Abstract
Context
Individuals with chronic spinal cord injury (SCI) are susceptible to central and visceral obesity and it’s metabolic consequences; consensus based guidelines for obesity management after SCI have not yet been stablished.
Objectives
To identify and compare effective means of obesity management among SCI individuals.
Methods
This systematic review included English and non-English articles, published prior to April 2017 found in the PubMed/Medline, Embase, CINAHL Psychinfo and Cochrane databases. Studies evaluating any obesity management strategy, alone or in combination, including: diet therapy, voluntary and involuntary exercise such as neuro-muscular electric stimulation (NMES), pharmacotherapy, and surgery, among individuals with chronic SCI were included. Outcomes of interest were reductions in waist circumference, body weight (BW), body mass index (BMI) and total fat mass (TFM) and increases in total lean body mass (TLBM) from baseline. From 3,553 retrieved titles and abstracts, 34 articles underwent full text review and 23 articles were selected for data abstraction. Articles describing weight loss due to inflammation, cancer or B12 deficiency were excluded. The Downs and Black reported poor to moderate quality of the studies.
Results
Bariatric surgery produced the greatest permanent weight reduction and BMI correction followed by combinations of physical exercise and diet therapy. Generally, NMES and pharmacotherapy improved TLBM and reduced TFM but not weight.
Conclusions
The greatest weight reduction and BMI correction was produced by bariatric surgery, followed by a combination of physical exercise and diet therapy. NMES and pharmacologic treatment did not reduce weight or TFM but increased in TLBM.
Keywords: Spinal cord injury, Obesity, Body composition, Management
Introduction
The chronic Spinal Cord Injury (SCI) population is vulnerable to obesity, due to concurrent development of sarcopenia and decreased metabolic rate, limited mobility, and diminished levels of activity.1 The prevalence of obesity among the individuals with SCI varies from 40 to 66% across studies.2 Obese individuals with SCI are susceptible to a wide range of health consequences.
Dangerous health consequences associated with obesity include, but are not limited to: an increased prevalence of angina pectoris, cerebrovascular accidents, breast cancer, carpal tunnel syndrome, cholecystitis/cholelithiasis, colon cancer, congestive heart failure, coronary artery disease, depression, glucose intolerance, diabetes mellitus, dyslipidemia, gout, nephrolithiasis, obstructive sleep apnea, osteoarthritis, peripheral vascular disease, pressure ulcers, reproductive dysfunction and social isolation.3 Adequate detection, monitoring and management of obesity in persons with SCI is of utmost importance in reducing all-cause mortality.
Detection of obesity in SCI
Obesity is defined as the excess accumulation of body fat, (more than 25% of body weight (BW) for men, and more than 30% for women,4 which is well characterized in the general population by Body Mass Index (BMI) due to the high correlation (r = 0.7–0.9) of BMI with fat mass. BMI is measured in (kg/m2) and is calculated by dividing an individual’s weight (kg) by his or her height squared (m2).5 The WHO has assigned BMI threshold values for the diagnosis of overweight (BMI 25.0–29.9) or obese (BMI ≥30) respectively.6
Although BMI is a good obesity screening tool in the general population, it’s application to individuals with chronic SCI is problematic as the decreases in lean mass and increases in fat mass do not necessarily result in changes in BW, a key component of the BMI calculation. In addition, measurement of height in individuals with chronic SCI is neither as feasible nor reproducible.7,8 Therefore, the BMI thresholds for individuals with chronic SCI have been lowered to 22 Kg/m2 to account for these measurement dilemmas.9
Total fat mass and its distribution to visceral rather than subcutaneous tissues is associated with Metabolic Syndrome, and other dangerous medical consequences of obesity.10,11 Abdominal obesity, specifically visceral adipose tissue (VAT) increases following SCI.12 The VAT threshold for obesity has been lowered from 130 cm2 in able-bodied13 to 100 cm2 for the SCI population.14
Waist circumference (WC) is an accurate means of predicting VAT in the able-bodied or general population. Persons with a WC > 88 cm for women and > 102 cm for men are defined as obese by the National Institute of Health (http://www.nhlbi.nih.gov). Although WC as a marker of VAT in SCI patients was arguable,7 newer studies have reported an increase in VAT and obesity-related cardiovascular disease risk in SCI population; with a disease- specific cut-point for obesity of WC ≥ 94cm.15,16
