Abstract
Background
Cardiorespiratory fitness training is commonly provided to manual wheelchair users (MWUs) in rehabilitation and physical activity programs, emphasizing the need for a reliable task-specific incremental wheelchair propulsion test.
Objective
Quantifying test-retest reliability and minimal detectable change (MDC) of key cardiorespiratory fitness measures following performance of a newly developed continuous treadmill-based wheelchair propulsion test (WPTTreadmill).
Methods
Twenty-five MWUs completed the WPTTreadmill on two separate occasions within one week. During these tests, participants continuously propelled their wheelchair on a motorized treadmill while the exercise intensity was gradually increased every minute until exhaustion by changing the slope and/or speed according to a standardized protocol. Peak oxygen consumption (VO2peak), carbon dioxide production (VCO2peak), respiratory exchange ratio (RERpeak), minute ventilation (VEpeak) and heart rate (HRpeak) were computed. Time to exhaustion (TTE) and number of increments completed were also measured. Intra-class correlation coefficients (ICC) were calculated to determine test-retest reliability. Standard error of measurement (SEM) and MDC90% values were calculated.
Results
Excellent test-retest reliability was reached for almost all outcome measures (ICC=0.91-0.76), except for RERpeak (ICC=0.58), which reached good reliability. TTE (ICC=0.89) and number of increments (ICC=0.91) also reached excellent test-retest reliability. For the main outcome measures (VO2peak and TTE), absolute SEM was 2.27 mL/kg/min and 0.76 minutes, respectively and absolute MDC90% was 5.30 mL/kg/min and 1.77 minutes, respectively.
Conclusion
The WPTTreadmill is a reliable test to assess cardiorespiratory fitness among MWUs. TTE and number of increments could be used as reliable outcome measures when VO2 measurement is not possible.
Keywords: Wheelchairs, Exercise test, Rehabilitation, Cardiorespiratory fitness
Introduction
A large proportion of individuals with neurological or lower limb impairments will use a manual wheelchair (MW) as a primary means of mobility. Independent and functional MW propulsion requires adequate physical capacity including good muscle strength and cardiorespiratory fitness.1 However, manual wheelchair users (MWUs) often adopt a sedentary lifestyle and have reduced physical capacity.2,3 Consequently, most MWUs have an impaired cardiorespiratory fitness level and are exposed to a higher risk of secondary cardiorespiratory or endocrine-metabolic complications than ambulatory individuals.4–7 Increasing physical capacity is an important objective for new MWUs during rehabilitation and for long-term MWUs living in the community. Thereafter encouraging them to participate in physical activity programs and to remain physically active is crucial.8,9
Cardiorespiratory fitness assessments among MWUs are essential to provide a personalized cardiorespiratory fitness training program for this population and to assess their impact. In most clinical and research settings, arm crank ergometers are typically used to test the cardiorespiratory fitness of MWUs.10,11 However, arm crank ergometer requirements considerably differ from those of MW propulsion. For example, arm crank movements are asynchronous and involve a continuous push and pull application of force, whereas MW propulsion is synchronous and includes push and recovery phases.12
Stationary rollers for MW propulsion can also be used to assess cardiorespiratory fitness.13–15 This device closely replicates the requirements of level-ground propulsion comparatively to the arm crank ergometer. However, the wheelchair is rigidly fixed on the rollers and it allows only for level-ground propulsion so trunk movements and their associated inertial effects are reduced compared to overground propulsion.16,17 Moreover, MWUs frequently encounter slopes such as access ramps and inclined surfaces in their everyday lives.18 During propulsion over slopes, the recruitment of the trunk muscles is increased and thus the energy requirements.19 Hence, using slope increments during the assessment of the cardiorespiratory fitness represents a strategy to increase workload and reach higher values.
A new treadmill-based MW propulsion progressive workload incremental test (WPTTreadmill) has recently been developed to assess the cardiorespiratory fitness of MWUs.20 During this task-specific incremental test, individuals propel their own MW over a motorized treadmill set at different speed and slope combinations. The exercise intensity is gradually increased every minute until exhaustion, by changing the slope or speed according to a standardized protocol. This test allows for an assessment of cardiorespiratory fitness during MW propulsion and closely replicates everyday activity of MWUs.
The objective of this study was to quantify test-retest reliability and minimal detectable change (MDC) of the WPTTreadmill in order to verify if MWUs can consistently perform the WPTTreadmill and to determine the smallest amount of change required between tests to confirm a true change (i.e., exceeding measurement error). To achieve this objective, the peak oxygen consumption (VO2peak) along with other cardiorespiratory responses, time to exhaustion (TTE) and number of increments measured during the performance of two WPTTreadmill tests conducted 2-7 days apart were compared. It was expected that the WPTTreadmill would be reliable (i.e., intra-class correlation coefficient (ICC) ≥ 0.75) and detect change (i.e., MDC ≤ 30%) when comparing VO2peak and other cardiorespiratory responses, TTE, and number of increments between the performance of two WPTTreadmill among MWUs.
Methods
Participants
Twenty-five MWUs (21 males and 4 females) participated in this study. The participants were individuals undergoing intensive inpatient functional rehabilitation (n=11) or living in the community (n=14). Participants undergoing rehabilitation were recruited at an installation of the CIUSSS du Centre-Sud-de-l’Île-de-Montréal–Site Institut de réadaptation Gingras-Lindsay-de-Montréal (IRGLM). Whereas participants living in the community were recruited from a list of individuals who previously participated in research project(s) and accepted to be contacted again. Each MWU underwent a clinical assessment performed by a physiotherapist to ensure eligibility for this study and to collect personal characteristics (age, type of injury, time since injury, level of physical activity) and anthropometric parameters (mass and height) (Table 1). Individuals were eligible to participate in this study if they used a MW as a primary means of mobility (i.e., at least 4 hours per day21) and were using both upper limbs to propel. Potential participants with medical contraindications to a cardiorespiratory fitness assessment and training according to American College of Sports Medicine (ACSM) standards22 or individuals who responded positively to at least one item on the Physical Activity Readiness Questionnaire (PAR-Q) and did not have medical clearance for physical activity23 were excluded from the study. Moreover, each participant completed the Wheelchair User’s Shoulder Pain Index (WUSPI)24 before beginning the experimental task in order to ensure the absence of significant shoulder pain during functional activities such as propelling a MW. Participants were excluded if their shoulder pain was greater than 5/10 for question 5 (“Pushing your chair for 10 minutes or more?”) and 6 (“Pushing up ramps or inclines outdoors?”) of the WUSPI. In addition, participants with any other type of pain or major musculoskeletal impairment or condition (cognitive or visual impairment) that could limit the performance of the experimental tasks were excluded from the study. The study was conducted at the Pathokinesiology Laboratory of the Centre for Interdisciplinary Research in Rehabilitation of Greater Montreal (CRIR) located at the CIUSSS-IRGLM. Ethical approval was obtained from the Research Ethics Committee of the CRIR (#943-0314). All participants reviewed and signed the informed consent form before entering the study.
