Abstract
Objectives
To determine the efficacy of functional electrical stimulation therapy assisted walking (FES-T) compared to a conventional aerobic and resistance training (CONV) with respect to bone biomarkers and lower extremity bone strength outcomes among adults with chronic motor incomplete spinal cord injury (SCI).
Design
Parallel group randomized controlled trial (www.clinicaltrials.gov - NCT0020196819).
Site
Tertiary academic rehabilitation centre in Canada.
Methods
Adults with chronic (≥18 months) motor incomplete SCI (C2-T12 AIS C-D) were consented and randomized to FES-T or CONV training for 45 minutes thrice-weekly for 4 months. Osteocalcin (OC), β-cross laps (CTX) and sclerostin were assessed at baseline, and 4 months. Similarly, total hip, distal femur and proximal tibia region bone mineral density (BMD) via DXA (4500A, Hologic Inc. Waltham, MA, USA) and tibia bone quality via pQCT (Stratec XCT-2000, Mezintecknik, Pforzheim, Germany) were assessed at baseline, 4, and 12 months. Between group differences were analyzed using repeated measures general linear models.
Results
Thirty-four participants (17 FES-T, 17 CONV) consented and were randomized, 27 participants completed the 4-month intervention and 12-month outcome assessments. Participants in the FES-T arm had a decrease in CTX and a significant increase in OC at intervention completion (P<0.05). Significant biomarker changes were not observed in the CONV group. No within or between group differences from baseline were observed in sclerostin or bone strength.
Conclusions
Four months of FES-T improved bone turnover (increase in OC and decrease in CTX) but not bone strength among individuals with chronic SCI. Future, long term FES-T may augment lower extremity bone strength.
Keywords: Spinal cord injury, Functional electrical stimulation, Osteocalcin, C-Telopepetide, Sclerostin, Bone density
Introduction
Sublesional osteoporosis and bone turnover
Sublesional osteoporosis (SLOP) is a common and established complication following motor complete spinal cord injury (SCI).1 The declines in bone mineral density (BMD) are most pronounced in the first year following injury, with individuals in the chronic stage of SCI experiencing as much as a 50% loss in sublesional bone mass of the hip and knee regions.2–4 Bone adapts to applied loads such that osteocytes detect strain and convert it to biochemical signals—a process known as mechanotransduction.5 These biochemical signals mediate changes in bone formation and resorption in response to mechanical forces, loading and the cellular environment.5 Following spinal cord injury, reductions in mechanical loading contribute to an increase in bone turnover particularly resorption—signalling deterioration of bone mass and bone quality (microarchitecture) and, consequently, an increased risk of fragility fracture.6 The severity of bone resorption and the changes in lower extremity bone mass are not as well described among patients with motor incomplete SCI.
Drug and rehab therapies that inhibit bone resorption or stimulate bone formation are appealing as potential therapies for individuals with SCI and SLOP.1,7 We have previously conducted a systematic review of the available drug and rehabilitation interventions for treatment of SLOP.1,7 Of the 16 rehabilitation intervention studies reviewed, no intervention led to sustained increases in hip or knee region BMD in participants with chronic SCI and SLOP.1 However, many prior studies evaluating the therapeutic effectiveness of rehabilitation interventions for augmenting bone mass have failed to include markers of bone turnover.1 The biomarkers, β-Crosslaps (CTX) and osteocalcin (OC) are frequently used in the assessment of bone turnover.8,9 CTX is a fragment of the collagen molecule released into bloodstream during bone resorption. OC is secreted by osteoblasts, and is a well-established bone biochemical marker, regulator of bone turnover and indicator of anabolic therapy.10
There has been recent interest in sclerostin as a candidate biomarker for osteoporosis after SCI.11 Sclerostin a glycoprotein secreted by osteocytes, after SCI has both anti-anabolic and catabolic effects on the bone remodelling pathways.5 Paralysis has been shown to upregulate sclerostin and thus, simultaneously both inhibit bone formation and stimulate bone resorption.12,13 Although sclerostin upregulation may contribute to bone loss with disuse, research is needed to understand its contributions to bone remodelling in response to mechanical loading.14 Few trials to date, with the exception of whole body vibration intervention trials (www.clinicaltrials.gov NCT02334410; NCT01225055; NCT02025179), have measured whether biomarkers or specifically, sclerostin is responsive to rehabilitation interventions among patients with chronic SCI and SLOP.
Functional electrical stimulation therapy assisted walking
Functional Electrical Stimulation (FES) involves applying electrical stimulation to muscles via implanted or surface electrodes to stimulate paralyzed or weak muscles to contract and produce functional movements (such as walking or cycling). FES devices are typically prescribed for long term use to augment muscle strength, cardiovascular fitness, or assist in restoring grasping and walking functions.8 Using a multichannel FES system, electrical impulses can be applied to several muscle groups in a coordinated fashion to facilitate a gait-like sequence of movement and enhance voluntary walking function in individuals with chronic incomplete SCI.15–17 Short-term use of a surface FES device or FES-Therapy (FES-T) has the capacity to promote neuroplasticity through stimulating reorganization and retraining of residual central nervous system function to improve walking ability and reducing dependence on the stimulus over time; benefits that are retained after therapy cessation.16,18–21
Treadmill training, with or without body weight support using a harness (BWSTT), is used to vary mechanical loading, assure step frequency and enhance walking ability after acute or chronic incomplete SCI.22 Our group has previously reported on the effects of body weight-support treadmill training (BWSTT) on muscle, bone and bone biomarkers following SCI.23 Twice-weekly BWSTT increased muscle cross-sectional area, however improvements in bone mass, bone geometry or bone biomarkers were not seen.23 Coupaud recently reported a case study demonstrating regional increments in lower extremity volumetric BMD (vBMD) following 7 months of treadmill training.24 Combining BWSTT with FES-T or “FES-T assisted walking” has the potential to emulate normal mechanical strains on bone during weight-bearing activity and stimulate bone formation through alterations in muscle activity that cannot be achieved with BWSTT alone. The overall purpose of this trial (NCT0020196819) was to determine if FES-T assisted walking for four months would reduce health complications (muscle, bone, spasticity) after SCI, and to determine if the hypothesized reductions in health complications were retained eight months after therapy cessation and resulted in improvements in life satisfaction and community participation. A secondary outcome was to determine if FES-T assisted walking improved walking competence in individuals with chronic, incomplete SCI. The effects of FES-T assisted walking on muscle density, quality of life, and walking competence have been previously reported.25–27 This manuscript describes the primary outcomes including the changes in the serum bone biomarkers (osteocalcin (OC), C-terminal telopeptide (CTX) and sclerostin),and bone strength including hip and knee region bone mass and tibia region vBMD following four months of thrice weekly FES-T assisted walking, compared to a CONV exercise program among individuals with chronic motor incomplete SCI. Given the duration of FES-T assisted walking, we hypothesized that bone formation markers (OC) would increase and bone resorption markers (CTX, Sclerostin) would decline to a greater degree in the FES-T assisted walking intervention arm as compared to CONV arm among adults with motor incomplete SCI. Fig. 1 is provided as a schematic diagram of a theoretical biomarker response to a rehab intervention (i.e. exercise, vibration, FES-T assisted walking, etc.). We further hypothesized that improvements in lower extremity bone mineral density and tibia bone quality would be observed at one year.
