Abstract
Introduction
Current tertiary Spinal Cord Injury (SCI) rehabilitation funding and rehabilitation length of stay (R-LOS) in most North American jurisdictions are linked to an individual’s impairment. Our objectives were to: 1) describe the impact of relevant demographic, impairment and medical complexity variables at rehabilitation admission on R-LOS among adult Canadians with traumatic SCI; and 2) identify factors which extend R-LOS.
Methods
Data from 1,376 adults with traumatic SCI were obtained via chart abstraction and administrative data linkage from 15 Rick Hansen SCI Registry sites (2004–2014). Variables included age, sex, neurological impairment (level, severity), rehabilitation onset days, R-LOS, Glasgow Coma Score (GCS) at admission, prior ventilation or endotracheal tube (Vent/ETT), or indwelling bladder catheter at acute discharge, pain interference score, intensive care unit (ICU) length of stay (LOS), and lower extremity motor scores (LEMS) at rehabilitation admission. Variables related to R-LOS in bivariate analysis were included in multivariate analysis to determine their impact on R-LOS.
Results
Prior Vent/ETT tube, indwelling bladder catheter, GCS, LEMS, and neurological impairment were related to R-LOS in bivariate analysis. Multivariate linear regression analyses identified five variables as significant predictors: age, Vent/ETT for >24 hours in acute care, indwelling bladder catheter at acute discharge, LEMS, and NLI/AIS subgroup at rehabilitation admission explained 32% of the variation in R-LOS (p<0.001).
Conclusions
Based on the enclosed formula, and knowledge of an individual’s age at injury, spinal cord impairment (level and severity), prior Vent/ETT, presence of an indwelling bladder catheter, and LEMS at admission, administrators and clinicians may readily identify patients for whom an extended R-LOS beyond conventional LOS targets is likely.
Keywords: Spinal cord injury, Length of stay, Rehabilitation, Outcome measures, Health system
Introduction
Rehabilitation is a key component in regaining independence, reducing complications and maximizing functional outcomes after sustaining a spinal cord injury (SCI) of traumatic etiology. According to the Canadian Institute of Health Information (CIHI), rehabilitation length of stay (R-LOS) is defined as the average number of days between a patient’s admission to and discharge from a rehabilitation facility.1 R-LOS is a direct indicator of the efficiency of health care delivery and is often used as a surrogate for the intensity of rehabilitation service delivery. Among 600 patients with traumatic SCI admitted to six US rehabilitation sites during the SCIRehab project, the time spent on therapy was recorded following each patient encounter. The total time spent during the patient’s stay and total minutes of treatment per week were calculated. The average R-LOS was 55 days, during which 180 hours of treatment were received.2 The total hours of treatment provided were primarily determined by R-LOS supporting the ongoing use of R-LOS as an important surrogate outcome.
While there is evidence that the average R-LOS for inpatient rehabilitation after SCI has become progressively shorter over the recent years,3 considerable variability in R-LOS exists across the world with many countries reporting a longer R-LOS than in the United States or Canada (see Table 1). This variability in R-LOS can be attributed in part to differences in the etiology of impairment deemed appropriate for inpatient rehabilitation admission, and variability in the available resources, intensity and quality of rehabilitation practice, and the choice of outcome measures used to determine readiness for discharge. A review of recent Canadian data from Ontario examined the economic impact of traumatic SCI across the continuum of care. Munce et al. identified tertiary rehabilitation as the primary driver for direct medical costs of care in the first year after injury. From 2003/04 to 2005/06, the average per person cost of rehabilitation was approximately three times the average per person costs of inpatient acute care.4 Thus, understanding individual patient factors and health system variables that influence R-LOS are essential for planning resource allocation.
Table 1.
Median/mean R-LOS by country for the traumatic SCI population
| Country | Centre | Period | Trauma (N) | Median R-LOS (IQR) | Mean R-LOS ± SD (days) |
|---|---|---|---|---|---|
| USA3 | Multi | 2010–2015 | 1446 | 35 | – |
| Australia25 | Single | 1993–1998 | 167 | 83 (IQR: 35–139) | – |
| Israel26 | Single | 1996–2002 | 25 | T= 102 ± 59 | |
| Italy27 | Multi | 1997–1999 | 684 | 122 | 135.5 |
| Japan28 | Single | 2000–2004 | 17 | 115.6 ± 35.8 | |
| Netherlands29 | Multi | 2000–2003 | 157 | 240 (IQR:164–322) | 272.9 ± 148.7 |
| Netherlands & Flanders30 | Multi | 2002–2007 | 207 | IQR: 116.0–307.0 | 227.6 ± 105.2 |
| Qatar31 | Single | 2008–2010 | 54 | 138.4 ± 114.1 | |
| Russia32 | Single | N/A | 50 | 46.9 ± 20.5 | |
| Saudi Arabia33 | Single | 2005–2008 | 495 | 58.8 ± 1.68 |
Note: SD=standard deviation; IQR=interquartile range.
In Canada, some jurisdictions have attempted to implement cost containing strategies by introduction of R-LOS targets.5 The National Rehabilitation Reporting System (NRS) reports the average length of stay (LOS) for each Rehabilitation Patient Grouping (RPG) for traumatic and non-traumatic SCI.6 The RPG methodology categorizes individual patient data submitted by participating organizations within the Canadian Institute of Health Information National Rehabilitation Reporting System. Across Canada, only the province of Ontario uses a Health-Based Allocation Model (HBAM) for evidence based distribution of funding based on the RPG most responsible for admission, age at admission and the individual patient’s admission motor and cognitive Functional Independence Measure (FIM) score. Upon client discharge, episodes are then weighted based on the client’s RPG and R-LOS, with the aim to encourage quality improvement in health outcomes.6 This HBAM methodology is predicated upon motor and cognitive FIM scores which are appropriate for measuring disease burden but have important floor and ceiling effects when predicting neurorecovery among patients with traumatic SCI.7
In recent years, R-LOS has been declining. The US SCI Model Systems database enables comparison of R-LOS over a 35-year period from 1973 to 2008.3 The Model Systems has reported substantial decreases in R-LOS across all impairment groups. Specific examples include, decreases in median R-LOS from 68 to 29 days for individuals with incomplete paraplegia, and decreases from 142 to 59 days for patients with motor complete tetraplegia.8 Shorter R-LOS has been associated with advances in rehabilitation service delivery, health system pressures and increased rates of rehospitalization.8 The reduced R-LOS has the potential for adverse health system outcomes in terms of resource expenditures. If reduced, R-LOS will trigger increased readmission rates or rising post discharge patient functional declines or multi-morbidity. Given the diversity of impairment and medical complexity within the SCI patient population, achieving an optimal R-LOS and service intensity should result in enhanced functional ability and social participation with low rates of readmission and multimorbidity.
