Abstract
The activation of the Wnt/β-catenin signaling pathway has been demonstrated to play important roles in breast carcinogenesis and to be associated with a poorer prognosis in breast cancer patients. However, genetic mutation is not the major reason for Wnt/β-catenin activation in breast cancer. Dishevelled-associated antagonist of β-catenin homolog 2 (DACT2) is a negative regulator of β-catenin and acts as a tumor suppressor in numerous cancer types; however, the expression change and potential role of DACT2 in breast cancer is unknown. The present study detected the expression and function of DACT2 in breast cancer progression. It was identified that the expression of DACT2 significantly decreased in breast cancer tissues compared with paired adjacent normal breast tissues. Additional investigation demonstrated that the hypermethylation of DACT2 gene promoter contributes to the loss of the gene in breast cancer. It was also demonstrated that DACT2 is a tumor suppressor in breast cancer and inhibits the proliferation and invasion of breast cancer cells by repressing the expression of β-catenin target genes associated with tumor growth and metastasis. The present study indicates that the loss of DACT2 may contribute to breast cancer progression and provides a promising therapeutic target for the treatment of breast cancer.
Keywords: breast cancer, dishevelled-associated antagonist of β-catenin homolog 2, methylation, Wnt/β-catenin
Introduction
Breast cancer accounts for ~30% of all cancers in women and is the most invasive form of cancer in women worldwide. Following lung cancer, breast cancer has become the second leading cause of cancer-associated mortality in women in developed nations (1). Thus, it is urgent to explore and define the mechanisms of breast cancer progression for the development of effective therapeutic and preventative methodologies.
The activation of Wnt/β-catenin signaling pathway has been demonstrated to play important roles in breast carcinogenesis and to be associated with a poorer prognosis in breast cancer patients (2–5). Upon activation of this signaling pathway, β-catenin accumulates and enters the nucleus where it binds transcription factors of the transcription factor/lymphoid enhancer-binding factor family and activates the transcription of target genes associating with breast cancer progression (1). It is widely accepted that genetic mutation is not the major contributing factor for β-catenin activation in breast cancer, thus the aberrant expression of signaling pathway components has become an area of intense focus for detecting the association between β-catenin activation and breast tumorigenesis (1,6). For example, numerous Wnt proteins, including Wnt2, Wnt7b, and Wnt10b, were identified to be upregulated in human breast carcinomas (6). It has also been reported that the receptors of WNT, such as frizzled (FZD) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), were upregulated and results in aberrant activation of the β-catenin signaling pathway in breast cancer (1).
Dishevelled-associated antagonist of β-catenin homolog (DACT) 2 is a member of the DACT protein family that interacts with dishevelled (Dvl), a key initiating factor of the Wnt signaling pathway (7). DACT2 interacts with Dvl and promotes the degradation of Dvl; thus, DACT2 is an inhibitor of the WNT/β-catenin signaling pathway (8,9). However, to the best of our knowledge, there have not yet been studies investigating the dysregulation of DACT2 and its effect on the signaling pathway in breast tumorigenesis. To explore the function and modes of expressional regulation of DACT2 in breast cancer, the present study assessed the status of the DACT2 gene in human breast cancer and assessed its function in breast carcinogenesis.
Materials and methods
Cell and tissue samples
Breast cancer MCF-7, MDA-MB-231 and MDA-MB-468 cell lines and the lentiviral vector packaging 293T/17 cell line were bought from the Cell Center of Institute of Basic Medical Science, Chinese Academy of Medical Science. Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C and 5% CO2.
In total, 20 patients with breast cancer were diagnosed at the Third Affiliated Hospital of the Harbin Medical University between August 2009 and November 2010, their breast cancer tissues and the paired adjacent normal breast tissues were collected subsequent to surgery and immediately stored in liquid nitrogen. The tissue slides of breast cancers and adjacent normal tissues were obtained from the Pathology Department of the hospital. The present study was approved by the Ethics Committee of Harbin Medical University. Written informed consent was obtained from all patients.