Figure 1.
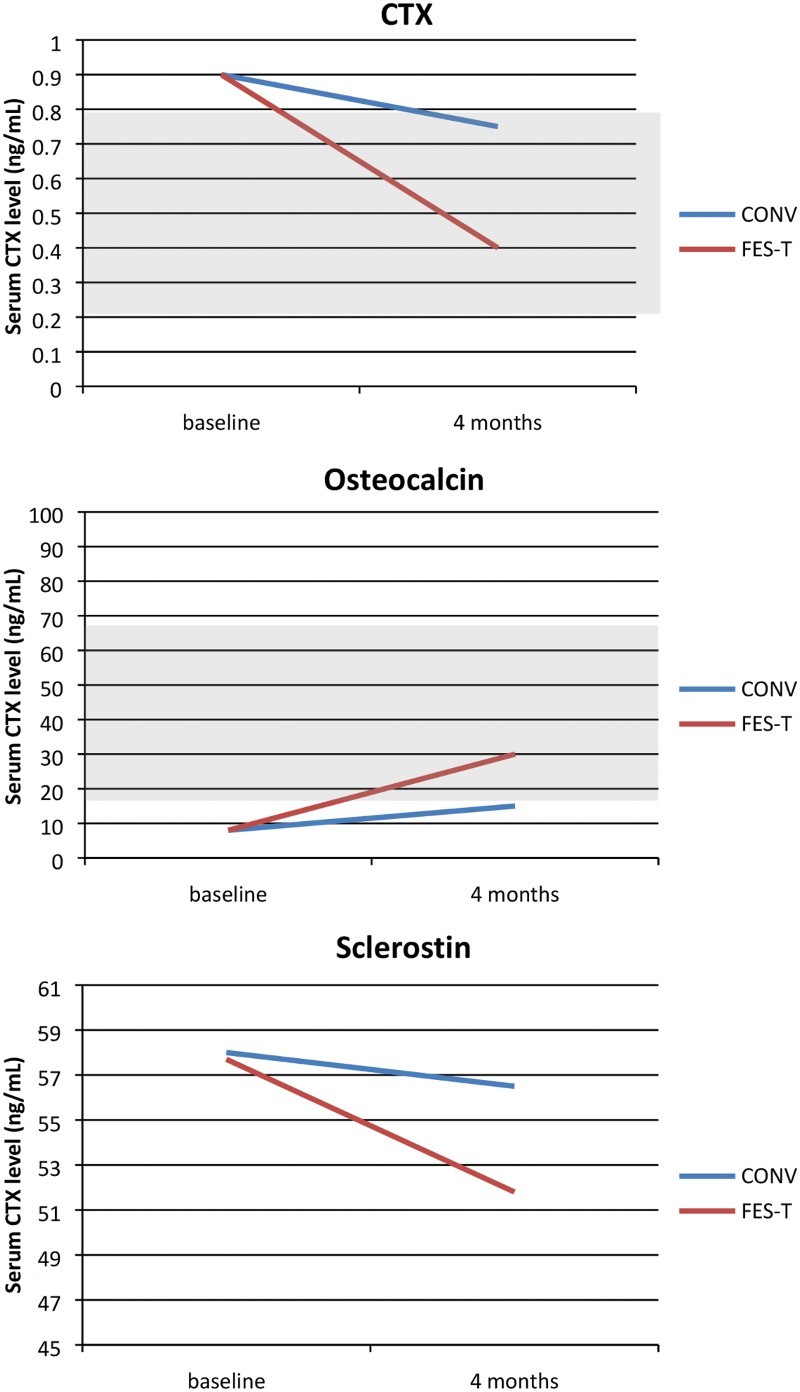
Schematic Diagram of Theoretical Biomarker Responses to an Intervention after SCI (Shaded areas represent schematic normal ranges for healthy men, age 30+). There are no published, consensus normal ranges for Sclerostin).
Methods
The trial was registered on www.clinicaltrials.gov (NCT0020196819). All study procedures were approved by Toronto Rehabilitation Institute’s Research Ethics Board. The study was a parallel group randomized controlled trial conducted between Mar 2005 and December 2010 (Figure 2). The study screening, recruitment, and consent procedures, inclusion and exclusion criteria (see Table 1 – Study Inclusion and Exclusion Criteria), randomization procedures, intervention and control activities and global study measures are described in detail elsewhere.25–27 In brief, participants were randomly assigned in a 1:1 allocation ratio to participate in either a 45 minute FES -T assisted walking program or a 45 minute supervised CONV exercise program of aerobic and resistance training, three times per week for 4 months. The randomization sequence was generated using randperm.m function in Matlab (The MathWorks, Natwick, MA, USA). Envelopes were prepared by a research assistant not involved in enrolling participants; each envelope contained a unique reference number. Each participant selected an unmarked, sealed envelope from a box. This reference number corresponded to another sealed envelope in a separate location that indicated the participant’s group allocation. Statistical power calculations were performed to ensure sufficient statistical power to test hypothesized changes in bone mineral density. We considered 0.6% per month to be a clinically significant difference in BMD change.28 Based on a 5% significance level, and an expected 20% drop out rate, a total of 17 participants per group were recruited. Participants were not blinded to their treatment allocation due to the nature of the interventions. The trial physician, dual-energy X-ray absorptiometry (DXA), peripheral quantitative computed tomography (pQCT) and biomarker outcome assessors were blind to the participants’ group allocation unless a serious adverse event occurred.
Figure 2.