In 2004, the Rick Hansen Spinal Cord Injury Registry (RHSCIR) was developed to examine the characteristics of adult Canadians with traumatic SCI admitted to acute and rehabilitation sites across the nation. RHSCIR contains individual’s demographic, impairment and functional outcomes, including some medical complication and health system factors that influence the duration and efficiency of rehabilitation service delivery. Given the variability in R-LOS internationally, and its’ importance in assuring quality rehabilitation care, it is imperative that we identify factors that influence R-LOS and predict a need for an extended R-LOS. The objectives of this manuscript are to: 1) identify and describe the relevant demographic, impairment and medical complexity variables evident at/before tertiary SCI rehabilitation admission and their association with R-LOS among adult Canadians with traumatic SCI; and, 2) to identify the factors which predict an extended R-LOS using a predictive equation.
Methods
Study participants were recruited from RHSCIR, a Canadian traumatic SCI registry, that receives data from 31 acute and rehabilitation facilities across Canada that treat individuals with new onset traumatic SCI.9 RHSCIR was initiated in 2004, to answer a priori research questions and to facilitate the implementation of best practices. The RHSCIR consent, enrolment and data collection procedures are described elsewhere.9 Any adult treated at a RHSCIR site for a new traumatic SCI was eligible for inclusion regardless of their neurological impairment. All RHSCIR sites obtained local Research Ethics Board (REB) approval prior to enrolling consented participants
The RHSCIR data elements to address the study objectives were selected by a group of specialists in Physical Medicine and Rehabilitation, from contributing RHSCIR sites, based on their clinical expertise and the available data elements. The authors selected a series of demographic, impairment, and medical complexity variables evident at or before rehabilitation admission that are clinically important predictors of R-LOS. Additionally, priority was placed on identifying medical comorbidities or surrogates outcomes for the medical comorbidities likely to extend R-LOS.
Analysis cohort
The analysis cohort was formed from adult RHSCIR participants with traumatic SCI who received rehabilitation care at a RHSCIR site, regardless of their neurological level of injury or injury severity.9 Participants were excluded if they did not have valid R-LOS data due to missing rehabilitation admission and discharge dates, or did not have a neurological examination performed as per the international standards for neurological classification of spinal cord injury (ISNCSCI)10 within 7 days of rehabilitation admission.
Demographic variables
Age, sex, education (less than high school, high school, higher education), employment (yes, no), pre-injury smoking status (current, former, never), living arrangement (alone, not alone), marital status (married/common law, widowed, divorced/separated, single), and Body Mass Index (BMI; ≥22 indicative of obesity in SCI)11 were collected and analyzed.
Impairment variables
Glasgow Coma Scale (GCS) at acute admission was collected as a surrogate for concurrent traumatic brain injury and analyzed as GCS ≤12 (moderate/severe) vs GCS >12 (mild to none).12 A detailed spinal neurological assessment was performed according to the ISNCSCI,10 including neurological level of injury (NLI) and the American Spinal Injury Association (ASIA) Impairment Scale (AIS), which measures severity of spinal cord injury from A (most severe) to D (least severe) and E (normal). For the purposes of this study, participants were grouped according to neurological level of injury and AIS into 8 subgroups, initially divided into four NLI subgroups including high cervical (C1-C4), low cervical (C5-T1), thoracic (T2-T10), and thoracolumbar (T11-S4-5), and then further subdivided by AIS as AIS A/B vs C/D. Lower extremity motor scores (LEMS) was included in the model as ≤10 vs >10; as LEMS at rehabilitation admission has been shown to be a potent predictor of ambulation.13
Medical complexity/co–morbidity variables
Variables related to medical complexity or comorbid conditions include: intensive care unit LOS (ICU-LOS), rehabilitation onset days (days from injury onset to a RHSCIR tertiary rehabilitation center admission) and inpatient R-LOS were documented, the latter two were obtained from NRS data. A history of acute care Vent/ETT was used as a surrogate for respiratory complexity. The presence or absence of a percutaneous endoscopic gastrostomy (PEG) tube during acute or rehabilitation care was used a surrogate for dysphagia.
Neuropathic pain, sufficiently severe to interfere with normal activities and rehabilitation participation/progression, was thought to be an important R-LOS predictor. The pain interference score, with 0 being no interference and 10 being complete interference, was collected on each of the three items: normal activity, quality of life, and sleep. These three scores were totaled and then dichotomized as a total interference score of ≤15 vs >15.
Difficulties implementing optimal neurogenic bowel and bladder management and long term Foley use14 were felt to be an important predictors of R-LOS (i.e. limited hand function, obesity precluding independent self-catheterization. etc.); thus, the presence or absence of an indwelling catheter at discharge from rehabilitation was the surrogate dichotomous variable selected. No similar surrogate for optimal neurogenic bowel care was identified from the RHSCIR data set.
Outcome
The outcome of interest is R-LOS which is the time from inpatient rehabilitation admission to discharge from the rehabilitation facility excluding any service interruptions.15
Statistical Analysis
Appropriate descriptive statistics were used to describe the demographic, impairment, medical complexity and R-LOS of the entire cohort (n= 1,445). Review of the national R-LOS data revealed significant outliers. The outliers were felt to be due to health system barriers rather than adverse outcomes; for example, lack of community housing for mechanically ventilated patients, lack of equipment receipt at the time of rehabilitation discharge, or inadequate community attendant care services. As a result, extreme outliers were removed from the cohort using Tukey’s rule16 to refine the cohort to 1376 participants. A sensitivity analysis was performed to compare the participant characteristics of those excluded due to extreme R-LOS and the final cohort.
