Abstract
OBJECTIVES
We examined whether multiple domains of baseline cognitive performance were associated with prospective physical activity (PA) adherence in the Lifestyle Interventions and Independence for Elders Pilot study (LIFE-P).
DESIGN, SETTING, PARTICIPANTS
The LIFE-P study was a single-blind, multicenter, randomized controlled trial of a PA intervention compared to a successful aging educational intervention in sedentary, mobility-limited older adults.
INTERVENTION
A 12-month structured, moderate-intensity, multi-modal PA program that included walking, resistance training, and flexibility exercises. For the first 2 months (adoption), 3 center-based exercise sessions (40–60 min) / week were conducted. During the next 4 months (transition), center-based sessions were conducted 2 times / week. The subsequent maintenance phase consisted of optional once-to-twice-per-week center-based sessions and home-based PA.
MEASUREMENTS
Tests of executive and global cognitive functioning, working memory and psychomotor speed were administered at baseline. Median test scores were used to dichotomize participants into low or high cognitive performance groups.
RESULTS
52 mobility-limited older adults (age: 76.9 ±5 yrs) were randomized to the PA arm of LIFE-P. Compared to participants with high cognitive performance, participants with low performance had similar PA adherence rates (all P ≥ 0.34). Furthermore, weak and non-significant univariate relationships were elicited between all measures of cognition and overall PA adherence levels (r values ranged: −0.20 to 0.12, P ≥ 0.12).
CONCLUSION
These data suggest that cognitive performance does not limit long-term PA adherence in mobility-limited older adults. Additional studies in larger cohorts are warranted to verify these findings.
Keywords: Cognition, physical activity, adherence, mobility-limited
Introduction
Participation in regular physical activity is now recognized as a critical health behavior for the prevention and management of chronic disease among older adults (1–4). While a significant amount of research has been conducted to explore factors related to the adoption and maintenance of physical activity in middle-aged and younger adults, few studies have examined the factors that influence physical activity participation among adults aged 65 years and older (1, 5, 6). Furthermore, little is known about the major determinants of adherence to physical activity among older adults during interventions over prolonged durations (> 6 months).
The Lifestyle Interventions and Independence for Elders Pilot study (LIFE-P) was conducted to examine the feasibility of conducting a large multi-center clinical trial on the effects of increasing physical activity in sedentary, older individuals at risk for mobility disability (7). Independent factors previously shown to influence adherence to this long-term (12 month) physical activity intervention include chronic disease burden and self-reported symptoms of chronic disease (6, 8). The potential influence of baseline cognitive function on subsequent adherence to the LIFE-P physical activity intervention was not examined in either of these previous investigations. However, recent studies have shown that older adults with lower cognitive function (reduced executive functioning) were less adherent to a 3-month exercise-based cardiac rehabilitation program. Importantly, participants with low adherence had poorer outcomes following their exercise intervention (9). Similarly, Tiedemann et al. demonstrated that impaired global cognitive function (assessed using the Mini-Mental State Examination) was a significant independent predictor of low physical activity adherence during a 6–12 month intervention among older retirement village residents (10).
The purpose of this study was to investigate whether measures of baseline cognitive function predict subsequent adherence to the LIFE-P physical activity intervention (PA). Data were examined from the cognitive sub-study of LIFE-P and four domains of cognitive function (global cognition, executive functioning, psychomotor speed and working memory) were evaluated (11). We hypothesized that, in mobility-limited older adults, lower levels of cognitive performance would be associated with lower levels of subsequent PA adherence.
Methods
Study design
The LIFE-P study was a single-blind, multicenter, randomized controlled trial of a PA intervention compared to a successful aging (SA) educational intervention in sedentary older adults. The study was designed to help plan a definitive phase 3 randomized controlled trial to examine the efficacy of a program of physical activity, compared with SA, on the incidence of major mobility disability in older adults. Complete descriptions of the LIFE-P study design and the primary and cognitive outcomes have been reported previously (7, 11, 12). Briefly, the study was conducted at four field centers across the United States (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University). The LIFE-P cognitive sub-study was conducted at Stanford University and Wake Forest University (11).
