Abstract
BACKGROUND
Inclisiran (ALN-PCSsc) is a long-acting RNA interference (RNAi) therapeutic agent that inhibits the synthesis of proprotein convertase subtilisin–kexin type 9 (PCSK9), a target for the lowering of low-density lipoprotein (LDL) cholesterol.
METHODS
In this phase 1 trial, we randomly assigned healthy volunteers with an LDL cholesterol level of at least 100 mg per deciliter in a 3:1 ratio to receive a subcutaneous injection of inclisiran or placebo in either a single-ascending-dose phase (at a dose of 25, 100, 300, 500, or 800 mg) or a multiple-dose phase (125 mg weekly for four doses, 250 mg every other week for two doses, or 300 or 500 mg monthly for two doses, with or without concurrent statin therapy); each dose cohort included four to eight participants. Safety, the side-effect profile, and pharmacodynamic measures (PCSK9 level, LDL cholesterol level, and exploratory lipid variables) were evaluated.
RESULTS
The most common adverse events were cough, musculoskeletal pain, nasopharyngitis, headache, back pain, and diarrhea. All the adverse events were mild or moderate in severity. There were no serious adverse events or discontinuations due to adverse events. There was one grade 3 elevation in the γ-glutamyltransferase level, which was considered by the investigator to be related to statin therapy. In the single-dose phase, inclisiran doses of 300 mg or more reduced the PCSK9 level (up to a least-squares mean reduction of 74.5% from baseline to day 84), and doses of 100 mg or more reduced the LDL cholesterol level (up to a least-squares mean reduction of 50.6% from baseline). Reductions in the levels of PCSK9 and LDL cholesterol were maintained at day 180 for doses of 300 mg or more. All multiple-dose regimens reduced the levels of PCSK9 (up to a least-squares mean reduction of 83.8% from baseline to day 84) and LDL cholesterol (up to a least-squares mean reduction of 59.7% from baseline to day 84).
CONCLUSIONS
In this phase 1 trial, no serious adverse events were observed with inclisiran. Doses of 300 mg or more (in single or multiple doses) significantly reduced levels of PCSK9 and LDL cholesterol for at least 6 months. (Funded by Alnylam Pharmaceuticals and the Medicines Company; ClinicalTrials.gov number, NCT02314442.)
An elevated level of low-density lipoprotein (LDL) cholesterol is a major risk factor for cardiovascular disease.1 Despite the use of statin therapy, alone or in combination with other lipid-lowering medications, many at-risk patients continue to have elevated levels of LDL cholesterol.2-4 Hence, there is a need for additional treatment options for lowering of the LDL cholesterol level to reduce cardiovascular risk.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) is a recently identified but well-validated target for LDL cholesterol–lowering therapy.5 This serine protease, which is expressed and secreted into the bloodstream predominantly by the liver, binds LDL receptors both intracellularly and extracellularly and promotes the lysosomal degradation of these receptors in hepatocytes,6,7 thereby increasing the circulating LDL cholesterol levels. PCSK9 loss-of-function mutations are associated with low circulating LDL cholesterol levels and diminished cardiovascular risk8,9 with no apparent negative health consequences.10
PCSK9-blocking antibodies, administered once or twice monthly, reduce circulating PCSK9 levels and lower LDL cholesterol levels.5,11,12 Preliminary data suggest that long-term treatment with such antibodies is associated with a lower incidence of cardiovascular events than placebo.13,14 However, PCSK9 antibodies have a short duration of effect, necessitating frequent subcutaneous injections.5,11,12
A recently discovered means of decreasing PCSK9 levels is the administration of small interfering RNA (siRNA) molecules.15 The siRNA molecules engage the natural pathway of RNA interference (RNAi) by binding intracellularly to the RNA-induced silencing complex (RISC), enabling it to cleave messenger RNA (mRNA) molecules encoding PCSK9 specifically. The cleaved mRNA is degraded and thus unavailable for protein translation, which results in decreased levels of the PCSK9 protein. A single siRNA-bound RISC is catalytic and cleaves many transcripts. This characteristic may be important during use of statins, which are known to up-regulate the production of PCSK9, potentially limiting the effectiveness of the drugs. The lipid nanoparticle ALN-PCS, an intravenous formulation of siRNA that inhibits PCSK9 synthesis, has been shown in a small phase 1 study to reduce the levels of both PCSK9 and LDL cholesterol in adult volunteers.15
Inclisiran (ALN-PCSsc) is a long-acting, subcutaneously delivered, synthetic siRNA directed against PCSK9 that is conjugated to triantennary N-acetylgalactosamine carbohydrates. These carbohydrates bind to abundant liver-expressed asialo-glycoprotein receptors, leading to inclisiran uptake specifically into hepatocytes.16 The siRNA was modified with a combination of phosphorothioate, 2′-O-methyl nucleotide, and 2′-fluoro nucleotide modifications to improve molecular stability.16 In preclinical studies involving non-human primates, doses of more than 3 mg per kilogram of body weight resulted in reductions of more than 80% in plasma PCSK9 levels and approximately 60% lowering of the serum LDL cholesterol level, with peak effects lasting more than 30 days, with a very slow return to baseline levels over a period of 90 to 120 days after administration (unpublished data). This phase 1 study assessed the safety, side-effect profile, and pharmacodynamic effects of inclisiran when it was administered subcutaneously in single or multiple doses in healthy volunteers who had an LDL cholesterol level of at least 100 mg per deciliter (2.60 mmol per liter) and in a small number of participants taking a stable dose of statin co-therapy.