DNA extraction and methylation-specific polymerase chain reaction (PCR) (MSP)
Genomic DNA extraction from cell lines, breast cancer tissues and bisulfite modification was performed as previously described (10). Briefly, cells and tissues were digested with lysis buffer containing proteinase K (Beyotime Institute of Biotechnology, Haimen, China), and then genomic DNA was precipitated using isopropanol and dissolved in nuclease-free water. For the modification of genomic DNA, DNA was denatured with 0.2 M NaOH, and then modified with 10 mM hydroquinone (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and 3 M sodium bisulfite (Sigma-Aldrich; Merck Millipore). Subsequent to purification, the modified DNA was treated with 0.3 M NaOH followed by ethanol precipitation. DNA was finally resuspended in nuclease-free water. The DNA polymerase, DNA ladder and Ethidium bromide were obtained from (Beijing Transgen Biotech Co., Ltd., Beijing, China). MSP was performed as previously described (10). MSP primers were designed around transcription start sites and the sequences of primers are listed in Table I. PCR products were analyzed with 2% agarose gels, and the lengths of methylated and unmethylated PCR products were identified to be 152 and 161 bp, respectively. Experiments were repeated in triplicate.
Table I.
Primers used in the present study.
| Primer name | Primer sequence (5′-3′) |
|---|---|
| DACT2 qPCR | |
| Forward | CGGTCGGTTGATGAGACTACT |
| Reverse | CAGGGCTCTGTCAAGATCACC |
| CyclinD1 qPCR | |
| Forward | GCTGCGAAGTGGAAACCATC |
| Reverse | CCTCCTTCTGCACACATTTGAA |
| MMP7 qPCR | |
| Forward | GAGTGAGCTACAGTGGGAACA |
| Reverse | CTATGACGCGGGAGTTTAACAT |
| GAPDH qPCR | |
| Forward | ATGGGGAAGGTGAAGGTCG |
| Reverse | GGGGTCATTGATGGCAACAATA |
| DACT2 ORF | |
| Forward | CGGGATCCGCCGCTCGTGGGGTTCGGGA |
| Reverse | CGACGCGTACCATGGTCATGACCTTCA |
| β-catenin reporter (wide) | |
| Forward | CGCGTAACTGACAGATCAAAGGGGGTAAGATCAAAGGGGGTAGTCAACTC |
| Reverse | TCGAGAGTTGACTACCCCCTTTGATCTTACCCCCTTTGATCTGTCAGTTA |
| β-catenin reporter (mutant) | |
| Forward | CGCGTAACTGACAGATCCCCTTTTTTAAGATCCCCTTTTTTAGTCAACTC |
| Reverse | TCGAGAGTTGACTAAAAAAGGGGATCTTAAAAAAGGGGATCTGTCAGTTA |
| MSP primers | |
| Methylated forward | GCGCGTGTAGATTTCGTTTTTCGC |
| Methylated reverse | AACCCCACGAACGACGCCG |
| Unmethylated forward | TTGGGGTGTGTGTAGATTTTGTTTTTTGT |
| Unmethylated reverse | CCCAAACCCCACAAACAACACCA |
qPCR, quantitative polymerase chain reaction; DACT2, dishevelled-associated antagonist of β-catenin homolog 2; MMP7, matrix metalloprotease 7; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MSP, methylation-specific polymerase chain reaction.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the harvested cell and tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Subsequent to quantification, the RNA was reversed to cDNA using M-MLV reverse transcript kit (Invitrogen; Thermo Fisher Scientific, Inc.), and the random primer was used as RT primer to synthesis cDNA. In brief, 5X RT buffer (4 µl), primer (0.1 µg), transcriptase (1 µl), 10 mM RT dNTPs (1 µl), RNA sample (1 µg) and DEPC-treated water (to make up 20 µl) mixture was incubated at 25°C for 5 min, 42°C for 30 min and 85°C for 5 min. All the reagents were obtained from Invitrogen; Thermo Fisher Scientific, Inc. RT-qPCR was performed using a Bio-Rad qPCR System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd., Dalian, China), according to the manufacturer's protocol. Gene relative expression is analyzed by the 2−∆∆Cq method (11). GAPDH was used as the endogenous controls for mRNA analysis. The primers used for qPCR are summarized in Table I. The experiment was repeated three times.
Western blot analysis
The cells were collected and treated with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Then, the cell lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Primary antibodies against the following proteins were used: mouse anti-human β-catenin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, 1:500, sc-59737); rabbit anti-human DACT2 (Abcam, Cambridge, MA, USA, 1:500, ab79042); and mouse-anti-human β-actin (Santa Cruz Biotechnology, Inc. 1:500, sc-130065). HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (bovine anti-mouse/rabbit, 1:3000, sc-2371/2370). Signal was detected using an enhanced chemiluminescence kit (EMD Millipore, Billerica, MA, USA), according to the manufacturer's protocol.
