Abstract
Context
Endocrine-metabolic disease (EMD) risk following spinal cord injury (SCI) is associated with significant multi-morbidity (i.e. fracture, diabetes, heart disease), mortality, and economic burden. It is unclear to what extent rehabilitation interventions can modify EMD risk and improve health status in community-dwelling adults with chronic SCI.
Objectives
To characterize rehabilitation interventions and summarize evidence on their efficacy/effectiveness to modify precursors to EMD risk in community-dwelling adults with chronic SCI.
Methods
Systematic searches of MEDLINE PubMed, EMBASE Ovid, CINAHL, CDSR, and PsychInfo were completed. All randomized, quasi-experimental, and prospective controlled trials comparing rehabilitation/therapeutic interventions with control/placebo interventions in adults with chronic SCI were eligible. Two authors independently selected studies and abstracted data. Mean differences of change from baseline were reported for EMD risk outcomes. The GRADE approach was used to rate the quality of evidence.
Results
Of 489 articles identified, 16 articles (11 studies; n=396) were eligible for inclusion. No studies assessed the effects of rehabilitation interventions on incident fragility fractures, heart disease, and/or diabetes. Individual studies reported that exercise and/or nutrition interventions could improve anthropometric indices, body composition/adiposity, and biomarkers. However, there were also reports of non-statistically significant between-group differences.
Conclusions
There was very low-quality evidence that rehabilitation interventions can improve precursors to EMD risk in community-dwelling adults with chronic SCI. The small number of studies, imprecise estimates, and inconsistency across studies limited our ability to make conclusions. A high-quality longitudinal intervention trial is needed to inform community-based rehabilitation strategies for EMD risk after chronic SCI.
Keywords: Endocrine and metabolic diseases, Exercise, Nutrition, Rehabilitation, Spinal cord injuries
Introduction
Endocrine-metabolic disease (EMD) risk is associated with adverse changes in body composition and cardiometabolic biomarkers in individuals with spinal cord injury (SCI). These changes often lead to multi-morbidity, specifically; fracture, diabetes and heart disease, in the chronic phase after injury (≥2 years). Significant bone and muscle losses1–4 alongside increases in fat mass and inflammatory stress5 in the first 3–6 months following motor-complete SCI are linked to an increased risk of sublesional osteoporosis6 and lower-extremity fragility fracture.7 Lower-extremity fracture risk among individuals with chronic SCI is estimated to be ∼25–46%, which is higher than 10-year fracture risk estimations in the general population.8 Lower-extremity fragility fractures contribute to an increased risk of morbidity and mortality, secondary complications (e.g. pressure sores), and significant hospitalization and attendant care costs.9 Individuals with SCI may also have a clustering of cardiometabolic risk factors, including but not limited to hyperlipidemia,10,11 reduced high-density lipoprotein cholesterol (HDL-C),12,13 insulin resistance,11 hypertension,14 and elevated C-reactive protein (CRP).15,16 Myocardial infarction, type II diabetes, stroke, and cardiac death have been shown to occur in individuals with SCI years before their age-matched non-SCI peers, without major differences in life expectancy.17
Declines in areal bone mineral density (BMD) occur rapidly at the hip, distal femur and proximal tibia between 1.1% to 47% within the first year3,6,18 and up to 73% by 24–84 months post-SCI.1,4,19–21 Reductions in trabecular and cortical volumetric BMD at the tibia occur progressively and persist for more than three years following SCI,4 compromising bone structure and increasing the annual risk of fragility fracture.22 Hip and knee region areal BMD are indicators of fracture risk, such that every one standard deviation (SD) decrease in femoral neck and distal femur areal BMD below the young adult mean is associated with up to two times greater risk of fracture.7,23 Additionally, declines in muscle cross-sectional area (CSA) (∼50% decrease), preferential atrophy of type I fibers, and increased fatty infiltration of muscle contribute to mitochondrial deficiency and sarcopenic obesity after SCI.9 Excessive visceral adipose tissue (VAT) is a known risk factor for insulin resistance, glucose intolerance, and cardiovascular disease,24 and is linked with all-cause mortality. Thus, rehabilitation interventions that specifically target improvements in BMD and bone turnover, and reduction in VAT and intramuscular fat infiltration may mitigate risk progression for fragility fractures, diabetes, and heart disease in individuals with chronic SCI.
Rehabilitation interventions after SCI typically involve neuromuscular stimulation and weight-bearing exercise along with nutritional modification to attenuate muscle and bone loss and cardiometabolic disease risk. Neuromuscular electrical stimulation (NMES) elicits muscle contractions in cyclic patterns at the lower-extremity (e.g. quadriceps, hamstrings, glutei) followed by a functional electrical stimulation (FES) training regimen, such as walking, cycling, or rowing. Passive or active weight-bearing activity (e.g. tilt-table, standing frame, robotic walking, body-weight supported treadmill training (BWSTT))25,26 and whole-body vibration27 have shown promising results for attenuating muscle atrophy, reducing fat mass, and improving BMD after SCI. Aerobic exercise training has been evaluated for efficacy/effectiveness to modify aerobic capacity and fitness, muscular performance, and to a lesser extent, cardiometabolic health.28,29 Modification of dietary intake is necessary for weight loss in individuals with SCI, due to the high prevalence of obesity and related secondary complications. Nutrition intervention may also be needed to address nutrient/vitamin deficiencies, with important implications for health status and chronic disease prevention. However, community-based rehabilitation interventions with the potential to modify the morbidity and mortality associated with EMD risk in the chronic phase of SCI remain elusive, with limited data and consensus on efficacy/effectiveness to improve outcomes of interest.
Few studies have focused on evaluating community-based strategies to combat modifiable precursors to EMD risk in individuals with chronic SCI, versus a larger body of evidence that has linked acute and sub-acute inpatient rehabilitation to functional recovery and neurorepair. It is unclear to what extent improving EMD risk translates to reductions in risk for fragility fractures, heart disease, and diabetes; in turn, these conditions impact functional capacity, social participation, and life satisfaction in community-dwelling adults living and aging with chronic SCI. Thus, there is an urgent need to identify inter-professional community-based rehabilitation solutions to reduce EMD risk expression. The objectives of this systematic review and scoping perspective were to characterize rehabilitation interventions and summarize evidence on their efficacy/effectiveness to modify precursors to EMD risk in community-dwelling adults with chronic SCI.
Methods
We used the framework proposed by Arksey and O’Malley30 to guide the methodology for Research Question 1. We used a systematic review approach in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement31 to guide the methodology for Research Question 2. The research questions and outcomes of interest are specified in Table 1. The RIISC team met in December 2015 (Toronto, ON, Canada) and February 2016 (Montreal, QC, Canada) and defined the key concepts, target population, interventions, and outcomes of interest, that are reflected in the two research questions. The RIISC team identified the need to evaluate rehabilitation interventions with high-level evidence to prevent or treat modifiable precursors to EMD risk in community-dwelling adults with chronic SCI.
Table 1.
Research questions and outcomes of interest.
| Research question | Outcomes of interest |
|---|---|
| 1. What types of rehabilitation interventions have been evaluated for efficacy/effectiveness in the prevention or treatment of precursors to EMD risk (i.e., fragility fractures, diabetes, heart disease) in community-dwelling adults with chronic SCI? | Types of rehabilitation interventions including:
|
| 2. What is the efficacy/effectiveness of rehabilitation interventions on precursors to EMD risk (i.e., fragility fractures, diabetes, heart disease) in community-dwelling adults with chronic SCI? | Effects on precursors related to EMD conditions:
|
Note: EMD = Endocrine-metabolic disease; SCI = Spinal cord injury; BMD = Bone mineral density.
