Abstract
A Ni-catalyzed method for arylboration is disclosed. The method allows for highly stereoselective arylboration of unactivated alkenes. The reactions utilize a simple Ni-catalyst and work with a broad range of alkenes and aryl bromides. The products represent useful intermediates for chemical synthesis due to the versatility of the C−B bond. Preliminary mechanistic details of the method are also disclosed.
Alkene difunctionalization processes are an important class of reactions for chemical synthesis.1 Of these methods, carboboration reactions are emerging as a versatile class of alkene difunctionalization processes because rapid molecular complexity can be established from simple olefin precursors.2,3 This is due to the diverse array of functional groups accessible from the carbon–boron bond, thus allowing for net carbofunctionalization (Scheme 1A).4
Scheme 1.
Strategies for Carboboration
Several classes of alkene carboboration reactions are known and vary based on the electrophile and catalyst(s) utilized. To achieve alkylboration,5 borylcarboxylation,6 or borylhydroxylalkylation,7 Cu- or Ni-catalysis has been employed. For incorporation of aryl groups, Cu/Pd-,8 Cu/Ni-,9 or Pd-catalysis10 has proven to be effective (Scheme 1B). The majority of these methods utilize activated alkenes (e.g., alkenyl arenes, vinylsilanes, strained alkenes), to increase the rate of a migratory insertion event prior to direct reaction between the electrophile and nucleophile (e.g., (Bpin)2 or [M]-Bpin). Carboboration of unactivated alkenes remains rare. Success has been achieved in the intra-5d and intermolecular5f,g alkylboration of terminal unactivated alkenes. In addition, intramolecular hydroxylalkylation of terminal unactivated alkenes has been developed.7a
Carboboration methods that allow for incorporation of aryl groups and utilize unactivated alkenes have not been developed. Furthermore, carboboration of 1,2-disubstituted unactivated alkenes is not known. Difunctionalization of 1,2-disubstituted unactivated alkenes is significant as the opportunity for establishing two new stereocenters becomes possible, thus allowing for rapid buildup of molecular complexity.
Our lab has taken an interest in arylboration reactions as a platform to achieve stereospecific Csp2−Csp3 cross-coupling.11 As noted above, this has led to the development of Cu/Pd-cooperative catalysis for arylboration of alkenylarenes, vinylsilanes, 1,3-dienes, and strained alkenes.8 To extend the scope of these processes, we sought to develop a method for arylboration of widely available unactivated alkenes. From this initiative we disclose a process for diastereoselective arylboration of unactivated alkenes catalyzed by a Ni-complex (Scheme 1C).12–16
Early efforts in our lab were directed toward developing a Pd/Cu-cooperative catalysis system for arylboration of cyclopentene.8 Under all conditions attempted, <10% yield of desired arylboration product was observed. The problems associated with this reaction were likely due to a slow migratory insertion of cyclopentene and β-hydride elimination of the putative alkyl metal intermediate. To overcome these issues, Ni-catalysis was investigated, as alkyl Ni-intermediates are less prone to β-hydride elimination.17 While early efforts were focused on investigation of Ni/Cu-cooperative catalysis,9 it was ultimately realized that the arylboration of cyclopentene with PhBr could be catalyzed by commercially available NiCl2(DME) to generate 1 as a single diastereomer (Table 1, entry 1). It is particularly noteworthy that these reactions are completely stereoselective, operate with near equimolar quantities of the alkene and aryl bromide, and occur at room temperature (or below in some cases).
Table 1.
Change from Standard Conditions

| |||
|---|---|---|---|
|
| |||
| entry | change from standard conditions | yield (%)a | dra |
| 1 | no change | 80 | >20:1 |
| 2 | NiCl2(PCy3)2 instead of NiCl2(DME) | <2 | – |
| 3 | NiCl2(phen) instead of NiCl2(DME) | <2 | – |
| 4 | NiCl2 instead of NiCl2(DME) | 65 | >20:1 |
| 5 | Phl instead of PhBr | 49 | >20:1 |
| 6 | PhOTf instead of PhBr | 76 | >20:1 |
| 7 | PhCl instead of PhBr | <2 | – |
Yield and dr determined by GC analysis with a calibrated internal standard.