Total body water (TBW), total lean body mass (TLBM), total fat mass (TFM) and extra cellular water (ECW) can be reasonably well predicted by bioelectric impedance analysis (BIA) in SCI population.17,18 Dual-Energy X-ray Absorptiometry (DEXA) is another valid and reliable method for estimation of body composition components and reference values have been defined from national health and nutrition examination survey (NHANES) in general population. Fat Mass Index (FMI) ≥ 9 kg/m2 or percentage body fat ≥ 25% for males and ≥ 13 kg/m2 or percentage body fat ≥35% for females indicates obesity.19 DEXA values are highly reproducible among wheel-chair athletes.20
Management of obesity in SCI
Numerous interventions are used to manage obesity in the general population; these mainly include: diet therapy, medical therapy, physical therapy or various exercise programs and bariatric surgeries
Application of these therapies to individuals with SCI is inappropriate due to their mobility impairment, lifestyle issues, greater sensitivity to anti-obesity agents and other health conditions, such as, pressure ulcer or septicemia.21
Diet Therapy:
The premise of all diets assumes lowering caloric intake below basic requirements for body organs/tissue and physical activity will result in weight loss. Low-calorie diets (LCDs) with 1000–1,200 kcal/day reduce weight by an average of 8% among the able-bodied population over 3–12 months. In contrast to LCD’s, Very low-calorie diets (VLCDs) of 400–500 kcal/day produce larger initial weight loss; however, the long-term (≥1 year) weight loss is equivalent to LCDs.22 Thus, healthy diets recommend decreasing caloric intake from fat to <30%, carbohydrate and protein ∼50% and 15%, respectively while increasing Fiber intake. LCDs and VLCDs may not deliver sufficient amounts of macro and micro-nutrients crucial for healthy body functioning.23 Individuals with chronic SCI may not respond to LCD’s due to sarcopenic obesity and greater protein requirements.
Physical Exercise:
The Resting Metabolic Rate (RMR) of skeletal muscle is relatively low although skeletal muscles comprises 40% - 50% of total BW, and dictates resting Total Energy Expenditure (TEE). Energy Expenditure in Physical Activity (EEPA) represents the most variable component of TEE. Lean Body Mass (LBM) is the main organ of the body in terms of daily EEPA.24 Both RMR and EEPA are decreased in chronic SCI due to reduced LBM and low levels of physical activity.25
Behavioral Modification:
Behavior therapy is one principal component of an “effective high-intensity lifestyle intervention” within the content of overweight and obesity programs which facilitates adherence to weight management recommendations (from Reduce CAD Risk in SCI). Common components of behavioral therapy for obesity include: self-monitoring, stimulus control, slow eating, goal settings, behavioral contracting, education, increasing physical activity and social supports.26 The key principle is to identify cravings and weaken or disconnect the triggering events that precipitate overeating.
Pharmacotherapy:
Pharmacological treatments for obesity are used as adjuvant therapy among the morbidly obese after lifestyle modification in order to maintain weight loss is achieved. Anti-obesity drugs either suppress appetite and/or stimulate thermogenesis (e.g. serotonergic: fenfluramine, chatecolaminergic: phentermine) and interact with intestinal fat absorption (orlistate). Older drugs include: dinitrophenol, aminorex, amphetamines, fenfluramine/ dexfenfluramine, phenylprpoanolamoine. Phentermine and diethylpropione are approved for short-term use (< three months) in the USA.
Surgical Therapy:
Weight-loss surgery is typically second or third line therapy after failure of lifestyle modifications in morbidly obese individuals. The National Institutes of Health consensus conference on gastrointestinal surgery for severe obesity reports weight-loss surgery may be an appropriate option for morbid obesity (BMI >40 kg/m2) or for those with moderate obesity (BMI>35 kg/m2) with two or more obesity related comorbidities.27 Bariatric procedures fall into three major categories: intestinal malabsorption, gastric restriction and combined malabsorption and restriction procedures. Mal-absorptive operations depend on rearrangement of the small intestine to decrease the functional length or efficiency of the intestinal mucosa for nutrient absorption. Restrictive procedures involve restriction of the amount of food entering the foregut.28
This systematic review aimed to identify and compare the effectiveness of diet therapy, physical exercises and newer modalities of passive exercises such as functional electrical stimulation (FES), pharmacological treatments and surgical therapy for management of obesity in individuals with chronic SCI. We considered five outcomes of importance including decrease in BW, BMI, total fat mass (TFM) and WC and TLBM.