Univariate analysis was conducted to explore and describe each of the aforementioned demographic, impairment and medical complexity variables individually. Bivariate analyses were performed to assess relevance of individual variables to R-LOS at RHSCIR rehabilitation participating centers as well as to compare participant characteristics between outliers and the final analysis cohort. Normally distributed continuous variables were compared using a t test between two groups or ANOVA for more than two groups. Similarly, for non-normally distributed variables, the Wilcoxon rank sum test or Kruskal–Wallis test were used as appropriate. Pearson correlation was used to examine linear relationship between two continuous variables. Associations between two categorical variables were assessed using a χ2 test unless the expected cell counts were smaller than 5, in which case Fisher’s exact test was used.
Multiple linear regression analysis was performed to determine the impact of statistically significant determinants (impairment, Vent/ETY or indwelling catheter and LEMS) in the bivariate analysis as well as the clinically relevant predictor of age on R-LOS. A Poisson regression and a negative binomial regression were also conducted as R-LOS could be a count variable. Goodness-of-fit tests were performed for all models and the Akaike Information Criterion was used for model selection. Associations with a p-value <0.05 were considered statistically significant. All analyses were performed using SAS software, Version 9.4 of the SAS System for Windows (Copyright © 2013. SAS Institute Inc., Cary, NC, USA).
Results
R-LOS
There were 2444 participants who received care at a RHSCIR rehabilitation center; 133 (5%) were excluded as R-LOS could not be calculated, 856 (35%) were excluded as they did not have an ISNCSCI examination performed within 7 days of rehabilitation admission, leaving a cohort of 1455. A total of 79 participants had a R-LOS greater than 224 days, and based on Tukey’s rule were considered outliers and were excluded from the analysis, leaving an analysis cohort of 1376. A histogram of LOS for the entire cohort and the analysis cohort with outliers removed is shown in Fig. 1; Fig. 2 displays a box plot of R-LOS; and Fig. 3 displays R-LOS based on the eight aforementioned NLI and AIS subgroups.
Figure 1.
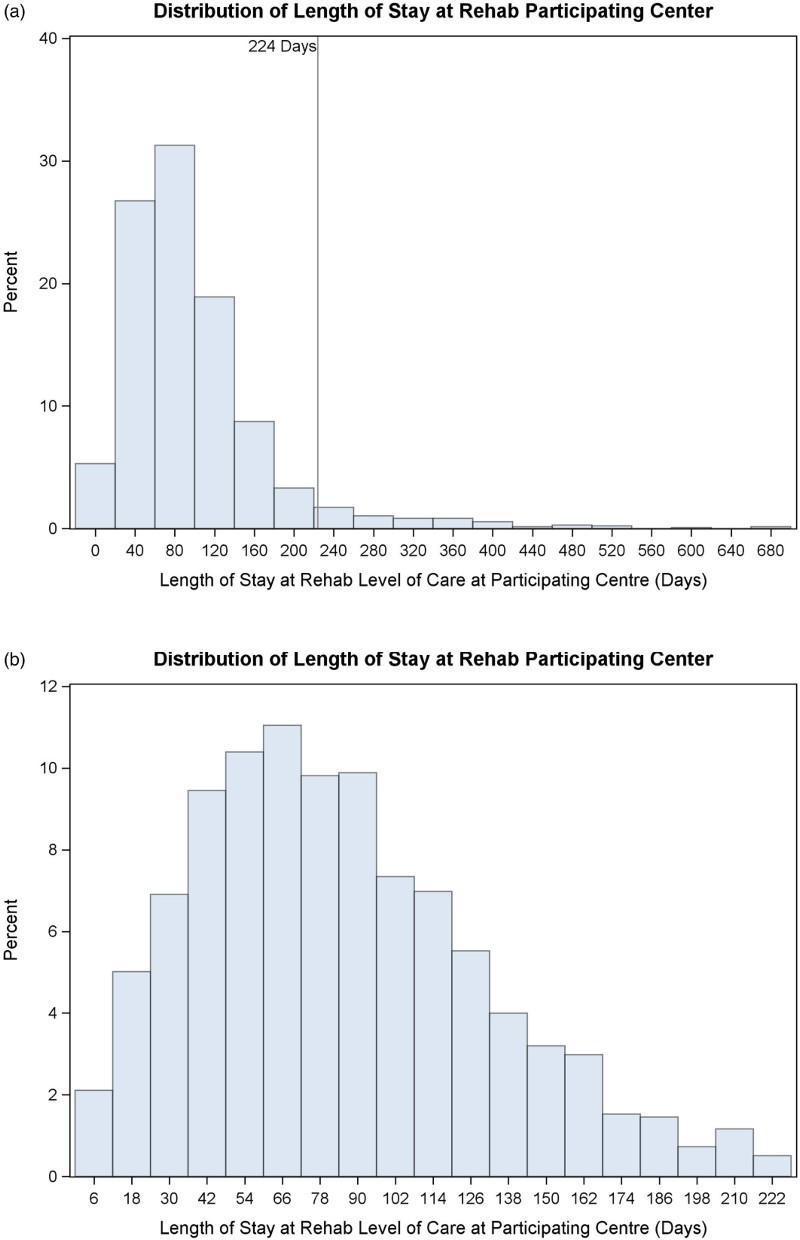
R-LOS (days) for A) initially identified cohort (n=1455) and B) the cohort with outliers removed (n=1376) as used for analysis.
Figure 2.