METHODS
STUDY DESIGN AND OVERSIGHT
We conducted this randomized, single-blind, placebo-controlled study in two stages — a single-dose phase (with ascending doses for sequential cohorts of patients), followed by a multiple-dose phase (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The trial had a prospectively defined adaptive design that allowed the modification, in subsequent cohorts, of dosing regimens, evaluation schedules, and follow-up duration on the basis of observations of the participants who had already been enrolled in the study and in accordance with decisions made by the safety review committee (see the Supplementary Appendix).
The study was sponsored by Alnylam Pharmaceuticals and the Medicines Company. The study protocol, available at NEJM.org, was designed by Alnylam Pharmaceuticals, the Medicines Company, and the principal investigators and was approved by the National Research Ethics Service Committee in London–Brent, United Kingdom. The study was performed at two contract research sites in the United Kingdom (Richmond Pharmacology and Covance). Data were collected by the investigators and analyzed by Covance and Alnylam Pharmaceuticals. All the authors interpreted the data, helped to prepare the manuscript, and made the decision to submit the manuscript for publication. Editorial assistance, funded by Alnylam Pharmaceuticals, was provided by Green-splash and Spencer Fontayne. All the authors vouch for the completeness, accuracy, and fidelity of this study to the protocol.
PARTICIPANTS
Men and women (18 to 60 years of age in the single-dose phase and 18 to 75 years of age in the multiple-dose phase) who had a serum LDL cholesterol level of at least 100 mg per deciliter and a fasting triglyceride level of less than 400 mg per deciliter (4.5 mmol per liter) were eligible. Participants taking statin therapy had to have been receiving a stable statin dose and regimen for at least 30 days before screening and had to have no planned changes during the study. Participants were not specifically instructed to maintain a stable dietary pattern. Participants with a history of cardiovascular disease, cerebrovascular disease, or diabetes mellitus were excluded except for those who were taking statins; such patients could be enrolled if they had noninsulin-dependent diabetes mellitus or controlled hypertension. The full eligibility criteria are provided in the Supplementary Appendix. All the participants provided written informed consent.
RANDOMIZATION AND STUDY TREATMENT
In the single-dose phase, six cohorts (with four participants each) were included. Participants in each cohort were randomly assigned in a 3:1 ratio to receive a subcutaneous injection of either inclisiran at a dose of 25, 100, 300, 500, or 800 mg (two cohorts for the 800-mg dose) or placebo.
In the multiple-dose phase, the participants in six cohorts (with four to eight participants each) were randomly assigned in a 3:1 ratio with the use of block sizes of four to receive inclisiran or placebo. One cohort received a dose of 125 mg once per week for 4 weeks, one cohort received a dose of 250 mg once every 2 weeks for 4 weeks, two cohorts (one of which was receiving statin therapy) received a dose of 300 mg once per month for 2 months, and two cohorts (one of which was receiving statin therapy) received a dose of 500 mg once per month for 2 months.
In the two phases, the first dose was administered on the day of randomization (day 0). Inclisiran or placebo (administered in sterile 0.9% normal saline) was injected subcutaneously into one or more sites of the abdomen; dose volumes of more than 1.5 ml were administered in two to three injections of equal volume. Inclisiran was supplied at a dose of 200 mg per milliliter of sterile solution. Participants were unaware of the assigned treatment because of syringe masking. No participant was included in more than one cohort.
EVALUATIONS
Participants were monitored as inpatients for 3 days, with day −1 considered to be the day before initial administration. Participants underwent randomization and treatment commenced on day 0. Participants were evaluated as outpatients for safety, side-effect profile, and pharmacodynamic end points at specified times throughout the study period (56 days for the single-dose phase, and ≤84 days for the multiple-dose phase). Pharmacodynamic end points were evaluated for an additional month after the completion of the safety and side-effect profile assessments.
Participants were considered to have completed the trial according to the protocol after their final planned safety and pharmacodynamic follow-up visit. If at that visit the most recent three LDL cholesterol levels averaged less than 80% of the baseline value, pharmacodynamic monitoring continued every 2 weeks for 1 month, and then every 4 weeks, until the more recent three LDL cholesterol measurements averaged 80% or more of the baseline value or until 180 days after the last dose of inclisiran or placebo, whichever came sooner.
The safety evaluation included clinical laboratory tests (hematologic, biochemical, coagulation, and urinalysis tests), vital signs (oral body temperature, blood pressure, heart rate, and respiration rate), physical examination, 12-lead electrocardiography (ECG), and adverse-event monitoring. Adverse events were graded with the use of the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, and were categorized with the use of the Medical Dictionary for Regulatory Activities, version 17.1.
Pharmacodynamic monitoring included plasma PCSK9 and serum LDL cholesterol measurements. PCSK9 assay methods are described in the Supplementary Appendix. The serum LDL cholesterol level was determined directly by means of beta-quantification (Medpace Reference Laboratories). The exploratory biomarkers that were evaluated included levels of total cholesterol, high-density lipoprotein (HDL) cholesterol, non-HDL cholesterol (total cholesterol level minus the HDL cholesterol level), apolipoprotein B, lipoprotein(a), and triglycerides.
STATISTICAL ANALYSIS
All the participants who received at least one dose of inclisiran or placebo were included in the safety analysis. All the participants who received all planned doses of the assigned regimen were included in the pharmacodynamics analysis. Descriptive statistics including counts, percentages, means, and standard deviations, as appropriate, are presented for the safety population.