Study Participants
Participants were recruited in the age range of 70–89 years. Additional inclusion criteria included a sedentary life style (< 20 min/wk spent in structured PA), able to walk 400 m within 15 minutes without sitting and without use of any assistive device, and Short Physical Performance Battery (SPPB) score 9 or less (of 12). Participants with severe heart failure, uncontrolled angina, severe pulmonary disease, severe arthritis, cancer requiring treatment in the past 3 years, Parkinson’s disease or other serious neurological disorders, life expectancy of less than 12 months, or a Mini-Mental State Examination score less than 21, or a diagnosis of dementia, were ineligible.
Eligible participants received detailed instructions for a 1-week to 2-week behavioral run-in, during which they were asked to self-monitor specific behaviors and to complete forms related to these behaviors. Participants who successfully completed the behavioral run-in received additional baseline assessments and were randomized to the study interventions via a web-based system. Of the 3141 persons who were initially screened by phone, a total of 424 (13.5%) were ultimately randomized to LIFE-P across the four field centers. For the cognitive sub-study, the first 50 participants at the Stanford University and Wake Forest University field centers were administered a cognitive assessment battery at baseline.
Physical Activity Intervention
The PA intervention consisted of a combination of aerobic, strength, balance, and flexibility exercises. The intervention was divided into three phases: adoption (weeks 1–8), transition (weeks 9–24), and maintenance (week 25 to the end of the trial). Each participant in the PA group received a 45-minute individualized, introductory session to describe the intervention and to provide individual counseling to optimize safety and participation. For the first 2 months (adoption), three center-based exercise sessions (40–60 min) per week were conducted in a supervised setting. During the next 4 months (transition), the number of center-based sessions was reduced (2/week) and home-based endurance/strengthening/flexibility exercises (≥3/week) were started. The subsequent maintenance phase consisted of the home-based intervention, optional once-to-twice-per-week center-based sessions, and monthly telephone contacts. The PA intervention included group-based behavioral counseling sessions (1 each week for the first 10 weeks) that focused on PA participation and disability prevention, and on encouraging participants to increase all forms of PA.
Outcome Measures
Cognitive Assessment Battery
The assessment battery was adapted from the Action to Control Cardiovascular Risk in Diabetes (ACCORD)—Memory in Diabetes trial (13). This battery was developed specifically for the purpose of incorporating cognitive assessment as a secondary outcome in a large cardiovascular clinical trial (ACCORD). The LIFE study investigators selected the cognitive assessment battery based on the numerous domains of cognition likely to be affected by the LIFE-P intervention, in addition to experience gained from the ACCORD study on the feasibility of administering this battery in a large clinical trial. The cognitive battery consisted of four primary components:
Modified Mini-Mental State Examination (3MSE) is a widely used measure of global cognitive functioning (14). The 3MSE is an expanded 100-point version of the original Folstein Mini-Mental State Examination (15).
Modified Stroop test (Stroop) was utilized as a measure of processing speed, cognitive flexibility, and inhibition or disinhibition. The Stroop test consists of three subtasks: color word naming, color naming, and naming of color words printed in a different color from the color word (interference component). Participant’s score on this test is the difference between scores on tests 2 and 3.
Digit Symbol Substitution Test (DSST) was used as a measure of psychomotor speed and working memory (16). The DSST has proven to be feasible in aging studies and large multicenter clinical trials (17). Participants are given a series of numbered symbols and then asked to draw the appropriate symbols below a list of random numbers. The score is the number of correctly made matches in 1 minute.
The Rey Auditory Verbal Learning Test (RAVLT), a test of short- and long-term verbal memory, was used to assess the ability to learn a list of 15 common words(18). The study participant is read this list five times, and after each time, he or she immediately recalls as many words as possible. Following the fifth recall, an interference list is presented after which the participant is asked to spontaneously recall words from the original list. Then, a 10-minute interval passes and he or she is asked again to remember spontaneously as many words as possible from the first list (delayed recall). Scoring is based on total correct words across all components.