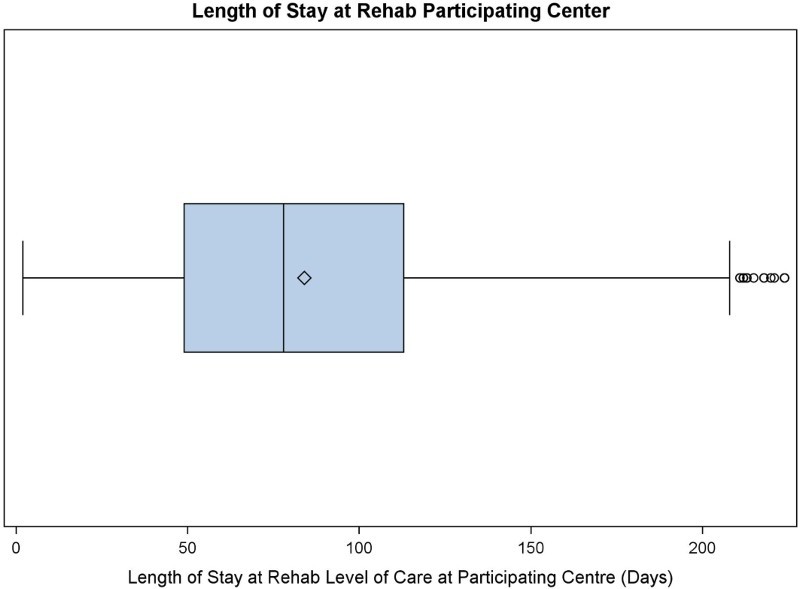
Box plot of R-LOS (days) among adults with traumatic SCI (2004–2014).
Figure 3.
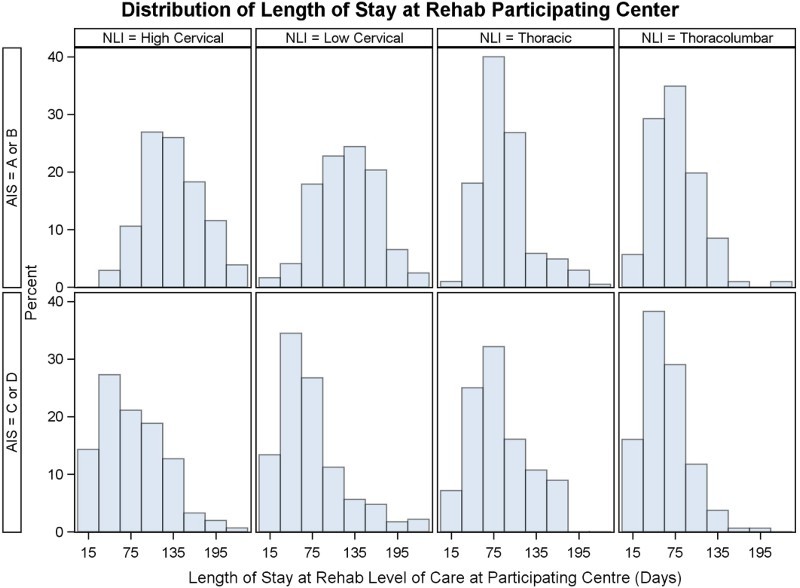
R-LOS (days) distribution based on the individual's neurological Level of injury and ASIA Impairment Scale category.
The median R-LOS was 78 days (IQR 64 days) preceded by a median 30-day rehabilitation onset. During the study time period, the number of RHSCIR rehabilitation sites increased from two to 15 (all regularly recruiting by 2011).
We compared the 79 participants classified as extreme R-LOS outliers with regards to R-LOS; the excluded participants did not differ with regards to age or gender. However, the excluded participants were more likely to live alone prior to injury onset (p = 0.0027), have more severe (AIS A/B) injuries (p < 0.0001), had longer rehabilitation onset days (p < 0.0001), and were more likely to have an indwelling catheter at discharge (p < 0.0001), and had received ventilation (p < 0.0001).
Cohort description
Of the 1376 in the analysis cohort, the mean age at injury onset was 45 (± 18.5), the majority (80%) of participants were married men, with at least a high school education. See Table 2 for a summary of participant, demographic and impairment data.
Table 2.
Patient, injury, and management details for patients with incomplete paraplegia (T2-S4/5, AIS C/D; n=218), incomplete tetraplegia (C1-T1, AIS C/D; n=540), complete paraplegia (T2-S4/5, AIS A/B; n=311), complete tetraplegia (C1-T1, AIS A/B; n=227), and the total analysis cohort (n=1376)
| Variable | Incomplete paraplegic n = 218 | Incomplete tetraplegia n = 540 | Complete paraplegic n = 311 | Complete tetraplegia n = 227 | Analysis cohort n = 1376 |
|---|---|---|---|---|---|
| Age at injury (years); mean (SD) | 42.7 (18.3) | 54.2 (17.2) | 36.7 (15.7) | 39.0 (16.5) | 45.2 (18.5) |
| Male; n (%) | 162 (74.3%) | 443 (82.0%) | 258 (83.0%) | 183 (80.6%) | 1107 (80.5%) |
| Marital status; n (%) | |||||
| Married/common law | 72 (45.9%) | 226 (55.9%) | 113 (44.5%) | 80 (48.8%) | 491 (50.2%) |
| Widowed | 7 (4.5%) | 25 (6.2%) | 2 (0.8%) | 3 (1.8%) | 37 (2.6%) |
| Divorced/separated | 9 (5.7%) | 48 (11.9%) | 14 (5.5%) | 10 (6.1%) | 81 (8.3%) |
| Single | 69 (44.0%) | 105 (26.0%) | 125 (49.2%) | 71 (43.3%) | 370 (37.8%) |
| Education; n (%) | |||||
| Less than high school | 41 (27.0%) | 118 (30.7%) | 69 (27.6%) | 35 (22.2%) | 263 (27.8%) |
| High school | 56 (36.8%) | 130 (33.8%) | 103 (41.2%) | 84 (53.2%) | 373 (13.8%) |
| Higher education | 55 (36.2%) | 137 (35.6%) | 78 (31.2%) | 39 (24.7%) | 309 (32.7%) |
| Employed at injury; n (%) | 103 (66.5%) | 212 (52.5%) | 185 (72.3%) | 116 (71.2%) | 616 (63.0%) |
| Pre-injury smoking status; n (%) | |||||
| Current | 36 (24.8%) | 87 (24.3%) | 62 (27.1%) | 33 (22.8%) | 218 (24.9%) |
| Former | 50 (34.5%) | 139 (38.8%) | 61 (26.6%) | 39 (26.9%) | 289 (32.9%) |
| Never | 59 (40.7%) | 132 (36.9%) | 106 (46.3%) | 73 (50.3%) | 370 (42.2%) |
| Pre-injury living setting; n (%) | |||||
| Own home/rent | 141 (95.9%) | 388 (97.2%) | 240 (98.4%) | 153 (98.1%) | 922 (97.5%) |
| Other | 6 (4.1%) | 11 (2.8%) | 4 (1.6%) | 3 (1.9%) | 24 (2.5%) |
| Pre-injury living situation; n (%) | |||||
| Alone | 36 (23.2%) | 91 (22.3%) | 45 (17.6%) | 24 (14.6%) | 196 (19.9%) |
| Not alone | 119 (76.8%) | 318 (77.8%) | 211 (82.4%) | 140 (85.4%) | 788 (80.1%) |
| Pre-injury BMI ≥ 22; n (%) | 112 (79.4%) | 309 (82.2%) | 198 (84.3%) | 122 (80.3%) | 741 (82.0%) |
| Mechanism of injury; n (%) | |||||
| Sports | 25 (11.8%) | 70 (13.2%) | 49 (15.9%) | 44 (19.4%) | 188 (14.7%) |
| Transport | 65 (30.7%) | 119 (22.4%) | 120 (38.8%) | 107 (47.1%) | 411 (32.1%) |
| Falls | 92 (43.3%) | 290 (54.5%) | 104 (34.7%) | 50 (22.0%) | 536 (41.9%) |
| Other | 30 (14.2%) | 53 (10.0%) | 36 (11.7%) | 26 (11.5%) | 145 (11.3%) |
| Rehabilitation onset (days); | |||||
| Mean (SD) | 34.3 (36.4) | 39.0 (41.2) | 35.0 (27.7) | 67.4 (49.3) | 42.3 (41.5) |