Lentiviral vector construction and packing
The coding sequence of DACT2 was amplified from cDNA of MCF7 cells by PCR as described previously (12), using the primers listed in Table I. This was then sequenced and digested with BamHI and MluI, followed by cloning into the pC-1 plasmid lined with the same enzyme as previously described (12). Lentiviral vectors were then prepared using the lentiviral vector packing kit (System Biosciences, Mountain View, CA, USA), according to the manufacturer's protocol. MDA-MB-468 cells (1×105 cells/well) were plated into 24-well plate, 10 µ of 1×108 IU/ml lentiviral vectors were added into 24-well plate and incubated for 12 h respectively. Then MDA-MB-468 cells infected with lentiviral vectors were transferred to a 25 cm2 flask and grown for at least 72 h prior to be sorted using a fluorescence-activated cell sorting Aria II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Luciferase assay
A β-catenin activity reporter plasmid was prepared by inserting the synthesized β-catenin-recognizing DNA sequence (Table I) into a pGL-3 basic vector (Promega Corporation, Madison, WI, USA). MCF7 cells were plated into 24-well plates to reach 50–70% confluency the following day. The cells were co-transfected with 0.4 µg pGL3-basic-based construct, 0.1 µg pRL-TK plasmid and 0.5 µg DACT2 overexpression plasmid or the control vector to evaluate the effect of DACT2 overexpression on β-catenin activity, or with 10 µl of 20 µM DACT2 small interfering RNA (siRNA) or negative control siRNA to evaluate the effect of DACT2 knockdown on β-catenin activity, using Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were then lysed and β-catenin activity was assessed using the dual-luciferase reporter assay system (Promega Corporation).
Transwell assay
Cell invasion was measured using the Biocoatmatrigel Invasion Chamber kit (BD Biosciences, Franklin Lakes, NJ, USA). The Matrigel-coated plates were rehydrated for 2 h, and then 2.5×104 cells were suspended in 500 ml DMEM medium and placed on the insert. Subsequently, 750 ml complete DMEM medium was added to the 24-well chamber. Cells were then incubated in 5% CO2 at 37°C for 36 h. Subsequently, non-invading cells on the upper surface of the membrane were scraped and invading cells were fixed with formaldehyde and then stained with crystal violet for counting.
Cell growth curve assay
For the cell proliferation assay, 2×105 cells were plated in a 6-well plate and cultured in complete medium at 37°C and 5% CO2. The cells were counted using cell counting chamber at time intervals and the cell growth curve was drawn according to the cell numbers at different time points.
Statistical analysis
Data were presented as mean ± standard deviation and subjected to one-way analysis of variance. Student's t test was used to compare the differences of gene level and invasive ability between two groups. One-way analysis of variance was used to test the effect of DACT2 on the proliferation of breast cancer cells. Multiple comparison between the groups was performed using the Student-Newman-Keuls method. SPSS software was used for statistical analysis (version 10.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
DACT2 frequently decreases in human breast cancer
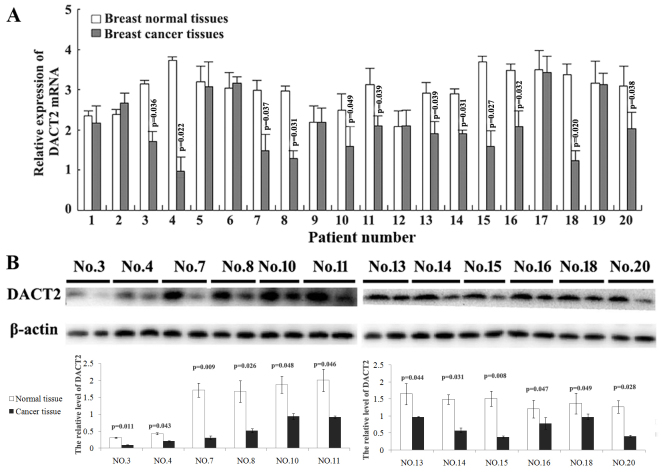
To learn the expression status of DACT2 in breast cancer, the present study first compared the mRNA expressional level of DACT2 in breast cancer tissues and their paired adjacent normal breast tissues using qPCR. It was identified that DACT2 mRNA was decreased in 12/20 detected breast cancer tissues (Fig. 1A). Furthermore, the expressional alters of DACT2 protein in these 12 breast cancer tissues was validated using western blot analysis. The present study demonstrated that DACT2 protein appeared to be decreased in these breast cancer tissues with DACT2 mRNA decreasing compared with their paired adjacent normal breast tissues (Fig. 1B). These results demonstrated that the decrease of DACT2 occurred in 60% of observed human breast cancer cases.