In each study phase, data from the participants in the placebo group were combined across cohorts for analysis. The mean percent reductions in the plasma PCSK9 level, as compared with baseline, at day 84 after the first dose of the study regimen were compared with those in the placebo group by means of a repeated-measures analysis of covariance (ANCOVA), including the baseline PCSK9 level as a covariate, and treatment-by-time interaction. The model used an autoregressive first-order covariance structure. This method was also used for the lipid data. A nominal P value of less than 0.05 was considered to indicate statistical significance. Analyses were performed with the use of SAS software, versions 9.2 and higher (SAS Institute). The complete statistical analysis plan is available with the protocol at NEJM.org.
RESULTS
PARTICIPANTS
A flowchart showing the randomization and follow-up of the participants is provided in Figure S2 in the Supplementary Appendix. Two participants (one in the placebo group and one in the inclisiran group) in the multiple-dose phase did not receive the assigned regimen according to the protocol and were excluded from the pharmacodynamic analyses. In addition, one participant in the placebo group underwent randomization but did not receive placebo and was thus excluded from both analyses; this participant was replaced in the trial. The characteristics of the participants at baseline, according to study phase and assigned group, in the safety population are shown in Table 1 and in Table S1 in the Supplementary Appendix.
Table 1.
Demographic and Clinical Characteristics of the Participants at Baseline (Safety Population).*
| Characteristic | Single-Dose Phase | Multiple-Dose Phase | ||||
|---|---|---|---|---|---|---|
| Placebo (N = 6) | Inclisiran (N = 18) | Placebo | Inclisiran | |||
| with statin (N = 4)† | without statin (N = 8) | with statin (N = 9)‡ | without statin (N = 24) | |||
| Age — yr | 48±14 | 46±10 | 58±3 | 51±14 | 54±16 | 51±12 |
| Male sex — no. (%) | 2 (33) | 17 (94) | 2 (50) | 6 (75) | 4 (44) | 17 (71) |
| Race — no. (%)§ | ||||||
| White | 4 (67) | 12 (67) | 4 (100) | 7 (88) | 6 (67) | 19 (79) |
| Other | 2 (33) | 6 (33) | 0 | 1 (12) | 3 (33) | 5 (21) |
| Weight — kg | 70.6±12.0 | 77.1±7.7 | 74.3±5.1 | 77.6±10.3 | 77.7±17.0 | 74.7±11.7 |
| Height — cm | 168±11 | 173±6 | 168±10 | 171±9 | 171±12 | 171±8 |
| LDL cholesterol — mg/dl | 131.5±19.3 | 163.0±32.9 | 143.1±89.7 | 131.5±20.9 | 143.4±29.8 | 139.3±32.3 |
| Triglycerides — mg/dl | 70.9±12.4 | 135.5±55.7 | 150.6±46.9 | 124.0±38.1 | 116.3±64.3 | 123.4±82.9 |
| PCSK9 — μg/liter | 279.0±99.5 | 275.4±58.2 | 460.7±56.3 | 276.2±58.7 | 451.8±132.2 | 317.1±66.8 |
Plus–minus values are means ±SD. Two participants (one in the placebo group and one in the inclisiran group) in the multiple-dose phase did not receive the assigned regimen according to the protocol and were excluded from the pharmacodynamic analyses but were included in the safety population. To convert the values for low-density lipoprotein (LDL) cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. PCSK9 denotes proprotein convertase subtilisin–kexin type 9.
Three participants were taking simvastatin at a dose of 40 mg per day, and one was taking pravastatin at a dose of 20 mg per day.
Four participants were taking atorvastatin at a dose of 40 mg per day, two at a dose of 20 mg per day, and one at a dose of 10 mg per day; one participant was taking simvastatin at a dose of 40 mg per day and one at a dose of 20 mg per day.
Race was self-reported.
SAFETY AND SIDE-EFFECT PROFILE
All the adverse events were mild or moderate (grade 1 or 2) in severity. There were no treatment discontinuations that were due to adverse events, and no serious adverse events were reported. In the single-dose phase, the most common adverse events (occurring in ≥5% of the participants in the inclisiran group) were cough, musculoskeletal pain, and nasopharyngitis (in 2 of 18 participants [11%] each) (Table S2 in the Supplementary Appendix). In the multiple-dose phase, the most common adverse events (occurring in ≥10% of the participants in the inclisiran group) were headache (in 6 of 33 participants [18%]), back pain and diarrhea (in 5 [15%] each), and nasopharyngitis (in 4 [12%]) (Table S3 in the Supplementary Appendix).
In the multiple-dose phase, one participant who received inclisiran at a dose of 500 mg and atorvastatin at a dose of 40 mg per day had elevated levels of γ-glutamyltransferase (grade 3) and alanine aminotransferase (grade 2). The bilirubin level was not elevated, and this event was asymptomatic. The enzyme elevations resolved on the discontinuation of statin therapy and recurred on the reintroduction of atorvastatin at a dose of 20 mg per day; these elevations were considered by the investigator to be related to statin therapy.
No participant had significant changes in the QT interval corrected for heart rate (QTc) as assessed by means of ECG (QTc of >500 msec according to Fridericia’s formula or change from baseline of >60 msec). No injection-site reactions were reported, according to the protocol definition of injection-site reaction as individual signs or symptoms at the injection site that met the CTCAE criteria beginning 4 or more hours after the receipt of the dose. However, four participants (one in the single-dose phase and three in the multiple-dose phase) reported having a delayed, mild, self-limiting rash that was localized to the injection site (grade 1). Three of these participants also reported mild, reversible hyperpigmentation after the rash (grade 1) at the injection site.