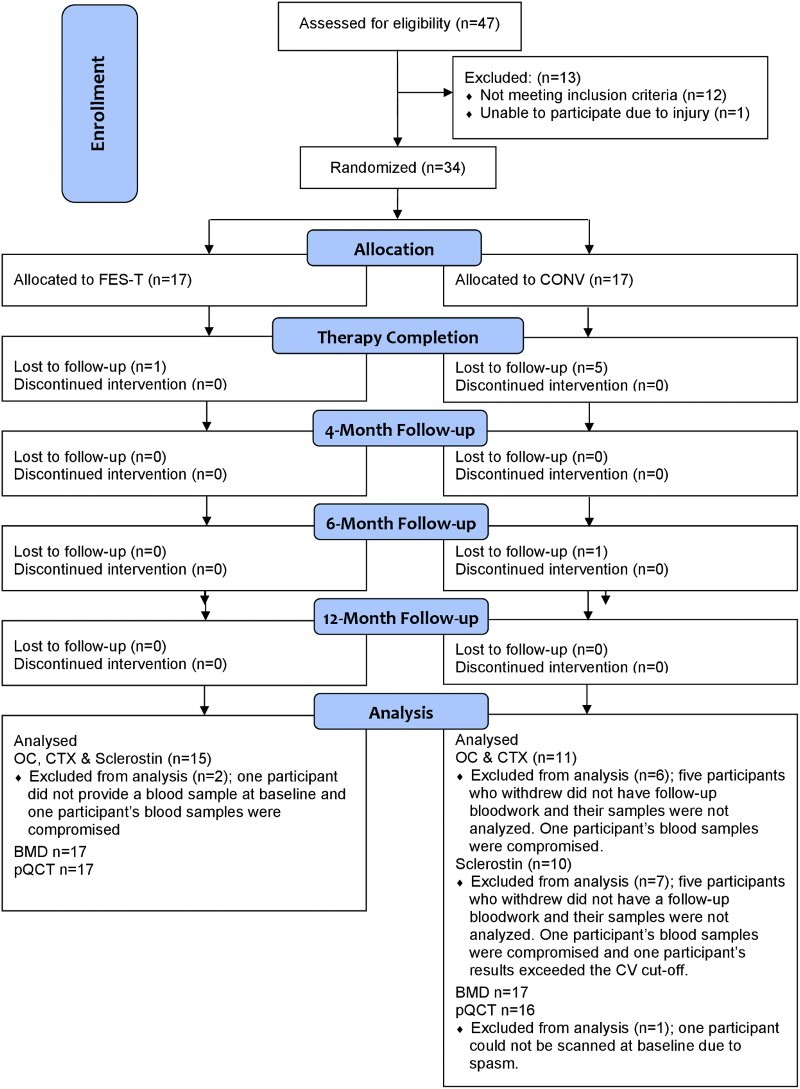
CONSORT Flow Diagram
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Participants
Thirty-four adults with chronic (≥18 months), traumatic, C2–T12 incomplete, (American Spinal Injury Association Impairment Scale [AIS] C or D) SCI were recruited. Neurological level of injury and AIS was assessed by a physiatrist in accordance to the International Standards for Neurological Classification of Spinal Cord Injury.29 Participants were excluded for contraindications to FES therapy.26
Intervention
Conventional Supervised Exercise Program
The control or CONV condition was comprised of a kinesiology supervised aerobic and resistance exercise program. The CONV training included 20–25 minutes of aerobic exercise (arm or leg bicycling, walking in parallel bars or on the treadmill) at a Borg rate of perceived exertion of 3–5. Resistance exercise included 2–3 sets of 12–15 repetition maximum resistance for muscles capable of voluntary contraction. The CONV group had an opportunity to exercise on a treadmill, if they were able to walk unassisted.
FES Therapy with BWSTT
FES was delivered using self-adhesive stimulation electrodes connected to two manually controlled Compex Motion, transcutaneous electric stimulators (Compex SA, Switzerland) which independently stimulated each leg. Electrodes were placed on the participant’s skin above the motor points corresponding to the muscles to be stimulated i.e. quadriceps, hamstrings, tibialis anterior and gastrocnemius. Depending on the muscle and participant, pulse amplitudes ranged from 8–125 mA, pulse durations ranged from 0–300 µs, and frequencies from 20–50 Hz. By changing the pulse duration, we were able to change the temporal activation of the muscle contraction with the amplitude adjusted for the strength of the participant’s muscle contraction. Typically, FES-T assisted walking participants were encouraged to produce the intended movement voluntarily, and after trying for a few seconds, FES was triggered to execute the movement for the participant. During movement execution, pulse duration generally ranged between 250–300µs. Therapists or assistants activated the stimulators using two independent push buttons shortly after heel-off but before toe-off phase of the gait cycle. Activation of the push button resulted in the delivery of a stimulation sequence to the targeted muscles in an open-loop control manner. FES-T was performed on a BWST Loko 70 (Woodway GmbH, Weil am Rhein, Germany). The apparatus included an overhead harness which can adjust the amount of body-weight support in 8 kg increments. Weight support varied across participants and decreased as therapy progressed. Walking exercises were performed at a speed selected by the person supervising the session with input from the participant. At times, manual assistance was applied to the participant’s lower extremities and low back region to facilitate normal gait. In the early stages of the intervention and with weaker participants, three physiotherapists and assistants were engaged in the delivery of treatment. Except for with weaker participants, personnel requirements decreased as the therapy progressed. Participants in either intervention arm (FES-T or CONV) who missed sessions were permitted to make them up. More detailed descriptions of the FES-T assisted walking and CONV treatment groups have been reported previously.25,26
Outcome Measures
Bone Biomarkers
Following collection, serum from centrifuged blood was stored in a -70°C freezer and later shipped on dry ice to the Hamilton Health Sciences Biochemistry Department for bulk analysis following study completion. The biomarkers described here vary from those described at trial registration due to amendments made to the protocol after study initiation. New advances in the biomarker field30 as well as budget constraints imposed by a need to extend the study timeline to meet recruitment targets, caused us to select alternate biomarkers and restrict the volume of biomarker data analysed. Serum biomarkers were collected at baseline, 4, 6, and 12 months; analysis of the baseline and four month biomarker data exhausted our remaining financial resources after trial completion. The lab services hired to complete the biomarker analysis chose to analyse data for all participants with at least two data collection time points; thus the five participants in the CONV arm who withdrew, did not have their baseline data analysed, in addition one participant’s sample per group (CONV and FES-T arm) was compromised and not reported.
Osteocalcin
OC was measured by radioimmunoassay. Normal range of OC is 24–70 ng/mL for men 18–30 years; 14–42 ng/mL for men 31–50 years; 14–46 ng/mL for men 51–70 years and 11–43 ng/mL for premenopausal women and 15–46 ng/mL for postmenopausal women.31 The intra and inter assay coefficients of variation are ∼8% in previous osteoporosis studies performed in our centre.