| Median (IQR) | 24.0 (26.0) | 27.0 (31.0) | 27.0 (24.0) | 55.0 (57.0) | 30.0 (34.0) |
| Rehabilitation Admission FIM; mean (SD) | 82.4 (15.1) | 66.4 (21.4) | 66.9 (10.5) | 50.5 (7.7) | 66.4 (18.6) |
Note: BMI = Body Mass Index, FIM = Functional Independence Measure.
Bivariate analysis
Neurological impairment (p < 0.0001), GCS at admission to acute care (p = 0.004), ventilation in acute care (p < 0.0001), total lower extremity motor scores or LEMS at admission to rehabilitation care (p < 0.0001), and indwelling catheter at discharge (p < 0.0001) were significantly related to total R-LOS. Sex (p = 0.5911), age at injury (p = 0.7648), pre-injury living arrangement (p = 0.2893), PEG tube (p = 0.7633), pain interference score (p=0.9410), and ICU-LOS (p = 0.7993) were not significantly related (Table 3).
Table 3.
Participant, injury, and management details for the analysis cohort (n = 1376) and their relationship to R-LOS. Neurological data was assessed within 7 days of admission to rehabilitation care. *Ventilation in acute care is defined as an ETT for > 24 hours or tracheostomy. AIS = ASIA Impairment Scale; ICU = intensive care unit; IQR = interquartile range; SD = standard deviation
| Variable | Analysis cohort n = 1376 | p-value |
|---|---|---|
| Age at injury (years); mean (SD) | 45.2 (18.5) | 0.7648 |
| Male; n (%) | 1107 (80.5%) | 0.5911 |
| Pre-injury living situation; n (%) | ||
| Alone | 201 (20.4%) | 0.2893 |
| Not alone | 829 (79.6%) | 0.0040 |
| Glasgow Coma Scale (GCS, n = 844) ≤ 12; n (%) | 60 (7.1%) | <0.0001 |
| Impairment (NLI and AIS subgroups) | ||
| High cervical (C1-C4) A/B | 104 (8.0%) | |
| High cervical (C1-C4) C/D | 308 (23.8%) | |
| Low cervical (C5-T1) A/B | 123 (9.5%) | |
| Low cervical (C5-T1) C/D | 232 (17.9%) | |
| Thoracic (T2-T10) A/B | 205 (15.8%) | |
| Thoracic (T2-T10) C/D | 56 (4.3%) | |
| Thoracolumbar (T11-S4/5) A/B | 106 (8.2%) | |
| Thoracolumbar (T11-S4/5) C/D | 162 (12.5%) | |
| Lower Extremity Motor Score ≤ 10; n (%) | 572 (45.0%) | <0.0001 |
| Ventilation in acute care*; n (%) | 316 (31.1%) | <0.0001 |
| PEG tube; n (%) | 22 (2.0%) | 0.7633 |
| Acute ICU length of stay (R-LOS, days, n = 321); mean (SD) | 82.8 (274.2) | 0.7993 |
| Pain interference score > 15; n (%) | 185 (21.3%) | 0.9410 |
| Rehabilitation R-LOS (days) | – | |
| Mean (SD) | 84.1 (46.0) | |
| Median (IQR) | 78 (64) | |
| Indwelling Catheter at Discharge; n (%) | 95 (10.0%) | <0.0001 |
Multivariate model
Multiple linear regression was preferred given the better goodness-of-fit and simpler interpretation compared to the Poisson and negative binomial regressions. The final model obtained included four significant predictors from the bivariate analysis, and age at injury based on relevance of age to R-LOS in prior publications. Although significant in the bivariate analysis, Glasgow Coma Scale was not a significant predictor after adjusting for other covariates in the model and was not included in the final model. A total of 816 patients with complete data were included in the model. See Table 4 for the results of the multivariate analysis. Age, injury neurology (level, AIS), ventilation, and presence of an indwelling catheter and LEMS were significantly associated with R-LOS. Patients with more severe impairment had a longer R-LOS than those with less severe injuries. In particular, compared to thoracolumbar, C, D patients, patients with high cervical, A, B, high cervical C, D, low cervical A, B stayed about 33 days (p < 0.0001), 12 days (p = 0.0164), 24 days (p = 0.0007) longer respectively. Prior ventilation in acute care and having an indwelling catheter increased R-LOS by 11 days (p = 0.0007) and 12 days (p = 0.0129) respectively. A 10 unit increase in age at injury increased the R-LOS approximately by 2 days. The model was significant (p < 0.0001), and the variables included in the modelling explain 32% of the variation in R-LOS.