Figure 1.
DACT2 levels are frequently decreased in human breast cancer tissues compared with normal tissues. (A) Detection of the mRNA level of DACT2 in breast cancer tissues and associated paired adjacent normal breast tissues, determined using quantitative polymerase chain reaction. (B) The change in DACT2 protein expression in 12 breast cancer tissues and paired adjacent tissues was detected using western blot analysis. N, normal tissue; C, cancer tissue; DACT2, dishevelled-associated antagonist of β-catenin homolog 2.
Hypermethylation of DACT2 promoter majorly contributes to the loss of DACT2 in human breast cancer
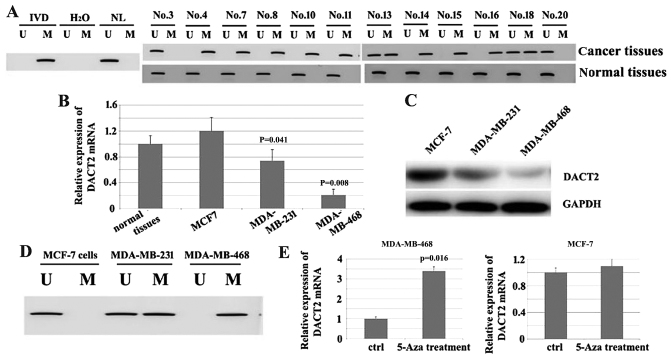
Since the decrease of DACT2 in breast cancer tissues occurred at mRNA and protein level, it was suspected that the decrease in DACT2 may result from the transcriptional inhibition. To test this hypothesis, MSP was used to assess the methylation status of DACT2 in these 12 cases of breast cancer tissues with DACT2 decrease (Fig. 2A). The results demonstrated that the promoter region of DACT2 was methylated in 10/12 tested breast cancer tissues, which indicated that promoter methylation of DACT2 may contribute to the loss of DACT2 expression in breast cancer patients. The expression of DACT2 was then screened in the 3 well-established breast cancer MCF-7, MDA-MB-231 and MDA-MB-468 cell lines; it was identified that DACT2 is significantly decreased in MDA-MB-468 cells at mRNA and protein levels compared with normal tissues. (Fig. 2B and C). Additionally, MSP was performed to identify if the loss of DACT2 in MDA-MB-468 resulted from the methylation of the gene promoter; the results demonstrated that the promoter region of DACT2 was methylated (Fig. 2D). To additionally confirm the association between the methylation of DACT2 gene promoter and the loss of DACT2 expression in the cell line 5-azacytidene (5-Aza) a DNA methylation transferase inhibitor that can induce the re-expression of methylated genes through de-methylation, was used to treat MDA-MB-468. The results demonstrated that DACT2 expression was induced in this cell line. In comparison, DACT2 expression was not significantly affected in the MCF-7 cell line (Fig. 2E). Overall, these results indicate that the loss or reduction of DACT2 in breast cancer may result from the methylation of the DACT2 promoter region.
Figure 2.
Hypermethylation of DACT2 promoter majorly contributes to the loss of DACT2 in human breast cancer. (A) MSP was used to assess the methylation status of DACT2 promoter in breast cancer tissues. (B) Detection of the DACT2 mRNA in 3 well-established breast cancer cell lines. (C) Detection of the DACT2 protein in 3 well-established breast cancer cell lines. (D) MSP was performed to assess the methylation status of DACT2 promoter in MDA-MB-468 cells. (E) 5-Aza induces the re-expression of DACT2 expression in MDA-MB-468 cells. DACT2, dishevelled-associated antagonist of β-catenin homolog 2; MSP, methylation-specific polymerase chain reaction; IVD, in vitro methylated DNA; NL, normal lymphocyte DNA; U, unmethylated; M, methylated; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; 5-Aza, 5-azacytidene; ctrl, control.