Table 1.
Description of participants
| Participants | Gender | Age | Height | Mass | BMI | Time since injury | Disorder | ASIA Neurological | WUSPI | |
|---|---|---|---|---|---|---|---|---|---|---|
| (Years) | (m) | (Kg) | (kg/m2) | (Years) | level | AIS* | /20 | |||
| 1 | M | 36.4 | 1.7 | 63.6 | 22 | 7.00 | SCI | C7 | B | 0 |
| 2 | M | 61.3 | 1.74 | 64.9 | 21.4 | 38.92 | SCI | T5 | A | 0 |
| 3 | M | 23.6 | 1.81 | 48.6 | 14.8 | 23.58 | Charcot-Marie-Tooth | -- | -- | 0 |
| 4 | M | 34.8 | 1.78 | 67.6 | 21.3 | 10.83 | SCI | T4 | A | 0 |
| 5 | F | 24.9 | 1.65 | 66 | 24.2 | 24.92 | Cerebral Palsy | -- | -- | 0 |
| 6 | M | 37.8 | 1.77 | 69 | 22.0 | 13.75 | SCI | T6 | D | 1.7 |
| 7 | M | 50.9 | 1.85 | 100 | 29.2 | 0.17 | Cauda equina | -- | -- | 0 |
| 8 | M | 40.1 | 1.81 | 86 | 26.3 | 9.67 | SCI | T6 | C | 0 |
| 9 | M | 57.8 | 1.73 | 83.5 | 27.9 | 25.75 | SCI | T12 | A | 5 |
| 10 | F | 28.4 | 1.71 | 52 | 17.8 | 0.42 | SCI | T12 | D | 0 |
| 11 | M | 27.5 | 1.77 | 47 | 15.0 | 0.33 | SCI | C6 | D | 0 |
| 12 | M | 35.4 | 1.92 | 82 | 22.2 | 10.08 | SCI | T12 | C | 0 |
| 13 | M | 56.7 | 1.72 | 78.88 | 26.7 | 1.33 | SCI | T7 | A | 3.5 |
| 14 | F | 45.8 | 1.67 | 90.5 | 32.5 | 0.33 | SCI | T4 | A | 4.4 |
| 15 | M | 38.9 | 1.85 | 82.6 | 24.1 | 3.75 | SCI | T10 | A | 2.7 |
| 16 | M | 28.8 | 1.8 | 70.5 | 21.8 | 0.75 | SCI | C7 | C | 0 |
| 17 | M | 62.6 | 1.74 | 79.2 | 26.2 | 0.58 | SCI | C5 | D | 0 |
| 18 | M | 42.4 | 1.77 | 62.4 | 19.9 | 0.42 | SCI | L5 | C | 0 |
| 19 | M | 22.3 | 1.77 | 73.8 | 23.6 | 0.00 | SCI | L5 | C | 0.4 |
| 20 | M | 28.7 | 1.78 | 64 | 20.2 | 0.08 | SCI | T5 | B | 0.6 |
| 21 | M | 28.6 | 1.7 | 77.1 | 26.7 | 3.00 | SCI | C8 | A | 2.3 |
| 22 | M | 25.1 | 1.75 | 61.3 | 20.0 | 0.08 | SCI | T12 | A | 0.1 |
| 23 | M | 18.4 | 1.83 | 70.1 | 20.9 | 0.08 | SCI | L1 | D | 0 |
| 24 | F | 26.1 | 1.61 | 63 | 24.3 | 2.42 | SCI | T6 | A | 0 |
| 25 | M | 32.4 | 1.92 | 90.2 | 24.5 | 8.08 | SCI | T6 | A | 0 |
| Mean | 35.3 | 1.77 | 71.75 | 22.96 | 7.64 | 0.8 | ||||
| SD | 14.9 | 0.08 | 13.29 | 4.18 | 10.84 | 1.5 | ||||
*A = No motor or sensory function is preserved below neurological level. B = Sensory function is preserved but motor function is not preserved below neurological level. C = Motor and sensory function is preserved below neurological level. AIS = American Spinal Injury Association Injury Scale. ASIA = American Spinal Injury Association. F = female. M = male. SD = standard deviation. T = thoracic. WUSPI = Wheelchair User’s Shoulder Pain Index. total score for question 5 (/10) and 6 (/10).
Study design
To assess the test-retest reliability of WPTTreadmil, participants performed the test on two separate occasions (i.e., first WPTTreadmill test (T1) and second WPTTreadmill test (T2)) within two to seven days. Both tests were performed at the same time of the day (± one hour) to limit the impact of the circadian rhythm on the measurement of cardiorespiratory responses.25 All participants were asked to avoid consuming caffeine or alcohol at least two hours prior to each test and were instructed to refrain from intense exercise the day before the test.26
Treadmill-based wheelchair propulsion test
The WPTTreadmill was performed on a dual-belt motorized treadmill (Bertec Corporation, Columbus, Ohio, United States) (width = 0.84 m; length = 1.8 m) adapted for safe MW propulsion. The MW was anchored with elastic bands to a bilateral frictionless gliding safety system to prevent excessive anteroposterior and rotational movements of the MW without altering the natural propelling strategies of each participant (Figure 1). In addition, one research associate supervised the participants closely to ensure safety and provide encouragement during the test. Prior to the test, participants had a two-minute warm-up on the motorized treadmill to become familiarized with each speed and slope included in the protocol. During the WPTTreadmil, the exercise workload gradually intensified by increasing the treadmill slope (0°, 1.7o, 2.9°, 3.6°, 4.8°, 5.8o, 7.1o) or the speed (0.6 m/s, 0.8 m/s and 1 m/s) every minute in a standardized manner (Table 2) as determined in a previous study.27 Participants were asked to propel their own MW until exhaustion. The test ended when the participants were unable to match the treadmill’s speed during MW propulsion (i.e., elastic bands were stretched and retain the wheelchair) or if any signs or symptoms for exercise intolerance developed.28 At the end of the test, heart rate and blood pressure were monitored for at least five minutes to ensure participants returned to clinically acceptable resting values.