Table 4.
Results of multivariate analysis on the effect of participant, injury, and management data on rehabilitation R-LOS (n = 861). CI = confidence interval. AIS = ASIA Impairment Scale
| Independent variable | Parameter | Standard error | 95% CI lower | 95% CI upper | p-value |
|---|---|---|---|---|---|
| Intercept | 50.0774 | 5.0942 | 40.0779 | 60.0769 | <.0001 |
| Age | 0.1785 | 0.0822 | 0.0173 | 0.3398 | 0.0301 |
| Injury neurology (level, AIS); n (%) | |||||
| High cervical (C1-C4) A/B | 33.2040 | 7.6553 | 18.1772 | 48.2308 | <0.0001 |
| High cervical (C1-C4) C/D | 11.9570 | 4.97701 | 2.2010 | 21.7129 | 0.0164 |
| Low cervical (C5-T1) A/B | 24.3632 | 7.1986 | 10.2330 | 38.4933 | <0.0007 |
| Low cervical (C5-T1) C/D | 3.5217 | 5.1223 | −6.5330 | 13.5764 | 0.4920 |
| Thoracic (T2-T10) A/B | −5.1770 | 6.5500 | −18.0342 | 7.6801 | 0.4295 |
| Thoracic (T2-T10) C/D | 11.6766 | 7.6545 | −3.3486 | 26.7017 | 0.1275 |
| Thoracolumbar (T11-S4/5) A/B | −3.8998 | 6.6225 | −16.8992 | 9.0997 | 0.5561 |
| Thoracolumbar (T11-S4/5) C/D | Baseline | – | – | – | – |
| Ventilation | |||||
| Yes | 10.6047 | 3.1180 | 4.4843 | 16.7250 | 0.0007 |
| No | |||||
| Indwelling catheter | |||||
| Yes | 12.0451 | 4.8327 | 2.5589 | 21.5314 | 0.0129 |
| No | |||||
| LEMS | |||||
| ≤10 | 36.2156 | 4.7964 | 26.8008 | 45.6305 | <.0001 |
| >10 | Baseline | – | – | – | – |
To facilitate the use of the obtained model for prediction, equation (1) was derived using the model parameter estimates (Table 4):
 |
(1) |
Using equation (1), the R-LOS for a patient with age 50, high cervical, ASIA Impairment Scale category A, with prior ventilation, an indwelling catheter and with LEMS less than or equal to 10 would be about 151 days while the R-LOS for a patient with the same age, thoracolumbar C, D, no ventilation, no indwelling catheter and LEMS greater than 10 would be about 59 days.
Discussion
There have been many health system advances and pressures, which have influenced SCI rehabilitation service delivery and R-LOS in Canada between 2004 and 2014. In particular, there have been substantial reductions in rehabilitation onset days and R-LOS for individuals with similar impairments. Further, the mean R-LOS has reduced from 90 to 79 days from 2004–2010 and 2011–2014, respectively among RHSCIR participants. Impairment subgroups derived from more severe NLI and AIS, LEMS≤10 and prior ventilation in acute care were associated with extended R-LOS. The identified model explains 32% of the variation in R-LOS.
The predictive equation obtained from the multivariable model has important health policy and clinical service implications as it allows for identification of subgroups of patients likely to have an extended R-LOS; an improvement over the current impairment only funding models or where the resources provided to rehabilitation programs are based on age, impairment and FIM subscores alone or in combination.
While the interim reductions in R-LOS may represent efficiencies in health system flow and/or significant operating cost savings, it is currently unclear, whether the long-term rehabilitation outcomes of individuals with SCI have been maintained, improved or declined in this same time period. Recently, there have been efforts internationally to promote the routine collection of the SCIM III and to identify impairment specific SCIM targets for rehabilitation outcomes among individuals with motor complete SCI by neurological level of injury.17,18
Age at injury onset and its impact on access to surgical intervention and rehabilitation outcome has been a source of controversy in the SCI literature. Although prior publications of Canadian data have demonstrated that the cost of surgical care for the elderly is higher,19 and that although the rehabilitation outcomes for elderly individuals are similar to younger patients, the R-LOS to obtain these functional gains was longer.20 Based on the weight of these prior reports, we felt it was prudent to continue to include age in the multivariable model.
Contrary to our assumptions and clinical experience, premorbid living environment and ICU-LOS were not predictors of R-LOS. Although premorbid living arrangements did not predict R-LOS, there is ample evidence in the literature that following rehabilitation discharge, living with family and having a strong social network are potent predictors of community integration.21
Failli et al. and others have reported an increased acute care LOS and adverse rehabilitation outcomes among patients with SCI who develop a systemic immune deficiency syndrome secondary to a pneumonia or urinary septicemia.22 Again, use of ICU-LOS as a surrogate predictor of infection/medical complexity as opposed to a direct measure of infection frequency and severity may explain the observed disconnect between our clinical experiences and the observed lack of association between ICU-LOS and R-LOS.
Similarly, GCS was used as a predictor for concurrent brain injury but was not a statistically significant predictor of R-LOS in the multivariate model although in the bivariate model there was a 16.5-day difference in median R-LOS (80 vs 96.5 days and a large IQR) for those based on the GCS threshold. Bradbury et al.23 have reported a ∼50% prevalence of co-morbid brain injury with spinal cord injury and described the impact on R-LOS. SCI patients with a concurrent TBI had a longer mean inpatient R-LOS of 138±70 days vs 100±41 days, with an associated greater economic burden in terms of R-LOS and service needs. An alternate surrogate for TBI such as the Galveston Orientation and Amnesia Test (GOAT) scores which provide an objective assessment with a standardized cut-off for the presence of post traumatic amnesia might be a more suitable future alternate.24
This study data provides RHSCIR network members a unique opportunity to revisit the data elements most predictive of R-LOS and elect to collect direct measures of medical complexity by incorporating new data elements to increase the clinical precision of R-LOS estimates in the future. Further, subgrouping the SCI population into the enclosed 8 subgroups in terms of understanding how demographic, impairment and medical complexity variables combine to influence R-LOS and rehabilitation resource allocation, will be important to include in provincial and national resource planning. For example, an individual with high C1-C4 ASIA Impairment Scale A. An individual with complete tetraplegia will require greater nursing, OT, and RT resources when compared to an individual with mid thoracic incomplete paraplegia and LEMS>10 who will require much less nursing resource and a higher intensity of physiotherapy and recreational therapy resources.