Methods
Data source
The PubMed/Medline, EMBASE, CINAHL, PSYCHINFO, Cochrane CENTRAL and Cochrane database for Systematic Review databases were reviewed to identify all potential trials published prior to April 2017 in any language. The search strategy in Ovid-Medline is attached as Appendix 1. The PRISMA Flow Diagram is shown in Figure 1.
Figure 1.
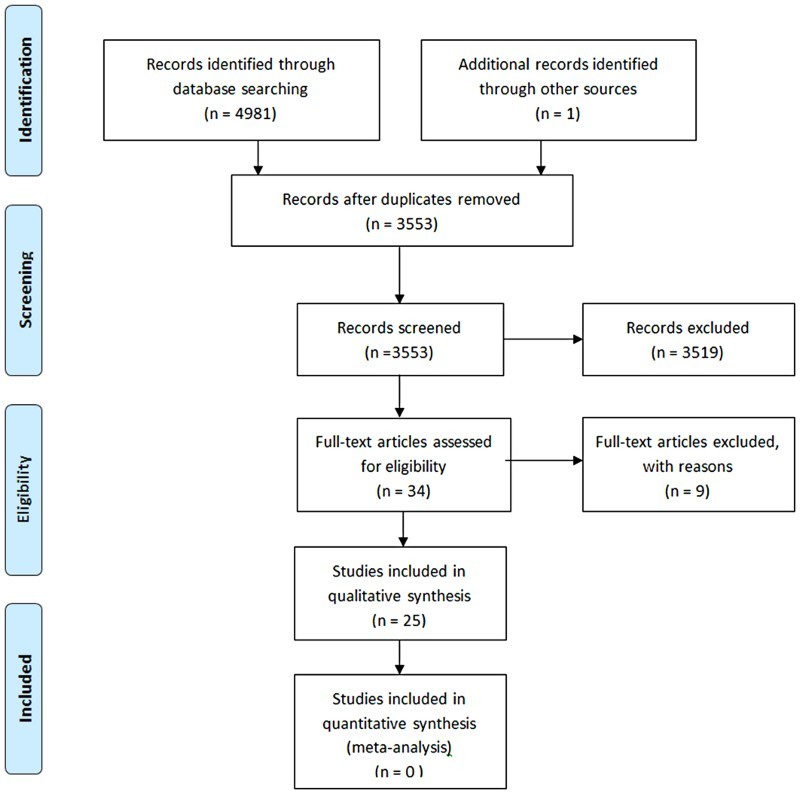
PRISMA Flow Diagram – study selection process.
Study selection
Inclusion and Exclusion Criteria: Articles evaluating any obesity management strategy alone or in combination including diet therapy, physical exercises, passive exercises like neuro-muscular electric stimulation (NMES), pharmacotherapy, and surgery, among individuals with chronic SCI were included. Outcomes of interest were declines in WC, BW, BMI and TFM and increases in TLBM.
Studies in which individuals had inflammation or any kind of neoplasms were excluded due to their role in unintended weight loss. Studies among participants with nutritional disturbances as a cause of SCI such as subacute combined degeneration due to vitamin B12 deficiency were also excluded.
Data extraction and quality assessment
Two reviewers (MHS, SMA) independently assessed the studies identified through the electronic searches. Discrepancy between reviewers was resolved by discussion, in the event of disagreement between the reviewers, a third reviewer was recruited prior to resolution. The quality of the selected studies was evaluated by Downs and Black (D&B) scale regardless of their designs.29 Because of the nature of the interventions, which are infeasible to blinding during study implementation, we chose the D&B tool to appraise article quality, a more comprehensive approach when compared to the logistics of blinding.
Results
In total there were 3,553 titles and abstracts reviewed based on the search strategy, 3,519 were removed due to being unrelated to the study objective. Thirty four articles met the inclusion criteria for full text review. We dismissed 9 articles due to lack of data regarding the outcomes of interest, and included the remaining 25 articles; six articles related to physical exercise modalities, 11 articles related to intervention with neuromuscular electric stimulation (NMES) or functional electric stimulation (FES), and eight articles related to pharmacotherapy (3), surgery (3) or diet therapy (1) (Fig. 1). The quality of the most studies based on Downs and Black appraisal tool29 was moderate (scale <20) to poor (scale <15) except for four RCT studies.
