Abstract
The aim of the present study was to investigate the clinical value of the preoperative neutrophil-to-lymphocyte ratio (NLR) and red blood cell distribution width (RDW) in the peripheral blood of colorectal carcinoma (CRC) patients. Clinical data obtained from 240 patients with CRC undergoing radical surgical resection in Shandong Provincial Hospital Affiliated to Shandong University (Jinan, Shandong, China) between January 2011 and April 2015 were retrospectively analyzed. Data were also collected from 110 patients with colon polyps and 48 healthy volunteers to serve as controls for comparative analysis. The clinicopathological characteristics of the patients in the low and high NLR and RDW groups were compared. The NLR and RDW values were compared prior to and following surgery. Kaplan-Meier analyses and Cox regression modeling were performed to predict overall survival (OS) and disease-free survival (DFS). The NLR and RDW levels in the CRC patients were markedly higher than those in the colon polyp patients and the healthy controls. The optimum NLR and RDW cutoff points for CRC were 2.06 and 13.45%, respectively. Significant differences were detected in tumor location, diameter, degree of differentiation, tumor depth, carcinoembryonic antigen and carbohydrate antigen 199 when comparing the high and low NLR groups (P<0.05). A high RDW was significantly associated with distant metastasis and older age in CRC patients. No significant difference was detected in the NLR and RDW levels of CRC patients prior to and following surgery (P>0.05). CRC patients with an increased RDW had significantly worse OS and DFS rates, particularly those with metastatic CRC (P<0.05). Patients with a high NLR exhibited a reduced DFS time in CRC (P=0.053), although this difference was not significant, and a significantly worse DFS time in metastatic CRC (P=0.047). In conclusion, it is convenient to use preoperative NLR and RDW to predict prognosis following surgery for patients with CRC.
Keywords: neutrophil-to-lymphocyte ratio, red blood cell distribution width, colorectal carcinoma, overall survival, disease-free survival
Introduction
Colorectal cancer (CRC) is one of the major causes of cancer-associated mortality and one of the most curable gastrointestinal cancers (1). It is therefore important to identify all the factors that may serve a role in the diagnosis and prognosis estimation of CRC such that timely diagnosis and treatment decisions may be made. A previous study demonstrated that the number of CRC patients may be reduced through routine screening (2), but the rates of screening for CRC remain low (2–4).
Cancer causes under-nutrition and chronic inflammation, and cancer-related inflammation has been reported to be a crucial factor in cancer progression and cancer-associated survival (5,6). In addition to the pathological characteristics of cancer, other non-cancer factors, including general health condition, may determine the outcomes of patients with cancer (7). The neutrophil-to-lymphocyte ratio (NLR) has been used as an indicator of the inflammatory-related response (8). The NLR is equivalent to the number of neutrophils divided by the number of lymphocytes. An elevated NLR has been reported to be a valuable predictive indicator of various cancer types, including epithelial ovarian, pancreatic, gastric and breast cancer (9–12). The red cell distribution width (RDW) is also a generally used laboratory indicator of inflammatory response (13). The RDW reflects the variation in erythrocyte size, and an increased RDW indicates anisocytosis (14). Previous studies have also demonstrated the role of an increased RDW, which predicts a worse overall survival (OS) rate and an increased disease-specific mortality rate in patients with chronic inflammatory diseases and certain cancer types (15–19), in addition to promoting the progression of cardiovascular and cerebrovascular diseases (20–22). However, few specific studies regarding the predictive value of NLR and RDW in patients with CRC have been reported (23,24). The present study systematically evaluated whether NLR and RDW elevations may serve potential roles as biomarkers of CRC activity. Any associations between the NLR or RDW and histopathological parameters in patients with CRC were identified, any differences in NLR and RDW prior to and following radical surgical resection were determined, and the prognostic importance of NLR and RDW in CRC patients was subsequently evaluated.
Materials and methods
Patients
Clinical data from 240 patients with CRC who had undergone radical surgical resection at Shandong Provincial Hospital Affiliated to Shandong University (Shandong, China) between January 2011 and April 2015 were analyzed retrospectively. Data from 110 patients with colon polyps and 48 healthy volunteers were also collected to serve as controls for comparative analysis. Following radical resection, patients were histopathologically diagnosed by two pathologists. Patients with CRC were enrolled according to the following inclusive criteria: CRC diagnosed by histopathology (the invasion of the mucosal muscle layer by cancer cells), radical resection under a microscope, blood parameters, and clinicopathological and follow-up results. Patients with anemia, hematological disorders, active infectious diseases, venous thrombosis diagnosed within the last 6 months, a history of blood transfusion within the last 3 months, a treatment history of asiderosis, hypertension, cardiac failure, autoimmune disorders and a history of other malignancies were excluded from the study. Blood parameters were detected within 1 week prior to surgery and 3 weeks after surgery. The complete blood count (including NLR and RDW) was detected using a hematology analyzer XE-2100 (Sysmex Corporation, Kobe, Japan). Tumor markers [carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9)] were measured within 1 month prior to surgery. CEA and CA19-9 were analyzed using a Cobas e601 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The high and low values of NLR or RDW were compared in terms of the CRC location, tumor diameter, Tumor-Node-Metastasis (TNM) stage (25), degree of differentiation, sex, age and tumor markers, as well as changes in the NLR and RDW prior to and following radical surgical resection. A total of 128 patients, including 54 patients with metastatic CRC, were followed up regularly through telephone interviews and patients received their last follow-up in June 2016. The first research end-point was OS time, which was defined as the time from the date of surgery to mortality from any cause. The second study end-point was disease-free survival (DFS) time, which was defined as the time from the date of surgery to the date of identification of disease recurrence, either radiological or histological. This study was approved by the Ethics Committee of the Shandong Provincial Hospital Affiliated to Shandong University. Written informed consent was obtained from all participants.