Several additional points regarding the optimized conditions are noteworthy: (1) Use of exogenous phosphine or nitrogen-based ligands are detrimental to the reaction (Table 1, entries 2–3). (2) While NiCl2 can be used, the yield is diminished (Table 1, entry 4). (3) Iodobenzene and phenyltriflate allowed for product formation (Table 1, entries 5–6), while chlorobenzene was unreactive (Table 1, entry 7).
A range of substituted alkenes functioned well under the reaction conditions (Scheme 2). Reactions of 3-substituted cyclopentene derivatives (4 and 6) proceeded with good diastereoselectivity (products 5 and 7). These reactions are notable in that three stereocenters are established. Arylboration of N-Boc pyrrole 8 functioned well to provide 9 in 66% isolated yield.
Scheme 2.
Evaluation of Various Alkenesa
aNMR yield refers to yield determined by 1H NMR analysis of the unpurified reaction mixture with an internal standard. Yield refers to yield of isolated product after silica gel column chromatography and is reported as the average of two or more experiments (0.5 mmol scale). The discrepancy between 1H NMR and yield of isolated product is due to a sometimes tedious separation between desired product and a common byproduct, ArBpin. bReaction run for 48 h at 4 °C. c3.0 equiv of ArBr were used. dNMR yield of the Bpin and yield of isolated alcohol after oxidation; see the Supporting Information (SI) for details.
The stereospecificity of the reaction was explored with acyclic alkenes 10 and 12. In each case, the product derived from a syn-arylboration was observed (products 11 and 13, respectively). Furthermore, it must be emphasized that regardless of the alkene utilized the reactions were completely stereoselective. The regioselectivity was investigated with alkenes 10, 12, 16, and 18. With a large disparity in steric size (i-Pr/Me), the highly selective formation of 11 and 13 were observed. Even with a small difference in size (n-Pr/Me), the reaction proceeded with 3:1 rr (product 17). The regioselectivity of the reaction with substrate 18 was 5:1, which suggests that proximal electronegative substituents can alter the selectivity.18 The reaction with 1-octene and vinylcyclohexane proceeded in good yield (products 23 and 25, respectively); however, in the former case, a mixture of regioisomers (4:1 rr) was observed with 23 being the major product. In both cases, ~20% of other isomeric products were also observed likely resulting from β-hydride elimination and reinsertion.19 These byproducts were not observed in any reaction involving 1,2-disubstituted alkenes. Activated alkenes such as styrene or vinylsilanes do not function well in this process,19 thus demonstrating the complementarity to related Cu/Pd-catalyzed arylboration.8
With respect to the aryl bromide component, electron-donating (product 33) and electron-withdrawing substituents (products 26–28) lead to formation of the products in good yield (Scheme 3). In general, reactions with more electron-deficient aryl bromides work better than reactions with electron-rich aryl bromides. Sterically hindered aryl bromides also function well (products 29 and 31). With respect to functional group tolerance, aryl chlorides (products 26, 27), an ester (product 28), tertiary amine (product 34), tertiary amide (product 35), and even a primary alcohol (product 32) allow for product formation. A vinyl bromide (product 30) and bromo indole (product 36) were also shown to function. In general, reaction with cyclopentene was one of the more challenging substrates likely due to the steric interaction involved with synthesis of the syn-arylboration product (vida infra). For example, synthesis of 37 from acyclic alkene required 1.5 equiv of ArBr, while 3.0 equiv of ArBr were necessary to generate 29 in good yield. With respect to the known limitations, ketones, nitriles, and use of some hetereoaromatic bromides (e.g., 3-bromopyridine, 3-bromofuran) did not allow for product formation.19,20
Scheme 3.
Evaluation of Various Aryl Bromidesa
aSee Scheme 2. bIsolated as the corresponding BF3K adduct. c3.0 equiv of ArBr were used. dNMR yield of the Bpin and yield of isolated alcohol after oxidation; see the SI for details.