Physical exercise intervention to manage obesity in SCI
Evidence for physical exercise intervention to improve body composition in SCI individuals included one RCT,30 five pre-post interventions31–35 and one case-report,36 for a total of 128 participants. These interventions consisted of arm ergometry, community-based exercise, activity-based therapy, therapeutic recreation such as bocce, canoeing, wheelchair rugby, and resistance training. The range of duration and frequency of the exercise interventions was from six weeks to six months and from 30 minutes once a week to 60 minutes five times per week. In the majority of studies, physical exercise alone did not decrease participants’ BW or BMI; however, it significantly reduced TFM and WC and increased TLBM (Table 1).
Table 1.
Initial values and amount of Changes in Anthropometric and Body Composition after Physical Exercise in Spinal Cord Injury individuals, Extracted from Selected Studies
| Parameter | Population | Intervention | BW (kg) | BMI (kg/m2) | TFM | TLBM | WC (cm) |
|---|---|---|---|---|---|---|---|
| Kim, D.I. 2015 RCT D&B=21 |
N=15 (T=8, C=7) Mean Age= 33.1±5.4 Injury level: C5-T11 ASIA: A-B |
Hand Bike Exercise, 60 minutes, 3 times/week for 6 weeks Intensity: Borg scale; 5–7 |
Initial: 64.8±10.5 Change: No data |
Initial: 22.0±3.7 Change: T: -0.2±0.2, C:+0.3±0.4 P<0.01 |
Initial: -39.0±13.7% Change: (%) T: -3.5±7.2 C:-0.1±1.9 P: 0.23 |
Initial: 20.2±5.0 Change: (Kg) T: +1.5±3.5 C: +0.3±0.9 P: 0.39 (NS) |
Initial: 88.3±13.1 Change: T:-2.6±1.7 C:+0.8±1.6 P:<0.01 |
| Radomski, M. 2011 Pre-Post intervention D&B=17 |
n=13 injury level: T1-T9 all complete |
Community-based program, Die/ Exercise, 12 Weeks |
Initial: 95.2 Change: -5.4 P=0.037 |
Initial: 34.1 Change: -1.2 P= 0.031 |
Skinfold body fat (%); Initial: 35.8 change:-3.2 P=0.013 |
No data | Initial: 115.5 cm Change: -10.6 cm P: 0.005 |
| Astorino T.A. 2015 Pre-Post intervention D&B=15 |
N=17 Mean Age=36.1±11.5 injury level: C4-L1 complete and incomplete |
Chronic activity-based therapy (ABT)1 a minimum of 2 days/week for 6 months, intensities ranging from 5 to 8 ml/kg/minute |
Initial: 76.5± 13.0 Change: +0.5 P:NS |
No Data | Initial: 31.2± 12.9% Change: +1% P: NS |
Initial:50.4±.09 -NS change in Incomplete injury -Decreased (−4.8 ± 5.8%) in complete injury (P<0.05) |
Initial: 92.5± 14.6 Change: -1.8 P: NS |
| Chen Y 2006 Pre-Post D&B=15 |
N=16 Age= 43.8 Injury leve: Tetra and Para AISA: A-C-D |
Time-calorie displacement diet + exercise + behavioral modification 12 weeks |
Initial: 97.4±17.8 Change: -3.5±3.1 P=0.0004 |
Initial: 34.3±4.5 Change: -1.3±1.2 P=0.0005 |
Initial: 41.4±11.2 Change: -2.9±4.6 P=0.05 |
Initial: 51.8±10.2 Change: -0.8±5.1 P=0.58 |
Initial: 117.4±17.1 Change: -4.1±5.0 P=0.005 |
| Neto, F.R. 2011 Pre-Post intervention D&B=13 |
N=53 Men Tetraplegia (C4-C8) n=20, Higher (T1-T6) n=15 and Lower (T7-L2) n=18 Paraplegia (PP) ASIA A-D |
Bocce, Canoeing, Physical therapy, Resistance training, Rugby, Swimming, Table tennis,Wheelchair basketball, Wheelchair, Repulsion physical conditioning 1–5 times/week 30–60 min/session |