Statistical analysis
SPSS statistical software version 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. All parameters are normally distributed and presented as the mean ± standard deviation (SD). Categorical variables are presented as frequencies. Receiver operating characteristic (ROC) curve analysis was used to determine the cutoff values for the NLR and the RDW, and to calculate the Youden index (YI), which was used to identify the optimal cutoff values for the NLR and the RDW (2.06 and 13.45%, respectively). All cases were divided into high and low NLR or RDW groups in terms of these cutoff values. Comparison between groups was evaluated using one-way analysis of variance and unpaired Student's t-tests. Multiple comparison between the groups was performed using Student-Newman-Keuls method. A χ2 test or Fisher's exact test was performed with ≥1 variables (<5) to analyze the differences between high and low NLR and RDW groups in terms of each clinicopathological characteristic. Logistic regression analysis was conducted to evaluate the clinicopathological factors that likely caused the increased NLR and RDW. Comparisons between NLR and RDW values prior to and following surgery were made using the Wilcoxon matched-pairs signed rank test. Kaplan-Meier analysis was used to calculate the OS and DFS time and the log-rank test was used to compare the survival rate curves. Significant indicators for survival determined in univariate analysis were introduced into the multivariate Cox proportional hazards model to establish independent prognostic indicators. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison between groups in terms of the RDW and NLR values
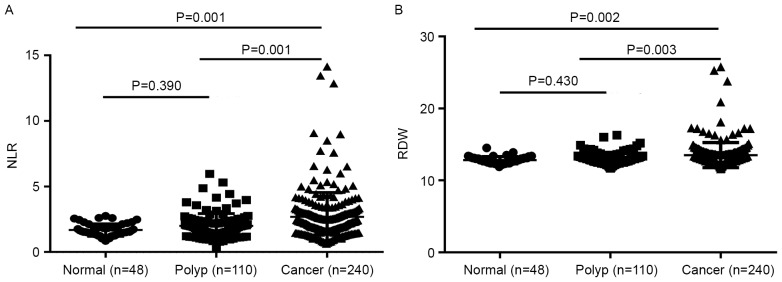
The mean ± SD of the NLR in the CRC, colon polyps and healthy control groups was 2.81±2.60, 1.99±0.94 and 1.68±0.48, respectively. The NLR in the CRC patients was significantly higher compared with that in the colon polyps patients and the healthy controls (P=0.001 and P=0.001, respectively). The colon polyps group demonstrated no significant difference in NLR compared with the healthy control group (P=0.390) (Fig. 1A). The mean ± SD of RDW in the CRC, colon polyps and healthy control groups were 13.51±1.74, 13.02±0.81 and 12.82±0.47%, respectively. The RDW in the CRC group was increased significantly compared with that of the colon polyps and the healthy control groups (P=0.003 and P=0.002, respectively). No significant difference in RDW was observed between the colon polyps patients and the healthy controls (P=0.430) (Fig. 1B).
Figure 1.
Comparison of groups in terms of (A) NLR values and (B) RDW values. Comparisons between the 3 groups were performed using analysis of variance. Data are expressed as the mean ± standard deviation. NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width.
Association between NLR or RDW and clinicopathological characteristics
The area under the curve of the NLR was 0.642 [95% confidence interval (CI), 0.582–0.703; P<0.001], and that of the RDW was 0.601 (95% CI, 0.539–0.663; P<0.001) (Fig. 2). When the YI was at its maximum (YI=0.256), the NLR was 2.06, the sensitivity was 58.3% and the specificity was 67.3%, revealing that the optimal cutoff value for NLR was 2.06. Thus, patients with CRC were divided into high NLR (≥2.06) and low NLR (<2.06) groups. When the RDW was 13.45%, the sensitivity was 38.8%, the specificity was 80.9% and the YI was at its maximum (YI=0.197). The patients with CRC were then divided into high RDW (≥13.45%) and low RDW (<13.45%) groups. The clinicopathological characteristics of the 2 groups were compared in terms of the NLR and the RDW (Table I). The high NLR group was associated with tumor location (colon), a larger tumor diameter, poor tumor differentiation, deeper tumor infiltration, and high CEA and CA19-9 levels (P<0.05). A high RDW was revealed to be associated with older age and distant metastases (P<0.05). The clinicopathological factors that likely caused the increased NLR and RDW were also evaluated following evaluation of other factors using logistic regression analysis. The results demonstrated that a larger tumor diameter and poor tumor differentiation were independent risk factors for increased NLR (P<0.05), while older age and distant metastases were independent risk factors for increased RDW (P<0.05) (Table II).
Figure 2.
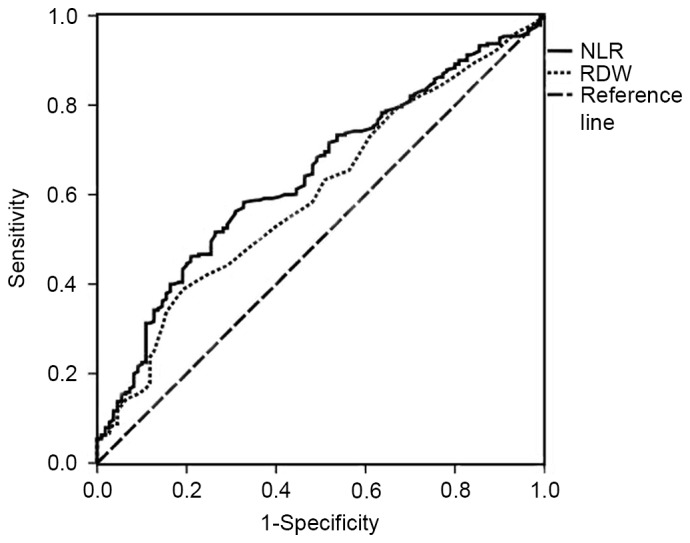
ROC curves grouped by NLR and RDW. The ROC for NLR is represented by a full line with an AUC of 0.642, sensitivity of 58.3% and specificity of 67.3% (P<0.001) and the ROC for the RDW is represented by a dotted line with an AUC of 0.601, sensitivity of 38.8% and specificity of 80.9% (P=0.002). ROC, receiver operating characteristic; NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; AUC, area under the curve.