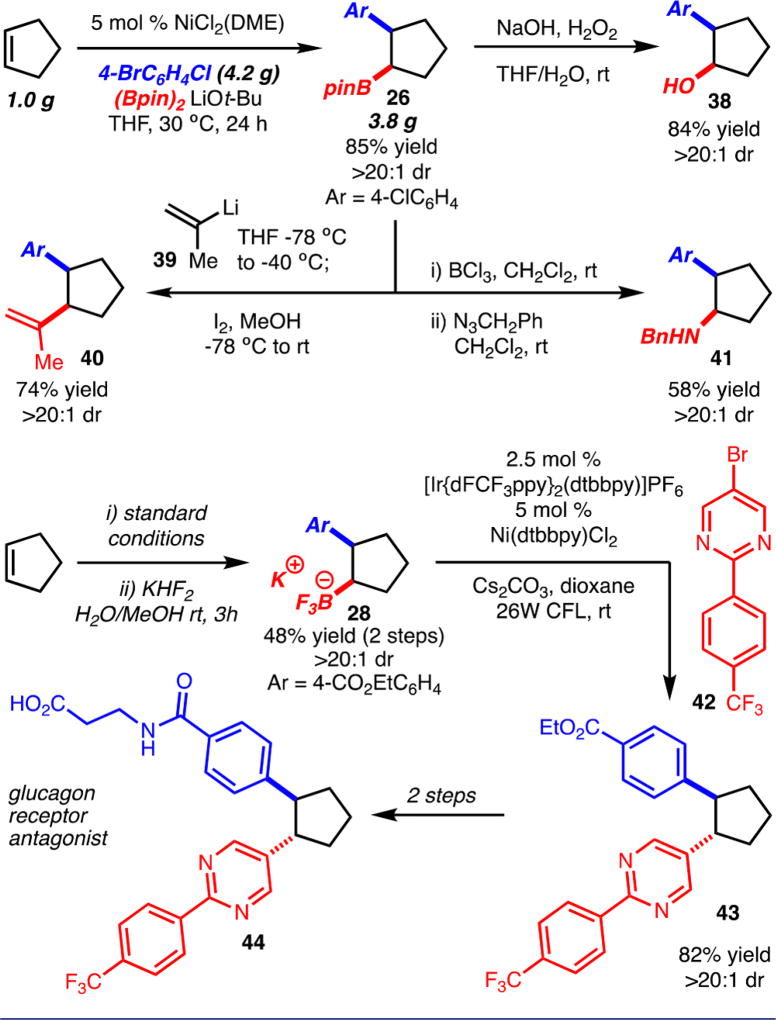
The reaction is also amenable to gram scale synthesis as illustrated in Scheme 4. The arylboration adducts could be readily converted to other compounds with control of stereochemistry through established protocols (Scheme 4, products 38, 40, and 41).4,21 The products of these reactions represent net hydroxyarylation, alkenylarylation, and aminoarylation of cyclopentene, respectively. In addition, an intermediate toward glucagon receptor antagonist 44 can be readily prepared by metallophotoredox cross-coupling of 28 (Scheme 4).22,23 In this example, the anti-diastereomer is generated due to the intermediacy of a secondary alkyl radical.
Scheme 4.
Gram Scale Reaction and Further Functionalization of Products
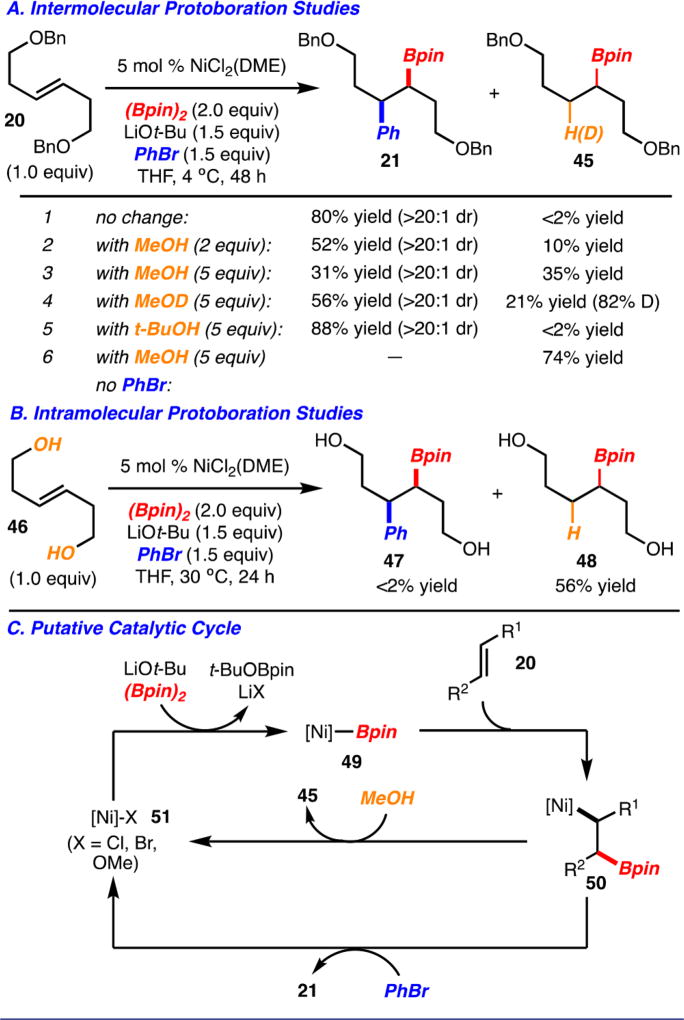
With respect to the mechanism, during our investigations it was observed that performing the arylboration of alkene 20 in the presence of MeOH (2 equiv) led to formation of 21 and adduct 45 (Scheme 5A). This observation led to the hypothesis that addition of [Ni]-Bpin 4916c to the alkene occurs to generate Ni-alkyl complex 50. This complex can undergo reaction with an ArBr to generate 21 or, in the presence of MeOH, undergo protonation to provide 45 according to the catalytic cycle shown in Scheme 3C. Further support for this catalytic cycle was found when addition of 5 equiv of MeOH (or 5 equiv MeOD) resulted in increased amounts of 45 relative to 21, whereas use of t-BuOH did not affect the outcome of the reaction. In addition, reaction of diol 46, which increases the proximity of acidic hydrogens near the [Ni]-alkyl bond in 50 and thus should give rise to increased amounts of protonation adducts, did indeed result in exclusive formation of 48 with <2% arylboration product 47 detected (Scheme 5B). It