DACT2 inhibits the proliferation and invasion of breast cancer cells
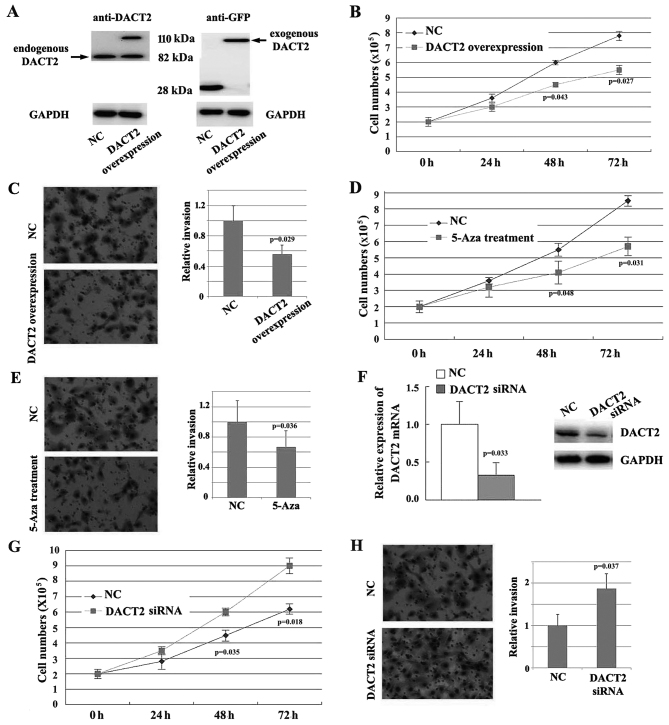
To learn the potential effect of DACT2 loss on the breast cancer progression, the present study evaluated the role of DACT2 in the proliferation and migration of breast cancer cells in vitro. Firstly, DACT2 was overexpressed in MDA-MB-468 cells using a lentiviral vector (Fig. 3A), and it was demonstrated that enforced expression of DACT2 in MDA-MB-468 cells inhibits the proliferation (Fig. 3B) and invasion (Fig. 3C) of the cells. Similarly, treatment with 5-Aza also inhibits the proliferation and invasion of the cells (Fig. 3D and E). The expression of DACT2 was knocked down in MDA-MB-231 cells using siRNA (Fig. 3F), and it was identified that the repression of DACT2 promotes the proliferation and invasion of MDA-MB-231 cells in vitro (Fig. 3G and H). Overall, these results suggest that DACT2 acts as a tumor suppressor and inhibits the progression of breast cancer.
Figure 3.
DACT2 inhibits the proliferation and invasion of breast cancer cells. (A) Detection of the overexpression of DACT2 in MDA-MB-468 cells. (B) Enforced expression of DACT2 in MDA-MB-468 cells inhibits cell proliferation. (C) Enforced expression of DACT2 in MDA-MB-468 cells inhibits cell invasion. (D) 5-Aza treatment inhibits the proliferation of cells. (E) 5-Aza treatment inhibits the invasion of cells. (F) Knockdown the expression of DACT2 in MDA-MB-231 cells by siRNA. (G) Repression of DACT2 promotes the proliferation of MDA-MB-231 cells. (H) Repression of DACT2 promotes the invasion of MDA-MB-231 cells. DACT2, dishevelled-associated antagonist of β-catenin homolog 2; 5-Aza, 5-azacytidene; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, negative control; siRNA, small interfering RNA.