Table I.
Association between NLR and RDW, and clinicopathological characteristics.
| Characteristics | Cases, n | NLR<2.06, n (%) | NLR≥2.06, n (%) | P-value | RDW<13.45, n (%) | RDW≥13.45, n (%) | P-value |
|---|---|---|---|---|---|---|---|
| Sex | 0.224 | 0.622 | |||||
| Male | 167 | 66 (65.3) | 101 (72.7) | 104 (70.7) | 63 (67.7) | ||
| Female | 73 | 35 (34.7) | 38 (27.3) | 43 (29.3) | 30 (32.3) | ||
| Age, years | 0.676 | 0.022 | |||||
| <60 | 94 | 38 (37.6) | 56 (40.3) | 66 (44.9) | 28 (30.1) | ||
| ≥60 | 146 | 63 (62.4) | 83 (59.7) | 81 (55.1) | 65 (69.9) | ||
| Tumor location | 0.046 | 0.457 | |||||
| Colon | 199 | 78 (77.2) | 121 (87.1) | 124 (84.4) | 75 (80.6) | ||
| Rectum | 41 | 23 (22.8) | 18 (12.9) | 23 (15.6) | 18 (19.4) | ||
| Tumor diameter, cm | <0.001 | 0.442 | |||||
| ≤4 | 95 | 55 (64.0) | 40 (33.9) | 65 (48.5) | 30 (42.9) | ||
| >4 | 109 | 31 (36.0) | 78 (66.1) | 69 (51.5) | 40 (57.1) | ||
| Differentiation | 0.004 | 0.799 | |||||
| Well | 7 | 2 (2.0) | 5 (3.6) | 5 (3.4) | 2 (2.2) | ||
| Moderate | 167 | 82 (81.2) | 85 (61.1) | 103 (70.1) | 64 (68.8) | ||
| Poor | 66 | 17 (16.8) | 49 (35.3) | 39 (26.5) | 27 (29.0) | ||
| Tumor depth | 0.012 | 0.634 | |||||
| T1 | 5 | 5 (5.0) | 0 (0.0) | 4 (2.7) | 1 (1.1) | ||
| T2 | 25 | 7 (6.9) | 18 (12.9) | 13 (8.8) | 12 (12.9) | ||
| T3 | 51 | 26 (25.7) | 25 (18.0) | 31 (21.1) | 20 (21.5) | ||
| T4 | 159 | 63 (62.4) | 96 (69.1) | 99 (67.3) | 60 (64.5) | ||
| Lymph node metastasis | 0.210 | 0.527 | |||||
| N0 | 119 | 44 (43.6) | 75 (54.0) | 77 (52.4) | 42 (45.2) | ||
| N1 | 66 | 29 (28.7) | 37 (26.6) | 39 (26.5) | 27 (29.0) | ||
| N2 | 55 | 28 (27.7) | 27 (19.4) | 31 (21.1) | 24 (25.8) | ||
| Distant metastasis | 0.395 | 0.023 | |||||
| M0 | 219 | 94 (93.1) | 125 (89.9) | 139 (94.6) | 80 (86.0) | ||
| M1 | 21 | 7 (6.9) | 14 (10.1) | 8 (5.4) | 13 (14.0) | ||
| pStage | 0.117 | 0.109 | |||||
| I | 22 | 10 (9.9) | 12 (8.6) | 15 (10.2) | 7 (7.5) | ||
| II | 91 | 31 (30.7) | 60 (43.2) | 60 (40.8) | 31 (33.3) | ||
| III | 106 | 53 (52.5) | 53 (38.1) | 64 (43.5) | 42 (45.2) | ||
| IV | 21 | 7 (6.9) | 14 (10.1) | 8 (5.4) | 13 (14.0) | ||
| CEA, ng/ml | 0.045 | 0.881 | |||||
| ≤5 | 100 | 49 (62.0) | 51 (47.2) | 61 (53.0) | 39 (54.2) | ||
| >5 | 87 | 30 (38.0) | 57 (52.8) | 54 (47.0) | 33 (45.8) | ||
| CA19-9, U/ml | 0.030 | 0.142 | |||||
| ≤39 | 155 | 71 (89.9) | 84 (77.8) | 99 (86.1) | 56 (77.8) | ||
| >39 | 32 | 8 (10.1) | 24 (22.2) | 16 (13.9) | 16 (22.2) |
P<0.05 was considered to indicate a statistically significant difference. Total patient numbers vary as pathological data regarding tumor diameter was only available from 204 patients and only 187 patients were examined for tumor markers, CEA and CA19-9. NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Table II.
Logistic regression analysis of NLR- and RDW-associated risk factors.