also appears that the formation of [Ni]-alkyl complex 50 does not require ArBr, as protonation adduct 45 was formed in the absence of aryl bromide (Scheme 5A).24 At this time, it is not clear how Ni-complex 50 undergoes reaction with ArBr, but it is possible an oxidative addition/reductive elimination sequence is involved.
Scheme 5.
Mechanistic Investigations
In summary, a new process for the Ni-catalyzed carboboration of unactivated alkenes is presented. Furthermore, the arylboration products can be readily transformed to other compounds to achieve net carbofunctionalization, thus allowing for complexity to be rapidly established from simple precursors. Finally, the likely presence of alkyl Ni-complex 50 opens up the possibility for further reaction development by investigating its potential reactivity in new transformations.
Supplementary Material
Acknowledgments
We thank Indiana University and the NIH (5R01GM114443) for generous financial support. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund.
Footnotes
ASSOCIATED CONTENT
The authors declare no competing financial interest.
References
- 1.For reviews, see: Saini V, Stokes BJ, Sigman MS. Angew. Chem. Int. Ed. 2013;52:11206. doi: 10.1002/anie.201303916.Coombs JR, Morken JP. Angew. Chem. Int. Ed. 2016;55:2636. doi: 10.1002/anie.201507151.
- 2.For reviews, see: Shimizu Y, Kanai M. Tetrahedron Lett. 2014;55:3727.Semba K, Fujihara T, Terao J, Tsuji Y. Tetrahedron. 2015;71:2183.Lazreg F, Nahra F, Cazin CSJ. Coord. Chem. Rev. 2015:293–294. 48.Neeve EC, Geier SJ, Mkhalid IAI, Westcott SA, Marder TB. Chem. Rev. 2016;116:9091. doi: 10.1021/acs.chemrev.6b00193.
- 3.For an alternative approach involving diboration followed by cross-coupling, see: Mlynarski SN, Schuster CH, Morken JP. Nature. 2013;505:386. doi: 10.1038/nature12781.