Study eligibility criteria for this review
Types of studies
Articles published in the peer-reviewed, scientific literature in English or in French in a full-text version in the year 2000 or later were considered for inclusion in this review. We excluded literature published before the year 2000 to identify the most applicable and best level of evidence related to rehabilitation interventions to reduce EMD risk after chronic SCI. Experimental randomized controlled trials (RCTs), quasi-experimental/non-randomized or prospective controlled trials or cohort studies using at least two groups with one exposed to a condition (Spinal Cord Injury Rehabilitation Evidence (SCIRE)- Levels 1 and 2) were included.32 All other study designs or types of articles, i.e. longitudinal single-group trials, prospective or cross-sectional observational studies, case studies, case series (N<5 subjects), clinical commentaries, reviews, editorials, interviews, lectures, legal cases, letters, newspaper articles, patient education handouts, or unpublished literature, were excluded.
Types of study participants
To be eligible for inclusion in the review, study participants must have been adults (18 years and older) with chronic SCI (a mean duration of injury ≥12 months), and resided in the community defined as a community-based independent living model typically after discharge from an institutionally-based physical restoration model. We included studies involving men and/or women with traumatic or non-traumatic SCI (American Spinal Injury Association Impairment Scale (AIS) A-D equivalent), and individuals whom were able or not able to ambulate.
Types of study interventions
Eligible rehabilitation interventions for EMD risk included: physical or occupational therapy; exercise or physical activity; mobility, transfer, or falls prevention training; NMES or FES; vibration; robotics/exoskeleton devices; nutritional prescription; and dietary supplementation, specifically, calcium, vitamin D, omega 3 fatty acid, alpha-lipoic acid or coenzyme Q10. Studies must have evaluated the efficacy/effectiveness of the intervention to modify precursors to EMD risk (i.e. fracture, diabetes, heart disease). Rehabilitation was defined in accordance with the Canadian SCI rehabilitation programs,32,33 SCI US Model System rehabilitation programs,34 and the World Health Organization.35
Types of outcome measures related to EMD risk
Eligible studies evaluated the effects of a rehabilitation or therapeutic intervention on EMD risk outcomes in adults with chronic SCI (Table 2). Primary outcomes included incident fragility fractures, diabetes mellitus, heart failure, myocardial infarction, hypertension, and adverse events related and unrelated to the intervention. Secondary outcomes included BMD, bone turnover markers, body composition/adiposity, bone microarchitecture and bone geometry, skeletal muscle and fat CSA, mass, or density, muscle fiber type composition, biomarkers of lipid and carbohydrate metabolism, diastolic and systolic blood pressure (DBP, SBP), anthropometric measures, and inflammatory, antioxidant, and other endocrine biomarkers.
Table 2.
Primary and secondary outcomes of interest related to endocrine-metabolic disease risk (EMD).
| Outcomes of interest |
| Primary outcomes |
|
| Secondary outcomes |
|
Note: HF = Heart failure; MI = Myocardial infarction; BMD = Bone mineral density; DXA = Dual energy X-ray absorptiometry; pQCT = Peripheral quantitative computed tomography; DBP = Diastolic blood pressure; SBP = Systolic blood pressure.
Electronic search for identification of studies
Systematic searches for peer-reviewed articles were conducted in the following licensed databases: MEDLINE PubMed, EMBASE Ovid, CINAHL, Cochrane Database of Systematic Reviews/Cochrane Clinical Trials Registry, and PsychInfo. All searches were conducted from database inception to May 16, 2016. The searches were limited to full-text articles in English and French. The search strategies used text and indexing terms to capture the key concepts: SCI, rehabilitation or therapeutic interventions, and outcomes related to precursors to EMD (i.e. fractures, diabetes, heart disease) (Supplementary Table 1). Concepts were combined using the Boolean Operator AND, and the search terms within each concept were combined with OR. Keywords were searched using truncation and phrase symbols when appropriate to ensure precise and comprehensive results. One final search strategy was used for each database.
Data collection and analysis
Selection of studies
Two team members (JG, AB, TC or other RIISC team members) independently reviewed the title, abstract, and descriptors of each identified citation and applied the eligibility criteria during Level 1 screening. If there was insufficient information to make an informed decision, the full-text article was retrieved and further screened for inclusion at Level 2. Two team members (JG, JA, or RE) then independently assessed all full-text articles for inclusion by applying the eligibility criteria again. Following Level 2 screening, disagreement was resolved through consensus or third-party adjudication. All abstract and full-text screening was performed using the Covidence online systematic review platform (Veritas Health Innovation Ltd, Australia, 2016).
Data extraction/management
Data were independently abstracted from each of the included studies by two team members (JG, AB, JA, TC, RE, or other RIISC team members) using the registered Covidence online systematic review platform (Veritas Health Innovation Ltd, Australia, 2016) (Table 3). Risk of bias was assessed independently by two review authors (JG, AB, JA, TC, RE, or other RIISC team members). The Cochrane Collaboration’s Risk of Bias tool was used to evaluate each study.36 Each study was reviewed for the presence or absence of each criterion, and coded for risk of bias as low, unclear/uncertain or high risk. Disagreements regarding abstraction and risk of bias were resolved by consensus or third-party adjudication.
Table 3.
Data extraction for research questions.
| Research questions | Data to be extracted |
|---|---|
| Descriptive information |
|
| Research question 1: interventions |
|
| Research question 2: efficacy/effectiveness |
|
Data analysis
Mean differences and SD values were reported for continuous outcomes. Risk ratios and the corresponding 95% confidence intervals (95% CI) would have been reported for binary outcomes, but there were none to report. Study results were grouped by type of intervention (exercise or nutrition). χ2 test and I2 statistic would have been used to quantify any unexplained heterogeneity, where an I2 of less than 25% was considered low heterogeneity, an I2 of 25 to 50% was considered moderate heterogeneity and an I2 greater than 50% was considered high heterogeneity.36 However, there was an insufficient number of studies to pool results of rehabilitation intervention with comparable outcomes using a random-effects approach (95% CI).36 In almost all cases, data were not pooled because of the heterogeneity across studies, the low number of studies, or the lack of available data within the selected articles. The GRADE approach was used to rate the quality of the body of evidence for each outcome of importance.36
Results
The search identified 9,623 records following removal of duplicates and articles published before 2000 (Fig. 1). After reviewing the titles and abstracts, 489 full-text articles were retrieved. Full-text screening identified 16 articles for inclusion in our synthesis.
Figure 1.