| NLR | RDW | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | B | SE | Wald | HR (95% CI) | P-value | B | SE | Wald | HR (95% CI) | P-value |
| Sex (male/female) | −0.182 | 0.453 | 0.160 | 0.834 (0.343–2.028) | 0.689 | 0.004 | 0.427 | 0.000 | 1.004 (0.435–2.318) | 0.992 |
| Age, years (<60/≥60) | 0.460 | 0.419 | 1.205 | 1.584 (0.697–3.602) | 0.272 | 0.669 | 0.285 | 5.526 | 1.953 (1.118–3.412) | 0.019 |
| Tumor location (colon/rectum) | −1.013 | 0.573 | 3.127 | 0.363 (0.118–1.116) | 0.077 | 0.022 | 0.526 | 0.002 | 1.022 (0.365–2.867) | 0.966 |
| Tumor diameter, cm (≤4/>4) | 1.793 | 0.430 | 17.375 | 6.010 (2.586–13.966) | <0.001 | 0.289 | 0.388 | 0.553 | 1.335 (0.623–2.859) | 0.457 |
| Differentiation (well+moderate/poor) | 0.501 | 0.237 | 4.465 | 1.651 (1.037–2.628) | 0.035 | 0.008 | 0.210 | 0.001 | 1.008 (0.667–1.522) | 0.971 |
| Depth of tumor (T1+T2/T3+T4) | −0.157 | 0.438 | 0.128 | 0.855 (0.362–2.017) | 0.721 | 0.041 | 0.623 | 0.004 | 1.042 (0.307–3.537) | 0.947 |
| Lymph node metastasis (N0/N1+N2) | 0.365 | 0.964 | 0.143 | 1.440 (0.218–9.522) | 0.705 | −1.026 | 1.511 | 0.461 | 0.358 (0.019–6.929) | 0.497 |
| Distant metastasis (M0/M1) | 0.631 | 0.591 | 1.140 | 1.879 (0.590–5.981) | 0.286 | 1.093 | 0.478 | 5.222 | 2.983 (1.168–7.615) | 0.022 |
| pStage (I+II/III+IV) | −1.041 | 0.999 | 1.085 | 0.353 (0.050–2.503) | 0.298 | 0.925 | 1.524 | 0.368 | 2.523 (0.127–50.060) | 0.544 |
| CEA, ng/ml (≤5/>5) | 0.666 | 0.417 | 2.545 | 1.946 (0.859–4.410) | 0.111 | −0.172 | 0.382 | 0.201 | 0.842 (0.397–1.785) | 0.654 |
| CA19-9, U/ml (≤39/>39) | 0.520 | 0.667 | 0.607 | 1.682 (0.455–6.223) | 0.436 | 0.415 | 0.557 | 0.555 | 1.514 (0.509–4.505) | 0.456 |
P<0.05 was considered to indicate a statistically significant difference. NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; B, partial regression coefficient; SE, standard error; Wald, square of B/SE.
Differences in the NLR and RDW prior to and following surgery
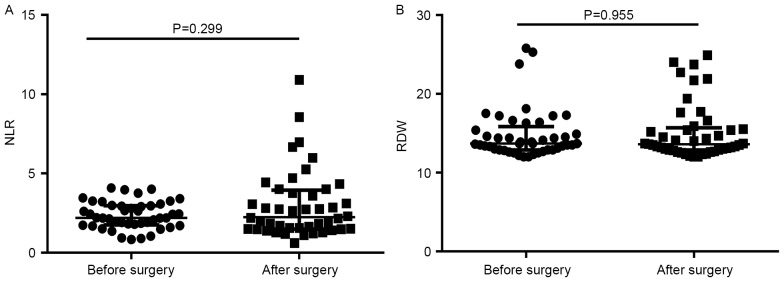
Differences in the NLR and RDW prior to and following surgery were analyzed in 45 patients with CRC. The median (interquartile range) values of the NLR and RDW prior to surgery were 2.20 (1.765–3.025) and 13.7% (12.90–15.85%), respectively, and following surgery were 2.30 (1.500–4.005) and 13.6% (12.85–15.70%), respectively. No significant differences in the NLR or RDW were observed prior to and following surgery (P=0.299 and P=0.955, respectively) (Fig. 3). Furthermore, the patients were divided into 2 groups according to the change in NLR and RDW values (the value following surgery divided by the value prior to surgery): NLR <1 and ≥1, and RDW <1 and ≥1. The association between the two groups and TNM stage was analyzed. These results demonstrated that no apparent association existed between changes in the NLR or RDW and TNM stage (Table III).
Figure 3.
Differences in the (A) NLR and (B) RDW values prior to and following surgery. Comparisons were made using the Wilcoxon matched-pairs signed rank test. Data are expressed as the median with the interquartile range. NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width.
Table III.
Association between the change in NLR and RDW values following surgery compared with those prior to surgery and TNM stage.
| Change in NLR | Change in RDW | |||||
|---|---|---|---|---|---|---|
| Characteristics | <1 (n=23) | ≥1 (n=22) | P-value | <1 (n=24) | ≥1 (n=21) | P-value |
| Depth of tumor | 0.699 | 0.443 | ||||
| T1+T2 | 5 (21.7) | 3 (13.6) | 3 (12.5) | 5 (23.8) | ||
| T3+T4 | 18 (78.3) | 19 (86.4) | 21 (87.5) | 16 (76.2) | ||
| Lymph node metastasis | 0.772 | 0.469 | ||||
| N0 | 4 (17.4) | 5 (22.7) | 6 (25.0) | 3 (14.3) | ||
| N1+N2 | 19 (82.6) | 17 (77.3) | 18 (75.0) | 18 (85.7) | ||
| Distance metastasis | 0.092 | 1.000 | ||||
| M0 | 18 (78.3) | 12 (54.5) | 16 (66.7) | 14 (66.7) | ||
| M1 | 5 (21.7) | 10 (54.5) | 8 (33.3) | 7 (33.3) | ||
| pStage | 1.000 | 1.000 | ||||
| I+II | 4 (17.4) | 3 (13.6) | 4 (16.7) | 3 (14.3) | ||
| III+IV | 19 (82.6) | 19 (86.4) | 20 (83.3) | 18 (85.7) | ||
NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; TNM, Tumor-Node-Metastasis.