DACT2 represses the expression of β-catenin target genes in breast cancer cells
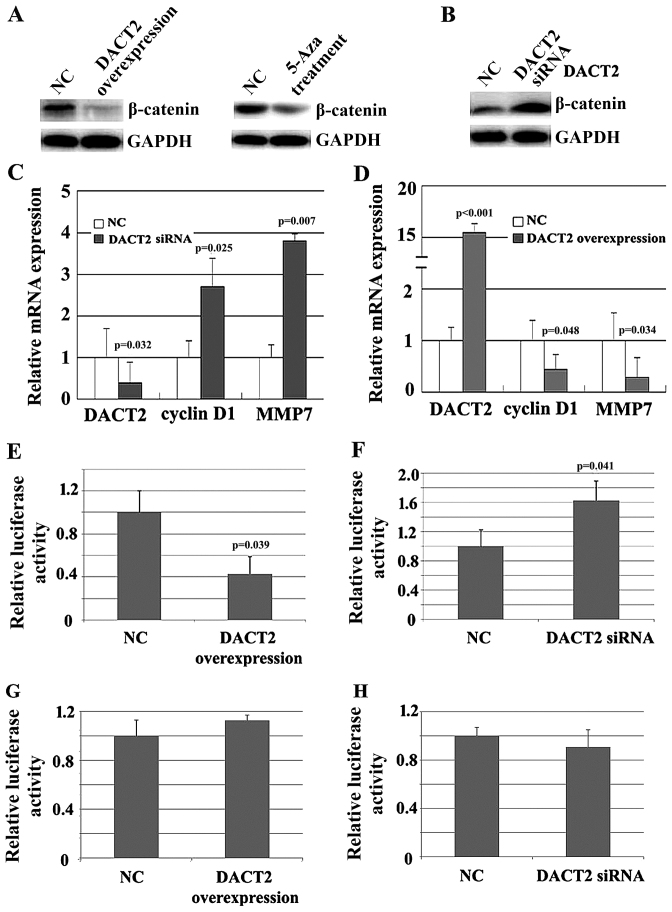
Since DACT2 promotes β-catenin degradation, the change in β-catenin expression due to alteration of DACT2 expression was evaluated in vitro. As expected, either the overexpression of DACT2 or 5-Aza treatment resulted in the decrease of β-catenin in MDA-MB-468 cells (Fig. 4A). By contrast, knockdown of DACT2 resulted in the elevated expression of β-catenin in MDA-MB-231 cells (Fig. 4B). Furthermore, the impact of DACT2 alteration on the expression of β-catenin target genes associated with cell proliferation and invasion was detected. qPCR results demonstrated that the knockdown of DACT2 in MDA-MB-231 cells elevated the mRNA level of cyclin D1 and matrix metalloproteinase 7 (Fig. 4C), while the overexpression of DACT2 attenuated the mRNA level of the two genes (Fig. 4D). Finally, β-catenin activity was detected using a luciferase reporter assay. As shown in Fig. 4E and F, knockdown of DACT2 elevated β-catenin activity, while overexpression of DACT2 attenuated β-catenin activity. Mutation of β-catenin DNA binding sites abolished the effect of DACT2 on β-catenin transcription activity (Fig. 4G and H). The present results suggested that DACT2 is a β-catenin signaling pathway inhibitor in breast cancer cells.
Figure 4.
DACT2 represses the expression of β-catenin target genes in breast cancer cells. (A) The overexpression of DACT2 or 5-Aza treatment resulted in the decrease of β-catenin in MDA-MB-468 cells. (B) The knockdown of DACT2 resulted in the elevated expression of β-catenin. (C) The knockdown of DACT2 in MDA-MB-231 cells elevated the mRNA level of cyclin D1 and MMP7. (D) The overexpression of DACT2 attenuated the mRNA level of cyclin D1 and MMP7. (E) The overexpression of DACT2 attenuated β-catenin reporter (wide) luciferase activity. (F) The knockdown of DACT2 elevated β-catenin reporter (wide) luciferase activity. (G) The overexpression of DACT2 did not affect β-catenin reporter (mutant) luciferase activity. (H) The knockdown of DACT2 did not affect β-catenin reporter (mutant) luciferase activity. DACT2, dishevelled-associated antagonist of β-catenin homolog 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP7, matrix metalloproteinase 7; siRNA, small interfering RNA; NC, negative control.
Discussion
In the absence of Wnt ligands, β-catenin is degraded in a proteasomal manner due to phosphorylation by a cytoplasmic complex consisting of Axin, adenomatous polyposis coli, casein kinase 1 and glycogen synthase kinase 3, and the signaling is suppressed (13). The binding of Wnt to its receptors FZD and LRP5/6 leads to the activation of Dvl, which in turn inhibits the degradation complex and promotes the accumulation of β-catenin, and thus the signaling is activated (14). Wnt/β-catenin signaling activation has been well established in breast carcinogenesis (2–5). Firstly, it has been identified that numerous Wnt proteins, such as Wnt2, Wnt7b, and Wnt10b, are upregulated in human breast carcinomas (6), and Wnt1 transgenic mice have been shown to exhibit mammary gland hyperplasia and an increase in adenocarcinomas (15). Secondly, it was demonstrated that receptors of Wnt are also upregulated in breast cancer. For example, FZD7 was identified to be overexpressed in breast cancer, and downregulation of FZD7 inactivates Wnt/β-catenin signaling and suppresses tumor formation (16). Overexpression of co-repressors LRP5 and LRP6 has been associated with the occurrence of breast cancer (17,18), and animal models demonstrated that the deletion of LRP5 or LRP6 delays mouse mammary tumor virus-Wnt1-induced tumor formation (17,19). Finally, certain inhibitors of this signaling pathway have been revealed to be downregulated in breast carcinoma. For example, the secreted proteins WNT inhibitory factor 1 (WIF1) and secreted frizzled-related protein (sFRP) bind Wnt proteins and thus inhibit their interaction with the FZD receptor. Additionally, it has been revealed that WIF1 and sFRP1-5 are silenced in several types of cancers, including breast cancer (20), whilst overexpression of sFRP markedly represses the development of breast cancer (21,22).