PCSK9 LEVELS
In the single-dose phase, inclisiran at a dose of 300 mg or more was associated with reductions from baseline in the PCSK9 level that were significant, as compared with placebo, at day 84 (Table 2 and Fig. 1A, and Table S4 in the Supplementary Appendix). The magnitudes of the reduction in the PCSK9 level were similar across the dose range of 300 to 800 mg (least-squares mean change, 69.9 to 74.5%); the largest reduction, 74.5%, occurred in the group that was treated with 300 mg of inclisiran. PCSK9 levels had returned to baseline values by day 180 after the receipt of the dose in the 25-mg and 100-mg cohorts. For inclisiran doses of 300 mg or more, the PCSK9 levels remained reduced, as compared with baseline, at day 180 after receipt of the dose (Fig. 1A, and Table S5 in the Supplementary Appendix).
Table 2.
Least-Squares Mean Percent Change from Baseline in Pharmacodynamic Variables at Day 84 in the Single-Dose Phase (Pharmacodynamic Population).*
| Variable | Placebo (N = 6) | Inclisiran
|
||||
|---|---|---|---|---|---|---|
| 25 mg (N = 3) | 100 mg (N = 3) | 300 mg (N = 3) | 500 mg (N = 3) | 800 mg (N = 6) | ||
| PCSK9 | ||||||
| Percent change (95% CI) | −0.6 (−24.2 to 30.4) | −46.6 (−65.4 to −17.8)† | −32.0 (−52.5 to −2.7) | −74.5 (−82.1 to −63.6)‡ | −69.9 (−78.9 to −57.0)‡ | −73.1 (−79.1 to −65.4)‡ |
| Difference (percentage points) | — | −46.0 | −31.4 | −73.9 | −69.3 | −72.5 |
|
| ||||||
| LDL cholesterol | ||||||
| Percent change (95% CI) | −10.9 (−26.0 to 7.1) | −21.5 (−41.3 to 5.0) | −36.7 (−50.2 to −19.4)† | −50.0 (−60.7 to −36.3)‡ | −50.6 (−61.3 to −36.9)‡ | −43.4 (−52.5 to −32.4)‡ |
| Difference (percentage points) | — | −10.6 | −25.8 | −39.1 | −39.7 | −32.5 |
| Percent change at group nadir§ | −10.9 | −25.3 | −38.9 | −50.0 | −59.0 | −52.8 |
|
| ||||||
| Total cholesterol | ||||||
| Percent change (95% CI) | −4.4 (−15.9 to 8.7) | −12.0 (−30.1 to 10.8) | −17.7 (−30.0 to −3.4) | −30.9 (−41.2 to −18.9)¶ | −27.1 (−38.2 to −14.0)† | −29.1 (−36.8 to −20.6)¶ |
| Difference (percentage points) | — | −7.6 | −13.3 | −26.5 | −22.7 | −24.7 |
|
| ||||||
| HDL cholesterol | ||||||
| Percent change (95% CI) | 13.6 (−9.2 to 42.1) | 7.3 (−22.0 to 47.7) | 17.9 (−9.2 to 53.1) | 36.8 (2.9 to 82.0) | 7.4 (−17.4 to 39.8) | 0.1 (−16.8 to 20.5) |
| Difference (percentage points) | — | −6.3 | 4.3 | 23.2 | −6.2 | −13.5 |
|
| ||||||
| Non-HDL cholesterol | ||||||
| Percent change (95% CI) | −11.7 (−23.7 to 2.3) | −19.8 (−36.1 to 0.6) | −28.4 (−40.8 to −13.5) | −48.9 (−57.8 to −38.2)‡ | −36.3 (−47.4 to −22.9)¶ | −37.0 (−44.9 to −28.0)¶ |
| Difference (percentage points) | — | −8.1 | −16.7 | −37.2 | −24.6 | −25.3 |
|
| ||||||
| Apolipoprotein B | ||||||
| Percent change (95% CI) | −15.3 (−31.4 to 4.6) | −11.3 (−36.8 to 24.5) | −26.5 (−43.3 to −4.7) | −47.1 (−59.0 to −31.9)¶ | −39.9 (−53.9 to −21.7)† | −37.5 (−47.7 to −25.2)† |
| Difference (percentage points) | — | 4.0 | −11.2 | −31.8 | −24.6 | −22.2 |
|
| ||||||
| Lipoprotein(a) | ||||||
| Percent change (95% CI) | 3.6 (−28.6 to 50.2) | −14.0 (−55.1 to 64.6) | −20.8 (−51.0 to 28.0) | −44.5 (−65.7 to −10.4)† | −35.5 (−60.1 to 4.3) | −21.3 (−45.0 to 12.6) |
| Difference (percentage points) | — | −17.6 | −24.4 | −48.1 | −39.1 | −24.9 |
The analysis was performed with the use of a baseline-adjusted analysis of covariance (ANCOVA). Differences shown in the inclisiran groups are with the placebo group. One participant in the placebo group and one in the 25-mg group did not attend the day 84 visit, and their data are not included in any of the analyses. The result for the LDL cholesterol level at day 84 for one participant in the 800-mg group was reported as “quantity not sufficient for analysis.” P values were calculated with the use of the repeated-measures ANCOVA. CI denotes confidence interval, and HDL high-density lipoprotein.