Survival analysis of prognostic factors
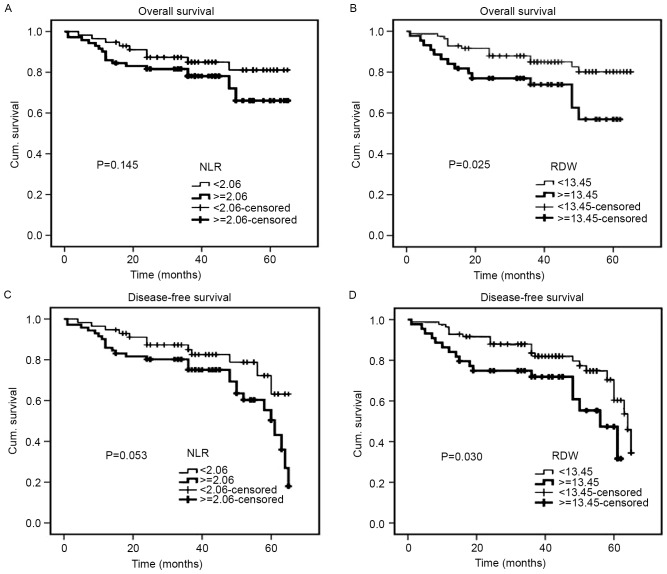
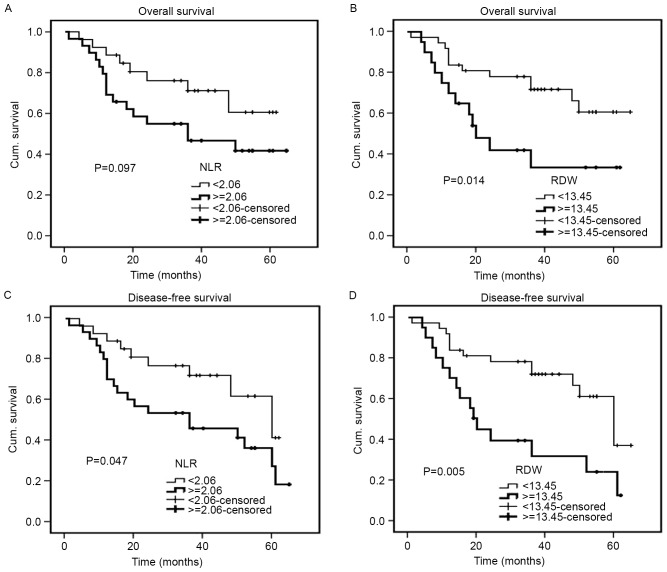
The median follow-up duration was 40 months (range, 1–65 months). The Kaplan-Meier cumulative survival curves for 128 patients with CRC are presented (Fig. 4). The mean OS time was 57.3 months in the low RDW group (95% CI, 53.5–61.1) and 46.9 months in the high RDW group (95% CI, 40.3–53.6). In addition, the mean DFS time was 55.1 months in the low RDW group (95% CI, 51.2–59.0) and 45.4 months in the high RDW group (95% CI, 38.8–52.0). The high RDW group exhibited an unfavorable OS time (P=0.025) and a shorter DFS time (P=0.030) compared with the low RDW group (Fig. 4B and D). With regards to the NLR, the mean OS and DFS times were 57.4 months and 55.7 months, respectively, in the low NLR group (95% CI, 52.8–62.0 and 50.9–60.4, respectively), and 52.0 months and 49.4 months, respectively, in the high NLR group (95% CI, 46.9–57.1 and 44.3–54.5, respectively). Therefore, the NLR was not significantly associated with OS or DFS (P=0.145 and P=0.053, respectively) (Fig. 4A and C). For 54 patients with metastatic CRC, an increased RDW was associated with a significantly shorter OS (P=0.014) and DFS (P=0.005), while an increased NLR was only associated with a significantly shorter DFS (P=0.047) (Fig. 5). For 128 patients with CRC, univariate analysis revealed that OS was significantly associated with RDW (P=0.030), degree of differentiation (P<0.001), pStage (P<0.001) (25), depth of tumor (P=0.028), lymph node metastasis (P<0.001), distant metastasis (P<0.001), CEA (P=0.031) and CA19-9 (P=0.008), while DFS demonstrated a similar association with RDW (P=0.035), tumor diameter (P=0.042), degree of differentiation (P<0.001), pStage (P<0.001), depth of tumor (P=0.009), lymph node metastasis (P=0.001), distant metastasis (P<0.001) and CEA (P=0.011). The multivariate analyses revealed that the degree of differentiation (P=0.003) and lymph node metastasis (P=0.001) may act as independent prognostic indicators of OS and DFS (Table IV). For 54 patients with metastatic CRC, univariate analysis revealed that OS was significantly associated with RDW (P=0.019), degree of differentiation (P=0.008), pStage (P=0.012), distant metastasis (P=0.012) and CA19-9 (P=0.046), while DFS exhibited a similar association with RDW (P=0.007), degree of differentiation (P=0.005), pStage (P=0.014), distant metastasis (P=0.014), CEA (P=0.041) and CA19-9 (P=0.037). The multivariate analyses revealed that RDW, the degree of differentiation and CA19-9 may act as independent prognostic indicators of OS and DFS in patients with metastatic CRC (Table V).
Figure 4.
Kaplan-Meier curves for OS and DFS of 128 patients with colorectal carcinoma. OS determined by (A) NLR and (B) RDW, and DFS determined by (C) NLR and (D) RDW. OS, overall survival; DFS, disease-free survival; NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; Cum, cumulative.
Figure 5.
Kaplan-Meier curves for OS and DFS of 54 patients with metastatic colorectal carcinoma. OS determined by (A) NLR and (B) RDW, and DFS determined by (C) NLR and (D) RDW. OS, overall survival; DFS, disease-free survival; NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; Cum, cumulative.
Table IV.
Univariate and multivariate Cox regression analyses for OS and DFS in 128 patients with colorectal cancer.