CTX
β-crosslaps (CTX) was measured on the Roche Elecsys® 2010 immunoassay analyzer using an electro chemiluminescence immunoassay (Roche Diagnostics GmbH, Indianapolis, IN, USA). In house validation and precision data conformed to acceptable standards and produced CV results comparable to this reported in the package insert. The normal range for CTX is 0.155–0.873 ng/mL for men 18–30 years; 0.093–0.630 ng/mL for men 31–50 years; 0.035–0.836 ng/mL for men 51–70 years and 0.025–0.573 ng/mL for premenopausal women and 0.104–1.008 ng/mL for postmenopausal women.32
Sclerostin
Sclerostin was analyzed using the BIOMEDICA sclerostin ELISA (Alpca Diagnostics, Salem, NH, USA). Assay characteristics on validation had a reportable range of 15.0 to 240 pmol/L. Three serum pools were run along with the assay and a commercial control. Within assay precision using the three pools (which had mean values of 40pmol/L) was 15–22%. A commercial control was also run with a manufacturer range of 50–92 pmol/L. Our mean value was 53.4 pmol/L with a coefficient of variation of 24%. Acceptable within run replicates were <12.5% and all samples fell within this range; except one participant results exceeded the CV cut off and was removed from the analysis.
Bone Mineral Density
Areal bone mineral density (aBMD) in g/cm2 was assessed by DXA (4500A, Hologic Inc., Waltham, MA, USA). The left hip (total hip), right distal femur, and right proximal tibia regions were scanned; in cases of severe spasticity, prior left hip pathology or regional surgery, or other contraindications, the alternate limb was scanned. The study site was equipped with a ceiling lift to transfer participants on and off the scanning table. Participants were positioned supine on the scanning table. The hip, distal femur and proximal tibia scans were analyzed using commercially available lumbar spine and total hip software from Hologic Inc. A lower extremity positioning device and knee aBMD protocol, whose reliability and accuracy have been previously described, was used to acquire and analyze the distal femur and proximal tibia scans.33
All scans were acquired and analyzed by an ISCD certified technologist in the Bone Density Lab at Lyndhurst Centre, Toronto Rehabilitation Institute. The technologist was blinded to group allocation. In a sample of 110 participants, the least significant change for DXA is 2% for the distal femur and 3% for the proximal tibia.
A pQCT scanner (XCT-2000, Stratec Mezintecknik, Pforzheim, Germany) was used to scan the distal end (4% site) and the tibia shaft (38% site) of the tibia to obtain information about trabecular bone and cortical bone, respectively. The right tibia was scanned, except in those participants with severe spasticity or other contraindications for which the left tibia was scanned. Bony landmarks at the medial condyle and medial malleolus were palpated, and a measuring tape used to measure the distance between the two points. A line corresponding to 38% of the tibia length, measuring proximally from the distal landmark was placed. The tibia distal endplate (anatomical reference point) was identified on a 30-mm coronal view of the joint line from the scout scan.
The ultradistal tibia scan site was automatically located proximal to the tibia distal endplate at 4% of the tibia length measuring proximally. The scanner then automatically repositions at the 38% line to measure vBMD and geometry at the tibia shaft. A single 2.5-mm slice was acquired at each site. A voxel size of 0.2 mm was used at the 4% site, while a voxel size of 0.5 mm was used at the 38% site. The same technologist performed all pQCT scans and was blind to group allocation. We have established pQCT precision in our setting among individuals with and without SCI; the root mean square coefficient of variation was ≤2% for all bone outcomes of interest.34 Analyses of the pQCT scans were performed using the manufacturer’s software (Stratec XCT-2000 version 5.50). Contour mode 3 and peel mode 2 with an outer threshold of 130 mg/cm3 and an inner threshold of 400 mg/cm3 was used in CALCBD mode to assess trabecular vBMD (TbvBMD, milligrams per cubic centimeter). Contour mode 1 and a threshold of 710 mg/cm3 were used in CORTBD mode to determine, Cortical Thickness (CTh, in millimeters) (in millimeters), stress strain index (SSI, mm4), and polar moment of inertia (PMI, mm4).35 SSI and PMI represent the ability of bone to resist bending and torsion, respectively. All bone outcomes DXA and pQCT were acquired and analysed at baseline 4 and 12 months.
Analysis
Demographic data, as well as baseline characteristics, were checked for normal distribution (Kolmogorov–Smirnov goodness of fit test) and were analyzed by parametric (χ2 test or Fisher’s exact test; Student’s t test) and non-parametric (Mann–Whitney U test) tests as applicable. A paired Student’s t test was used to assess within group differences in serum biochemical bone markers. The change of each serum bone marker (OC, CTX and Sclerostin) from the baseline was also calculated, and the mean change from the baseline was compared between FES-T assisted walking and CONV groups using independent student t test if data were normally distributed, or the Mann-Whitney U test where data were not normally distributed. In the event of missing biochemical marker or bone density data at a follow-up time point, the baseline values were substituted for the missing value. Biomarker within group comparisons were one-sided and were performed at a P = 0.05 level of significance based on our a priori hypotheses. Differences in the mean changes in bone mass or bone quality (DXA and pQCT) values in the FES T and CONV groups were analysed by repeated-measures ANOVA. Between group comparisons were 2-sided and were performed at a P = 0.05 level of significance. All analyses were performed as intention to treat using SPSS version 22 (IBM, Armonk, NY, USA).
RESULTS
Thirty-four individuals consented to participation and were randomized, Seventeen participants were allocated to each treatment arm (FES-T or CONV). Sixteen individuals completed the FES-T assisted walking, while 12 completed the CONV program (Fig. 1). Five participants in the CONV group and one participant in the FES-T assisted walking group were lost to follow-up (drop out n=3, relocation n=1, and medical removal n=2). Participants in the FES-T assisted walking and CONV groups attended a mean of 43.5 and 44.5 training sessions, respectively. Participants in the FES-T assisted walking and CONV groups were similar at baseline (Table 2).
Table 2.