- 4.Sandford C, Aggarwal VK. Chem. Commun. 2017;53:5481. doi: 10.1039/c7cc01254c. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ito H, Kosaka Y, Nonoyama K, Sasaki Y, Sawamura M. Angew. Chem. Int. Ed. 2008;47:7424. doi: 10.1002/anie.200802342. [DOI] [PubMed] [Google Scholar]; (b) Ito H, Toyoda T, Sawamura M. J. Am. Chem. Soc. 2010;132:5990. doi: 10.1021/ja101793a. [DOI] [PubMed] [Google Scholar]; (c) Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. J. Am. Chem. Soc. 2010;132:11440. doi: 10.1021/ja103783p. [DOI] [PubMed] [Google Scholar]; (d) Kubota K, Yamamoto E, Ito H. J. Am. Chem. Soc. 2013;135:2635. doi: 10.1021/ja3104582. [DOI] [PubMed] [Google Scholar]; (e) Yoshida H, Kageyuki I, Takaki K. Org. Lett. 2013;15:952. doi: 10.1021/ol4001526. [DOI] [PubMed] [Google Scholar]; (f) Kageyuki I, Yoshida H, Takaki K. Synthesis. 2014;46:1924. [Google Scholar]; (g) Su W, Gong T-J, Lu X, Xu M-Y, Yu C-G, Xu Z-Y, Yu H-Z, Xiao B, Fu Y. Angew. Chem. Int. Ed. 2015;54:12957. doi: 10.1002/anie.201506713. [DOI] [PubMed] [Google Scholar]; (h) Kageyuki I, Osaka I, Takaki K, Yoshida H. Org. Lett. 2017;19:830. doi: 10.1021/acs.orglett.6b03820. [DOI] [PubMed] [Google Scholar]; (i) Radomkit S, Liu Z, Closs A, Mikus MS, Hoveyda AH. Tetrahedron. 2017;73:5011. doi: 10.1016/j.tet.2017.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher TW, McClain EJ, Hamilton TG, Perrone TM, Kroner KM, Donohoe GC, Akhmedov NG, Petersen JL, Popp BV. Org. Lett. 2016;18:6428. doi: 10.1021/acs.orglett.6b03326. [DOI] [PubMed] [Google Scholar]
- 7.(a) Crotti S, Bertolini F, Macchia F, Pineschi M. Org. Lett. 2009;11:3762. doi: 10.1021/ol901429g. [DOI] [PubMed] [Google Scholar]; (b) Cho HY, Morken JP. J. Am. Chem. Soc. 2008;130:16140. doi: 10.1021/ja806113v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yamamoto E, Kojima R, Kubota K, Ito H. Synlett. 2016;27:272. [Google Scholar]; (d) Green JC, Joannou MV, Murray SA, Zanghi JM, Meek SJ. ACS Catal. 2017;7:4441. doi: 10.1021/acscatal.7b01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semba K, Nakao Y. J. Am. Chem. Soc. 2014;136:7567. doi: 10.1021/ja5029556.Smith KB, Logan KM, You W, Brown MK. Chem. - Eur. J. 2014;20:12032. doi: 10.1002/chem.201404310.Logan KM, Smith KB, Brown MK. Angew. Chem. Int. Ed. 2015;54:5228. doi: 10.1002/anie.201500396.Logan KM, Brown MK. Angew. Chem. Int. Ed. 2017;56:851. doi: 10.1002/anie.201609844.Chen B, Cao P, Yin X, Liao Y, Jiang L, Ye J, Wang M, Liao J. ACS Catal. 2017;7:2425.Smith KB, Brown MK. J. Am. Chem. Soc. 2017;139:7721. doi: 10.1021/jacs.7b04024.Sardini SR, Brown MK. J. Am. Chem. Soc. 2017;139:9823. doi: 10.1021/jacs.7b05477.For allylboration see: Jia T, Cao P, Wang B, Lou Y, Yin X, Wang M, Liao J. J. Am. Chem. Soc. 2015;137:13760. doi: 10.1021/jacs.5b09146.