| Univariate (OS) | Multivariate (OS) | Univariate (DFS) | Multivariate (DFS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| NLR (<2.06/≥2.06) | 1.781 | 0.806–3.938 | 0.154 | 1.923 | 0.976–3.788 | 0.059 | ||||||
| RDW (<13.45/≥13.45) | 2.273 | 1.082–4.774 | 0.030 | 2.011 | 1.052–3.846 | 0.035 | ||||||
| Sex (male/female) | 1.672 | 0.781–3.579 | 0.186 | 1.561 | 0.811–3.007 | 0.183 | ||||||
| Age, years (<60/≥60) | 1.974 | 0.839–4.647 | 0.119 | 1.312 | 0.676–2.548 | 0.422 | ||||||
| Tumor location (colon/rectum) | 1.529 | 0.619–3.776 | 0.357 | 1.439 | 0.629–3.292 | 0.388 | ||||||
| Tumor diameter, cm (≤4/>4) | 2.313 | 0.302–17.683 | 0.419 | 4.686 | 1.057–20.777 | 0.042 | ||||||
| Differentiation (well/moderate/poor) | 4.534 | 2.132–9.641 | <0.001 | 4.398 | 1.631–11.860 | 0.003 | 3.544 | 1.907–6.588 | <0.001 | 3.887 | 1.649–9.165 | 0.002 |
| Depth of tumor (T1/T2/T3/T4) | 3.991 | 1.161–13.715 | 0.028 | 3.067 | 1.320–7.128 | 0.009 | ||||||
| Lymph node metastasis (N0/N1/N2) | 2.364 | 1.530–3.654 | <0.001 | 2.734 | 1.521–4.914 | 0.001 | 1.850 | 1.299–2.634 | 0.001 | 1.817 | 1.112–2.968 | 0.017 |
| Distant metastasis (M0/M1) | 8.282 | 2.823–24.302 | <0.001 | 6.633 | 2.527–17.407 | <0.001 | ||||||
| pStage (I/II/III/IV) | 3.861 | 2.118–7.041 | <0.001 | 2.377 | 1.712–4.364 | <0.001 | ||||||
| CEA, ng/ml (≤5/>5) | 2.900 | 1.101–7.637 | 0.031 | 2.849 | 1.268–6.402 | 0.011 | ||||||
| CA19-9, U/ml (≤39/>39) | 4.041 | 1.452–11.250 | 0.008 | 2.205 | 0.823–5.911 | 0.116 | ||||||
Univariate analysis was performed using the Kaplan-Meier analysis model and the log-rank test and values of P<0.05 in the univariate analysis were entered into a multivariate analysis, which was performed using the Cox proportional hazards models with the forward likelihood method. OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Table V.
Univariate and multivariable Cox regression analyses for OS and DFS in 54 patients with metastatic colorectal cancer.
| Univariate (OS) | Multivariate (OS) | Univariate (DFS) | Multivariate (DFS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| NLR (<2.06/≥2.06) | 2.012 | 0.859–4.712 | 0.107 | 2.158 | 0.980–4.752 | 0.056 | ||||||
| RDW (<13.45/≥13.45) | 2.628 | 1.170–5.901 | 0.019 | 2.755 | 1.194–6.361 | 0.018 | 2.742 | 1.313–5.724 | 0.007 | 2.684 | 1.258–5.725 | 0.011 |
| Sex (male/female) | 1.419 | 0.629–3.199 | 0.399 | 1.606 | 0.771–3.346 | 0.206 | ||||||
| Age, years (<60/≥60) | 1.781 | 0.705–4.499 | 0.222 | 1.993 | 0.847–4.691 | 0.114 | ||||||
| Tumor location (colon/rectum) | 1.692 | 0.619–4.623 | 0.305 | 1.603 | 0.591–4.350 | 0.354 | ||||||
| Tumor diameter, cm (≤4/>4) | 1.383 | 0.180–10.644 | 0.755 | 2.811 | 0.629–12.573 | 0.176 | ||||||
| Differentiation (well/moderate/poor) | 3.187 | 1.357–7.481 | 0.008 | 2.933 | 1.239–6.947 | 0.014 | 3.017 | 1.397–6.514 | 0.005 | 2.605 | 1.193–5.686 | 0.016 |
| Depth of tumor (T1/T2/T3/T4) | 1.263 | 0.370–4.307 | 0.709 | 0.958 | 0.355–2.585 | 0.933 | ||||||
| Lymph node metastasis (N0/N1/N2) | 1.265 | 0.574–2.785 | 0.560 | 1.196 | 0.576–2.483 | 0.631 | ||||||
| Distant metastasis (M0/M1) | 4.065 | 1.365–12.111 | 0.012 | 3.472 | 1.281–9.415 | 0.014 | ||||||
| pStage (I/II/III/IV) | 4.065 | 1.365–12.111 | 0.012 | 3.472 | 1.281–9.415 | 0.014 | ||||||
| CEA, ng/ml (≤5/>5) | 1.578 | 0.950–2.621 | 0.078 | 1.600 | 1.020–2.509 | 0.041 | ||||||
| CA19-9, U/ml (≤39/>39) | 1.906 | 1.011–3.595 | 0.046 | 2.240 | 1.180–4.252 | 0.014 | 1.883 | 1.039–3.411 | 0.037 | 2.061 | 1.139–3.731 | 0.017 |
Univariate analysis was performed using the Kaplan-Meier analysis model and the log-rank test and values of P<0.05 in the univariate analysis were entered into a multivariate analysis, which was performed using the Cox proportional hazards models with the forward likelihood method. OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Discussion
CRC is one of the most common cancers and the fourth highest cause of cancer-associated mortality in the world (26). In China, with the increasing number of risk factors, including an aging population and changes in dietary habits (e.g., reduced fiber intake), CRC has become the fifth most common cancer in the country (27).
Tumor-associated inflammatory cytokines and mediators may mediate inflammatory responses, which lead to tumor growth, infiltration and metastasis (28). Previous studies have demonstrated that blood parameters, including C-reactive protein, albumin, hemoglobin, mean corpuscular volume, NLR, white blood cell count and RDW, are significantly associated with the host inflammatory response and the poorer nutritional status induced by numerous types of cancer, partially through acting as predictors of disease progression and prognosis (29–31). Firstly, elevated neutrophils facilitate tumor proliferation, migration and vasculogenesis. Secondly, lymphocytes can promote cytotoxic cell activation and cytokine production, which inhibit tumor proliferation and migration. Thus, low lymphocyte levels destroy the antitumor immune response and result in a poorer prognosis. Therefore, NLR reflects the balance between the pro-tumor inflammatory response and the antitumor immune response (32). Furthermore, several studies have reported that an increased preoperative NLR is associated with the activated inflammatory response, advanced stage and poorer survival in patients with CRC, non-small cell lung cancer and hepatocellular carcinoma (23,33–35). RDW has been used as an early indicator of increased oxidative stress and disorders in iron deficiency anemia and iron mobilization, and its increase is associated with inflammation markers, including C-reactive protein and interleukin-6 (36,37). Despite previous studies that have evaluated the clinical value of RDW as a prognostic indicator in patients with impaired cardiometabolic function and active inflammation, there is limited data available concerning the potential use of RDW as a biomarker of cancer growth and metastatic activity in solid cancer types (20,36). A previous study indicated that a high preoperative RDW could be used to predict the long-term survival rate of patients with lung cancer (38). Another study reported that for patients with symptomatic multiple myeloma, a preoperative increase in RDW may reflect worse progression-free survival (39). Albayrak et al (19) demonstrated that an increased RDW was significantly associated with an elevated risk of progressing into advanced prostate cancer. RDW has been gradually used to predict inflammatory status and tumor stress.