Figure 1.
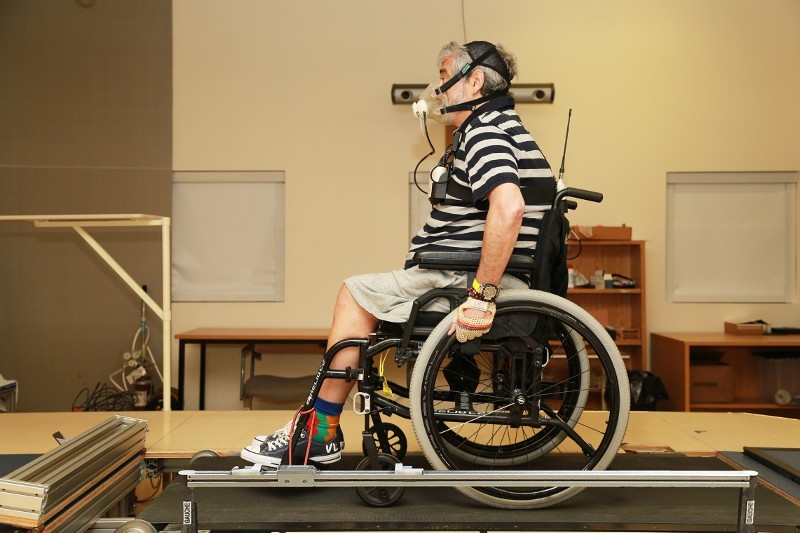
Experimental setting.
Table 2.
Cardiorespiratory fitness wheelchair test protocol
| Increments (60 sec) |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slopes (o) | 0o | 0° | 0° | 1.7° | 1.7° | 2.9° | 2.9° | 3.6° | 4.8° | 4.8° | 4.8° | 5.8° | 5.8° | 7.1° | 7.1° |
| Speeds (m/s) | 0.6 | 0.8 | 1.0 | 0.6 | 0.8 | 0.6 | 0.8 | 0.8 | 0.6 | 0.8 | 1.0 | 0.8 | 1.0 | 0.8 | 1.0 |
Instrumentation and main outcome measures
During the WPTTreadmill, each participant was equipped with a portable gas analysis system (Cosmed K4b2; Cosmed, Rome, Italy), previously calibrated according to manufacturer recommendations, in order to measure their cardiorespiratory responses. This system allows breath-by-breath measurement of oxygen uptake (VO2, mL/kg/min and mL/min), carbon dioxide production (VCO2, mL/min), minute ventilation (VE, L/min) and respiratory exchange ratio (RER). The Cosmed K4b2 is a valid and reliable system for measuring gas exchange during exercise.29 Heart rate (HR, bpm) was monitored using the Polar® Soft Strap heart rate monitor (Polar FT4; Polar, Lachine, Canada) placed around the chest whereas O2 saturation was monitored using an earlobe pulse oximeter (Nonin model 8500; Nonin, Minnesota, USA). Participants were asked to rate their perceived muscular exertion (RPEmuscle) and their perceived cardiorespiratory exertion (RPEcardio) separately, using the Modified Borg Scale (/10).30
Peak values of VO2 (VO2peak), VCO2 (VCO2peak), HR (HRpeak), RER (RERpeak) and VE (VEpeak) were determined using the peak 20-second average over the test. Moreover, RPEmuscle, RPEcardio, time to exhaustion (TTE) expressed in minutes and number of increments completed were measured at the end of each test. Exertion was considered to be maximal if participants had reached RER > 1.1 or if a plateau in VO2 was reached (change < 2.1 mL/kg/min) with an increase in exercise intensity.31 VO2peak and TTE were defined as the main outcome measures in this study.
Statistical analysis
Descriptive statistics were calculated for demographics, clinical characteristics and outcome measures and were expressed as a mean (standard deviation (SD)). The Shapiro-Wilk test was used to confirm the normal distribution of the data (P < 0.05) prior to performing the student paired t-test (or the Wilcoxon test when appropriate) to compare outcome measures obtained during T1 and T2. To assess the test-retest reliability, intra-class coefficients (ICC) for absolute agreement with a 95% confidence interval were performed. ICC values were considered to be excellent if > 0.75, fair to good between 0.40–0.75 and poor if < 0.40.32 Standard error of measurements (SEM) were then calculated before determining the MDC of each outcome measure. Absolute MDC (MDC90%−abs) was calculated (MDC = SEM * z90% * 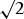 ) to determine the magnitude of the absolute change required to detect a difference that would represent a "real" change, exceeding the measurement error between the two trials. In order for the MDC to be easily interpreted from a clinical point of view, it was expressed as a percentage (MDC90%-rel). Bland-Altman plots were used to determine the limits of agreement between the main outcome measures obtained during T1 and T2. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics 23.0 for Windows.
) to determine the magnitude of the absolute change required to detect a difference that would represent a "real" change, exceeding the measurement error between the two trials. In order for the MDC to be easily interpreted from a clinical point of view, it was expressed as a percentage (MDC90%-rel). Bland-Altman plots were used to determine the limits of agreement between the main outcome measures obtained during T1 and T2. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics 23.0 for Windows.