Baseline Characteristics of the Study Subjects in FES-T and CONV group. The values are shown as mean (standard deviation) or Median (IQR) as noted
| N | FES-T | N | CONV | P-value* | |
|---|---|---|---|---|---|
| Sex – % (no. of males) | 17 | 82.53 (14) | 17 | 70.59 (12) | |
| Age – Mean (SD) | 17 | 56.59 (14.0) | 17 | 54.06 (16.5) | 0.63 |
| BMI (kg/m2) (SD) | 17 | 27.01 (4.28) | 17 | 26.65 (4.82) | 0.82 |
| AIS - % | 17 | 16 | |||
| C | 6 | 35.3% | 7 | 43.8% | 0.44 |
| D | 11 | 64.7% | 9 | 56.3% | |
| Time post injury – Medan (IQR) | 17 | 5 (6.6) | 17 | 5 (18) | 0.96* |
| Spinal Cord Independent Measure (SCIM) - Median (IQR) | 17 | 52 (34) | 17 | 69.5 (31) | 0.24* |
| Fracture History % | 9 | 52.9% | 5 | 29.4% | 0.15 |
| Left Total Hip BMD (g/cm2) (SD) | 17 | 0.89 (0.21) | 17 | 0.79 (0.20) | 0.65 |
| Current Calcium Use – Mean (SD) | 15 | 11 (68.8) | 12 | 8 (66.7) | 0.63 |
| Current Vitamin D Use – Mean (SD) | 15 | 10 (66.7) | 12 | 3 (25.0) | 0.52 |
| Current Bisphosphonate Use – Mean (SD) | 16 | 3 (18.8) | 12 | 6 (66.7) | 0.53 |
*Two-sided P-value
Specifically, there were no significant differences between treatment groups at baseline with regards to OC, CTX or sclerostin (not shown). There were no significant between group changes in OC or CTX from baseline to four months (Table 3). Post intervention, the mean serum concentration of osteocalcin was significantly elevated in the FES-T assisted walking group, but no significant change was observed in the CONV group (four months). Conversely, the mean serum CTX was decreased in the FES-T assisted walking group, with no significant changes in the CONV group (Table 4). The mean serum concentrations of sclerostin were not significantly different within or between groups. The effect size for OC and CTX was small (0.15, and 0.16, respectively) and for sclerostin was medium (0.25).
Table 3.
Mean change and SD of bone biomarkers from baseline (4-month – baseline). The values are shown as mean change (standard deviation)
| Bone Marker | Mean Change and SD | P-Value* | |
|---|---|---|---|
| FES-T Group | CONV Group | ||
| CTX | 0.039 (0.09) | 0.024 (0.1) | 0.68 |
| Osteocalcin | 1.07 (1.92) | 0.65 (3.44) | 0.72 |
| Sclerostin | 1.47 (5.28) | -1.71 (16.90) | 0.56 |
*Two-sided P-value
Table 4.
Bone biomarker outcomes at baseline and after 4 month in FES-T assisted walking and CONV groups. The values are shown as mean (standard deviation)
| Group | Bone Marker | N | Baseline | 4 Month | P-value | |
|---|---|---|---|---|---|---|
| FES-T | CTX ng/mL | Group | 15 | 0.26 (0.15) | 0.24 (0.17) | 0.05* |
| Male | 12 | 0.28 (0.15) | 0.31 (0.17) | 0.11** | ||
| Female | 3 | 0.17 (0.17) | 0.22 (0.18) | 0.06** | ||
| Osteocalcin µg/mL | Group | 15 | 16.70 (6.51) | 17.77 (6.23) | 0.02* | |
| Male | 12 | 17.7 (6.80) | 18.87 (6.40) | 0.50** | ||
| Female | 3 | 12.5 (3.04) | 13.40 (3.20) | 0.06** | ||
| Sclerostin pmol/L | Group | 15 | 52.87 (16.78) | 54.34 (20.13) | 0.11** | |
| Male | 12 | 53.27 (16.46) | 54.55 (20.93) | 0.24** | ||
| Female | 3 | 51.30 (21.81) | 53.50 (20.66) | 0.06** | ||
| CONV | CTX ng/mL | Group | 11 | 0.24 (0.21) | 0.27 (0.18) | 0.26** |
| Male | 6 | 0.36 (0.23) | 0.35 (0.20) | 0.50** | ||
| Female | 5 | 0.10 (0.02) | 0.16 (0.10) | 0.04** | ||
| Osteocalcin µg/mL | Group | 11 | 20.10 (8.33) | 20.74 (8.62) | 0.28* | |
| Male | 6 | 25.38 (7.53) | 25.36 (7.97) | 0.46** | ||
| Female | 5 | 13.76 (3.25) | 15.20 (6.00) | 0.25** | ||
| Sclerostin pmol/L | Group | 10 | 58.28 (12.43) | 61.06 (13.52) | 0.19* | |
| Male | 6 | 63.60 (10.78) | 67.26 (15.04) | 0.17** | ||
| Female | 5 | 52.96 (12.68) | 54.86 (9.46) | 0.25** |
There were no significant between group differences in aBMD of the total hip, distal femur or proximal tibia, or vBMD of the tibia 4% site or 38% site or bone architecture outcomes assessed by pQCT (PMI, SSI, CTh) at any time point (Table 5A and 5B).
Table 5A.
DXA and pQCT outcomes used in intention to treat analyses for the FES-T and CONV groups at baseline, 4 months and 12 months. The values are shown as mean change (standard deviation). N=17 in each group with last observation carried forward in cases of missing data
| FES-T | P-value* | CONV | P-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | 12 months | Baseline | 4 months | 12 months | |||
| 17 | 17 | 17 | 17 | 17 | 17 | |||
| aBMD | ||||||||
| Left Total Hip (g/cm2) | 0.89 (0.20) |
0.88 (0.20) |
0.88 (0.20) |
0.42 | 0.86 (0.24) |
0.87 (0.23) |
0.88 (0.23) |
0.02 |
| Left Distal Femur (g/cm2) | 0.89 (0.16) |
0.87 (0.15) |
0.87 (0.14) |
0.12 | 0.81 (0.18) |
0.80 (0.18) |
0.81 (0.17) |
0.62 |
| Left Proximal Tibia (g/cm2) | 0.71 (0.18) |
0.71 (0.15) |
0.69 (0.17) |
0.24 | 0.68 (0.19) |
0.66 (0.19) |
0.67 (0.19) |
0.27 |
| 17 | 17 | 17 | 17 | 17 | 17 | |||
| pQCT | ||||||||
| 38% TbvBMD (mg/cm3) | 87.69 (17.11) |
91.13 (17.45) |
89.06 (20.43) |
0.09 | 88.45 (27.17) |
90.82 (21.45) |
93.09 (31.62) |
0.03 |
| 38% CTh (mm) | 3.88 (0.89) |
3.88 (0.96) |
3.75 (0.78) |
0.04 | 4.27 (1.19) |
4.18 (1.08) |
3.91 (0.94) |
0.15 |
| 38% Cortical Density | 1089.31 (37.48) |
1087.62 (34.42) |
1082.0 (36.85) |
0.10 | 1106.73 (34.04) |
1102.36 (37.78) |
1100.00 (26.22) |
0.04 |
| PMI (mm4) | 58558.03 (18002.27) |
58557.63 (18377.84) |
59377.30 (20364.38) |
0.02 | 44640.33 (16882.90) |
43396.61 (16863.27) |
43774.16 (17166.62) |
0.08 |
| SSI | 2866.34 (778.20) |
2902.53 (789.56) |
2925.79 (829.38) |
0.05 | 2429.00 (739.62) |
2389.76 (743.69) |
2359.34 (716.79) |
0.17 |
| 4% Tbv BMD (mg/cm3) | 201.99 (35.65) |
189.43 (53.96) |
200.51 (35.89) |
0.05 | 171.40 (52.47) |
166.54 (52.47) |
169.35 (51.00) |
0.18 |
Least Significant Change for DXA is 2% for the Distal Femur and 3% for the Proximal Tibia.