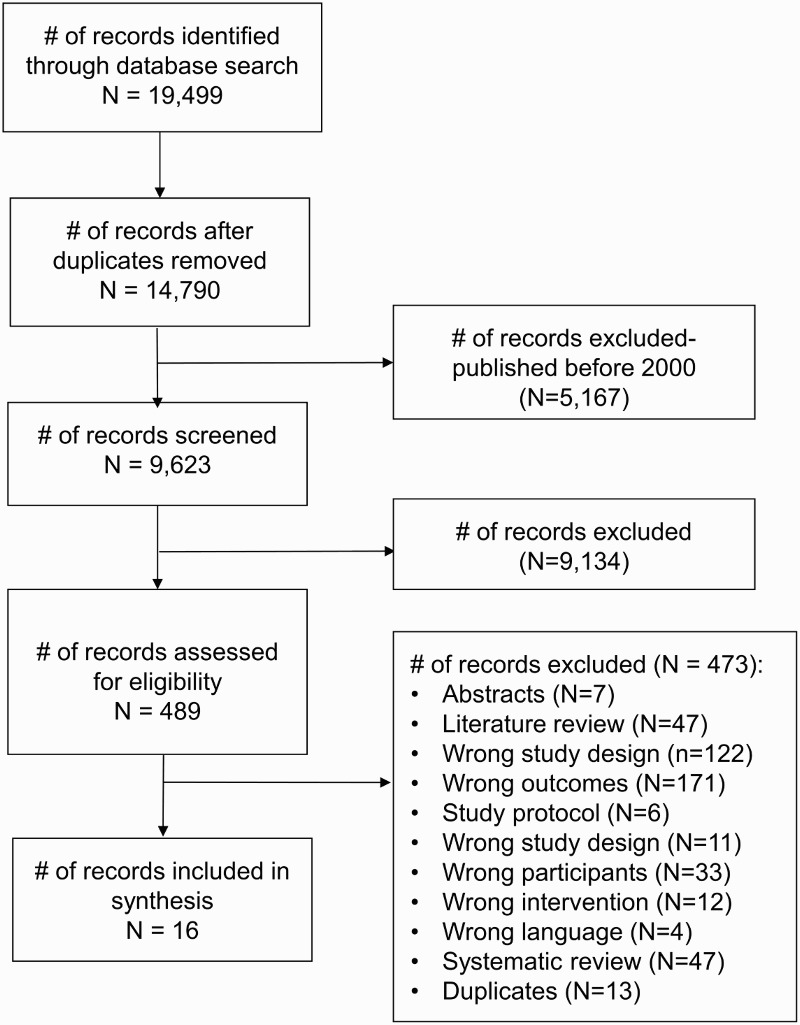
PRISMA study flow diagram.
Included studies
Sixteen full-text English articles (11 studies) with a total of 396 participants were eligible for inclusion. Of these eleven studies, four exercise studies and three nutrition studies included women. Four studies were from North America,37–41 two studies were from Europe,42–44 four studies were from Asia,45–49 and one study was from South America.50–52 Low risk of bias was found across most domains for 9 articles, and unclear/high risk of bias was found across most domains for 7 articles (Tables 4 and 5).
Table 4.
Summary of risk of bias from randomized or quasi/non-randomized controlled trials of exercise/physical activity interventions to modify EMD risk in community-dwelling adults with chronic SCI.
| First Author, Year | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Bakkum 201542 | Low | Low | High | Low | High | Low |
| de Abreu 200650 | High | High | High | Unclear | Low | Low |
| de Abreu 200852 | High | High | High | Unclear | Low | Unclear |
| de Abreu 200951 | High | High | High | Unclear | Low | High |
| Giangregorio 201237 | Low | Low | High | Low | Low | Low |
| Gorgey 201239 | Low | Unclear | High | Unclear | Low | Low |
| Gorgey 201338 | Low | Unclear | High | Unclear | Low | Low |
| Kim 201545 | Low | Low | High | Unclear | Low | Low |
| Ordonez 201343 | Unclear | Low | High | Unclear | Unclear | High |
| Rosety-Rodriguez 201444 | Unclear | Low | High | Unclear | Unclear | Low |
| Totosy de Zepetnek 201540 | Low | Low | High | Unclear | Low | Low |
Note: EMD = Endocrine-metabolic disease; SCI = Spinal cord injury.
Table 5.
Summary of risk of bias from randomized or quasi/non-randomized controlled trials of nutrition interventions to modify EMD risk in community-dwelling adults with chronic SCI.
| First Author, Year | Sequence generation | Allocation concealment | Blinding of participants & personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Allison 201541 | Low | Low | High | Unclear | Low | Low |
| Mohammadi 201546 | Low | Low | Low | Unclear | Low | Low |
| Sabour 201548 | Low | Unclear | Low | Unclear | Low | Low |
| Sabour 201549 | Low | Unclear | Unclear | Unclear | Unclear | Unclear |
| Sabour 201247 | Low | Unclear | Unclear | Unclear | Low | Unclear |
Note: EMD = Endocrine-metabolic disease; SCI = Spinal cord injury.
Excluded studies
Four hundred and seventy-three full-text journal articles identified during the search were not included in the review. The majority were excluded because they did not measure outcomes relevant to EMD risk (n=171) or were not an RCT or quasi/non-RCT (n=122). Six articles were study protocols; 47 articles were literature reviews; and 47 articles were systematic reviews. Seven abstracts/conference proceedings were excluded. Twelve articles did not focus on an eligible intervention. Thirty-three articles studied individuals with SCI (<1 year post-injury) of whom were participating in an in-patient tertiary rehabilitation. Four articles were neither English nor French.
Study participant characteristics
All studies had chronic SCI (≥1 year post-injury) as an inclusion criterion, but the mean duration of injury (years) varied across studies. One study each included participants with mean duration of injury between 1–2 years38,39 and 2–5 years.43,44 Five and four studies included participants with a mean duration of injury between 5–10 years37,40,45,46,50–52 and >10 years, respectively.41,42,47–49 Three studies were in individuals with complete SCI only,43,44,46,50–52 two studies were in motor complete SCI only,38,39,45 one study was in incomplete SCI only,37 and five studies were in both complete and incomplete SCI.40–42,47–49 Four studies had injury level as an inclusion criterion: cervical only,50–52 cervical/thoracic only,37 thoracic/lumbar only43,44 or cervical/thoracic/lumbar.38,39 Seven studies did not specify injury level as an inclusion criterion.40–42,45–49 Two studies stated individuals must have the ability to ambulate;37,50–52 whereas, two studies included only individuals whom were wheelchair-dependent.38,39,42 Studies primarily included men (336/396 participants).38,39,43,44,46,50–52 However, seven studies included women, but to a lesser extent (60/396 participants).37,40–42,45,47–49 Five studies specified that the SCI must be of traumatic causes.37–39,43,44,47–49 Detailed participant characteristics and eligibility criteria are provided in Tables 6 and 7 and Supplementary Tables 2 and 3.
Table 6.
Summary of results from randomized or quasi/non-randomized controlled trials of exercise/physical activity interventions to modify EMD risk in community-dwelling adults with chronic SCI.