P<0.05 for the pairwise comparison with placebo.
P<0.001 for the pairwise comparison with placebo.
The group nadir was defined as the largest mean percent reduction from the baseline value during the study.
P<0.01 for the pairwise comparison with placebo.
Figure 1. Change in Plasma Levels of Proprotein Convertase Subtilisin–Kexin Type 9 (PCSK9), According to Study Group and Dose Cohort.
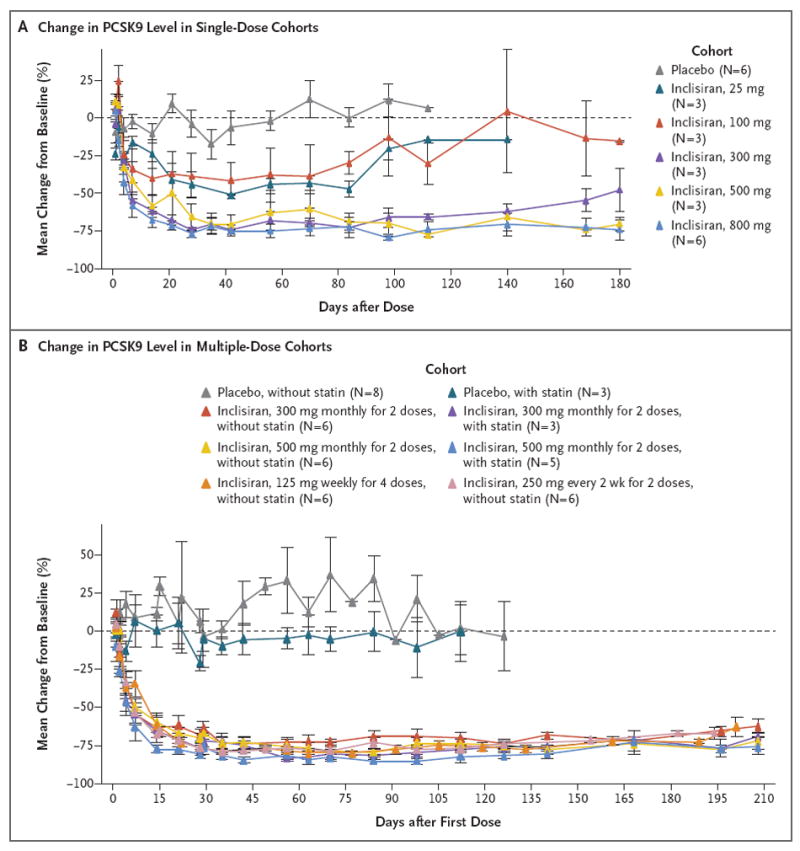
Shown are the effects (mean percentage changes from baseline) of single or multiple doses of inclisiran or placebo on plasma levels of PCSK9 over time. Baseline values were the average of all the study measurements obtained before the first dose. For the single-dose cohorts, the data for the placebo group include all the participants; for the multiple-dose cohorts, the data for the placebo group are presented according to whether the participants were or were not taking a stable baseline dose of statin cotherapy. I bars represent standard errors. For at least one cohort, only one value (for one participant) is shown at some time points. Participants were followed beyond the last planned visit only if the low-density lipoprotein cholesterol level had not returned to 80% of the baseline value by that time. In the single-dose phase, inclisiran or placebo was administered in one dose on day 0. In the multiple-dose phase, the first dose was administered on day 0 and subsequent doses at the indicated intervals. The 300-mg and 500-mg doses were administered as two monthly doses, the 125-mg dose was administered as four weekly doses, and the 250-mg dose was administered once every 2 weeks for 4 weeks.
In the multiple-dose phase, all the inclisiran regimens were associated with reductions from baseline in the PCSK9 level that were significant, as compared with placebo, at day 84 after receipt of the first dose (Table 3 and Fig. 1B, and Table S6 in the Supplementary Appendix). The magnitude of the reductions in the PCSK9 level were similar across all the inclisiran cohorts, with the least-squares mean change from baseline ranging from 71.8 to 83.8% at day 84 after receipt of the first dose; the largest reduction, 83.8%, was observed in the group that received 500 mg of inclisiran once per month for 2 months as well as statin cotherapy. Levels of PCSK9 remained reduced, as compared with baseline, in all the inclisiran cohorts at day 196 after receipt of the first dose (Fig. 1B, and Table S7 in the Supplementary Appendix).
Table 3.