Measures of adherence
Attendance at center-based physical activity sessions was reported as the percentage of attended sessions relative to the total number of possible sessions in each study phase, excluding facility closings (e.g., holidays, weather emergencies, etc.). During maintenance, adherence was also assessed by completion of the home activity logs.
Statistical Analysis
Data analysis was performed using SAS statistical software (Version 9.4, SAS Institute Inc., Cary, North Carolina). Statistical significance was set at P ≤ 0.05 and results are reported as mean ± SD. Univariate correlation analyses (Pearson and Spearman’s rank) were performed to examine the associations between baseline measures of cognitive function and subsequent PA adherence. Separate analyses were conducted for each of the adoption, transition and maintenance phases of the intervention. In the adoption and transition phases, percent attendance per participant was calculated by dividing the number of sessions attended by the expected number of sessions. For the maintenance phase, the total number of sessions attended was used as the index of PA adherence. To further examine the influence of baseline cognition on subsequent PA adherence, independent samples T tests were used to determine whether group differences in PA adherence were evident for participants with low or high baseline cognition performance in the adoption, transition, and the average of adoption and transition phases of PA. The median test score on the respective cognitive assessment was used as the cut point to define low and high cognitive performance, and differences were adjusted for gender and site. Linear regression models were used to examine the relationships between baseline cognitive function and home log completion rates throughout PA.
Results
A total of 102 participants were administered the cognitive battery at the baseline examination in LIFE-P. From these participants a total of 52 participants were randomized to the PA intervention. The baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of PA participants (n = 52)
| Variable | |
|---|---|
| Age, (yrs) | 76.9 ± 4.5 |
| BMI (kg/m2) | 28.9 ± 5.2 |
| Female gender, n (%) | 36 (72) |
| SPPB score | 7.9 ± 1.2 |
| 3MSE (min – max) | 89.9 ± 6.2 (69–99) |
| DSST | 45.0 ± 12.4 (13–73) |
| Stroop | 40.1 ± 21.5 (9–128) |
| RAVLT | 6.2 ± 3.2 (0–13) |
Values are mean ± SD. BMI: body mass index; SPPB: Short Physical Performance Battery; 3MSE: Modified Mini-Mental State Examination; DSST: Digit Symbol Substitution Test; Stroop: Modified Stroop test; RAVLT: Rey Auditory Verbal Learning Test.
Results of the correlation analysis between the four domains of cognitive performance and subsequent level of PA adherence are provided in Table 2 and Figure 1. No significant relationships were found between any measure of cognitive function and subsequent level of PA adherence at any phase in LIFE-P (all P ≥ 0.12). Figure 1 displays scatterplots of the relationships between the four cognitive performance domains and PA adherence (combined for first 6 months of intervention).
Table 2.
Correlation coefficients between domains of cognitive performance and subsequent adherence to PA during LIFE–P
| Variable | Adoption phase adherence (%) | Transition phase adherence (%) | Maintenance phase adherence (number of sessions) |
|---|---|---|---|
| 3MSE | −0.22 (P = 0.12) | −0.07 (P = 0.62) | −0.06 (P = 0.86) |
| DSST | −0.21 (P = 0.14) | −0.11 (P = 0.45) | −0.06 (P = 0.70) |
| Stroopˆ | 0.05 (P = 0.72) | 0.06 (P = 0.70) | −0.04 (P = 0.77) |
| RAVLTˆ | −0.15 (P = 0.29) | 0.03 (P = 0.85) | −0.13 (P = 0.37) |
= Spearman’s rank correlation coefficient
Figure 1.