| Author, Year, Design | Methods | Results |
|---|---|---|
| Bakkum 201542
RCT Netherlands |
Participants: 18 men and 1 woman, age: 28–65 years with chronic SCI (≥10 years post-injury), wheelchair dependent Intervention: Hybrid FES cycling (n=9)- 2 d/wk (18–32 min/session), 16 weeks Comparator: Hand cycling (n=10)- 2 d/wk (18–32 min/session), 16 weeks Outcomes: WC; BP via manometer; lipid and carbohydrate metabolism, and inflammatory markers via blood analysis; visceral adiposity via DXA Time-points: Baseline and 16 weeks |
|
| de Abreu 200650
Non-randomized controlled trial Brazil |
Participants: 21 men, mean±SD age 32±8 yr with chronic complete tetraplegia Intervention: NMES-30–50% BWS treadmill training (n=10)- 2 times/week, 20 min/session, 24 weeks Comparator: Conventional physical therapy (n=20), 2 d/wk, 24 weeks Outcomes: Bone turnover markers via serum and urinary assay; lumbar spine, proximal femur, total femur BMD via DXA Time-points: Baseline and 24 weeks |
|
| de Abreu 200852
Non-randomized controlled trial Brazil |
Participants: 15 men, mean±SD age 32±8 yr with chronic complete tetraplegia (C4-C7), mean±SD DOI 66±48 months Intervention: NMES-30–50% BWSTT (n=8)-2 d/wk, 20 min/session, 24 weeks Comparator: Conventional physical therapy (n=20), 2 d/wk, 24 weeks Outcomes: Quadriceps CSA via MRI Time-points: Baseline and 24 weeks |
|
| de Abreu 200951
Non-randomized controlled trial Brazil |
Participants: 15 men, mean±SD age 32±8 yr with chronic complete tetraplegia (C4-C7), mean±SD DOI 66±48 months Intervention: NMES-30–50% BWS treadmill training (n=8)- 2 times/week, 20 min/session, 24 weeks Comparator: Conventional physical therapy (n=20), 2 d/wk, 24 weeks; followed by 30–50% BWSTT (without NMES), 24 weeks Outcomes: Quadriceps CSA via MRI Time-points: Baseline, 6 months, 12 months (controls only) |
|
| Giangregorio 201237
RCT Canada |
Participants: 26 men and 9 women, mean age 55±15 yr with chronic traumatic, incomplete SCI (mean±SD DOI 10±10 yr, C2-T12) Intervention: FES-BWSTT (n=17), 3 d/wk, 45 min/session for 16 weeks Comparator: Active control (n=17), tailored progressive exercise program 3 times/week, 45 min/session for 16 weeks Outcomes: Calf muscle and fat CSA via pQCT; total body, leg fat and lean mass via DXA Time-points: Baseline and 12 months |
|
| Gorgey 201239
Pilot RCT United States |
Participants: 9 adult men, age: 18–50 yr with chronic traumatic motor complete SCI (>1-yr post-injury, C5-L1), wheelchair reliant Intervention: NMES-assisted lower-extremity resistance training, 2 d/wk + diet (45% carbohydrates, 30% fat, 25% protein) (n=5) for 12 weeks Comparator: Diet (45% carbohydrates, 30% fat, 25% protein) (n=4) for 12 weeks Outcomes: Anthropometric outcomes; thigh, knee muscle CSA, thigh, trunk SAT CSA, trunk VAT CSA, intramuscular fat via MRI; total, trunk, leg fat and fat-free mass via DXA; lipid and carbohydrate metabolic markers via blood analysis Time-points: Baseline and 12 weeks |
|
| Gorgey 201338
Pilot RCT United States |
Participants: 7 adult men, age: 18–50 yr with chronic traumatic motor complete SCI (>1-yr post-injury, C5-L1), wheelchair dependent Intervention: NMES-assisted lower-extremity resistance training, 2 d/wk + diet (45% carbohydrates, 30% fat, 25% protein) (n=4) for 12 weeks Comparator: Diet (45% carbohydrates, 30% fat, 25% protein) (n=3) for 12 weeks Outcomes: Thigh and trunk muscle CSA by MRI Time-points: Baseline and 12 weeks |
|
| Kim 201545
RCT Korea |
Participants: 9 men and 6 women, mean age 33±5 yr with chronic SCI (AIS A/B, 2–16 yr post-injury, C5-T11) Intervention: Indoor hand cycle ergometry (n=8), 3 d/wk, 6 weeks Comparator: Control (n=7) Outcomes: Anthropometric outcomes; body composition via BIA; lipid and carbohydrate metabolic markers via blood sample analysis Time-points: Baseline and 6 weeks |
|
| Ordonez 201343
RCT Spain |
Participants: 17 men, age: 20–35 years with chronic traumatic complete SCI below T5 (4–5 years post-injury) Intervention: Arm cycle ergometry (n=9), 3 d/wk, 35–55 min/session, 12 weeks Comparator: Control (n=8) Outcomes: Anthropometric outcomes; antioxidant, lipid and protein oxidation markers via blood sample analysis Time-points: Baseline and 12 weeks |
|
| Rosety-Rodriguez 201444
RCT Spain |
Participants: 17 men, age: 20–35 years with chronic traumatic complete SCI below T5 (4–5 years post-injury) Intervention: Arm cycle ergometry (n=9), 3 d/wk, 35–55 min/session, 12 weeks Comparator: Control (n=8) Outcomes: WC; testosterone, estradiol, luteinizing hormone, and follicular stimulating hormone via blood sample analysis Time-points: Baseline and 12 weeks |
|
| Totosy de Zepetnek 201540
RCT Canada |
Participants: 21 men and 2 women, mean age 41±12 years with chronic SCI (AIS A-C, mean DOI 12±10 years, C3-T11) Intervention: Physical activity guidelines training (n=12), aerobic + resistance exercise 2 d/wk, 60 min/session for 16 weeks Comparator: Active control (n=11) Outcomes: Blood pressure via manometer; Hb1ac and lipid profile via blood sample analysis; anthropometric outcomes; total body fat and lean mass, and visceral adiposity via DXA; adiponectin, leptin, and inflammatory cytokines via blood sample analysis Time-points: Baseline and 16 weeks |
|
EMD= Endocrine-metabolic disease; SCI = Spinal cord injury; DOI = Duration of injury; AIS = American Spinal Injury Association impairment scale; RCT = Randomized controlled trial; MD= Mean difference of change or percentage change from baseline; ES = Effect size; NMES = Neuromuscular electrical stimulation; FES = Functional electrical stimulation; BWSTT = Body weight-supported treadmill training; BMI = Body mass index; WC = Waist circumference; VAT = Visceral adipose tissue; SAT = Subcutaneous adipose tissue; DBP = Diastolic blood pressure; IL-6 = Interleukin=6; IL-10 = Interleukin-10; CSA = Cross-sectional area; IMF = Intermuscular fat; DXA = Dual energy X-ray absorptiometry; MRI = Magnetic resonance imaging; BIA = Bioelectrical impedance analysis; HOMA-IR= Homeostatic model assessment of insulin resistance; Hb1ac = Glycated hemoglobin; AUC = Area under the curve.
Table 7.
Summary of results from randomized or quasi/non-randomized controlled trials of nutrition interventions to modify EMD risk in community-dwelling adults with chronic SCI.
| First author, Year | Methods | Results |
|---|---|---|
| Allison 201541
RCT Canada |
Participants: 10 men and 10 women, mean age 48.7±13.9 yr with chronic SCI (4–37 yr post-injury) between C2-L4 Intervention: Anti-inflammatory diet (n=12) for 12 weeks Comparator: Control (n=8) Outcomes: Inflammatory markers and blood glucose via blood sample analysis Time-points: Baseline and 12 weeks |
|
| Mohammadi 201546
Double-blind RCT Iran |
Participants: 58 men, age 30–50 yr with chronic complete SCI (1–10 yr post-injury) Intervention: Alpha-lipoic acid supplementation (n=28) for 14 months Comparator: Placebo (n=30) Outcomes: Lipid profile and glucose via blood analysis Time-points: Baseline and 14 months |
|
| Sabour 201548
Double-blind RCT Iran |
Participants: 85 men and 19 women, mean age 51.1±13.4 yr (treatment) and 54.1±11.7 yr (placebo) with chronic traumatic SCI (>1-yr post-injury) Intervention: Omega-3 polyunsaturated fatty acid supplementation (n=54) for 14 months Comparator: Placebo (n=50) Outcomes: Lipid profiles and blood glucose via blood sample analysis, anthropometric outcomes Time-points: Baseline and 14 months |
|
| Sabour 201549
Double-blind RCT Iran |
Note: Participants, intervention, comparator (see above) Outcomes: Leptin, adiponectin via blood sample analysis Time-points: Baseline, 6, and 14 months |
|
| Sabour 201247
Double-blind RCT Iran |
Participants: 69 men and 13 women, mean age 40±15 yr (treatment) and 38±12 yr (placebo) with chronic traumatic SCI (>1-yr post-injury) Intervention: n-3 polyunsaturated fatty acids + calcium w/ vitamin D (n=43) for 16 weeks Comparator: Placebo + calcium w/ vitamin D (n=39) Outcomes: Inflammatory markers via blood analysis Time-points: Baseline and 16 weeks |
|
EMD= Endocrine-metabolic disease; SCI = Spinal cord injury; RCT = Randomized controlled trial; MD= Mean difference of change or percentage change from baseline; TRP/LNAA = Trytophan/large neutral amino acids; IL-β = Interleukin-beta; IFN-ϒ = Interfeuron-gamma; BMI = Body mass index; WC = Waist circumference; BP = Blood pressure
Research Question 1: Study Interventions
There was considerable diversity in the frequency, intensity, time, type, length, setting, and co-interventions of the rehabilitation interventions, and comparator groups (Tables 6 and 7, Supplementary Tables 2 and 3).