Least-Squares Mean Percent Change from Baseline in Pharmacodynamic Variables at Day 84 after the First Dose in the Multiple-Dose Phase (Pharmacodynamic Population).*
| Variable | Placebo (N = 11) | Inclisiran
|
|||||
|---|---|---|---|---|---|---|---|
| 300 mg, with Statin (N = 3) | 300 mg, without Statin (N = 6) | 500 mg, with Statin (N = 5) | 500 mg, without Statin (N = 6) | 125 mg, without Statin (N = 6) | 250 mg, without Statin (N = 6) | ||
| PCSK9 | |||||||
| Percent change (95% CI) | 16.9 (−2.4 to 40.0) | −79.9 (−85.4 to −72.5)† | −71.8 (−77.4 to −64.8)† | −83.8 (−87.3 to −79.3)† | −81.5 (−85.2 to −76.9)† | −77.4 (−81.9 to −71.8)† | −75.7 (−80.5 to −69.6)† |
| Difference (percentage points) | — | −96.8 | −88.7 | −100.7 | −98.4 | −62.1 | −92.6 |
|
| |||||||
| LDL cholesterol | |||||||
| Percent change (95% CI) | −14.2 (−30.2 to 5.5) | −45.1 (−61.6 to −21.4)‡ | −59.7 (−68.7 to −48.1)† | −53.2 (−64.5 to −38.3)§ | −51.7 (−62.5 to −37.8)§ | −39.8 (−51.1 to −25.9) | −52.2 (−62.9 to −38.4)§ |
| Difference (percentage points) | — | −30.9 | −45.5 | −39.0 | −37.5 | −49.3 | −38.0 |
| Percent change at group nadir¶ | −14.2 | −51.6 | −59.7 | −58.8 | −53.9 | −45.0 | −55.5 |
|
| |||||||
| Total cholesterol | |||||||
| Percent change (95% CI) | −7.6 (−15.5 to 1.0) | −24.9 (−35.8 to −12.0)‡ | −40.4 (−46.7 to −33.4)† | −30.4 (−38.4 to −21.3)† | −27.0 (−34.7 to −18.3)§ | −23.8 (−31.9 to −14.7) | −34.5 (−41.4 to −26.7)† |
| Difference (percentage points) | — | −17.3 | −32.8 | −22.8 | −19.4 | −20.9 | −26.9 |
|
| |||||||
| HDL cholesterol | |||||||
| Percent change (95% CI) | 0.6 (−6.0 to 7.6) | 10.8 (−1.5 to 24.6) | 11.7 (2.7 to 21.4) | 5.3 (−3.9 to 15.3) | 12.8 (3.8 to 22.6)‡ | 12.9 (3.8 to 22.7) | 4.1 (−4.2 to 13.1) |
| Difference (percentage points) | — | 10.2 | 11.1 | 4.7 | 12.2 | −4.1 | 3.5 |
|
| |||||||
| Non-HDL cholesterol | |||||||
| Percent change (95% CI) | −10.6 (−21.3 to 1.6) | −35.7 (−48.7 to −19.3)‡ | −56.9 (−63.3 to −49.4)† | −46.2 (−54.9 to −35.8)† | −45.1 (−53.3 to −35.5)† | −36.9 (−46.3 to −25.9) | −45.3 (−53.4 to −35.8)† |
| Difference (percentage points) | — | −25.1 | −46.3 | −35.6 | −34.5 | −26.2 | −34.7 |
|
| |||||||
| Apolipoprotein B | |||||||
| Percent change (95% CI) | −12.8 (−23.2 to −1.0) | −37.2 (−49.8 to −21.4)‡ | −52.4 (−59.4 to −44.2)† | −41.8 (−51.1 to −30.7)† | −46.4 (−54.3 to −37.2)† | −33.3 (−43.1 to −21.8) | −46.5 (−54.3 to −37.3)† |
| Difference (percentage points) | — | −24.4 | −39.6 | −29.0 | −33.6 | −24.5 | −33.7 |
|
| |||||||
| Lipoprotein(a) | |||||||
| Percent change (95% CI) | −6.0 (−26.7 to 20.4) | −30.7 (−54.3 to 5.1) | −19.2 (−40.0 to 8.8) | −42.7 (−59.7 to −18.4)‡ | −27.4 (−45.8 to −2.7) | −22.7 (−28.4 to −16.5)‡ | −28.1 (−46.4 to −3.4) |
| Difference (percentage points) | — | −24.7 | −13.2 | −36.7 | −21.4 | −24.7 | −22.1 |
The analysis was performed with the use of a baseline-adjusted analysis of covariance (ANCOVA). Differences shown in the inclisiran groups are with the placebo group. Data for the 11 participants in the placebo group were pooled (3 participants were taking a stable dose of statins, and 8 were not). The 300-mg and 500-mg doses were administered as two monthly doses, the 125-mg dose was administered as four weekly doses, and the 250-mg dose was administered as once every 2 weeks for two doses. Values are from day 91 for the 125-mg group. P values were calculated with the use of the repeated-measures ANCOVA.
P<0.001 for the pairwise comparison with placebo.
P<0.05 for the pairwise comparison with placebo.
P<0.01 for the pairwise comparison with placebo.
The group nadir was defined as the largest mean percent reduction from the baseline value during the study.
LDL CHOLESTEROL LEVELS
In the single-dose phase, reductions from baseline in the LDL cholesterol level that were significant, as compared with placebo, were observed at day 84 after receipt of inclisiran doses of 100 mg or more (Table 2 and Fig. 2A). At these doses, the least-squares mean reductions in the LDL cholesterol level ranged from 36.7 to 50.6%; the largest reduction, 50.6%, was observed in the group that received 500 mg of inclisiran. The LDL cholesterol levels returned toward baseline values at 180 days after receipt of the dose in the 25-mg and 100-mg cohorts, whereas the levels remained reduced, as compared with baseline, until at least 180 days after receipt of inclisiran doses of 300 mg or more (Fig. 2A). Additional details, including the absolute LDL cholesterol levels, are provided in Tables S4 and S8 in the Supplementary Appendix.
Figure 2. Effects on Serum Levels of Low-Density Lipoprotein (LDL) Cholesterol, According to Study Group and Dose Cohort.