*Within group P-value
Further, there were no significant within group mean changes in aBMD of the FES-T group (Table 5A and B). Post-hoc sensitivity analyses, adjusting for confounding variables (i.e. bisphosphonate, calcium use, etc.) did not alter the data significantly from that presented in Table 5A and B.
Table 5B.
DXA and pQCT outcomes used in per protocol analyses for the FES-T and CONV groups at baseline, 4 months and 12 months. The values are shown as mean change (standard deviation)
| FES-T | P-value* | CONV | P-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | 12 months | Baseline | 4 months | 12 months | |||
| 17 | 16 | 16 | 17 | 12 | 11 | |||
| aBMD | ||||||||
| Left Total Hip (g/cm2) | 0.90 (0.20) |
0.88 (0.20) |
0.89 (0.20) |
0.41 | 0.86 (0.21) |
0.87 (0.23) |
0.90 (0.21) |
0.06 |
| Left Distal Femur (g/cm2) | 0.89 (0.15) |
0.87 (0.15) |
0.87 (0.14) |
0.12 | 0.82 (0.17) |
0.80 (0.18) |
0.82 (0.17) |
0.64 |
| Left Proximal Tibia (g/cm2) | 0.71 (0.17) |
0.71 (0.15) |
0.69 (0.17) |
0.24 | 0.69 (0.18) |
0.66 (0.19) |
0.68 (0.19) |
0.26 |
| 17 | 16 | 16 | 15 | 10 | 9 | |||
| pQCT | ||||||||
| 38% TbvBMD (mg/cm3) | 87.72 (16.58) |
91.16 (17.50) |
89.16 (20.45) |
0.08 | 89.93 (25.38) |
89.56 (22.33) |
95.70 (34.53) |
0.08 |
| 38% CTh (mm) | 3.88 (0.75) |
3.83 (0.85) |
3.68 (0.77) |
0.11 | 4.14 (0.91) |
4.21 (0.98) |
4.08 (0.84) |
0.11 |
| 38% Cortical Density | 1088.54 (36.30) |
1087.64 (34.61) |
1082.12 (36.91) |
0.10 | 1098.64 (40.98) |
1105.25 (38.57) |
1103.54 (27.14) |
0.14 |
| PMI (mm4) | 58333.45 (174556.20) |
58557.61 (19377.85) |
59377.26 (20364.39) |
0.04 | 49819.34 (17455.20) (16882.90) |
43197.68 (17761.83) (16863.27) |
40926.83 (15467.87) |
0.12 |
| SSI | 2861.80 (753.78) |
2902.50 (789.57) |
2925.80 (829.37) |
0.05 | 2597.79 (737.50) |
2396.07 (783.60) |
2274.88 (712.59) |
0.16 |
| 4% Tbv BMD (mg/cm3) | 202.36 (35.52) |
189.43 (53.96) |
200.51 (35.89) |
0.05 | 172.91 (48.10) |
167.19 (49.79.47) |
181.41 (47.70) |
0.24 |
Least Significant Change for DXA is 2% for the Distal Femur and 3% for the Proximal Tibia.
*Within group P-value
DISCUSSION
The current study suggests that FES-T assisted walking may stimulate bone formation in individuals with incomplete SCI. However, the changes observed were significant in within, and not between group comparisons, and did not translate into meaningful between group differences in indices of bone strength. Our findings suggest that short term (i.e. 4 months) FES-T assisted gait training does not modify bone strength in individuals with chronic SCI.
A priori, we hypothesized that significant between group differences in bone strength indices would be detectable at 12 months, but not at four months, given that one bone turnover cycle is 3–6 months in duration.36 A number of prior published studies, have investigated the therapeutic benefits of FES for maintaining and/or increasing lower extremity bone mass.37 The majority of studies examining the effects of FES-biking38 or FES-rowing39 on bone outcomes have demonstrated regional increases in bone mass using stimulation thresholds well above those used during this FES-T assisted walking intervention. To date, there is a lack of evidence supporting rehabilitation interventions for treatment of SLOP, with the exception of FES-cycle ergometry and FES-rowing which increase lower extremity BMD over stimulated areas; however, these gains in bone density are not sustained with therapy cessation.40 Further, there is inconclusive evidence for reciprocating gait orthoses, long leg braces, passive standing or physical activity as a treatment for SLOP after SCI.40 A majority of these studies did not include biomarkers as primary or secondary outcomes.
One of the greatest challenges in interpreting the enclosed biomarker data is the current lack of normative data for the general or SCI population. Bone turnover levels vary with time after SCI and are distinct from levels in the general population. SCI-specific normative data and reference ranges in the acute, sub-acute and chronic phases of injury are needed to interpret changes depending on time post injury. Further, biomarkers may provide insight into time periods when interventions might elicit optimal therapeutic responses.
Osteocalcin values for all participants in the study fell within the normal range for the biomarker. The normal range for CTX varies depending on age and participants had values that fell within the published ranges, except one. We observed that CTX was significantly decreased compared to baseline, and osteocalcin levels were significantly increased in the FES-T assisted walking group upon completion of 4 months of therapy. This finding was consistent with our biomarker hypothesis, and the within group changes suggest that long term FES-T assisted walking may be a potential therapy for SLOP among patients with chronic motor incomplete spinal cord injury (AIS C-D). However, they should be interpreted with caution given the absence of a significant between group differences in biomarkers. Serum and urine biomarkers provide useful insight into bone metabolism, at specific time points post-injury, and are an effective tool for monitoring response to therapy.1,9, 41 Higher serum osteocalcin levels strongly correlate with increases in bone mineral density (BMD) and bone strength during anabolic drug therapy for osteoporosis.42 For this reason, osteocalcin is often used as a biomarker for identifying effective or potentially effective therapy for osteoporosis and is appropriate for evaluating the potential effectiveness of rehabilitation interventions for augmenting bone strength among patients with SLOP after SCI. However, only a limited number of case series in SCI have included biomarkers as primary or secondary outcomes43–45 without consensus regarding which markers are most sensitive and specific to the desired outcome.