Therefore, in the present study, the clinical value of NLR and RDW in patients with CRC was detected. Karaman et al (40) reported that NLR may be used to distinguish neoplastic from non-neoplastic colon polyps, as the NLR was revealed to be elevated in neoplastic polyps. Ay et al (24) observed that a significantly higher RDW was detected in patients with CRC compared with that in individuals with colon polyps. In the present study, the NLR and RDW values were higher in patients with CRC compared with those in patients with colon polyps and healthy controls, which is consistent with the results of the aforementioned study. At present, the mechanism underlying this effect has not been confirmed. It is generally believed that the onset of CRC begins with an infection or an inflammatory response. NLR and RDW are sensitive indicators that reflect the activation of the inflammatory system and are involved in the inflammatory response (41). Neutrophils remodel the extracellular matrix to promote tumor growth and invasion, and inhibit lymphocytes from killing the malignant tumor cells (42). RDW is a sensitive and specific indicator of early iron deficiency and malnutrition in CRC (43). Therefore, when the NLR and the RDW are elevated in CRC, the body's defense mechanism is weakened and the barrier against malignant cells is destroyed, ultimately leading to a poor survival prognosis. This concept is consistent with the results of the present study.
In the present study, the cutoff values for NLR and RDW were determined to be 2.06 and 13.45%, respectively, using the ROC curve. CRC patients with an elevated NLR (NLR ≥2.06) appeared to exhibit more clinicopathological characteristics associated with advanced conditions, including a larger tumor diameter, poor tumor differentiation, deeper tumor infiltration, and high CEA and CA19-9 levels (P<0.05). A higher RDW was also detected in patients with clinicopathological features associated with advanced conditions, including older age and distant metastasis (P<0.05). Furthermore, the logistic regression analysis revealed that a larger tumor diameter and poor tumor differentiation were independent risk factors associated with an increased NLR (P<0.05), while older age and distant metastases were independent risk factors associated with an increased RDW (P<0.05).
Few studies have compared the changes in the NLR and RDW prior to and following surgery; however, no significant difference was observed and there was no apparent association between the changes in the NLR and RDW and TNM stage in the present study.
The Kaplan-Meier cumulative survival rates for OS and DFS demonstrated that a high RDW value indicated significantly shorter OS and DFS times in the 128 CRC patients and in the 54 patients with metastases. The high NLR group had no association with OS or DFS in the 128 patients with CRC. Furthermore, an increased NLR was indicative of a significantly shorter DFS time for the 54 patients with metastatic CRC. These results revealed that RDW serves an important role in predicting the survival of patients with CRC, particularly those with metastatic CRC.
Furthermore, univariate and multivariate analyses indicated that, for CRC, only lymph node metastasis and the degree of differentiation were independent prognostic indicators for OS and DFS. The potential use of NLR and RDW as independent prognostic indicators for CRC was not demonstrated in the present study. Patients with metastasis were analyzed separately in order to reduce bias and it was revealed that for metastatic CRC, RDW may act as an independent prognostic indicator for OS and DFS, which has also been confirmed in previous studies. Zou et al (41) reported that the NLR acted as an independent prognostic indicator in patients with CRC. Furthermore, Malietzis et al (44) demonstrated that the preoperative NLR could be an independent prognostic indicator for patients with CRC. Shibutani et al (45) reported that the preoperative NLR was a simple biomarker and indicator of poor prognosis for CRC following surgery. Zhao et al (46) detected that patients with hepatocellular carcinoma exhibiting high preoperative RDW values had significantly poorer survival compared with those with low levels of RDW.
There were a number of limitations to the present study. As is the case for the majority of retrospective studies, there may have been unavoidable errors in the data collection. In addition, the number of subjects in the present study was relatively small and the follow-up duration was not that long. Thus, the findings of this study should be validated in further investigations with larger subject sizes and longer follow-up durations.
Pre-operative NLR and RDW values are simple and conveniently measured biomarkers of clinical diagnosis and prognostic assessment in patients with CRC. NLR and RDW may function as novel indicators that precisely predict the prognosis in patients with CRC, particularly in patients with metastatic CRC.
Acknowledgements
This study was supported by grants obtained from the National Natural Science Foundation of China (grant nos. 81000731 and 81202307) and the Promoted Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (grant no. BS2010YY045).