DACT family proteins were initially identified in Xenopus (8), and were identified to suppress Wnt/β-catenin signaling activity through interacting with or degrading Dvl, the initial activating factor of the signaling pathway (7,9). The human DACT protein family has 3 members, and the encoding genes of DACT1, 2 and 3 are located on human chromosome 14q22.3, 6q27 and 19q13.32, respectively (7,23). Previously, studies have suggested that human DACT is frequently silenced in human cancers. For example, deletion of chromosome 6q, where the DACT2 gene is located, is one of the most frequent chromosomal aberrations in human tumors (24,25). In addition to deletion, epigenetic modifications of DACT genes were also reported. For instance, DACT1 and DACT2 were reported to be methylated in hepatocellular carcinoma, oral squamous, gastric cancer, nasopharyngeal carcinoma, thyroid cancer, colon cancer and lung cancer (10,26–31). In addition, DACT3 was identified to have histone modifications in colorectal cancer (25,26,32). However, the function and expressional regulation of DACT in human breast cancer is largely unknown. In the present study, the expression of DACT2 was significantly decreased due to promoter methylation in breast cancer.
To assess the expression status of DACT2 in breast cancer cell lines and tissues, RT-qPCR and the western blot analysis was used. The results show that DACT2 was frequently silenced in breast cancer tissues and cell lines. MSP analysis of these DACT2-silent breast cancer tissues and cell line demonstrated promoter hypermethylation of the DACT2 gene. Furthermore, the DNMT inhibitor 5-AZA induced the re-expression of DACT2 in DACT2-silent breast cancer cells. These results indicated that the loss of DACT2 in breast cancer cells largely results from the methylation of the DACT2 promoter region.
DACT2 was previously identified to bind Dvl and promote Dvl degradation in a lysosome-dependent manner, thus stabilizing the β-catenin degradation complex and decreasing soluble β-catenin (9). Additionally, it was also identified that DACT2 could inhibit β-catenin activity by directly and firmly binding β-catenin in the cytoplasm (32). Consistently, the present study demonstrated that the knockdown of endogenous DACT2 increases and overexpression of DACT2 inhibits β-catenin target gene expression and β-catenin/TCF reporter luciferase activity in the cell lines studied. Furthermore, the current study evaluated the potential roles of DACT2 in breast cancer progression. The effect of DACT2 on breast cancer cell proliferation was evaluated by a cell growth curve assay, and the effect on invasion of cancer cells was evaluated by Transwell assay. The present results demonstrated that DACT2 overexpression inhibits breast cancer cell proliferation and invasion, while the knockdown of DACT2 promotes the proliferation and invasion of breast cancer cells. The current data has demonstrated that DACT2 acts as a tumor suppressor in breast cancer.
In summary, the present study demonstrated that DACT2 was frequently silent in breast cancer, and the methylation of the DACT2 gene promoter largely contributes to the silencing of the gene in human breast cancer. It was also identified that DACT2 acts as a tumor suppressor in breast cancer by inhibiting Wnt/β-catenin activation and repressing cancer cell proliferation and invasion. The present study indicates that the loss of DACT2 may contribute to breast cancer progression and provides a promising therapeutic target for the treatment of breast cancer.