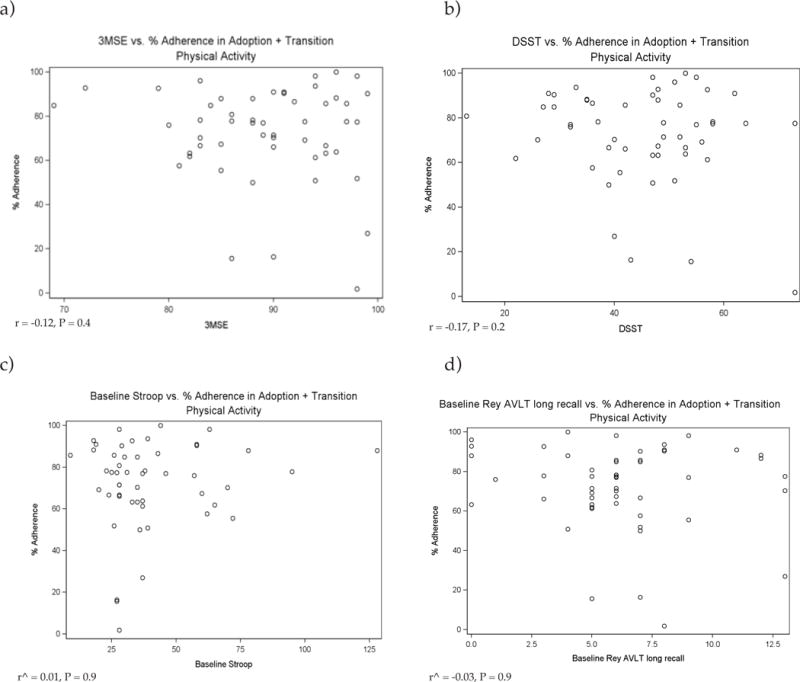
Relationships between cognitive performance domains and PA adherence (combined for first 6 months of intervention)
ˆ = Spearman’s rank correlation coefficient
Table 3 compares group differences in subsequent PA adherence according to low or high baseline cognitive performance (participants dichotomized into groups based on median score on each measure). The median cut point scores for the four respective cognitive performance domains were as follows: 3MSE: 91.0; DSST: 48.0; Stroop: 33.0; RAVLT: 6.0. Compared to participants with high cognitive performance at baseline, lower cognitive performance was not associated with a significant reduction in PA adherence throughout the adoption or transition phases of the intervention (all P ≥ 0.34). Similarly, no association between any domain of cognitive function and the number of home logs completed was evident (data not shown).
Table 3.
Baseline measures of cognitive performance (dichotomized into Low (n = 31) and High (n = 21) groups based on median score) vs. PA adherence
| 3MSE | Stroop | DSST | RAVLT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phaseˆ | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
| Transition | 64.5 ± 25.5 | 67.1 ± 29.6 | 0.49 | 64.2 ± 28.3 | 65.8 ± 26.1 | 0.61 | 65.1 ± 26.4 | 66.1 ± 28.3 | 0.87 | 68.7 ± 20.3 | 60.9 ± 34.6 | 0.65 |
| Adoption | 82.2 ± 15.2 | 76.1 ± 24.1 | 0.34 | 77.7 ± 22.9 | 80.9 ± 15.3 | 0.41 | 81.1 ± 15.2 | 77.7 ± 24.4 | 0.45 | 81.3 ± 17.1 | 77.4 ± 22.4 | 0.92 |
| Combined | 72.5 ± 19.2 | 72.1 ± 24.5 | 0.82 | 70.4 ± 25.0 | 73.3 ± 17.3 | 0.44 | 72.9 ± 19.3 | 71.5 ± 24.4 | 0.79 | 74.6 ± 16.3 | 69.1 ± 27.1 | 0.78 |
Values mean ± SD, P values (Low vs. High) are adjusted for clinical site and gender; ˆAdoption phase of the intervention is weeks 1–8; ˆTransition phase of the intervention is weeks 9–24; ˆCombined is the adoption + transition (weeks 1–24)
Discussion
The major finding of this study is that cognitive performance did not limit the adoption of, and participation in, a 12 month intervention of multi-modal physical activity in LIFE-P. We demonstrated that, within this population of mobility-limited older adults with mild cognitive deficits, baseline cognitive performance assessed across multiple domains including executive functioning, global cognition and short and long term working memory, was not predictive of subsequent adherence to PA.