Results
Of the twenty-five participants, twenty-two participants had a spinal cord injury (SCI), one participant had cauda equine syndrome, one had Charcot-Marie-Tooth disease and one had cerebral palsy. Most participants were men (21/25) and the mean age was 35.3 ± 14.9 years. Eleven participants were physically active (i.e., were participating in physical activity at least three times per week for more than 30 minutes22) while the others were not physically active. None of the participants had previously completed a maximal cardiorespiratory fitness test using upper limbs. Participant characteristics are summarized in Table 1.
All participants performed the WPTTreadmill twice within 4.60 ± 2.02 days and no adverse events occurred during the tests. All participants successfully completed the WPTTreadmill since they met at least one of the criteria for the maximal exercise test. Actually, all participants reached R ≥ 1.1 (mean R for T1 = 1.49 ± 0.23 and mean R for T2 = 1.46 ± 0.27), except for one participant during T2 who reached 0.98 but reached a VO2 plateau (i.e., change in VO2 with an increase in intensity of less than 2.1 ml/kg/min). During this study, the test was stopped by the research team for only one participant because his oxygen saturation level fell below the threshold of 88%.22 His results were kept since he had met maximal exertion criteria when the test was stopped. The results obtained during the first WPTTreadmill and the second WPTTreadmill are shown in Table 3. Due to equipment issues during the tests, HR was successfully recorded for only 13/25 participants. There were no significant differences between T1 and T2 for any outcomes measures (P ≥ 0.297) (Table 3).
Table 3.
Mean values for T1 and T2
| T1 Mean (SD) | T2Mean (SD) | P value | ||
|---|---|---|---|---|
| VO2/kgpeak (mL/kg/min) | n = 25 | 17.90 (5.28) | 18.24 (6.00) | 0.608 |
| TTE (min) | n = 25 | 8.92 (2.42) | 8.99 (2.24) | 0.740 |
| VO2peak (mL/min) | n = 25 | 1287.29 (425.55) | 1297.92 (424.28) | 0.819 |
| VCO2peak (mL/min) | n = 25 | 1663.17 (536.02) | 1625.95 (651.74) | 0.658 |
| HRpeak (bpm) | n = 13 | 167.23 (21.92) | 171.00 (17.22) | 0.528 |
| RERpeak | n = 25 | 1.49 (0.23) | 1.46 (0.27) | 0.482 |
| VEpeak (L/min) | n = 25 | 69.19 (20.62) | 70.73 (24.64) | 0.584 |
| RPEmuscle (/10) | n = 25 | 8.48 (1.48) | 8.08 (1.85) | 0.179 |
| RPEcardio (/10) | n = 25 | 7.16 (1.99) | 7.24 (2.07) | 0.784 |
| Increments | n = 25 | 8.84 (2.46) | 8.80 (2.24) | 0.846 |
SD = standard deviation
In order to quantify the reliability of the outcome measures obtained during T1 and T2, ICC values were calculated. ICC for the main outcome measures of VO2 and the TTE were excellent, with values of 0.835 (95% confidence interval (CI) 0.662–0.924; P < 0.000) and 0.891 (95% CI 0.768-0.950; P < 0,000), respectively (Table 4). ICC for the secondary outcome measures were good to excellent (ICC 0.583-0.909). SEM and the MDC expressed in absolute and relative values for each outcome measure are shown in Table 4.
Table 4.
Intra-class correlation coefficients and minimal detectable change between T1 and T2
| ICC | P value | SEM | MDC90%-abs | MDC90%-rel | |||
|---|---|---|---|---|---|---|---|
| VO2/kgpeak (mL/kg/min) | All participants | n = 25 | 0.835 [0.662–0.924] | 0.000 | 2.27 | 5.30 | 29.35% |
| MWUs-rehabilitation | n = 9 | 0.776 [0.326–0.944] | 0.003 | 2.23 | 5.21 | 31.54% | |
| MWUs-community | n = 11 | 0.877 [0.617–0.965] | 0.000 | 2.15 | 5.01 | 25.27% | |
| TTE (min) | All participants | n = 25 | 0.891 [0.768–0.950] | 0.000 | 0.76 | 1.77 | 19.75% |
| MWUs-rehabilitation | n = 9 | 0.931 [0.465–0.986] | 0.000 | 0.41 | 0.95 | 12.26% | |
| MWUs-community | n = 11 | 0.854 [0.556–0.958] | 0.000 | 0.85 | 1.99 | 20.16% | |
| VO2peak (mL/min) | All participants | n = 25 | 0.859 [0.705–0.935] | 0.000 | 157.93 | 368.52 | 28.51% |
| MWUs-rehabilitation | n = 9 | 0.645 [0.053–0.906] | 0.023 | 175.75 | 410.10 | 36.35% | |
| MWUs-community | n = 11 | 0.895 [0.671–0.970] | 0.000 | 159.78 | 372.84 | 25.56% | |
| VCO2peak (mL/min) | All participants | n = 25 | 0.764 [0.534–0.889] | 0.000 | 287.04 | 669.80 | 40.73% |
| MWUs-rehabilitation | n = 9 | 0.741 [0.181–0.936] | 0.009 | 333.64 | 778.53 | 49.75% | |
| MWUs-community | n = 11 | 0.749 [0.329–0.925] | 0.002 | 249.62 | 582.47 | 34.15% | |
| HRpeak (bpm) | All participants | n = 13 | 0.559 [0.038–0.841] | 0.021 | 12.69 | 29.62 | 17.55% |
| MWUs-rehabilitation | |||||||
| MWUs-community | |||||||
| RERpeak | All participants | n = 25 | 0.583 [0.251–0.792] | 0.001 | 0.16 | 0.38 | 25.72% |
| MWUs-rehabilitation | n = 9 | 0.850 [0.495–0.963] | 0.001 | 0.11 | 0.26 | 17.79% | |