R-LOS remains a complex and elusive construct and an important surrogate for rehabilitation intensity. In recent years, R-LOS in Canada has been primarily driven by impairment specific funding, rather than an individual’s medical complexity or prognosis for functional recovery. With reductions in R-LOS, individuals with SCI often require extended outpatient care to optimize functional recovery and manage secondary health conditions (e.g. pressure ulcer, urinary tract infection, or neuropathic pain). Given that R-LOS is the largest cost driver to the Canadian health care system,4 it is imperative that we accurately predict R-LOS based on their demographic, impairment and medical comorbidity and implement strategies to mitigate the impact of these variables on patient outcome and health system cost.
Limitations
The enclosed predictive model of R-LOS is built on variables which are direct demographic and impairment measures and surrogate measures of rehabilitation and medical complexity. Direct measures of relevant health system variables and medical complexity may yield a more potent multivariate model. The time frame upon which the enclosed results are based, covers a period of rapid health system transformation and advances in SCI Care and reductions in R-LOS which are not reflected in our model. In addition, introduction of Alternate Level of Care (ALC) designations and coding of service interruptions have impacted standard procedures for coding rehabilitation admission and discharge and service interruptions within the NRS. There are health system variables that also influence R-LOS that we did not account for nor include in our multivariable model.
Conclusion
The study results indicate that five variables: age, prior Vent/ETT tube for >24 hours in acute care, indwelling catheter, LEMS within 7 days of rehabilitation admission, and NLI/AIS subgroups at rehabilitation admission, explain 32% of the variation in R-LOS. This enclosed predictive equation may be used to identify patients with SCI at risk for an extended R-LOS, well beyond the mean NRS R-LOS by RCG at rehabilitation admission. These data elements including the NLI-AIS subgroups may be used to help inform the design of future predictive models identifying individuals at risk for an extended R-LOS.
Disclaimer statements
Contributors None.
Funding None.
Declaration of interest None.
Conflict of interest None.
Ethics approval None.
ORCID
B. Catharine Craven http://orcid.org/0000-0001-8234-6803
References
- 1.Canadian Institute for Health Information DAD/HMDB Inpatient Hospitalizations: Volumes, Length of Stay, and Standardized Rates | CIHI. https://www.cihi.ca/en/types-of-care/hospital-care/dadhmdb-inpatient-hospitalizations-volumes-length-of-stay-and. Published 2011. Accessed January 13, 2017.
- 2.Whiteneck G, Gassaway J, Dijkers M, Backus D, Charlifue S, Chen D, et al The SCIRehab project: treatment time spent in SCI rehabilitation. Inpatient treatment time across disciplines in spinal cord injury rehabilitation. J Spinal Cord Med 2011;34(2):133–48. doi: 10.1179/107902611X12971826988011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Spinal Cord Injury Statistical Center Annual Report for the Spinal Cord Injury Model Systems. Birmingham; 2008. https://www.nscisc.uab.edu/PublicDocuments/reports/pdf/2008NSCISCAnnualStatisticalReport-CompletePublicVersion.pdf. Accessed January 13, 2017.
- 4.Munce SE, Wodchis WP, Guilcher SJ, Couris CM, Verrier M, Fung K, et al Direct costs of adult traumatic spinal cord injury in Ontario. Spinal Cord 2013;51(1):64–9. doi: 10.1038/sc.2012.81 [DOI] [PubMed] [Google Scholar]
- 5.Burns A, Yee J, Flett HM, Guy K, Cournoyea N.. Impact of benchmarking and clinical decision making tools on rehabilitation length of stay following spinal cord injury. Spinal Cord 2013;51(2):165–9. doi: 10.1038/sc.2012.91 [DOI] [PubMed] [Google Scholar]
- 6.Ontario Ministry of Health & Long GT Health System Funding Reform. http://www.health.gov.on.ca/en/pro/programs/ecfa/funding/hs_funding_qa.aspx. Accessed January 1, 2017.
- 7.Anderson K, Aito S, Atkins M, et al Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med 2007;31(2):133–44. doi: 10.1080/10790268.2008.11760704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eastwood EA, Hagglund KJ, Ragnarsson KT, et al Medical rehabilitation length of stay and outcomes for persons with traumatic spinal cord injury—1990–1997. Arch Phys Med Rehabil 1999;80(11):1457–63. doi: 10.1016/S0003-9993(99)90258-7 [DOI] [PubMed] [Google Scholar]
- 9.Noonan VK, Kwon BK, Soril L, Fehlings MG, Hurlbert RJ, Townson A, et al The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord 2012;50(1):22–7. doi: 10.1038/sc.2011.109 [DOI] [PubMed] [Google Scholar]
- 10.Burns S, Biering-Sørensen F, Donovan W, Graves DE, Jha A, Johansen M, et al International Standards for Neurological Classification of Spinal Cord Injury, Revised 2011. Top Spinal Cord Inj Rehabil 2012;18(2):85–99. doi: 10.1310/sci1801-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughton GE, Buchholz C, Martin Ginis K, Goy RE.. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord 2009;47(10):757–62. doi: 10.1038/sc.2009.33 [DOI] [PubMed] [Google Scholar]
- 12.Kortbeek JB, Al Turki S, Ali J, Antoine JA, Bouillon B, Brasel K, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma 2008;64(June):1638–50. doi: 10.1097/TA.0b013e3181744b03 [DOI] [PubMed] [Google Scholar]
- 13.Scivoletto G, Di Donna V.. Prediction of walking recovery after spinal cord injury. Brain Res Bull 2009;78(1):43–51. doi: 10.1016/j.brainresbull.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Wilde MH, Brasch J, Getliffe K, Brown KA, McMahon JM, Smith JA, et al Study on the use of long-term urinary catheters in community-dwelling individuals. J Wound Ostomy Cont Nurs 2010;37(3):301–10. doi: 10.1097/WON.0b013e3181d73ac4 [DOI] [PubMed] [Google Scholar]
- 15.Canadian Institute for Health Information Inpatient Rehabilitation in Canada.; 2006. https://secure.cihi.ca/free_products/NRS_2006_IRC_EN.pdf. Accessed January 15, 2017.