Although our primary observations were contrary to our initial hypothesis, there are important considerations associated with our investigation. Our null findings suggest that inherent aspects of the LIFE-P study design may have been important for limiting the potential influence of cognitive performance and subsequent participation in PA. In particular, the PA intervention was complimented with weekly closed-group behavioral counseling sessions that focused on physical activity adherence and the prevention of physical disability. Such behavioral group sessions have been shown to be effective for older adults in promoting commitment to physical activity and as a strategy to cope with the process of physical disablement (19). Previous studies that have identified cognitive function as a significant determinant of activity adherence in cardiac rehabilitation patients and among older persons in retirement villages did not include any additional behavioral counseling sessions to support physical activity adherence (9, 10). In addition, throughout LIFE-P, a Lifestyle Resource Core of behavioral scientists, geriatricians and exercise therapists closely monitored PA attendance, reviewed adherence issues and disseminated strategies to problem-solve and consistently promote strong participation in PA. The use of participant proxy representatives in LIFE-P may have also diminished any overriding influence of cognitive performance deficits on subsequent PA attendance.
Several other factors could have influenced the present study findings. Due to the small sample size, the current analysis may have been underpowered to detect a meaningful relationship between baseline cognition and subsequent physical activity adherence. In addition, the 12 month duration of PA may not have been of sufficient duration for any potential baseline cognitive impairments to manifest into PA non-adherence. We also did not evaluate the influence of numerous covariates (e.g. education, dietary intake, comorbidities etc.) that may have influenced any potential association between cognitive performance and physical activity adherence in this study (20–22). In addition, previous studies have demonstrated that the relationship between cognition and physical activity adherence may be mediated through self-efficacy (23). The results of this study are also limited to older adults who successfully completed a behavioral run-in prior to study enrollment and were motivated to volunteer for a long-term randomized controlled trial comparing two distinct behavioral interventions. In addition, the external validity of our findings are further limited as we used distribution based cut-points, rather than established population-based norm values, to dichotomize participants into low vs. high cognitive performance groups.
Conclusion
The results of the study demonstrate that, among mobility limited older adults, cognitive performance does not limit the subsequent adoption of, and participation in, a year-long PA intervention (LIFE-P). Study design aspects, including inherent components of the PA intervention that targeted physical activity adherence, may have influenced any potential relationship between cognitive function and prospective activity adherence. These findings are both positive and encouraging as they demonstrate that older adults with mobility limitations, and some with coexisting cognitive performance deficits, can successfully adhere to a long-term multi modal exercise intervention. Additional investigations, in studies with larger sample sizes and longer durations of PA (24), are warranted to further examine cognitive function as a determinant of physical activity adherence in mobility-limited older adults.
Appendix. Research Investigators for Pilot Phase of LIFE
|
Cooper Institute, Dallas, TX:
|
| Steven N. Blair, P.E.D. – Field Center Principal Investigator |