| MWUs-community | n = 11 | 0.033 [-0.535–0.533] | 0.544 | 0.22 | 0.50 | 36.41% | |
| VEpeak (L/min) | All participants | n = 25 | 0.817 [0.628–0.915] | 0.000 | 9.63 | 22.46 | 32.11% |
| MWUs-rehabilitation | n = 9 | 0.808 [0.398–0.952] | 0.002 | 10.43 | 24.34 | 36.64% | |
| MWUs-community | n = 11 | 0.835 [0.517–0.952] | 0.000 | 8.28 | 19.33 | 23.84% | |
| RPEmuscle (/10) | All participants | n = 25 | 0.619 [0.312–0.810] | 0.000 | 1.03 | 2.40 | 28.99% |
| MWUs-rehabilitation | n = 9 | 0.415 [-0.269–0.828] | 0.117 | 1.41 | 3.30 | 39.54% | |
| MWUs-community | n = 11 | 0.913 [0.714–0.976] | 0.000 | 0.48 | 1.12 | 14.19% | |
| RPEcardio (/10) | All participants | n = 25 | 0.755 [0.517–0.884] | 0.000 | 0.99 | 2.32 | 32.25% |
| MWUs-rehabilitation | n = 9 | 0.721 [0.140–0.930] | 0.012 | 1.36 | 3.18 | 51.16% | |
| MWUs-community | n = 11 | 0.915 [0.715–0.977] | 0.000 | 0.40 | 0.93 | 12.39% | |
| Increments | All participants | n = 25 | 0.909 [0.805–0.959] | 0.000 | 0.70 | 1.64 | 18.57% |
| MWUs-rehabilitation | n = 9 | 0.914 [0.478–0.989] | 0.000 | 0.34 | 0.79 | 10.33% | |
| MWUs-community | n = 11 | 0.876 [0.622–0.965] | 0.000 | 0.74 | 1.74 | 17.94% |
ICC = intra-class correlation coefficient; SEM = standard error of measurement; MDC90%-abs = absolute minimal detectable change; MDC90%-rel = relative minimal detectable change; MWUs = manual wheelchair users
VO2 obtained during T2 tended to be slightly greater (+ 0.34 ± 3.28 mL/kg/min) on average, but the difference was considerably smaller than the MDC of 5.30, and the 95% limit of agreement (i.e., 1.96 SD) for the difference ranges between 6.77 and -6.09 mL/kg/min (Figure 2A). Moreover, all but one data point fell within the limit of agreement and the data points were equally distributed above and below the mean difference line (i.e., absence of systematic error). TTE obtained during T2 tended to be slightly greater (+ 0.07 ± 1.10 minutes) on average, but was lower than the MDC of 1.77 minutes, and the 95% limit of agreement (2.23 to -2.09 minutes) (Figure 2B). All data points fell within the limit of agreement and the data points were equally distributed above and below the mean difference line.
Figure 2.
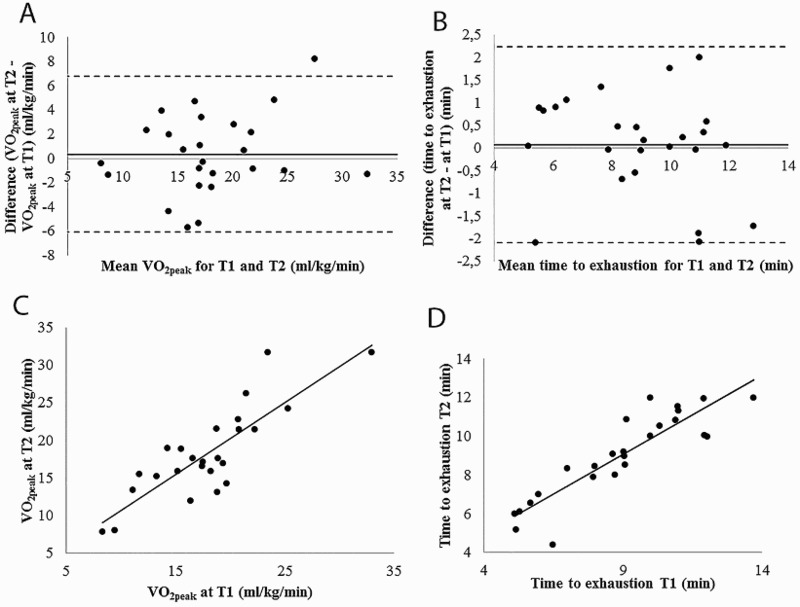
Bland-Altman plot graphs for VO2peak (A) and TTE (B) between T1 and T2 and correlation graphs for VO2peak (C) and TTE (D).
Participants who were recruited during their intensive inpatient functional rehabilitation program had similar cardiorespiratory fitness values than those recruited in the community (P = 0.064-0.915). Moreover, the reliability values for each group are presented in Table 4.
Discussion
The aim of this study was to quantify the test-retest reliability and the MDC of cardiorespiratory fitness outcome measures obtained via a new treadmill-based MW propulsion progressive workload incremental test for MWUs (i.e., WPTTreadmill). The results of this study suggest that the WPTTreadmill is a reliable test for rehabilitation and physical activity professionals to quantify cardiorespiratory fitness of MWUs and, consequently, to propose personalized rehabilitation or physical activity programs. Moreover, this study measured the absolute and relative MDC in order to help professionals in decision making. Furthermore, this test may be useful for measuring or comparing the impact or effectiveness of various rehabilitation or physical activity programs targeting cardiorespiratory fitness.
Feasibility of the proposed test
All participants have reached maximal exercise intensity since they all have met at least one criteria characterizing VO2peak. No participant had to stop the test before reaching their maximal capacity. In our sample, all participants who had a tetraplegia successfully completed the test and had a tendency to reach slightly lower peak values than individuals with paraplegia (i.e., mean VO2peak for tetraplegia = 15.35 ± 4.68 ml/kg/min versus 18.28 ± 6.01 ml/kg/min for participants with paraplegia). The experience in MW propulsion doesn't appear to impact the results of the test since there was no statistical difference between individuals undergoing rehabilitation (i.e., with recent SCI and little experience in MW propulsion) and participants living in the community (i.e., with longer experience in MW propulsion). Moreover, most of the ICC, SEM and MDC values are comparable for individuals in rehabilitation and those living in the community. Hence, the proposed test can be completed by most MWUs, including those with high thoracic or cervical SCI having basic wheelchair skills and with stable cardiovascular and autonomic functions.