Exercise Interventions
Seven studies involved an exercise intervention37–40,42–45,50–52 with reported exercise frequency of 2 times/week38,39,42,45,50–52 or 3 times/week.37,40,43,44 All seven exercise studies reported a specific intensity based on rating of heart rate, perceived exertion, or 1-repetition maximum.37–40,42–45,50–52 Exercise interventions were of a short or moderate length: 4–12 weeks38,39,43–45 and 16–24 weeks,37,40,42,50–52 with no intervention >24 weeks in length. All exercise studies were delivered in an outpatient rehabilitation facility, clinical research setting, or community centre.37–40,42–45,50–52 Study supervision varied with one study each led by a physiotherapist,50–52 trainer/exercise instructor,42,45 kinesiologist,37 and research staff.43,44 Three studies did not clarify the type and level of supervision.38–40 Two studies reported adherence data, wherein 88% and 98% of the 16-week intervention sessions were completed.37,40 Three studies reported 100% adherence.38,39,42,45 However, no data were provided in those studies, and the interventions were ≤16 weeks in length. One study did not report their adherence data, and stated that only participants whom attended 90% of intervention sessions were eligible for study inclusion,43,44 while another failed to report adherence altogether.50–52
Nutrition interventions
Four studies focused on a nutrition intervention or supplementation.41,46–49 One study involved an anti-inflammatory diet, excluding foods with high glycemic indices, and provision of daily supplements with anti-inflammatory benefits (e.g. omega-3, chlorella, coenzyme-Q10) and vegetable-based protein powder.41 One study involved alpha-lipoic acid supplementation.46 Two studies involved omega-3 polyunsaturated fatty acid supplementation.47–49 All nutrition interventions were held in a clinical research environment or community centre. Only one intervention reported supervision by a dietician.41 One study reported 89% adherence (70–100%) to their 12-week anti-inflammatory intervention.41 One study reported 80% adherence to omega-3 supplementation over 16 weeks47; whereas, another study did not report adherence to 14-month omega-3 supplementation.48,49 One study excluded participants if their adherence was <80% over 14-month alpha lipoic acid supplementation.46
Research Question 2a: Outcomes
The outcomes and associated measures evaluated from the included studies, and their duration of follow-up after baseline assessment are reported in Tables 6 and 7, and in Supplementary Tables 2 and 3.
No studies measured incident fragility fractures, heart disease, and/or diabetes. Four studies reported on adverse events.37,40,41,47 Limited data on BMD and bone turnover markers were identified, with only one study reporting on lumbar (L1-L4) spine, proximal femur, and total hip BMD via DXA and bone turnover markers via serum and urinary assays.50 No studies reported on bone microarchitecture and bone geometry measured by peripheral quantitative computed tomography (pQCT). Body composition, muscle CSA, and adiposity data were more frequently reported; yet, the measures (DXA n=4 studies; pQCT, n=1 study; BIA, n=1 study; or MRI, n=2 studies), sites (whole body versus regional), and outcome units (absolute versus percentages) varied across studies.37–40,42,45,51,52 No studies measured muscle fiber type composition.
Lipid profiles,39,40,42,45,47 glucose metabolism/insulin resistance,39,42,45,46,48 waist circumference,40,42,44–46 and BMI39,40,43,45,46 were the most frequently reported cardiometabolic risk outcomes. Resting SBP and DBP,40,42,46 body weight,39,46 and anthropometry index43 were reported on to a lesser extent. Five studies measured inflammatory markers via blood serum analysis.40–42,46,47 Two studies measured plasma leptin and adiponectin.40,49 Individual studies measured endocrine-metabolic biomarkers, including: glycated hemoglobin (Hb1ac) and plasminogen activator inhibitor-1 (PAI-1),40 malondialdehyde as a marker of lipid peroxidation,43 plasmatic carbonyl group level as a marker of protein metabolism,43 erythrocyte GPX activity,43 total antioxidant status,43 follicular stimulating hormone,44 luteinizing hormone,44 testosterone,44 estradiol,44 amino acids (tryptophan, phenylalanine, tyrosine)41 and branched-chain amino acids (valine, leucine, isoleucine) via blood serum analysis.41
Research Question 2b: Efficacy/Effectiveness of Interventions
A summary of the results related to the efficacy/effectiveness of rehabilitation interventions to modify EMD risk in community-dwelling adults with chronic SCI was reported in Tables 6 and 7. The results of the higher quality studies ≥16 weeks (low risk of bias, adequate follow-up and sample size) were reported below.
Primary outcomes
Since no studies measured incident fragility fractures, diabetes, and/or heart disease, the efficacy/effectiveness of rehabilitation interventions to modify the incidence of EMD clinical end-points in community-dwelling adults with chronic SCI was not estimable. Adverse events were reported for two 16-week exercise intervention studies. Seven adverse events were reported during a FES-BWSTT intervention, including bruising/blistering in groin area (n=2); loss of footing on treadmill, no fall (n=1), fall (n=1), sharp pain in left heel/ankle (n=1), pain/discomfort in the hip/groin area (n=1), and one was unknown. Five adverse events were reported in the control group (fainting/loss of consciousness, pectoral muscle strain, swollen knees, left elbow pain, dizziness/ringing ears, n=1 each).37 One adverse event unrelated to the intervention was reported during physical activity guideline training.40 No adverse events were reported in association with the 12-week anti-inflammatory diet.41 Gastrointestinal problems were reported in two participants taking omega-3 supplementation for 16 weeks47 and an unknown number of individuals taking omega-3 supplementation for 14 months.48,49 No other studies reported adverse events.
BMD and bone turnover markers
de Abreu et al. evaluated the efficacy of a 24-week NMES-treadmill training intervention (2 times/week, 20 min/session) to modify outcomes related to BMD and bone turnover outcomes.50 However, de Abreu et al. did not statistically evaluate between-group differences for these outcomes, and therefore, the effects of rehabilitation interventions on BMD and bone turnover markers in community-dwelling adults with chronic SCI were not estimable.
Body composition/adiposity
There was very low-quality evidence that rehabilitation interventions can improve body composition/adiposity in community-dwelling adults with chronic SCI (downgraded due to imprecise estimates, important inconsistency, and limitations to study quality). For DXA-measured outcomes, Totosy de Zepetnek et al. observed a significant interaction effect (group x time) for whole body mass (data values not reported, effect size=1.07, P = 0.03), fat mass (data values not reported, effect size=1.00, P = 0.04), and VAT (data values not reported, effect size=1.02, P = 0.04) after 16 weeks adhering to the physical activity guideline training.40 However, Totosy de Zepetnek et al. found non-significant changes in lean mass or leg fat mass.40 Giangregorio et al. found non-significant differences in DXA-measured whole body fat mass and lean mass, and leg lean mass in intention-to-treat and per-protocol analyses after 16 and 52 weeks.37 For pQCT-measured outcomes, Giangregorio et al. found a significant between-group difference for pQCT-measured calf muscle CSA (intervention mean change±SD: 212±517 mm2 versus control mean change±SD: –136±268 mm2, P = 0.026) after 52 weeks of FES-BWSTT intervention, but not after 16 weeks (P = 0.083).37 Also, there were no significant interaction (group x time) or main effects (time) for pQCT-measured calf fat CSA (P > 0.05) in intention-to-treat and per-protocol analyses.37 No studies evaluated the efficacy of rehabilitation interventions to change muscle fiber type composition.