- 9.Semba K, Ohtagaki Y, Nakao Y. Org. Lett. 2016;18:3956. doi: 10.1021/acs.orglett.6b01675. [DOI] [PubMed] [Google Scholar]
- 10.(a) Yang K, Song Q. Org. Lett. 2016;18:5460. doi: 10.1021/acs.orglett.6b02527. [DOI] [PubMed] [Google Scholar]; (b) Yang K, Song Q. J. Org. Chem. 2016;81:1000. doi: 10.1021/acs.joc.5b02564. [DOI] [PubMed] [Google Scholar]
- 11.For reviews see: Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. doi: 10.1021/cr100327p.Swift EC, Jarvo ER. Tetrahedron. 2013;69:5799. doi: 10.1016/j.tet.2013.05.001.Wang C-Y, Derosa J, Biscoe MR. Chem. Sci. 2015;6:5105. doi: 10.1039/c5sc01710f.Cherney AH, Kadunce NT, Reisman SE. Chem. Rev. 2015;115:9587. doi: 10.1021/acs.chemrev.5b00162.
- 12.For a mechanistically distinct Ni-catalyzed intramolecular carboboration of alkynes see: Yamamoto A, Suginome M. J. Am. Chem. Soc. 2005;127:15706. doi: 10.1021/ja055396z.Daini M, Yamamoto A, Suginome M. Asian J. Org. Chem. 2013;2:968.
- 13.For recent examples of Ni-catalyzed hydroarylation and hydroalkylation see: Lu X, Xiao B, Zhang Z, Gong T, Su W, Yi J, Fu Y, Liu L. Nat. Commun. 2016;7:11129. doi: 10.1038/ncomms11129.Green SA, Matos JLM, Yagi A, Shenvi RA. J. Am. Chem. Soc. 2016;138:12779. doi: 10.1021/jacs.6b08507.He Y, Cai Y, Zhu S. J. Am. Chem. Soc. 2017;139:1061. doi: 10.1021/jacs.6b11962.
- 14.For recent examples of dicarbofunctionalization of alkenes by Nicatalysis see: García-Domínguez A, Li Z, Nevado C. J. Am. Chem. Soc. 2017;139:6835. doi: 10.1021/jacs.7b03195.Shrestha B, Basnet P, Dhungana RK, KC S, Thapa S, Sears JM, Giri R. J. Am. Chem. Soc. 2017;139:10653. doi: 10.1021/jacs.7b06340.Derosa J, Tran VT, Boulous MN, Chen JS, Engle KM. J. Am. Chem. Soc. 2017;139:10657. doi: 10.1021/jacs.7b06567.
- 15.For a review regarding Ni-catalysis see: Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299. doi: 10.1038/nature13274.
- 16.For representative examples of Ni-catalyzed reactions involving diboron reagents see: Hirano K, Yorimitsu H, Oshima K. Org. Lett. 2007;9:5031. doi: 10.1021/ol702254g.Sumida Y, Yorimitsu H, Oshima K. Org. Lett. 2008;10:4677. doi: 10.1021/ol801982d.Dudnik AS, Fu GC. J. Am. Chem. Soc. 2012;134:10693. doi: 10.1021/ja304068t.(d) Reference 7a.(e) Reference 7b.
- 17.Lin, Liu L, Fu Y, Luo S-W, Chen Q, Guo Q-X. Organometallics. 2004;23:2114. [Google Scholar]
- 18.Xi Y, Hartwig JF. J. Am. Chem. Soc. 2016;138:6703. doi: 10.1021/jacs.6b02478. [DOI] [PubMed] [Google Scholar]
- 19.See the Supporting Information for details.
- 20.The moderate yield in some cases can be attributed to incomplete conversion.
- 21.Hupe E, Marek I, Knochel P. Org. Lett. 2002;4:2861. doi: 10.1021/ol0262486. [DOI] [PubMed] [Google Scholar]
- 22.Primer DN, Karakaya I, Tellis JC, Molander GA. J. Am. Chem. Soc. 2015;137:2195. doi: 10.1021/ja512946e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee ECY, Tu M, Stevens BD, Bian J, Aspnes G, Perreault C, Sammons MF, Wright SW, Litchfield J, Kalgutkar AS, Sharma R, Didiuk MT, Ebner DC, Filipski KJ, Brown J, Atkinson K, Pfefferkorn JA, Guzman-Perez A. Bioorg. Med. Chem. Lett. 2014;24:839. doi: 10.1016/j.bmcl.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 24.Protoboration and arylboration of 18 resulted in formation of the products in similar regioisomeric ratios (5:1 rr), thus supporting the hypothesis that a common intermediate is generated prior to reaction with PhBr or MeOH. See the SI for additional details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.