References
- 1.King TD, Suto MJ, Li Y. The Wnt/β-catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 2012;113:13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–223. doi: 10.1159/000098207. [DOI] [PubMed] [Google Scholar]
- 3.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 4.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Beta-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression; Proc Natl Acad Sci USA; 2000; pp. 4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 7.Fisher DA, Kivimäe S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–2630. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- 8.Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. doi: 10.1016/S1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 9.Teran E, Branscomb AD, Seeling JM. Dpr acts as a molecular switch, inhibiting Wnt signaling when unphosphorylated, but promoting Wnt signaling when phosphorylated by casein kinase Idelta/epsilon. PLoS One. 2009;4:e5522. doi: 10.1371/journal.pone.0005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y, Yan W, Liu X, Jia Y, Cao B, Yu Y, Lv Y, Brock MV, Herman JG, Licchesi J, et al. DACT2 is frequently methylated in human gastric cancer and methylation of DACT2 activated Wnt signaling. Am J Cancer Res. 2014;4:710–724. [PMC free article] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Zhang J, Gao L, McClellan S, Finan MA, Butler TW, Owen LB, Piazza GA, Xi Y. MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell Death Differ. 2012;19:378–386. doi: 10.1038/cdd.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol Cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Du W, Liu X, Fan G, Zhao X, Sun Y, Wang T, Zhao R, Wang G, Zhao C, Zhu Y, et al. From cell membrane to the nucleus: An emerging role of E-cadherin in gene transcriptional regulation. J Cell Mol Med. 2014;18:1712–1719. doi: 10.1111/jcmm.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- 17.Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One. 2009;4:e6594. doi: 10.1371/journal.pone.0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 20.Willemsen RH, de Kort SW, van der Kaay DC, Hokken-Koelega AC. Independent effects of prematurity on metabolic and cardiovascular risk factors in short small-for-gestational-age children. J Clin Endocrinol Metab. 2008;93:452–458. doi: 10.1210/jc.2007-1913. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98:1147–1156. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11:R32. doi: 10.1186/bcr2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh M, Katoh M. Identification and characterization of human DAPPER1 and DAPPER2 genes in silico. Int J Oncol. 2003;22:907–913. [PubMed] [Google Scholar]
- 24.Girard L, Zöchbauer-Müller S, Virmani AK, Gazdar AF, Minna JD. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60:4894–4906. [PubMed] [Google Scholar]
- 25.Steinemann D, Gesk S, Zhang Y, Harder L, Pilarsky C, Hinzmann B, Martin-Subero JI, Calasanz MJ, Mungall A, Rosenthal A, et al. Identification of candidate tumor-suppressor genes in 6q27 by combined deletion mapping and electronic expression profiling in lymphoid neoplasms. Genes, Chromosomes Cancer. 2003;37:421–426. doi: 10.1002/gcc.10231. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG, Guo M. Epigenetic regulation of DACT2, a key component of the Wnt signalling pathway in human lung cancer. J Pathol. 2013;230:194–204. doi: 10.1002/path.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Yang Y, Liu X, Herman JG, Brock MV, Licchesi JD, Yue W, Pei X, Guo M. Epigenetic regulation of the Wnt signaling inhibitor DACT2 in human hepatocellular carcinoma. Epigenetics. 2013;8:373–382. doi: 10.4161/epi.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Dong Y, Zhang Y, Wang X, Xu L, Yang S, Li X, Dong H, Xu L, Su L, et al. DACT2 is a functional tumor suppressor through inhibiting Wnt/β-catenin pathway and associated with poor survival in colon cancer. Oncogene. 2015;34:2575–2585. doi: 10.1038/onc.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z, Herman JG, Brock MV, Sheng J, Zhang M, Liu B, Guo M. Methylation of DACT2 promotes papillary thyroid cancer metastasis by activating Wnt signaling. PLoS One. 2014;9:e112336. doi: 10.1371/journal.pone.0112336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Zhang Y, Fan Y, Sun K, Su X, Du Z, Tsao SW, Loh TK, Sun H, Chan AT, et al. Characterization of the nasopharyngeal carcinoma methylome identifies aberrant disruption of key signaling pathways and methylated tumor suppressor genes. Epigenomics. 2015;7:155–173. doi: 10.2217/epi.14.79. [DOI] [PubMed] [Google Scholar]
- 31.Schussel JL, Kalinke LP, Sassi LM, de Oliveira BV, Pedruzzi PA, Olandoski M, Alvares LE, Garlet GP, Trevilatto PC. Expression and epigenetic regulation of DACT1 and DACT2 in oral squamous cell carcinoma. Cancer Biomark. 2015;15:11–17. doi: 10.3233/CBM-140436. [DOI] [PubMed] [Google Scholar]
- 32.Kivimäe S, Yang XY, Cheyette BN. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33. doi: 10.1186/1471-2091-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]