Cardiometabolic risk-related outcomes
There was very low-quality evidence that rehabilitation interventions can improve cardiometabolic risk-related outcomes in individuals with chronic SCI (downgraded due to imprecise estimates, important inconsistency, and limitations to study quality). For lipid profile outcomes (triglycerides, HDL-C, LDL-C, C/HDL-C ratio), Totosy de Zepetnek et al. found no statistically significant interaction (group x time) or main effects (time) after 16 weeks.40 Totosy de Zepetnek et al. also reported no statistically significant interaction (group x time) or main effects (time) for SBP and DBP after 16 weeks.40 However, Totosy de Zepetnek et al. found a significant between-group difference for BMI (intervention mean change: –0.3 kg/m2 versus control mean change: 0.9 kg/m2, P = 0.02) and waist circumference (intervention mean change: -1.0 cm versus control mean change: 3.5 cm, P = 0.02) after 16 weeks.40
Inflammatory, antioxidant, and other endocrine biomarkers
There was very low-quality evidence that rehabilitation interventions can improve inflammatory, antioxidant, and other endocrine biomarkers in community-dwelling adults with chronic SCI (downgraded due to imprecise estimates, important inconsistency, and limitations to study quality).
Allison and Ditor demonstrated a significant interaction (group x time) effect for plasma tryptophan/large neutral amino acids ratio (intervention mean change: 41.8 versus control mean change: –35.1, Cohen’s d=0.90, P = 0.04) over the 12-week anti-inflammatory intervention.41 There were no significant interaction or between-group effects for any other serum amino acids after 12 weeks. Allison and Ditor found a significant interaction (group x time) effect for the composite score of pro-inflammatory cytokines (P=0.04), interleukin-1-beta (intervention mean change: –0.6 pg/mL versus control mean change: 0 pg/mL, P = 0.04), and interfeuron-gamma (intervention mean change: –17.9 pg/mL versus control: 21.5 pg/mL, P = 0.03).41 There were no significant interaction or between-group effects for any other inflammatory markers after 12 weeks. Totosy de Zepetnek et al. found no significant interaction (group x time) and main effects (time) for metabolic biomarkers (Hb1ac, PAI-1, leptin, and adiponectin) (P > 0.05) after 16 weeks.40 Totosy de Zepetnek et al. found no significant interaction (group x time) or main effects (time) for inflammatory biomarkers (tumour necrosis factor-alpha, interleukin-6) (P > 0.05) after 16 weeks.40
Discussion
Our systematic review and scoping perspective provides a comprehensive synthesis of 11 RCTs or quasi/prospective controlled trials from 16 articles that evaluated the effects of rehabilitation interventions on EMD risk among adults with chronic SCI. Despite the plethora of rehabilitation interventions, few studies have been adequately powered or of sufficient duration to determine efficacy for reducing EMD risk in the chronic phase after injury (≥2 years).37,40,41 No trials assessed the efficacy/effectiveness of exercise or nutrition interventions to reduce incident fragility fractures, diabetes, or heart disease, and therefore, the effect was not estimable. However, there was potentially a lower likelihood of detecting incidence of the primary outcomes across studies, due to the smaller sample sizes and shorter study timeframes (<12 months follow-up). Among the studies with similar outcomes and adequate duration of follow-up, not all studies were affirmative for changes in EMD risk, contrary to the investigators’ a priori hypotheses. Overall, there was very low-quality evidence that rehabilitation interventions can improve secondary outcomes related to EMD risk, especially BMD and bone turnover markers. Some adverse events were directly associated with the interventions, yet reporting of harms was negligible, inconsistent or absent. Adherence to interventions varied across studies; adherence appeared higher among studies that were shorter-term (≤16 weeks) and included structured supervision by health professionals with exercise/nutrition expertise. Also, there was a lack of evidence regarding the effects of vibration and robotic treadmill/over-ground interventions to modify precursors to EMD risk.
Despite identifying numerous studies of rehabilitation interventions targeting EMD risk in individuals with chronic SCI, few interventions made high-quality inferences and had adequate duration of follow-up and sample size.37,40,41 Giangregorio et al. demonstrated that FES-BWSTT was not associated with changes in whole-body and regional body composition in individuals with incomplete SCI after 4 months.37 However, lower-extremity muscle CSA was maintained after 12-month follow-up. Alternatively, Totosy de Zepetnek et al. found that physical activity guidelines counseling was linked to favorable changes in whole-body fat mass and VAT in adults with chronic SCI after 4 months, but not other cardiometabolic risk-related outcomes.40 Both exercise interventions were of similar length and sample size (n=23–37) with a focus on weight-bearing, moderate-to-vigorous aerobic training (2–3 times/week, 20–45 min/session) and had acceptable adherence levels (88–98%).37,40 However, the physical activity guidelines counseling intervention also involved upper-body strengthening exercises (≥2 times/week), which likely contributed to greater improvements in adiposity. Allison and Ditor showed favourable changes in inflammatory and serum amino acid biomarkers following a 12-week anti-inflammatory diet in individuals with chronic SCI,41 with adequate adherence (89%). Future RCTs or prospective controlled trials should consider implementing similar exercise and nutrition interventions for longer durations in larger samples and conducting a broad evaluation of the effects of community-based rehabilitation interventions on EMD risk outcomes.
Our review highlights the lack of consensus in body composition/adiposity measures and cardiometabolic biomarkers across studies, and the need to determine SCI-specific clinometric properties for these outcomes. Although muscle atrophy and fat accumulation have been well-documented after motor-complete SCI,2,5 relevant thresholds or degrees of muscle loss or fat infiltration associated with EMD risk have yet to be established. Standard BMI and waist circumference cut-off values used in the able-bodied population underestimate obesity in individuals with SCI.53 While the amount of VAT is correlated with waist circumference in able-bodied individuals, an increase in VAT is not correlated with an increase in waist circumference in individuals with SCI.54 As well, there is limited data to inform the sensitivity of bone turnover and cardiometabolic biomarkers55 to change in individuals with chronic SCI, which would be useful in assessing intervention effectiveness. Also, BMD and/or bone microarchitecture data are mostly absent from the included studies, likely due to measurement challenges (e.g. availability and cost of equipment, infrastructure, and expertise to support image analysis) and longer follow-up required.
The high/unclear risk of bias due to poor methodological rigor and small sample sizes of the included studies downgraded the quality of the evidence on the effects of rehabilitation interventions on EMD risk in individuals with chronic SCI. All exercise studies had a high risk of experimental and performance bias due to the inability to blind participants or personnel delivering the intervention. All studies were rated as unclear/high risk for bias for at least one other quality assessment criterion (i.e. allocation concealment, blinding of outcome assessment); issues which could be readily addressed. Several studies demonstrated possible selective reporting bias, such that two or more articles were published from the same dataset, and specific outcomes were reported in multiple instances, which may reflect system or funding pressures on authors. Although most studies were transparent in reporting incomplete outcome data (i.e. reasons for exclusion and loss to follow-up), the majority did not discuss how missing data were addressed, or more importantly whether the primary analysis was intent-to-treat or per-protocol. The quality of reporting data was not consistent for all outcomes, limiting our capacity to pool data and make conclusions. Few studies reported a sample size calculation.37,42 All studies, but one,48,49 had less than 100 participants, and only three studies had more than 50 participants (all dietary supplementation interventions).
