arrest in hepatocytes and a reduction in liver binucleation: The role of p53 and p21. FASEB J. 2000;14:1073–1082. doi: 10.1096/fasebj.14.9.1073. [DOI] [PubMed] [Google Scholar]
- 22.Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16. doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 24.Yeh PY, Chuang SE, Yeh KH, Song YC, Ea CK, Cheng AL. Increase of the resistance of human cervical carcinoma cells to cisplatin by inhibition of the MEK to ERK signaling pathway partly via enhancement of anticancer drug-induced NF kappa B activation. Biochem Pharmacol. 2002;63:1423–1430. doi: 10.1016/S0006-2952(02)00908-5. [DOI] [PubMed] [Google Scholar]
- 25.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr, el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- 27.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 28.Shankar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–186. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- 29.Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: Up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther. 2007;6:1387–1399. doi: 10.1158/1535-7163.MCT-06-0521. [DOI] [PubMed] [Google Scholar]
- 30.Wang XN, Zhang HJ, Ren DM, Ji M, Yu WT, Lou HX. Lobarialides A-C, antifungal triterpenoids from the lichen Lobaria kurokawae. Chem Biodivers. 2009;6:746–753. doi: 10.1002/cbdv.200800054. [DOI] [PubMed] [Google Scholar]
- 31.Liu YQ, Gao FB, Jiang HM, Niu LL, Bi YL, Young CY, Yuan HQ, Lou HX. Induction of DNA damage and ATF3 by retigeric acid B, a novel topoisomerase II inhibitor, promotes apoptosis in prostate cancer cells. Cancer Lett. 2013;337:66–76. doi: 10.1016/j.canlet.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Liu YQ, Ji Y, Li XZ, Tian KL, Young CY, Lou HX, Yuan HQ. Retigeric acid B-induced mitophagy by oxidative stress attenuates cell death against prostate cancer cells in vitro. Acta Pharmacol Sin. 2013;34:1183–1191. doi: 10.1038/aps.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu YQ, Hu XY, Lu T, Cheng YN, Young CY, Yuan HQ, Lou HX. Retigeric acid B exhibits antitumor activity through suppression of nuclear factor-kappaB signaling in prostate cancer cells in vitro and in vivo. PLoS One. 2012;7:e38000. doi: 10.1371/journal.pone.0038000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Wang M, Wang D, Li X, Wang W, Lou H, Yuan H. Malformin A1 promotes cell death through induction of apoptosis, necrosis and autophagy in prostate cancer cells. Cancer Chemother Pharmacol. 2016;77:63–75. doi: 10.1007/s00280-015-2915-4. [DOI] [PubMed] [Google Scholar]
- 36.Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013;32:894–903. doi: 10.1016/j.clnu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Kim MK, James J, Annunziata CM. Topotecan synergizes with CHEK1 (CHK1) inhibitor to induce apoptosis in ovarian cancer cells. BMC Cancer. 2015;15:196. doi: 10.1186/s12885-015-1231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zdraveski ZZ, Mello JA, Farinelli CK, Essigmann JM, Marinus MG. MutS preferentially recognizes cisplatin-over oxaliplatin-modified DNA. J Biol Chem. 2002;277:1255–1260. doi: 10.1074/jbc.M105382200. [DOI] [PubMed] [Google Scholar]
- 39.Ko H, Jeong MH, Jeon H, Sung GJ, So Y, Kim I, Son J, Lee SW, Yoon HG, Choi KC. Delphinidin sensitizes prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5 and causing caspase-mediated HDAC3 cleavage. Oncotarget. 2015;6:9970–9984. doi: 10.18632/oncotarget.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin SW, Park JW. Ursolic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Biochim Biophys Acta. 2013;1833:723–730. doi: 10.1016/j.bbamcr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Park SJ, Park SH, Kim JO, Kim JH, Park SJ, Hwang JJ, Jin DH, Jeong SY, Lee SJ, Kim JC, et al. Carnitine sensitizes TRAIL-resistant cancer cells to TRAIL-induced apoptotic cell death through the up-regulation of Bax. Biochem Biophys Res Commun. 2012;428:185–190. doi: 10.1016/j.bbrc.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 42.Charette N, De Saeger C, Horsmans Y, Leclercq I, Stärkel P. Salirasib sensitizes hepatocarcinoma cells to TRAIL-induced apoptosis through DR5 and survivin-dependent mechanisms. Cell Death Dis. 2013;4:e471. doi: 10.1038/cddis.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA, Lim JH, Kwon TK, Choi KS. Monensin, a polyether ionophore antibiotic, overcomes TRAIL resistance in glioma cells via endoplasmic reticulum stress, DR5 upregulation and c-FLIP downregulation. Carcinogenesis. 2013;34:1918–1928. doi: 10.1093/carcin/bgt137. [DOI] [PubMed] [Google Scholar]
- 44.Lin T, Ding Z, Li N, Xu J, Luo G, Liu J, Shen J. 2-Tellurium-bridged β-cyclodextrin, a thioredoxin reductase inhibitor, sensitizes human breast cancer cells to TRAIL-induced apoptosis through DR5 induction and NF-κB suppression. Carcinogenesis. 2011;32:154–167. doi: 10.1093/carcin/bgq234. [DOI] [PubMed] [Google Scholar]