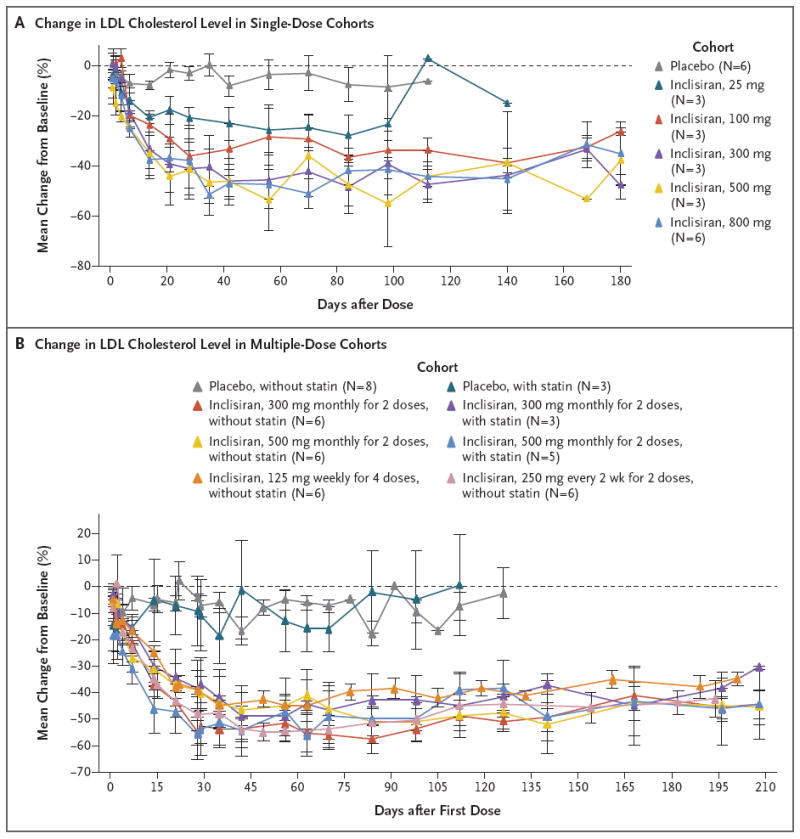
Shown are the effects (mean percentage changes from baseline) of inclisiran or placebo on the serum levels of LDL cholesterol over time. Baseline values were the average of all the study measurements taken before the first dose. For the single-dose cohorts, the data for the placebo group include all the participants; for the multiple-dose cohorts, the data for the placebo group are presented according to whether the participants were or were not taking a stable baseline dose of statin cotherapy. I bars represent standard errors. For at least one cohort, only one value (for one participant) is shown at some time points. In the single-dose phase, inclisiran or placebo was administered in one dose on day 0. In the multiple-dose phase, the first dose was administered on day 0 and subsequent doses at the indicated intervals. The 300-mg and 500-mg doses were administered as two monthly doses, the 125-mg dose was administered as four weekly doses, and the 250-mg dose was administered once every 2 weeks for two doses.
In the multiple-dose phase, reductions in the LDL cholesterol level that were significant, as compared with placebo, were observed at day 84 after receipt of the first dose for all inclisiran regimens except for the cohort that received 125 mg weekly for 4 weeks. Reductions ranged from a least-squares mean change of 45.1 to 59.7%, with the largest reduction, 59.7%, occurring in the group that received 300 mg of inclisiran monthly for 2 months (Table 3 and Fig. 2B). The LDL cholesterol levels remained reduced, as compared with baseline, in all the inclisiran cohorts at day 196 after receipt of the first dose (Fig. 2B). Additional details, including the absolute LDL cholesterol levels, are provided in Tables S6, S7, and S9 in the Supplementary Appendix.
EXPLORATORY ANALYSES
In both the single-dose and multiple-dose phases, decreases in the levels of total cholesterol, non-HDL cholesterol, and apolipoprotein B were noted in participants treated with inclisiran (Tables 2 and 3, and Tables S4, S5, S7, S8, and S9 in the Supplementary Appendix). The reductions in these variables from baseline to day 84 after receipt of the first dose were significant, as compared with placebo, for single or multiple doses of 250 mg or more.
DISCUSSION
In this study, treatment with inclisiran, a subcutaneously administered RNAi therapeutic agent targeting PCSK9 to reduce LDL cholesterol levels, resulted in no treatment discontinuations due to adverse events and no serious adverse events at the doses we studied. All the adverse events were mild or moderate in severity.
Single doses of inclisiran of 300 mg or more and all the multiple-dose regimens that we studied were associated with reductions of circulating levels of both PCSK9 and LDL cholesterol at 84 days after receipt of the first dose. We observed reductions in the PCSK9 level of up to 83.8% and in the LDL cholesterol level of up to 59.7%. We also observed lowering of the serum LDL cholesterol level when inclisiran was administered to patients taking stable doses of statin therapy. These findings add to previous nonclinical17 and clinical15,18,19 evidence that supports the ability of RNAi therapeutic agents in general to inhibit the synthesis of liver-derived target proteins.
The results of this study also add to the clinical evidence from anti-PCSK9 antibody trials that supports PCSK9 as a therapeutic target for significant lowering of the LDL cholesterol level.15,20-24 The magnitude of the lowering of the LDL cholesterol level that we found with inclisiran was generally similar to that observed previously with anti-PCSK9 antibodies20-24 or intensive statin therapy.25 However, inclisiran differs mechanistically from anti-PCSK9 antibodies. Whereas anti-PCSK9 antibodies bind to extracellular PCSK9 (produced from any tissue) and prevent its interaction with the LDL receptor, inclisiran inhibits the synthesis of PCSK9 protein specifically in the liver.