| Timothy Church, M.D., Ph.D., M.P.H. – Field Center Co-Principal Investigator |
| Jamile A. Ashmore, Ph.D. |
| Judy Dubreuil, M.S. |
| Georita Frierson, Ph.D. |
| Alexander N. Jordan, M.S. |
| Gina Morss, M.A. |
| Ruben Q. Rodarte, M.S. |
| Jason M. Wallace, M.P.H. |
|
National Institute on Aging |
| Jack M. Guralnik, M.D., Ph.D. – Co-Principal Investigator of the Study |
| Evan C. Hadley, M.D. |
| Sergei Romashkan, M.D., Ph.D. |
|
Stanford University, Palo Alto, CA |
| Abby C. King, Ph.D. – Field Center Principal Investigator |
| William L. Haskell, Ph.D. – Field Center Co-Principal Investigator |
| Leslie A. Pruitt, Ph.D. |
| Kari Abbott-Pilolla, M.S. |
| Karen Bolen, M.S. |
| Stephen Fortmann, M.D. |
| Ami Laws, M.D. |
| Carolyn Prosak, R.D. |
| Kristin Wallace, M.P.H. |
|
Tufts University |
| Roger Fielding, Ph.D. |
| Miriam Nelson, Ph.D. |
| Dr. Reid and Dr. Fielding’s contribution is partially supported by the U.S. Dept. of Agriculture, under agreement No. 58-1950-4-003. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. This research was also supported by the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679) and the Boston Rehabilitation Outcomes Center Grant (1R24HD065688-01A1). |
|
University of California, Los Angeles, Los Angeles, CA |
| Robert M. Kaplan, Ph.D., M.A. |
|
VA San Diego Healthcare System and University of California, San Diego, San Diego, CA |
| Erik J. Groessl, Ph.D. |
|
University of Florida, Gainesville, FL |
| Marco Pahor, M.D. – Principal Investigator of the Study |
| Michael Perri, Ph.D. |
| Connie Caudle |
| Lauren Crump, M.P.H |
| Sarah Hayden |
| Latonia Holmes |
| Cinzia Maraldi, M.D. |
| Crystal Quirin |
|
University of Pittsburgh, Pittsburgh, PA |
| Anne B. Newman, M.D., M.P.H. – Field Center Principal Investigator |
| Stephanie Studenski, M.D., M.P.H. – Field Center Co-Principal Investigator |
| Bret H. Goodpaster, Ph.D., M.S. |
| Nancy W. Glynn, Ph.D. |
| Erin K. Aiken, B.S. |
| Steve Anthony, M.S. |
| Sarah Beck (for recruitment papers only) |
| Judith Kadosh, B.S.N., R.N. |
| Piera Kost, B.A. |
| Mark Newman, M.S. |
| Jennifer Rush, M.P.H. (for recruitment papers only) |
| Roberta Spanos (for recruitment papers only) |
| Christopher A. Taylor, B.S. |
| Pam Vincent, C.M.A. |
| Diane Ives, M.P.H |
| The Pittsburgh Field Center was partially supported by the Pittsburgh Claude D. Pepper Center P30 AG024827. |
|
Wake Forest University, Winston–Salem, NC |
| Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator |
| Peter Brubaker, Ph.D. |
| Jamehl Demons, M.D. |
| Curt Furberg, M.D., Ph.D. |
| Jeffrey A. Katula, Ph.D., M.A. |
| Anthony Marsh, Ph.D. |
| Barbara J. Nicklas, Ph.D. |
| Jeff D. Williamson, M.D., M.P.H. |
| Rose Fries, L.P.M. |
| Kimberly Kennedy |
| Karin M. Murphy, B.S., M.T. (ASCP) |
| Shruti Nagaria, M.S. |
| Katie Wickley-Krupel, M.S. |
|
Data Management, Analysis and Quality Control Center (DMAQC) |
| Michael E. Miller, Ph.D. – DMAQC Principal Investigator |
| Mark Espeland, Ph.D. – DMAQC Co-Principal Investigator |
| Fang–Chi Hsu, Ph.D. |
| Walter J. Rejeski, Ph.D. |
| Don P. Babcock, Jr., P.E. |
| Lorraine Costanza |
| Lea N. Harvin |
| Lisa Kaltenbach, M.S. |
| Wei Lang, Ph.D. |
| Wesley A. Roberson |
| Julia Rushing, M.S. |
| Scott Rushing |
| Michael P. Walkup, M.S. |
| The Wake Forest University Field Center is, in part, supported by the Claude D. Older American Independence Pepper Center #1 P30 AG21332. |
|
Yale University |
| Thomas M. Gill, M.D. |
| Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging. |
| The Lifestyle Interventions and Independence for Elders (LIFE-P) Pilot Study is funded by a National Institutes on Health/National Institute on Aging Cooperative Agreement |
| #UO1 AG22376 and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. |
Footnotes
Ethical Standards: This study was in accordance with the Declaration of Helsinki for human studies.
Trial Registration: clinicaltrials.gov Identifier: NCT00116194.