Reliability of the proposed test
As expected, the WPTTreadmill is reliable since the main outcome measures yielded excellent reliability coefficients (i.e., ICC ≥ 0.75). Moreover, all other outcome measures measured on two separate occasions had good to excellent reliability coefficients. Most outcome measures had a small MDC, except for VEpeak, RPEcardio, which had a relatively small MDC (i.e., MDC = 32.11% and 32.25%, respectively) and VCO2peak, which was the outcome measure with the smallest MDC (MDC = 40.73%). Finally, the VO2peak values found in this study were similar to other findings previously reported in the literature,1,4,33,34 indicating that this sample was representative of a MWU population with a SCI.
When compared to reliability coefficients for cardiorespiratory fitness tests among MWUs in other studies, the WPTTreadmill had better test-retest reliability. Hol et al. studied the reliability of a 6-minute arm crank test among individuals with SCI who were MWUs and found an ICC of 0.81 for VO2 at a steady state,33 whereas Bulthuis et al. found a test-retest ICC of 0.76 for VO2peak during a submaximal arm crank ergometer test.26
The small outcome measure differences found between the two tests can be explained in part by measurement errors inherent to the use of the Cosmed K4b2 system. In fact, measurement errors range between 1.24% and 12.06%, depending on the variable studied and the protocol used.29 Moreover, day-to-day variability in strength, coordination, concentration and/or motivation of the participants could have affected their performance during the tests and impacted test-retest reliability to some extent.35 Other potential factors, such as time of the day, testing protocol and equipment used, were controlled as much as possible to minimize their impact on reliability and measurement error. Since consumption of stimulants such as coffee, tobacco or alcohol can affect VO2peak and other outcome measures of the test,36 participants were asked to refrain from taking those substances two hours before the test. However, some factors are still difficult to control such as verbal encouragement, which may have differed between each trial and between participants. Moreover, the reaction of participants to those encouragements could not be controlled and may have differed among participants.35 Finally, level of fatigue could have affected performance among participants and may have been influenced by many factors such as quality of sleep, level of activity during the day, stress, etc. In order to reduce the effect of fatigue, participants were asked not to exercise at moderate to high intensity the day prior to the trial.
The Bland-Althman plots demonstrated a mean difference close to zero for VO2peak and TTE (i.e., 0.34 ± 3.28 mL/kg/min and 0.07 ± 1.10 minutes, respectively). Moreover, no familiarization effect was observed for VO2peak and TTE since there was no tendency for either the first test or the second test to have higher values. This can be explained by the familiarization period allowed prior to the test during which participants propelled their own MW at each speed and over each slope included in the test. Moreover, since participants used their own personal MW during the test, a familiarization effect related to the MW or body positioning was less likely to occur.
Standard error of measurement and minimal detectable change of the newly developed continuous treadmill-based wheelchair propulsion test
Only a few studies have reported SEM values for exercise tests developed for MWUs33,37 and only one has calculated the MDC for VO2peak.37 Bloemen et al. found a SEM of 1.87 mL/kg/min and a MDCabs95% of 5.18 mL/kg/min (MDCrel95% ≈ 22.38%) for a graded wheelchair propulsion test performed on a roller among children with spina bifida.37 Stoller O. et al. found a SEM of 2 mL/kg/min for VO2peak with a MDCabs95% of 5.6 mL/kg/min (MDCrel95% of 36%) during a maximal cardiorespiratory test among individuals with early stroke using a feedback-controlled robotics-assisted treadmill exercise.38 Vancampfort D. et al. found a MDCabs95% of 6.5 mL/kg/min (MDCrel95% ≈ 18.81%) among ambulatory individuals with schizophrenia during a Astrand–Rhyming cycle ergometer test.39 Moreover, a study of 39 studies on reliability of VO2peak measurements using different protocols and exercise models among ambulatory participants reported an average SEM of 2.58 mL/kg/min.40 All those results are similar to those found in this study since the SEM for VO2peak was 2.27 mL/kg/min and the MDCabs90% was 5.30 mL/kg/min (MDCrel90% of 29.35%). However, the relative MDC found in this study was slightly higher than in the other studies, which may be explained by a lower mean VO2peak in our study (mean VO2peak = 18.07 ± 5.59 mL/kg/min vs 22.8 ± 6.6 mL/kg/min37 and 34.6 ± 8.7 mL/kg/min39). Finally, no studies have reported SEM or MDC for TTE.
Translating outcomes into clinical practice
Measuring direct VO2peak using a gas exchange analyser is not often possible in clinical settings since such devices are expensive and require specific user training. Moreover, the time constraints experienced by many rehabilitation or physical activity professionals limit the use of direct measurements of gas exchange for cardiorespiratory fitness assessments. Interestingly, the results of the present study emphasize that cardiorespiratory fitness can be accurately estimated using only TTE and number of increments completed during the WPTTreadmill since their test-retest reliability coefficients are excellent. Of course, measurement of direct VO2peak should be encouraged whenever possible since it is the gold standard for cardiorespiratory fitness41 and allows for comparison with normative values. Moreover, direct measurement of VO2peak with a gas exchange analyser allows clinicians and researchers to measure many other valuable outcome measures such as RER, VE and respiration rate. These outcome measures would provide highly relevant comprehensive measures of cardiorespiratory fitness for MWUs.
Study limitations
The first limitation of the present study is its small sample size. According to Altman, a sample size of 50 or more is considered adequate to assess agreement parameters.42 However considering the difficulty with recruiting MWUs, our sample of 25 participants appears sufficient, and is greater than previous studies reporting on reliability. Even though participants tried each speed and each slope during the familiarization period, they did not perform the test itself before the measurements were taken as proposed by Currell K and Jeukendrup AE.35 Using the comparison between a second and third test may have reduced the coefficient of variability by almost 2%.35
Conclusion
In summary, the WPTTreadmill is a reliable test for assessing cardiorespiratory fitness among MWUs. This test can be used with confidence in both clinical and research settings since both direct (i.e., Cosmed K4b2 gas analyser) and indirect (i.e., TTE, number of increments) measures of VO2peak yielded excellent reliability. Moreover, it is a safe test that can be used for inpatient and outpatient individuals since no adverse events occurred. Measuring the validity and responsiveness of the WPTTreadmill in future studies is recommended to strengthen the level of evidence before recommending its use as a clinical measurement instrument to quantify cardiovascular fitness in MWUs.