We observed that sclerostin levels were elevated (above the normal range) at 58.28 ± 12.43pmol/mL and 52.87±16.78pmol/L in CONV and FES-T assisted walking groups respectively at baseline. A prior study among men with acute SCI reported elevated sclerostin levels. On the contrary, sclerostin was observed to be reduced in men with chronic SCI and established osteoporosis.21,22 In theory, sclerostin levels should be highest in those with the most severe SLOP; however, this outcome was not observed by Morse et al. who reported that sclerostin levels were proportional to lower extremity BMD for those with chronic SCI. The authors hypothesized that disuse following SCI causes sclerostin levels to rise initially, but as SLOP is established and fewer osteocytes are present to secrete sclerostin, lower levels of sclerostin are observed in chronic SCI. Based on this assumption, Morse and colleagues have suggested that sclerostin is an attractive biomarker of SLOP severity in chronic SCI, given that it is easily measured and correlates with lower extremity BMD. This hypothesis would suggest differences in osteoporosis severity between the two groups following the four-month intervention.
A greater understanding of the natural history of changes in bone biomarkers, muscle density, and bone strength may assist in identification of candidate SLOP therapies. Importantly, in the FES-T assisted walking and CONV intervention groups, we did not see interim declines in bone mass or adverse changes in bone architecture. This is particularly cogent given the frequency of prior fracture reported by the trial participants at baseline in the treatment groups (n=9 FES-T and n=5 CONV) and case reports of fracture during robotic treadmill training.46,47
Limitations
With our combined assessment of bone biomarkers, aBMD and vBMD, this manuscript provides a comprehensive assessment of the effect of FES-T assisted walking versus CONV therapy on bone turnover and bone strength outcomes in chronic motor incomplete SCI. We caution regarding generalization of the study results based on restricted use of our current FES-T protocol, the limited sample size (n=34), and our growing knowledge regarding the use of the selected biomarkers (CTX, OC and Sclerostin) in incomplete SCI.
Further limitations should be acknowledged. Retrospectively, the study was not adequately powered for the bone biomarkers and bone strength indices as the changes in bone mass were near equivalent to the least significant change, a larger sample size or longer duration of intervention was required. We did not plan to include gender differences in our analyses. We did not meet our recruitment targets in a timely manner, and as a result over extended our study budget, thereby, limiting our ability to complete the planned biomarker analysis. Future trials of this nature should include recruitment milestones.48
CONCLUSIONS
The FES-T assisted walking and CONV interventions were well tolerated by patients with low bone mass and prior fracture at baseline. FES-T assisted walking among individuals with chronic incomplete SCI had beneficial effects on OC and CTX biomarkers responsible for bone formation and bone resorption when compared to baseline. Based on these results, FES-T assisted walking should not be expected to treat SLOP or modify bone strength among individuals with SCI in the short term. However, interval changes in sclerostin (clinically important, but not statistically significant) in the FES-T assisted walking group suggests therapeutic potential requiring prospective evaluation in a longer duration intervention using biomarkers and bone strength indices with established clinimetric properties (i.e., normal range, reliability, validity, responsiveness) for patients with chronic SCI.
ORCID
B. Catharine Craven http://orcid.org/0000-0001-8234-6803
S. Mohammad Alavinia http://orcid.org/0000-0002-5503-9362
Lindsie A. Blencowe http://orcid.org/0000-0003-2187-7656
Naaz Desai http://orcid.org/0000-0001-7999-7488
Milos R. Popovic http://orcid.org/0000-0002-2837-2346
Disclaimer statements
Contributors None.
Funding None.
Conflict of interest None.
Ethics approval None.
References
- 1.Craven BC, Giangregorio L, Robertson L, Delparte JJ, Ashe MC, Eng JJ.. Sublesional Osteoporosis Prevention, Detection, and Treatment: A Decision Guide for Rehabilitation Clinicians Treating Patients with Spinal Cord Injury. Crit Rev Phys Rehabil Med 2008;20(4):277–321. doi: 10.1615/CritRevPhysRehabilMed.v20.i4.10 [DOI] [Google Scholar]
- 2.Dudley-Javoroski S, Shields RK.. Regional cortical and trabecular bone loss after spinal cord injury. J Rehabil Res Dev 2012;49(9):1365–76. doi: 10.1682/JRRD.2011.12.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, et al. . Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 2004;34(5):869–80. doi: 10.1016/j.bone.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Biering-Sorensen F, Bohr HH, Schaadt OP.. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest 1990;20(3):330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x [DOI] [PubMed] [Google Scholar]
- 5.Baron R, Kneissel M.. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013;19(2):179–92. doi: 10.1038/nm.3074 [DOI] [PubMed] [Google Scholar]
- 6.Brandi ML. Microarchitecture, the key to bone quality. Rheumatology (Oxford) 2009;48 Suppl 4:iv3–8. doi: 10.1093/rheumatology/kep273 [DOI] [PubMed] [Google Scholar]
- 7.Craven BC, Robertson LA, McGillivray CF, Adachi JD.. Detection and Treatment of Sublesional Osteoporosis Among Patients with Chronic Spinal Cord Injury: Proposed Paradigms. Top Spinal Cord Inj Rehabil 2009;14(4):1–22. doi: 10.1310/sci1404-1 [DOI] [Google Scholar]
- 8.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev 2005;26(4):97–122. [PMC free article] [PubMed] [Google Scholar]
- 9.Seibel MJ. Biochemical markers of bone turnover part II: clinical applications in the management of osteoporosis. Clin Biochem Rev 2006;27(3):123–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. . Endocrine regulation of energy metabolism by the skeleton. Cell 2007;130(3):456–69. doi: 10.1016/j.cell.2007.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglino RA, Lazzari AA, Garshick E, Morse LR.. Spinal cord injury-induced osteoporosis: pathogenesis and emerging therapies. Curr Osteoporos Rep 2012;10(4):278–85. doi: 10.1007/s11914-012-0117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR.. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone 2012;51(3):600–5. doi: 10.1016/j.bone.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, et al. . Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res 2012;27(2):352–9. doi: 10.1002/jbmr.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galea GL, Lanyon LE, Price JS.. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovic MR, Curt A, Keller T, Dietz V.. Functional electrical stimulation for grasping and walking: indications and limitations. Spinal Cord 2001;39(8):403–12. doi: 10.1038/sj.sc.3101191 [DOI] [PubMed] [Google Scholar]
- 16.Thrasher TA, Flett HM, Popovic MR.. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord 2006;44(6):357–61. doi: 10.1038/sj.sc.3101864 [DOI] [PubMed] [Google Scholar]