References
- 1.Brassington GS, Atienza AA, Perczek RE, DiLorenzo TM, King AC. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. Am J Prev Med. 2002;23(2 Suppl):80–6. doi: 10.1016/s0749-3797(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 2.Williamson J, Pahor M. Evidence regarding the benefits of physical exercise. Arch Intern Med. 2010;170(2):124–5. doi: 10.1001/archinternmed.2009.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of Structured Physical Activity on Prevention of Major Mobility Disability in Older Adults: The LIFE Study Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2014 doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Investigators LS. Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(11):1157–65. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 5.King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adults. A critical review and recommendations. American journal of preventive medicine. 1998;15(4):316–33. doi: 10.1016/s0749-3797(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 6.Rejeski WJ, Miller ME, King AC, Studenski SA, Katula JA, Fielding RA, et al. Predictors of adherence to physical activity in the Lifestyle Interventions and Independence for Elders pilot study (LIFE-P) Clin Interv Aging. 2007;2(3):485–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Rejeski WJ, Fielding RA, Blair SN, Guralnik JM, Gill TM, Hadley EC, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26(2):141–54. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Fielding RA, Katula J, Miller ME, Abbott-Pillola K, Jordan A, Glynn NW, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007;39(11):1997–2004. doi: 10.1249/mss.0b013e318145348d. [DOI] [PubMed] [Google Scholar]
- 9.Kakos LS, Szabo AJ, Gunstad J, Stanek KM, Waechter D, Hughes J, et al. Reduced executive functioning is associated with poorer outcome in cardiac rehabilitation. Prev Cardiol. 2010;13(3):100–3. doi: 10.1111/j.1751-7141.2009.00065.x. [DOI] [PubMed] [Google Scholar]
- 10.Tiedemann A, Sherrington C, Lord SR. Predictors of exercise adherence in older people living in retirement villages. Prev Med. 2011;52(6):480–1. doi: 10.1016/j.ypmed.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Williamson JD, Espeland M, Kritchevsky SB, Newman AB, King AC, Pahor M, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64(6):688–94. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–65. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 13.Williamson JD, Miller ME, Bryan RN, Lazar RM, Coker LH, Johnson J, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99(12A):112i–22i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48(8):314–8. [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. «Mini-mental state». A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Salthouse TA. The role of memory in the age decline in digit-symbol substitution performance. Journal of gerontology. 1978;33(2):232–8. doi: 10.1093/geronj/33.2.232. [DOI] [PubMed] [Google Scholar]
- 17.Launer LJ, Oudkerk M, Nilsson LG, Alperovitch A, Berger K, Breteler MM, et al. CASCADE: a European collaborative study on vascular determinants of brain lesions. Study design and objectives. Neuroepidemiology. 2000;19(3):113–20. doi: 10.1159/000026246. [DOI] [PubMed] [Google Scholar]
- 18.Estevez-Gonzalez A, Kulisevsky J, Boltes A, Otermin P, Garcia-Sanchez C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: comparison with mild cognitive impairment and normal aging. Int J Geriatr Psychiatry. 2003;18(11):1021–8. doi: 10.1002/gps.1010. [DOI] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Brawley LR, Ambrosius WT, Brubaker PH, Focht BC, Foy CG, et al. Older adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22(4):414–23. doi: 10.1037/0278-6133.22.4.414. [DOI] [PubMed] [Google Scholar]
- 20.Liu CK, Leng X, Hsu FC, Kritchevsky SB, Ding J, Earnest CP, et al. The impact of sarcopenia on a physical activity intervention: the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P) The journal of nutrition, health & aging. 2014;18(1):59–64. doi: 10.1007/s12603-013-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crichton GE, Elias MF, Davey A, Alkerwi A, Dore GA. Higher Cognitive Performance Is Prospectively Associated with Healthy Dietary Choices: The Maine Syracuse Longitudinal Study. The journal of prevention of Alzheimer’s disease. 2015;2(1):24–32. doi: 10.14283/jpad.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubuc MM, Barbat-Artigas S, Karelis AD, Aubertin-Leheudre M. Relationship between the Level of Education and Functional Capacity in Active Elderly Adults. The Journal of frailty & aging. 2014;3(3):148–52. doi: 10.14283/jfa.2014.16. [DOI] [PubMed] [Google Scholar]
- 23.McAuley E, Mullen SP, Szabo AN, White SM, Wojcicki TR, Mailey EL, et al. Self-regulatory processes and exercise adherence in older adults: executive function and self-efficacy effects. American journal of preventive medicine. 2011;41(3):284–90. doi: 10.1016/j.amepre.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fielding RA, Rejeski WJ, Blair S, Church T, Espeland MA, Gill TM, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(11):1226–37. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]