The pharmacodynamic profile of inclisiran also differs substantially from that of the anti-PCSK9 antibodies that have been studied to date.26,27 The effect on the PCSK9 and LDL cholesterol levels persisted for at least 180 days after the initiation of treatment, with little variation over the 6-month period after the receipt of the first dose. Our data suggest that inclisiran has the potential to provide effective management of hypercholesterolemia with administration every 3 or 6 months, as compared with the recommended regimens of administration once or twice monthly for the currently approved antibodies. Further evaluation of safety and the potential for inclisiran administration every 3 or 6 months is currently under way in a phase 2 trial (ORION ClinicalTrials.gov number, NCT02597127).
The limitations of the study should be considered carefully. First, although the trial was randomized and placebo-controlled, it was a single-blind trial and included only a limited number of participants in order to obtain an initial assessment of safety and pharmacodynamics. Therefore, the results must be regarded as preliminary and be confirmed in larger clinical trials of longer duration. Second, the sample included mainly healthy participants with relatively normal lipid profiles, albeit with a baseline LDL cholesterol level of at least 100 mg per deciliter. A limited number of patients taking statins were enrolled, and the coadministration of nonstatin LDL cholesterol–lowering agents was not investigated. In addition, in the multiple-dose phase, subsequent doses of inclisiran were administered well within the period of maximal pharmacodynamic activity of the first dose. Also, the cumulative doses of the multiple-dose regimens (500 to 1000 mg) were similar to the most effective single doses studied (300 to 800 mg). This situation may explain the apparently moderate incremental effects of increasing doses on the levels of PCSK9 and LDL cholesterol.
In conclusion, in this small phase 1 study, single or multiple doses of inclisiran, an RNAi therapeutic agent targeting PCSK9, were administered over a 1-month period. No serious adverse events occurred, and consistent, sustained reductions in the circulating PCSK9 and LDL cholesterol levels were observed.
Supplementary Material
Acknowledgments
Supported by Alnylam Pharmaceuticals and the Medicines Company.
Drs. Fitzgerald, Borodovsky, Bettencourt, Strahs, Clausen, Vaishnaw, and Simon report being employees of, and Ms. White, being a contractor to, Alnylam Pharmaceuticals; Drs. Fitzgerald and Borodovsky, being named inventors on a pending patent related to the composition of matter for inclisiran (U.S. patent number, US2016/0017335, held by Alnylam Pharmaceuticals); Dr. Horton, receiving fees for serving on advisory boards from Aegerion Pharmaceuticals, Pfizer, and Merck, consulting fees from Novartis, Sanofi, and Regeneron Pharmaceuticals, and holding stock options in Catabasis Pharmaceuticals; Dr. Kauffman, receiving consulting fees from Alnylam Pharmaceuticals; and Drs. Wijngaard and Kallend, being employees of the Medicines Company.
We thank the staff of Greensplash and Robert Marlowe of Spencer Fontayne for editorial assistance with an earlier version of the manuscript.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Barkas F, Liberopoulos EN, Kostapanosnos MS, Liamis G, Tziallas D, Elisaf M. Lipid target achievement among patients with very high and high cardiovascular risk in a lipid clinic. Angiology. 2015;66:346–53. doi: 10.1177/0003319714535073. [DOI] [PubMed] [Google Scholar]
- 3.Jameson K, Zhang Q, Zhao C, et al. Total and low-density lipoprotein cholesterol in high-risk patients treated with atorvastatin monotherapy in the United Kingdom: analysis of a primary-care database. Curr Med Res Opin. 2014;30:655–65. doi: 10.1185/03007995.2014.890926. [DOI] [PubMed] [Google Scholar]
- 4.Jones PH, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1(6):e001800. doi: 10.1161/JAHA.112.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper AJ, Burnett JR. Anti-PCSK9 therapies for the treatment of hypercholesterolemia. Expert Opin Biol Ther. 2013;13:429–35. doi: 10.1517/14712598.2012.748743. [DOI] [PubMed] [Google Scholar]
- 6.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–43. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mousavi SA, Berge KE, Leren TP. The unique role of proprotein convertase subtilisin/kexin 9 in cholesterol homeostasis. J Intern Med. 2009;266:507–19. doi: 10.1111/j.1365-2796.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(Suppl):S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007;193:445–8. doi: 10.1016/j.atherosclerosis.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:40–51. doi: 10.7326/M14-2957. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 14.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair JK, Willoughby JL, Chan A, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–61. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 17.Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–20. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 19.Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–17. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 20.Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98. doi: 10.1016/j.jacc.2012.08.986. [DOI] [PubMed] [Google Scholar]
- 21.Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 22.Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–18. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 23.Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21. doi: 10.1016/j.amjcard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Rouleau J. Improved outcome after acute coronary syndromes with an intensive versus standard lipid-lowering regimen: results from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial. Am J Med. 2005;118(Suppl 12A):28–35. doi: 10.1016/j.amjmed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Henne KR, Ason B, Howard M, et al. Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement. J Pharmacol Exp Ther. 2015;353:119–31. doi: 10.1124/jpet.114.221242. [DOI] [PubMed] [Google Scholar]
- 27.Egom EE, Hafeez H. Biochemistry of statins. Adv Clin Chem. 2016;73:127–68. doi: 10.1016/bs.acc.2015.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