- 16.Tukey J. Exploratory Data Analysis. In: Exploratory Data Analysis. Addison-Wesley; 1977:43–4. [Google Scholar]
- 17.Alexander MS, Anderson KD, Biering-Sorensen F, Blight AR, Brannon R, Bryce TN, et al Outcome measures in spinal cord injury: recent assessments and recommendations for future directions. Spinal Cord 2009;47(8):582–91. doi: 10.1038/sc.2009.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aidinoff E, Front L, Itzkovich M, Bluvshtein V, Gelernter I, Hart J, et al Expected spinal cord independence measure, third version, scores for various neurological levels after complete spinal cord lesions. Spinal Cord 2011;49(8):893–896. doi: 10.1038/sc.2011.32 [DOI] [PubMed] [Google Scholar]
- 19.Furlan JC, Craven BC, Fehlings MG.. Surgical Management of the Elderly With Traumatic Cervical Spinal Cord Injury: A Cost-Utility Analysis. Neurosurgery 2016;0(0):1–8. [DOI] [PubMed] [Google Scholar]
- 20.Furlan JC, Hitzig SL, Craven BC.. The influence of age on functional recovery of adults with spinal cord injury or disease after inpatient rehabilitative care: a pilot study. Aging Clin Exp Res 2013;25(4):463–71. doi: 10.1007/s40520-013-0066-1 [DOI] [PubMed] [Google Scholar]
- 21.Guilcher SJT, Craven BC, Lemieux-Charles L, Casciaro T, McColl MA, Jaglal SB.. Secondary health conditions and spinal cord injury: an uphill battle in the journey of care. Disabil Rehabil 2013;35(11):894–906. doi: 10.3109/09638288.2012.721048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Failli V, Kopp M, Gericke C, Martus P, Klingbeil S, Brommer B, et al Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 2012;135(11):3238–50. doi: 10.1093/brain/aws267 [DOI] [PubMed] [Google Scholar]
- 23.Bradbury CL, Wodchis WP, Mikulis DJ, Pano EG, Hitzig SL, McGillivray CF, et al Traumatic brain injury in patients with traumatic spinal cord injury: clinical and economic consequences. Arch Phys Med Rehabil 2008;89(12 Suppl):S77–84. doi: 10.1016/j.apmr.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 24.Galveston Orientation and Amnesia Test | ERABI http://www.abiebr.com/set/17-assessment-outcomes-following-acquiredtraumatic-brain-injury/galveston-orientation-and. Accessed January 15, 2017.
- 25.Tooth L, McKenna K, Geraghty T.. Rehabilitation outcomes in traumatic spinal cord injury in Australia: functional status, length of stay and discharge setting. Spinal Cord 2003;41(4):220–30. doi: 10.1038/sj.sc.3101433 [DOI] [PubMed] [Google Scholar]
- 26.Ronen J, Itzkovich M, Bluvshtein V, Thaleisnik M, Goldin D, Gelernter I, et al Length of stay in hospital following spinal cord lesions in Israel. Spinal Cord 2004;42(6):353–8. doi: 10.1038/sj.sc.3101590 [DOI] [PubMed] [Google Scholar]
- 27.Pagliacci MC, Celani MG, Zampolini M, Spizzichino L, Franceschini M, Baratta S, et al An Italian survey of traumatic spinal cord injury. The Gruppo Italiano Studio Epidemiologico Mielolesioni study. Arch Phys Med Rehabil 2003;84(9):1266–75. doi: 10.1016/S0003-9993(03)00234-X [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama O, Sakuma F, Itoh R, Sashika H.. Paraplegia after aortic aneurysm repair versus traumatic spinal cord injury: functional outcome, complications, and therapy intensity of inpatient rehabilitation. Arch Phys Med Rehabil 2006;87(9):1189–94. doi: 10.1016/j.apmr.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 29.Post MWM, Dallmeijer AJ, Angenot ELD, van Asbeck FW, van der Woude LH V.. Duration and functional outcome of spinal cord injury rehabilitation in the Netherlands. J Rehabil Res Dev 2004;42(3sup1):75. doi: 10.1682/JRRD.2004.10.0133 [DOI] [PubMed] [Google Scholar]
- 30.Osterthun R, Post M, van Asbeck F, Society D-FSC.. Characteristics, length of stay and functional outcome of patients with spinal cord injury in Dutch and Flemish rehabilitation centres. Spinal Cord 2009;47(4):339–44. doi: 10.1038/sc.2008.127 [DOI] [PubMed] [Google Scholar]
- 31.Venkatachalam L, Yazeedi W Al, George LA.. Predictors of the length of stay of inpatients in rehabilitation setting after traumatic spinal cord injury. Open Access Scientific Reports 2012;1(2). 2012.
- 32.Fromovich-Amit Y, Biering-Sørensen F, Baskov V, Juocevicius A, Hansen HV, Gelernter I, et al Properties and outcomes of spinal rehabilitation units in four countries. Spinal Cord 2009;47(8):597–603. doi: 10.1038/sc.2008.178 [DOI] [PubMed] [Google Scholar]
- 33.Al-jadid MS, Robert AA.. An analysis of the length of stay in traumatic and non- traumatic spinal cord injured patients. Saudi Med J. 2010;31(5):555–9. [PubMed] [Google Scholar]