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale - Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 3.Byers T, Levin B, Rothenberger D, Dodd GD, Smith RA. American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer: Update 1997. American Cancer Society Detection and Treatment Advisory Group on Colorectal Cancer. CA Cancer J Clin. 1997;47:154–160. doi: 10.3322/canjclin.47.3.154. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: Screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–877. doi: 10.1016/S0002-9270(00)00851-0. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. (In English) [PubMed] [Google Scholar]
- 9.Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J Gastroenterol. 2015;21:2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 12.Aliustaoglu M, Bilici A, Ustaalioglu BB, Konya V, Gucun M, Seker M, Gumus M. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;27:1060–1065. doi: 10.1007/s12032-009-9335-4. [DOI] [PubMed] [Google Scholar]
- 13.Yesil A, Senates E, Bayoğlu IV, Erdem ED, Demirtunc R, Kurdas OA. Red cell distribution width: A novel marker of activity in inflammatory bowel disease. Gut Liver. 2011;5:460–467. doi: 10.5009/gnl.2011.5.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Jánoskuti L. Red cell distribution width in heart failure: Prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function and nutritional state. Am Heart J. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Lorente L, Martin MM, Abreu-Gonzalez P, Solé-Violán J, Ferreres J, Labarta L, Díaz C, González O, García D, Jiménez A, Borreguero-León JM. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PLoS One. 2014;9:e105436. doi: 10.1371/journal.pone.0105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang R, Yang C, Wu K, Cao S, Liu Y, Su R, Xiong Y, Huang A, Wu C. Red cell distribution width as a potential index to assess the severity of hepatitis B virus-related liver diseases. Hepatol Res. 2014;44:E464–E470. doi: 10.1111/hepr.12342. [DOI] [PubMed] [Google Scholar]
- 17.Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Matsumoto Y, Koyama M, Okada N, Nakashima N, Masuya D, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 2013;8:e80240. doi: 10.1371/journal.pone.0080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirne C, Grossi G, Pinato DJ, Burlone ME, Mauri FA, Januszewski A, Oldani A, Minisini R, Sharma R, Pirisi M. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis. 2015;47:488–494. doi: 10.1016/j.dld.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Albayrak S, Zengin K, Tanik S, Bakirtas H, Imamoglu A, Gurdal M. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev. 2014;15:7781–7784. doi: 10.7314/APJCP.2014.15.18.7781. [DOI] [PubMed] [Google Scholar]
- 20.Söderholm M, Borné Y, Hedblad B, Persson M, Engström G. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: A population-based cohort study. PLoS One. 2015;10:e124957. doi: 10.1371/journal.pone.0124957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin I, Karabulut A, Kaya A, Güngör B, Avcı İİ, Okuyan E, Can MM, Sığırcı S, Ayça B, Dinçkal MH. Increased level of red cell distribution width is associated with poor coronary collateral circulation in patients with stable coronary artery disease. Turk Kardiyol Dern Ars. 2015;43:123–130. doi: 10.5543/tkda.2015.24819. [DOI] [PubMed] [Google Scholar]
- 22.Huang YL, Hu ZD, Liu SJ, Sun Y, Qin Q, Qin BD, Zhang WW, Zhang JR, Zhong RQ, Deng AM. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS One. 2014;9:e104861. doi: 10.1371/journal.pone.0104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088–1097. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ay S, Eryilmaz MA, Aksoy N, Okus A, Unlu Y, Sevinc B. Is early detection of colon cancer possible with red blood cell distribution width? Asian Pac J Cancer Prev. 2015;16:753–756. doi: 10.7314/APJCP.2015.16.2.753. [DOI] [PubMed] [Google Scholar]
- 25.Wittekind C. 2010 TNM system: On the 7th edition of TNM classification of malignant tumors. Pathologe. 2010;31:331–332. doi: 10.1007/s00292-010-1349-3. (In German) [DOI] [PubMed] [Google Scholar]
- 26.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 27.Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40:281–285. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 29.Song ZB, Lin BC, Li B, He CX, Zhang BB, Shao L, Zhang YP. Preoperative elevation of serum C-reactive protein as an indicator of poor prognosis for early-stage esophageal squamous cell carcinoma. Kaohsiung J Med Sci. 2013;29:662–666. doi: 10.1016/j.kjms.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang LJ, Fu JH, Wang JY. Prognostic value of preoperative mean corpuscular volume in esophageal squamous cell carcinoma. World J Gastroenterol. 2013;19:2811–2817. doi: 10.3748/wjg.v19.i18.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan D, Zhu K, Li K, Yan R, Jia Y, Dang C. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol. 2014;110:333–340. doi: 10.1002/jso.23651. [DOI] [PubMed] [Google Scholar]
- 32.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 33.Kayadibi H, Sertoglu E, Uyanik M, Tapan S. Neutrophil-lymphocyte ratio is useful for the prognosis of patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:9631–9632. doi: 10.3748/wjg.v20.i28.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu J, Yue D, Zhang B, Wang C. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: Based on a large cohort study. PLoS One. 2015;10:e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141:4395–4402. [PubMed] [Google Scholar]
- 36.Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: Results from the national health and nutrition examination survey. Indian Heart J. 2012;64:380–387. doi: 10.1016/j.ihj.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karabulut A, Uzunlar B. Correlation between red cell distribution width and coronary ectasia in the acute myocardial infarction. Clin Appl Thromb Hemost. 2012;18:551–552. doi: 10.1177/1076029611436198. [DOI] [PubMed] [Google Scholar]
- 38.Warwick R, Mediratta N, Shackcloth M, Shaw M, McShane J, Poullis M. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:108–113. doi: 10.1093/ejcts/ezt275. [DOI] [PubMed] [Google Scholar]
- 39.Lee H, Kong SY, Sohn JY, Shim H, Youn HS, Lee S, Kim HJ, Eom HS. Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int. 2014;2014:145619. doi: 10.1155/2014/145619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karaman H, Karaman A, Erden A, Poyrazoglu OK, Karakukcu C, Tasdemir A. Relationship between colonic polyp type and the neutrophil/lymphocyte ratio as a biomarker. Asian Pac J Cancer Prev. 2013;14:3159–3161. doi: 10.7314/APJCP.2013.14.5.3159. [DOI] [PubMed] [Google Scholar]
- 41.Zou ZY, Liu HL, Ning N, Li SY, DU XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11:2241–2248. doi: 10.3892/ol.2016.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emir S, Aydin M, Can G, Bali I, Yildirim O, Öznur M, Yildiz ZD, Sözen S, Gürel A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur Rev Med Pharmacol Sci. 2015;19:3613–3618. [PubMed] [Google Scholar]
- 43.Spell DW, Jones DV, jr, Harper WF, David Bessman J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28:37–42. doi: 10.1016/j.cdp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 45.Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–3294. [PubMed] [Google Scholar]
- 46.Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark. 2016;16:507–512. doi: 10.3233/CBM-160591. [DOI] [PubMed] [Google Scholar]