Participant selection or inclusion, and study design eligibility influenced the quality and validity of our results. Variability within or between samples in sex, SCI severity (Neurological Level and ASIA Impairment Scale), duration of injury, comorbidities, ambulatory status, and medication use for osteoporosis, diabetes or another metabolic dysfunction, may have affected the observed effects and generalizability of the data. More studies evaluating intervention effects in women with chronic SCI as a single group or as a subgroup of a larger cohort, and in correlation with duration of injury are necessary to make any conclusive inferences. Also, the review was restricted to randomized/quasi-RCTs, and prospective controlled studies in an attempt to summarize the best evidence for our research questions (SCIRE– Level 1 and 2).32 We acknowledge that our review excludes a significant portion of the lower-level evidence on effects of community-based rehabilitation interventions to modify EMD risk in individuals with chronic SCI. Our exhaustive search will enable production of a similar review focusing on the pre-post trials, case-control studies, and case series (SCIRE– Level 3 and 4) to identify intervention opportunities, EMD risk outcomes sensitive to change, and gaps for future research.
In conclusion, there was very low-quality evidence that rehabilitation interventions can modify our secondary outcomes related to EMD risk (body composition, biomarkers) and no evidence that rehabilitation interventions can alter the incidence of EMD clinical end-points (fragility fractures, diabetes, heart disease) in community-dwelling adults with chronic SCI. The small number of studies, and heterogeneity in study design (participants, interventions, and outcomes) profoundly limited our ability to pool outcomes or make generalizable conclusions. Evidence regarding the effects of rehabilitation interventions on EMD risk after chronic SCI, particularly for women, is scarce. Adequately powered, high-quality, prospective exercise and nutrition interventions preferably RCTs (≥12 months) are needed to evaluate their effects alone or in combination for mitigating multi-morbidity associated with EMD risk in a representative sample of adults with chronic SCI. To date, superiority, equivalence, and non-inferiority trial designs have rarely been used and no design tools/algorithms exist to identify the best possible intervention for a given individual at a specific time. Future research should investigate whether multi-modal and inter-professional community-based rehabilitation solutions contribute to change in health outcomes across tissues, and quantify these effects on EMD risk, including biomarkers.
Supplementary Material
Acknowledgements
We acknowledge the contributions of members of the Rehabilitation Interventions for Individuals with a SCI in the Community Research Team (RIISC Team) whom many engaged in different stages and to different extents during the preparation of this manuscript. In particular, we thank Krista Best, Cindy Gauthier, Jean-François Lemay, Masae Miyatani, and Mohammad Alavinia for assisting with abstract screening, data extraction, and/or quality assessment. We are greatly appreciative of Maureen Pakosh for performing the systematic search of electronic databases. We also acknowledge Eleni Patsakos for her administrative and project coordination duties.
Disclaimer statements
Contributors None.
Funding This work was supported by the Ontario Neurotrauma Foundation (ONF) and the Réseau provincial de recherche en adaptation-réadaptation du Québec (REPAR) through the grant entitled “Rehabilitation based research and knowledge translation activities to modify health risks for individuals living with chronic spinal cord injury” (2015-SCI-REPAR-1010). Dr. Jenna Gibbs is supported by a Canadian Institutes of Health Research Fellowship Award. Dr. Tomas Cervinka is supported by a Fellowship Salary Support Award of Spinal Cord Injury-Ontario.
Declarations of Interest The authors report no declarations of interest.
Conflicts of interest None.
Ethics approval None.
Supplementary material
Supplementary Material for this article available here: 10.1080/10790268.2017.1350341
ORCID
B. Catharine Craven http://orcid.org/0000-0001-8234-6803
References
- 1.Biering-Sorensen F, Bohr HH, Schaadt OP.. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest 1990;20(3):330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x [DOI] [PubMed] [Google Scholar]
- 2.Castro MJ, Apple DF Jr., Hillegass EA, Dudley GA.. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 1999;80(4):373–8. doi: 10.1007/s004210050606 [DOI] [PubMed] [Google Scholar]
- 3.Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V.. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord 2000;38(1):26–32. doi: 10.1038/sj.sc.3100905 [DOI] [PubMed] [Google Scholar]
- 4.de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E.. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord 2005;43(2):96–101. doi: 10.1038/sj.sc.3101685 [DOI] [PubMed] [Google Scholar]
- 5.Gorgey AS, Dudley GA.. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007;45(4):304–9. [DOI] [PubMed] [Google Scholar]
- 6.Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, et al. Osteoporosis after spinal cord injury. J Orthop Res 1992;10(3):371–8. doi: 10.1002/jor.1100100309 [DOI] [PubMed] [Google Scholar]
- 7.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M.. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 2001;39(4):208–14. doi: 10.1038/sj.sc.3101139 [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L.. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 1998;36(11):790–6. doi: 10.1038/sj.sc.3100648 [DOI] [PubMed] [Google Scholar]
- 9.Pelletier CA, Dumont FS, Leblond J, Noreau L, Giangregorio L, Craven BC.. Self-report of one-year fracture incidence and osteoporosis prevalence in a community cohort of canadians with spinal cord injury. Top Spinal Cord Inj Rehabil 2014;20(4):302–9. doi: 10.1310/sci2004-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers J, Lee M, Kiratli J.. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 2007;86(2):142–52. doi: 10.1097/PHM.0b013e31802f0247 [DOI] [PubMed] [Google Scholar]
- 11.Bauman WA, Spungen AM.. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77. doi: 10.1080/10790268.2001.11753584 [DOI] [PubMed] [Google Scholar]
- 12.Gilbert O, Croffoot JR, Taylor AJ, Nash M, Schomer K, Groah S.. Serum lipid concentrations among persons with spinal cord injury - a systematic review and meta-analysis of the literature. Atherosclerosis 2014;232(2):305–12. doi: 10.1016/j.atherosclerosis.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 13.Libin A, Tinsley EA, Nash MS, Mendez AJ, Burns P, Elrod M, et al. Cardiometabolic risk clustering in spinal cord injury: results of exploratory factor analysis. Top Spinal Cord Inj Rehabil. 2013;19(3):183–94. doi: 10.1310/sci1903-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R.. Cardiovascular disease risk factors in persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med 2010;42(3):272–8. doi: 10.2340/16501977-0510 [DOI] [PubMed] [Google Scholar]
- 15.Liang H, Mojtahedi MC, Chen D, Braunschweig CL.. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil 2008;89(1):36–41. doi: 10.1016/j.apmr.2007.08.121 [DOI] [PubMed] [Google Scholar]
- 16.Garshick E, Stolzmann KL, Gagnon DR, Morse LR, Brown R.. Systemic inflammation and reduced pulmonary function in chronic spinal cord injury. PM R 2011;3(5):433–9. doi: 10.1016/j.pmrj.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–16. doi: 10.1038/sj.sc.3101729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garland DE, Adkins RH, Kushwaha V, Stewart C.. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 2004;27(3):202–6. doi: 10.1080/10790268.2004.11753748 [DOI] [PubMed] [Google Scholar]
- 19.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF.. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 2000;27(2):305–9. doi: 10.1016/S8756-3282(00)00326-4 [DOI] [PubMed] [Google Scholar]
- 20.de Bruin ED, Dietz V, Dambacher MA, Stussi E.. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil 2000;14(2):145–52. doi: 10.1191/026921500670532165 [DOI] [PubMed] [Google Scholar]