Initial: 68.3±12.4 -Total change: NS -Increased in TP 0.8 ±1.5, P<0.05 -Decreased in PP, −1.0±1.8; P<0.05 |
No data | Initial: 15.2±7.1 Kg Change: −0.5±1.3 Kg P<0.05 *Significant decrease only in PP group |
Initial: 53.1±6.8 Kg Change: 0.7±1.2 Kg P<0.05 |
No data |
| Gorla, J. I. 2016 Pre-Post intervention D&B=12 |
N=13 Mean Age= 26.6±6.0 Tetraplegia ASIA: A-B-C |
Wheelchair Rugby 4 times/week (aerobic and anaerobic) average intervention time 8.1±2.5 months |
Initial: 64.2±6.2 Change: -0.9 P:NS |
Initial:20.6±1.9 Change:-1.2% P=0.731 (NS) |
Initial: 15.191±4.603 Change: -3.0% P=0.016 |
Initial: 46.759±4.831 change: +2.0% P=0.308 (NS) |
No data |
| Dolbow, David R 2010 Case report D&B=11 |
N=1 62 yo Male Injury level:T8-9 ASIA A Paraplegic |
Arm Crank Exercise Five times/week 16 Weeks 60 min/session Workload:20–40 |
Initial: 79.4 Change: -3 |
Initial: 28.1 Change: -1.0 |
Initial: 28.05 Change: -2.79 |
Initial: 51.06 Change: -0.12 |
No data |
Activity-based therapy (ABT): This exercise modality is of high volume (>6 hours/week) and typically includes dynamic resistance training, FES, body weight-supported treadmill training, and load bearing and/or standing. T: trial, C control, NS: not significant; TP: tetraplegia; PP: paraplegia; D&B: Downs & Black
NMES intervention to manage obesity in SCI
Available evidence for NMES as a treatment of obesity in SCI population includes two RCTs,37,38 six pre-post studies39–44 and 3 case-reports,45–47 for a total of 128 individuals. Duration and frequency of NMES and FES interventions in the selected studies ranged from six weeks to 6 months, and from once a week to five times per week, respectively. Regarding the effect of NMES, one study evaluated eight weeks of five days per week, therapy for one hour per day, and reported a significant decrease on BW; the other 10 studies showed no change or non-significant changes. Two pre-post studies of six and eight weeks duration reported significant decreases in TFM (-3.5% and -1.9%) and 3 case-reports reported similar effects (from -1.2 to -1.3%), however, the other 4 pre-post studies and 2 RCTs failed to show any significant change in TFM. All studies except two RCTs (Giangregorio 2012 and Gorgey 2012) reported a noticeable increase in TLBM by NMES interventions (from 1.16 to 4.08 kg) (Table 2).
Table 2.
Initial values and amount of Changes in Anthropometric and Body Composition after Neuro-Muscular Electric Stimulation (NMES) in Spinal Cord Injury individuals
| Parameter | Population | Intervention | BW (kg) | BMI (kg/m2) | TFM | TLBM (kg) | WC (cm) |
|---|---|---|---|---|---|---|---|
| Giangregorio, L 2012, RCT D&B=23 |
N=34 (T:17, C:17) Age: 56.6±14 Injury level: C2-T12; AISA C-D |
FES walking/conventional exercise program 16 Weeks, |
Initial: 81.3±13.1 Change: T: no data C: no data |
Initial:26.75 Change: T: no data C: no data |
Initial: 25.4±9.5 Kg Change: T: -1.3 C: +0.8 P: NS |
Initial: (leg) 14.9±5.7 Kg Change: T: +2.2 C: -0.3 P: NS |
No Data |
| Gorgey, A. 2012 RCT D&B=19 |
N=9 males Age = 35± 9 Injury level: C5-T11 AISA: A-B |
NME resistance training, 12 Weeks, twice a week | Initial:74±14 Change: Kg T: +1 C:-1 P: NS |
Initial:21±5 Change: T: 0 C: 0 P: NS |