- 17.Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisne D, Beillot J, et al. . Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine (Phila Pa 1976) 2000;25(4):501–8. doi: 10.1097/00007632-200002150-00018 [DOI] [PubMed] [Google Scholar]
- 18.Thrasher TA, Popovic MR.. Functional electrical stimulation of walking: function, exercise and rehabilitation. Ann Readapt Med Phys 2008;51(6):452–60. doi: 10.1016/j.annrmp.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J, et al. . Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair 2010;24(2):152–67. doi: 10.1177/1545968309347681 [DOI] [PubMed] [Google Scholar]
- 20.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82(6):818–24. doi: 10.1053/apmr.2001.23752 [DOI] [PubMed] [Google Scholar]
- 21.Ladouceur M, Barbeau H.. Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: longitudinal changes in maximal overground walking speed. Scand J Rehabil Med 2000;32(1):28–36. doi: 10.1080/003655000750045712 [DOI] [PubMed] [Google Scholar]
- 22.Yang JF, Musselman KE.. Training to achieve over ground walking after spinal cord injury: a review of who, what, when, and how. J Spinal Cord Med 2012;35(5):293–304. doi: 10.1179/2045772312Y.0000000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, et al. . Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 2005;43(11):649–57. doi: 10.1038/sj.sc.3101774 [DOI] [PubMed] [Google Scholar]
- 24.Coupaud S, Jack LP, Hunt KJ, Allan DB.. Muscle and bone adaptations after treadmill training in incomplete Spinal Cord Injury: a case study using peripheral Quantitative Computed Tomography. J Musculoskelet Neuronal Interact 2009;9(4):288–97. [PubMed] [Google Scholar]
- 25.Giangregorio L, Craven C, Richards K, Kapadia N, Hitzig SL, Masani K, et al. . A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on body composition. J Spinal Cord Med 2012;35(5):351–60. doi: 10.1179/2045772312Y.0000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia N, Masani K, Craven BC, Giangregorio LM, Hitzig SL, Richards K, et al. . A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014;37(5):511–24. doi: 10.1179/2045772314Y.0000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hitzig SL, Craven BC, Panjwani A, Kapadia N, Giangregorio LM, Richards K, et al. . Randomized trial of functional electrical stimulation therapy for walking in incomplete spinal cord injury: effects on quality of life and community participation. Top Spinal Cord Inj Rehabil 2013;19(4):245–58. doi: 10.1310/sci1904-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eser PC, Donaldson Nde N, Knecht H, Stussi E.. Influence of different stimulation frequencies on power output and fatigue during FES-cycling in recently injured SCI people. IEEE Trans Neural Syst Rehabil Eng 2003;11(3):236–40. doi: 10.1109/TNSRE.2003.817677 [DOI] [PubMed] [Google Scholar]
- 29.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. . International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, et al. . Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem 2009;42(10-11):929–42. doi: 10.1016/j.clinbiochem.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 31.Roche N-MID Osteocalcin. Cobas Roche Diagnostics Packaging Insert. Volume 14 2011. [Google Scholar]
- 32.Roche, Beta Crosslaps/serum Cobas Roche Diagnostics Packaging Insert. Version 12, 2011.
- 33.Moreno JC, Protocol for Using Dual Photon Absorptiometry to Measure Bone Mineral Density of the Distal Femur and Proximal Tibia 2001; McMaster University: Hamilton, Ontario. [Google Scholar]
- 34.Giangregorio L, Lala D, Hummel K, Gordon C, Craven BC.. Measuring apparent trabecular density and bone structure using peripheral quantitative computed tomography at the tibia: precision in participants with and without spinal cord injury. J Clin Densitom 2013;16(2):139–46. doi: 10.1016/j.jocd.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 35.Ashe MC, Khan KM, Kontulainen SA, Guy P, Liu D, Beck TJ, et al. . Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int 2006;17(8):1241–51. doi: 10.1007/s00198-006-0110-5 [DOI] [PubMed] [Google Scholar]
- 36.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 2010;11(4):219–27. doi: 10.1007/s11154-010-9153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbs J, Gagnon DH, Berquist AJ, Arel J, Cervinka T, El-Kotob R, Maltais DB, Wolfe D, Craven BC.. Rehabilitation Interventions to modify endocrine-metabolic disease risk in Individuals with chronic Spinal cord injury living in the Community (RIISC): A systematic review and scoping perspective. J Spinal Cord Med This Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B.. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 2000;81(8):1090–8. doi: 10.1053/apmr.2000.7170 [DOI] [PubMed] [Google Scholar]
- 39.Gibbons RS, Shave RE, Gall A, Andrews BJ.. FES-rowing in tetraplegia: a preliminary report. Spinal Cord 2014;52(12):880–6. doi: 10.1038/sc.2014.159 [DOI] [PubMed] [Google Scholar]
- 40.Ashe MC, Craven C, Eng JJ, Krassioukov A, the SRT Prevention and treatment of bone loss after a spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 2007;13(1):123–145. doi: 10.1310/sci1301-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seibel MJ. Clinical application of biochemical markers of bone turnover. Arq Bras Endocrinol Metabol 2006;50(4):603–20. doi: 10.1590/S0004-27302006000400006 [DOI] [PubMed] [Google Scholar]
- 42.Canalis E, Giustina A, Bilezikian JP.. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 2007;357(9):905–16. doi: 10.1056/NEJMra067395 [DOI] [PubMed] [Google Scholar]
- 43.Mechanick JI, Liu K, Nierman DM, Stein A.. Effect of a convenient single 90-mg pamidronate dose on biochemical markers of bone metabolism in patients with acute spinal cord injury. J Spinal Cord Med 2006;29(4):406–12. doi: 10.1080/10790268.2006.11753890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B, Mechanick JI, Nierman DM, Stein A.. Combined calcitriol-pamidronate therapy for bone hyperresorption in spinal cord injury. J Spinal Cord Med 2001;24(4):235–40. doi: 10.1080/10790268.2001.11753580 [DOI] [PubMed] [Google Scholar]
- 45.Bauman WA, Zhang RL, Morrison N, Spungen AM.. Acute suppression of bone turnover with calcium infusion in persons with spinal cord injury. J Spinal Cord Med 2009;32(4):398–403. doi: 10.1080/10790268.2009.11754393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith E. Proximal tibia fracture in a patient with incomplete spinal cord injury associated with robotic treadmill training. Spinal Cord Ser Cases 2016;2:16010. doi: 10.1038/scsandc.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippo TR, De Carvalho MC, Carvalho LB, de Souza DR, Imamura M, Battistella LR.. Proximal tibia fracture in a patient with incomplete spinal cord injury associated with robotic treadmill training. Spinal Cord 2015;53(12):875–6. doi: 10.1038/sc.2015.27 [DOI] [PubMed] [Google Scholar]
- 48.Lee YJ. Interim recruitment goals in clinical trials. J Chronic Dis 1983;36(5):379–89. doi: 10.1016/0021-9681(83)90170-4 [DOI] [PubMed] [Google Scholar]