- 21.Eser P, Frotzler A, Zehnder Y, Denoth J.. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil 2005;86(3):498–504. doi: 10.1016/j.apmr.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 22.Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15(3):180–9. doi: 10.1007/s00198-003-1529-6 [DOI] [PubMed] [Google Scholar]
- 23.Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, et al. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int 2014;25(1):177–85. doi: 10.1007/s00198-013-2419-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien LC, Gorgey AS.. Skeletal muscle mitochondrial health and spinal cord injury. World J Orthop 2016;7(10):628. doi: 10.5312/wjo.v7.i10.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E.. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil 1999;80(2):214–20. doi: 10.1016/S0003-9993(99)90124-7 [DOI] [PubMed] [Google Scholar]
- 26.Alekna V, Tamulaitiene M, Sinevicius T, Juocevicius A.. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord 2008;46(11):727–32. doi: 10.1038/sc.2008.36 [DOI] [PubMed] [Google Scholar]
- 27.Ji Q, He H, Zhang C, Lu C, Zheng Y, Luo XT, et al. Effects of whole-body vibration on neuromuscular performance in individuals with spinal cord injury: A systematic review. Clin Rehabil 2016. [DOI] [PubMed] [Google Scholar]
- 28.Rimaud D, Calmels P, Devillard X.. Training programs in spinal cord injury. Ann Readapt Med Phys 2005;48(5):259–69. doi: 10.1016/j.annrmp.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 29.Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL.. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord 2011;49(11):1103–27. doi: 10.1038/sc.2011.62 [DOI] [PubMed] [Google Scholar]
- 30.Arksey H, O'Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8(1):19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 32.Wolfe DL, Hsieh JT, Mehta S.. Rehabilitation practices and associated outcomes following spinal cord injury. Spinal Cord Injury Rehabilitation Evidence. 2010. [Google Scholar]
- 33.Craven C, Verrier M, Balioussis C, Wolfe DL, Hsieh J, Noonan V, et al. Rehabilitation environmental scan atlas: capturing capacity in Canadian SCI rehabilitation. Rick Hansen Institute, Vancouver, BC: 2012. [Google Scholar]
- 34.Tate DG, Boninger ML, Jackson AB.. Future directions for spinal cord injury research: recent developments and model systems contributions. Arch Phys Med Rehabil 2011;92(3):509–15. doi: 10.1016/j.apmr.2010.07.243 [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization International Classification of Functioning, Disability and Health: ICF: World Health Organization; 2001. [Google Scholar]
- 36.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giangregorio L, Catharine C, Richards K, Kapadia N, Hitzig SL, Masani K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on body composition. J Spinal Cord Med 2012;35(5):351–60. doi: 10.1179/2045772312Y.0000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorgey AS, Dolbow DR, Cifu DX, Gater DR.. Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J Electromyogr Kinesiol 2013;23(4):977–84. doi: 10.1016/j.jelekin.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 39.Gorgey AS, Mather KJ, Cupp HR, Gater DR.. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 2012;44(1):165–74. doi: 10.1249/MSS.0b013e31822672aa [DOI] [PubMed] [Google Scholar]
- 40.de Zepetnek JOT, Pelletier CA, Hicks AL, MacDonald MJ.. Following the physical activity guidelines for adults with spinal cord injury for 16 weeks does not improve vascular health: a randomized controlled trial. Arch Phys Med Rehabil 2015;96(9):1566–75. doi: 10.1016/j.apmr.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 41.Allison DJ, Ditor DS.. Targeting inflammation to influence mood following spinal cord injury: a randomized clinical trial. J Neuroinflammation 2015;12(1):1. doi: 10.1186/s12974-015-0425-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakkum AJ, Paulson TA, Bishop NC, Goosey-Tolfrey VL, Stolwijk-Swüste JM, van Kuppevelt DJ, et al. Effects of hybrid cycle and handcycle exercise on cardiovascular disease risk factors in people with spinal cord injury: a randomized controlled trial. J Rehabil Med 2015;47(6):523–30. doi: 10.2340/16501977-1946 [DOI] [PubMed] [Google Scholar]
- 43.Ordonez FJ, Rosety MA, Camacho A, Rosety I, Diaz AJ, Fornieles G, et al. Arm-cranking exercise reduced oxidative damage in adults with chronic spinal cord injury. Arch Phys Med Rehabil 2013;94(12):2336–41. doi: 10.1016/j.apmr.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 44.Rosety-Rodriguez M, Rosety I, Fornieles G, Rosety JM, Elosegui S, Rosety MA, et al. A short-term arm-crank exercise program improved testosterone deficiency in adults with chronic spinal cord injury. Int Braz J Urol 2014;40(3):367–72. doi: 10.1590/S1677-5538.IBJU.2014.03.10 [DOI] [PubMed] [Google Scholar]
- 45.Kim D-I, Lee H, Lee B-S, Kim J, Jeon JY.. Effects of a 6-week indoor hand-bike exercise program on health and fitness levels in people with spinal cord injury: a randomized controlled trial study. Arch Phys Med Rehabil 2015;96(11):2033–40. e1. doi: 10.1016/j.apmr.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 46.Mohammadi V, Khalili M, Eghtesadi S, Dehghani S, Jazayeri S, Aghababaee S, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: a clinical trial. Spinal Cord 2015;53(8):621–4. doi: 10.1038/sc.2015.35 [DOI] [PubMed] [Google Scholar]
- 47.Sabour H, Larijani B, Vafa MR, Hadian MR, Heshmat R, Meybodi HA, et al. The effects of n-3 fatty acids on inflammatory cytokines in osteoporotic spinal cord injured patients: A randomized clinical trial. J Res Med Sci 2012;17(4). [PMC free article] [PubMed] [Google Scholar]
- 48.Sabour H, Javidan AN, Latifi S, Shakeri H, Arman F, Larijani B, et al. Does consumption of Omega-3 polyunsaturated fatty acids affect lipid profile and fasting blood glucose in patients with traumatic spinal cord injury? A double-blinded randomized clinical trial. Top Clin Nutr 2015;30(4):333–43. [Google Scholar]
- 49.Sabour H, Norouzi Javidan A, Latifi S, Shidfar F, Heshmat R, Emami Razavi S-H, et al. Omega-3 fatty acids’ effect on leptin and adiponectin concentrations in patients with spinal cord injury: A double-blinded randomized clinical trial. J Spinal Cord Med 2015;38(5):599–606. doi: 10.1179/2045772314Y.0000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho D, Garlipp C, Bottini P, Afaz S, Moda M, Cliquet Jr A.. Effect of treadmill gait on bone markers and bone mineral density of quadriplegic subjects. Braz J Med Biol Res 2006;39(10):1357–63. doi: 10.1590/S0100-879X2006001000012 [DOI] [PubMed] [Google Scholar]
- 51.de Abreu DCC, Cliquet Jr A, Rondina JM, Cendes F.. Electrical stimulation during gait promotes increase of muscle cross-sectional area in quadriplegics: a preliminary study. Clin Orthop Relat Res 2009;467(2):553–7. doi: 10.1007/s11999-008-0496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Abreu DCC, Junior AC, Rondina JM, Cendes F.. Muscle hypertrophy in quadriplegics with combined electrical stimulation and body weight support training. Int J Rehabil Res 2008;31(2):171–5. doi: 10.1097/MRR.0b013e3282fc0fa4 [DOI] [PubMed] [Google Scholar]
- 53.Ravensbergen HJC, Lear SA, Claydon VE.. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma 2014;31(3):292–300. doi: 10.1089/neu.2013.3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorgey AS, Mather KJ, Poarch HJ, Gater DR.. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J Spinal Cord Med 2011;34(1):99–109. doi: 10.1179/107902610X12911165975106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warburton DE, Sproule S, Krassioukov A, Eng JJ.. Cardiovascular health and exercise following spinal cord injury. Top Spinal Cord Inj Rehabil 2007;13(1):98–122. doi: 10.1310/sci1301-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control Prevention International classification of diseases ninth revision clinical modification (ICD-9-CM). 2013. [Google Scholar]
- 57.American Medical Association International Classification of Diseases; 10th Revision Clinical Modification (ICD-10-CM). 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