Initial: 23.3±9 Change: Kg T:-0.7 C: -1 P: NS |
Initial:51.8±8 Change: Kg T: = +0.5 C: -0.4 P: NS |
No data |
| Kim, D.I. 2014 Pre-Post D&B=15 |
N=12, Injury level: C6-L1 AISA: A-B-C |
FES rowing 6 Weeks, 5 days/w monophasic rectangular phase, 30Hz, 10–140mA |
Initial: 70.2±12.5 Change: no data P: NS |
Initial: 23.4±3.7 Change: -0.4 P: 0.058 |
Initial: 23.9±8.5% Change: -3.5% P=0.028 |
Initial: 50.4±9.4% Change: +2.9% P=0.001 |
Initial: 83.9±9.9 Change: -2.1 P=0.059 |
| Carty, A. 2013 Cohort (Pre-Post) D&B=14 |
N=14 Injury lev:T4-11 ASIA: A-B |
Subtetanic NMES on lower limb muscles, 8 weeks; 1h/d, 5d/w | Initial: 74.81 ± 3.94 Change: -1.41 P:0.001 |
Initial: 26.32± Change: +0.1 P: 0.69 (NS) |
Initial: 33.76±6.62% Change: -0.6% P: 0.15 (NS) |
Initial:13.65 ± 2.91 Kg Change: +1.16 Kg P<0.001 |
No Data |
| Griffin, L. 2009, Pre-Post D&B=14 |
N=18, mean=40 Injury level: C4-T7 Complete and incomplete |
FES cycling 50 Hz 10 weeks, 2–3 times/week |
Initial: 153Ib Change: -4.54Ib P: NS |
No Data | Initial:50.42Ib Change: +1.36 P: NS |
Initial: 96.8Ib Change: +3.2 P<0.05 |
No Data |
| Liu, C. 2007 Pre-Post D&B=14 |
N=18 Mean=40±11.3 Injury level: C4-L1 AISA: A-B-C-D |
FES cycling 8 Weeks |
Initial: 73.8±13.9 Change: +1.2 P: NS |
Initial: 25.4±3.9 Change:+0.3 P: NS |
Initial: 18.6±8.6 Change: +0.1 P: NS |
Initial: 51.6±7.1 Change: +1.2 P<0.05 |
No Data |
| Skold, C. 2002 Pre-Post D&B=14 |
N=15, males Age: 33 AISA: A-B |
FES cycling 6 months, 3 times/week |
No change | No data | No change | 10% Increase | No data |
| Hjeltnes, 1997 Pre-Post D&B=14 |
N=5, male age: 35±3 Injury level: C5-7, ASIA A |
ESLC: Electrically Stimulated Leg Cycling 8 Weeks, |
Initial: 76.6±4.7 Change: No Data |
Initial: 22.23±1.34 Change: No Data |
Initial: 29.7±2.6% Change: -1.9% P<0.05 |
Initial: 66.2±2.6% Change:+2.0 P<0.05 |
No Data |
| Gorgey, A.S. 2016, case report D&B=11 |
N=1, 33 year old male, Injury level T6, AISA A | Surface NMES + ankle weight, 10 weeks, once a week | No change | No change | Initial: No data Change: -1.3% |
Initial: No Data Change: -5.0% |
No Data |
| Dolbow, D.R. 2014, Case Report D&B=11 |
N=1, 60 year old female Injury levl: T6 ASIA: A |
FES cycling, 12 Months, 2.9 sessions/week | No change | No change | Initial: 36.79 Change: -1.2% |
Initial: 39.22 Change: +3.01 (7.7%) | No Data |
| Dolbow, D.R. 2012, Case Report D&B=11 |
N=1, 64 year old male, motor complete C5 injury | Home-based FES cycling 9 Weeks, 3 sessions/week, distance from 3.98 to 9 km. 93% compliance |
No change | No change | Initial: 29.6 Change:-1.2% |
Initial: 48.94 Change: +4.08 (8.3%) |
No Data |
T: trial, C: control, NS: not significant, TP: tetraplegia, PP: paraplegia
Pharmacotherapy and Surgery to manage obesity in SCI
No pharmacotherapy study reported a decrease in TFM among SCI participants.48–50 In an RCT, a 5–10 mg testosterone patch for 12 months did not result in changes in TFM and TLBM, although it showed an increase in BW in the treatment group. In contrast oral Alpha-Lipoic Acid for 12 weeks resulted in a significant decrease in BW, BMI and WC in the treatment group compared with controls (Table 3).
Table 3.