Disclaimer statements
Contributors Special thanks to Michel Goyette and Philippe Gordou for their technical support and assistance during the preparation and realization of this study.
Funding Cindy Gauthier is supported by a doctoral scholarship from the Canadian Institutes of Health Research (CIHR). This study was funded by the partnership between l’Ordre professionnel de la physiothérapie du Québec (OPPQ) and the Réseau provincial de recherche en adaptation-réadaptation (REPAR) (Grant 2014-15#2). The equipment and material required for the research completed at the Pathokinesiology Laboratory was financed by the Canada Foundation for Innovation (CFI).
Declaration of interest: None.
Conflicts of interest None.
Ethics approval Ethical approval was obtained from the Research Ethics Committee of the CRIR (#943–0314).
References
- 1.van der Scheer JW, de Groot S, Tepper M, Gobets D, Veeger DH, van der Woude LH.. Wheelchair-specific fitness of inactive people with long-term spinal cord injury. J Rehabil Med. 2015;47(4):330–7 doi: 10.2340/16501977-1934 [DOI] [PubMed] [Google Scholar]
- 2.Tawashy AE, Eng JJ, Krassioukov AV, Miller WC, Sproule S.. Aerobic exercise during early rehabilitation for cervical spinal cord injury. Phys Ther. 2010;90(3):427–37 doi: 10.2522/ptj.20090023 [DOI] [PubMed] [Google Scholar]
- 3.van den Berg-Emons RJ, Bussmann JB, Haisma JA, Sluis TA, van der Woude LH, Bergen MP, et al. A prospective study on physical activity levels after spinal cord injury during inpatient rehabilitation and the year after discharge. Arch Phys Med Rehabil. 2008;89(11):2094–101 doi: 10.1016/j.apmr.2008.04.024 [DOI] [PubMed] [Google Scholar]
- 4.Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Bussmann JB.. Physical capacity in wheelchair-dependent persons with a spinal cord injury: A critical review of the literature. Spinal Cord. 2006;44(11):642–52 doi: 10.1038/sj.sc.3101915 [DOI] [PubMed] [Google Scholar]
- 5.Phillips WT, Kiratli BJ, Sarkarati M, Weraarchakul G, Myers J, Franklin BA, et al. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998;23(11):641–716 doi: 10.1016/S0146-2806(98)80003-0 [DOI] [PubMed] [Google Scholar]
- 6.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–16 doi: 10.1038/sj.sc.3101729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers J, Lee M, Kiratli J.. Cardiovascular disease in spinal cord injury: An overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–52 doi: 10.1097/PHM.0b013e31802f0247 [DOI] [PubMed] [Google Scholar]
- 8.Dallmeijer AJ, van der Woude LH, Hollander AP, van As HH.. Physical performance during rehabilitation in persons with spinal cord injuries. Med Sci Sports Exerc. 1999;31(9):1330–5 doi: 10.1097/00005768-199909000-00015 [DOI] [PubMed] [Google Scholar]
- 9.Pelletier CA. Incorporating physical activity into the rehabilitation process after spinal cord injury. Appl Physiol Nutr Metab. 2014;39(4):513 doi: 10.1139/apnm-2013-0482 [DOI] [Google Scholar]
- 10.Price MJ, Bottoms L, Smith PM, Nicholettos A.. The effects of an increasing versus constant crank rate on peak physiological responses during incremental arm crank ergometry. J Sports Sci. 2011;29(3):263–9 doi: 10.1080/02640414.2010.525520 [DOI] [PubMed] [Google Scholar]
- 11.Battikha M, Sa L, Porter A, Taylor JA.. Relationship between pulmonary function and exercise capacity in individuals with spinal cord injury. Am J Phys Med Rehabil. 2014;93(5):413–21 doi: 10.1097/PHM.0000000000000046 [DOI] [PubMed] [Google Scholar]
- 12.Torhaug T, Brurok B, Hoff J, Helgerud J, Leivseth G.. Arm crank and wheelchair ergometry produce similar peak oxygen uptake but different work economy values in individuals with spinal cord injury. Biomed Res Int. 2016;2016(5481843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Berg R, de Groot S, Swart KM, van der Woude LH.. Physical capacity after 7 weeks of low-intensity wheelchair training. Disabil Rehabil. 2010;32(26):2244–52 doi: 10.3109/09638288.2010.535688 [DOI] [PubMed] [Google Scholar]
- 14.Bougenot MP, Tordi N, Betik AC, Martin X, Le Foll D, Parratte B, et al. Effects of a wheelchair ergometer training programme on spinal cord-injured persons. Spinal Cord. 2003;41(8):451–6 doi: 10.1038/sj.sc.3101475 [DOI] [PubMed] [Google Scholar]
- 15.Grange CC, Bougenot MP, Groslambert A, Tordi N, Rouillon JD.. Perceived exertion and rehabilitation with wheelchair ergometer: Comparison between patients with spinal cord injury and healthy subjects. Spinal Cord. 2002;40(10):513–8 doi: 10.1038/sj.sc.3101353 [DOI] [PubMed] [Google Scholar]
- 16.Vanlandewijck Y, Theisen D, Daly D.. Wheelchair propulsion biomechanics: Implications for wheelchair sports. Sports Med. 2001;31(5):339–67 doi: 10.2165/00007256-200131050-00005 [DOI] [PubMed] [Google Scholar]
- 17.Mason B, Lenton J, Leicht C, Goosey-Tolfrey V.. A physiological and biomechanical comparison of over-ground, treadmill and ergometer wheelchair propulsion. J Sports Sci. 2014;32(1):78–91 doi: 10.1080/02640414.2013.807350 [DOI] [PubMed] [Google Scholar]
- 18.Hurd WJ, Morrow MM, Kaufman KR, An KN.. Wheelchair propulsion demands during outdoor community ambulation. J Electromyogr Kinesiol. 2009;19(5):942–7 doi: 10.1016/j.jelekin.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howarth SJ, Polgar JM, Dickerson CR, Callaghan JP.. Trunk muscle activity during wheelchair ramp ascent and the influence of a geared wheel on the demands of postural control. Arch Phys Med Rehabil. 2010;91(3):436–42 doi: 10.1016/j.apmr.2009.10.016 [DOI] [PubMed] [Google Scholar]
- 20.Gauthier C, Grangeon M, Ananos L, Brosseau R, Gagnon DH.. Quantifying cardiorespiratory responses resulting from speed and slope increments during motorized treadmill propulsion among manual wheelchair users. Ann Phys Rehabil Med. 2017;60(5):281–8 doi: 10.1016/j.rehab.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 21.Sonenblum SE, Sprigle SH, Martin JS.. Everyday sitting behavior of full-time wheelchair users. J Rehabil Res Dev. 2016;53(5):585–98 doi: 10.1682/JRRD.2015.07.0130 [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. , Swain DP, Brawner CA.. Acsm's resource manual for guidelines for exercise testing and prescription. 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. xv, 862 p. [Google Scholar]
- 23.Thomas S, Reading J, Shephard RJ.. Revision of the physical activity readiness questionnaire (par-q). Can J Sport Sci. 1992;17(4):338–45 [PubMed] [Google Scholar]
- 24.Curtis KA, Roach KE, Applegate EB, Amar T, Benbow CS, Genecco TD, et al. Reliability and validity of the wheelchair user's shoulder pain index (wuspi). Paraplegia. 1995;33(10):595–601 doi: 10.1038/sc.1995.126 [DOI] [PubMed] [Google Scholar]
- 25.Hill DW. Effect of time of day on aerobic power in exhaustive high-intensity exercise. J Sports Med Phys Fitness. 1996;36(3):155–60 [PubMed] [Google Scholar]
- 26.Bulthuis Y, Drossaers-Bakker W, Oosterveld F, van der Palen J, van de Laar M.. Arm crank ergometer is reliable and valid for measuring aerobic capacity during submaximal exercise. J Strength Cond Res. 2010;24(10):2809–15 doi: 10.1519/JSC.0b013e3181e31242 [DOI] [PubMed] [Google Scholar]
- 27.Gauthier C, Grangeon M, Ananos L, Brosseau R, Gagnon D.. Development of a new cardiorespiratory fitness test during treadmill propulsion using graded slope and speed increments in manual wheelchair users. Annales de Réadaptation et de Médecine Physique. 2017;(accepted for publication) [Google Scholar]
- 28.American College of Sport Medicine Acsm's resource manual for guidelines for exercise testing and prescription. 5th ed: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 29.Duffield R, Dawson B, Pinnington HC, Wong P.. Accuracy and reliability of a cosmed k4b2 portable gas analysis system. J Sci Med Sport. 2004;7(1):11–22 doi: 10.1016/S1440-2440(04)80039-2 [DOI] [PubMed] [Google Scholar]
- 30.Wilson RC, Jones PW.. A comparison of the visual analogue scale and modified borg scale for the measurement of dyspnoea during exercise. Clin Sci (Lond). 1989;76(3):277–82 doi: 10.1042/cs0760277 [DOI] [PubMed] [Google Scholar]
- 31.Duncan GE, Howley ET, Johnson BN.. Applicability of vo2max criteria: Discontinuous versus continuous protocols. Med Sci Sports Exerc. 1997;29(2):273–8 doi: 10.1097/00005768-199702000-00017 [DOI] [PubMed] [Google Scholar]
- 32.Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil. 2000;81(12 Suppl 2):S15–20 doi: 10.1053/apmr.2000.20619 [DOI] [PubMed] [Google Scholar]
- 33.Hol AT, Eng JJ, Miller WC, Sproule S, Krassioukov AV.. Reliability and validity of the six-minute arm test for the evaluation of cardiovascular fitness in people with spinal cord injury. Arch Phys Med Rehabil. 2007;88(4):489–95 doi: 10.1016/j.apmr.2006.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen TW, Dallmeijer AJ, Veeger DJ, van der Woude LH.. Normative values and determinants of physical capacity in individuals with spinal cord injury. J Rehabil Res Dev. 2002;39(1):29–39 [PubMed] [Google Scholar]
- 35.Currell K, Jeukendrup AE.. Validity, reliability and sensitivity of measures of sporting performance. Sports Med. 2008;38(4):297–316 doi: 10.2165/00007256-200838040-00003 [DOI] [PubMed] [Google Scholar]
- 36.Medicine ACS. Acsm's health-related physical fitness assessment manual: Wolters Kluwer Health; 2013. [Google Scholar]
- 37.Bloemen MA, de Groot JF, Backx FJ, Westerveld RA, Takken T.. Arm cranking versus wheelchair propulsion for testing aerobic fitness in children with spina bifida who are wheelchair dependent. J Rehabil Med. 2015;47(5):432–7 doi: 10.2340/16501977-1944 [DOI] [PubMed] [Google Scholar]
- 38.Stoller O, de Bruin ED, Schindelholz M, Schuster-Amft C, de Bie RA, Hunt KJ.. Cardiopulmonary exercise testing early after stroke using feedback-controlled robotics-assisted treadmill exercise: Test-retest reliability and repeatability. J Neuroeng Rehabil. 2014;11:145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vancampfort D, Guelinckx H, De Hert M, Stubbs B, Soundy A, Rosenbaum S, et al. Reliability and clinical correlates of the astrand-rhyming sub-maximal exercise test in patients with schizophrenia or schizoaffective disorder. Psychiatry Res. 2014;220(3):778–83 doi: 10.1016/j.psychres.2014.08.049 [DOI] [PubMed] [Google Scholar]
- 40.Vickers R. Measurement error in maximal oxygen uptake tests. In: Center NHR , editor. San Diego supported by the U.S. Army Medical Research and Materiel Command; 2003. p. 1–23. [Google Scholar]
- 41.Heine M, Verschuren O, Kwakkel G.. Validity of oxygen uptake efficiency slope in patients with multiple sclerosis. J Rehabil Med. 2014;46(7):656–61 doi: 10.2340/16501977-1825 [DOI] [PubMed] [Google Scholar]
- 42.Altman DG. Practical statistics for medical research: Taylor & Francis; 1990. [Google Scholar]