Initial values and amount of Changes in Anthropometric and Body Composition after Pharmacotherapya and Surgeryb in Spinal Cord Injury individuals
| Parameter | Population | Intervention | BW (kg) | BMI (kg/m2) | TFM (Kg) | TLBM (kg) | WC (cm) |
|---|---|---|---|---|---|---|---|
|
aBauman, W.A 2011 RCT D&B=27 |
N=22 Men Age: 43±6 AISA: A-B-C |
Testosterone patch, 5–10mg 12 Months |
Initial: 82.9±12.6 Change: T: +3.2 C:+0.1 P: NS |
Initial T: 25 ± 3 |
Initial: 29.8±10.6 Change: T:+0.3 C:+1.5 P:NS |
Initial: 49.6±7.6 Change: T:+3.5 C:-1.4 P:NS |
No Data |
|
aMohammadi, V 2015 RCT D&B=23 |
N=58 Men No more data |
alpha-Lipoic Acid, Orally 12 Weeks |
Initial: 77.11±14.58 Change: T:−3.7±4.4 C: +0.38±1.4 P=0.0001 |
Initial: 27.77±4.33 Change: T:−1.0±0.6 C:0.09±0.6 P=0.0001 |
No Data | No Data | Initial T: 101.79 Change T:−3.7± 2.7, C:0.4±1.4) P=0.0001 |
|
aHallstead, L.S. 2010, Prospective repeated-measures D&B=15 |
N= 10 Men Age: 32.5 Injury level:C4–8 AISA: A-B |
20 mg, Orally Oxandrolone 8 Weeks |
Initial: 78.8 | Initial : 23.8 | Initial : 26.7 Change: -1.5% P: NS |
Initial T:48.7±8.5 Change: +0.9% P=0.02 |
No Data |
|
bLutrzykowski, M. 2008 Case report D&B=13 |
N=2 (Only 1 SCI) Age: 49 |
Open duodenal switch procedure 4 years follow up |
Initial: 134 Change: -65 |
Initial: 47.7 Change: −22.7 (-91.6%) |
No Data | No Data | Initial: 101.8±10.9 Change: −3.7±2.7 |
|
bGros Herguido,N 2014 Case report D&B=11 |
N=2 Males Age: 37 and 47 |
Laparoscopic gastric bypass, 24 months follow up | Initial: 150 Change:-66 |
Initial: 44.08 kg Change: -21 kg |
No Data | No Data | Initial: 140 Change: -53 |
|
bWong, S. 2013 Case report D&B=11 |
N=1, Age: 28 Injury level: T12 AISA: C |
Roux-en-Y gastric bypass followed 7 months |
Initial: 180.3 Change:-66 |
Initial:59.8 kg After 7m: 49.8 Change: -10 kg -16.7% |
No Data | No Data | Initial:165 Change :-19 (-11.5%) |
|
bAlaedeen, D.I. 2006 Case Report D&B=11 |
N=1 Male Age=51 Injury level: T7 |
Roux-en-Y gastric bypass |
Initial: 169 Change:-52 |
Initial:48 kg Change: -15 |
No Data | No Data | No Data |
Among all intervention modalities to manage obesity after SCI, surgery showed a significant effect in TFM reduction (from -10 to -22.7 Kg); however, all four studies were case-reports,51–54 and were appraised as low-quality with a high potential for bias (Table 3).
Discussion
The present review indicates bariatric surgeries including restrictive and mal-absorptive procedures produced the greatest sustained weight loss and BMI correction in individuals with chronic SCI. However, these surgical interventions are only indicated for morbidly obese patients (BMI > 40). Similar to the general non-SCI population, bariatric surgery provides durable treatment of obesity and obesity-associated morbidities. Moreover, surgery is not a simple “cure” for obesity. An individual’s commitment to dietary and lifestyle changes are necessary for successful long-term outcomes. Surgical interventions for obesity are invasive with many anticipated complications and/or long term side effects, including: protein-calorie malnutrition, vitamin B12 deficiency, folate, calcium and vitamin D insufficiency, post-surgical infection, emboli and anastomosis leak.55–61 These side effects are of utmost importance in individuals with SCI as they are at risk of protein calorie malnutrition. Energy and calcium intake are shown to be lower as active handicapped individuals,62 also very low intakes of vitamins C, D, E, folic acid, pantothenic acid, biotin, potassium and iron are reported for the SCI population.63 On the other hand, it seems that larger amounts of Vitamins A, C and E and Copper and Zinc are required due to the high incidence of infections and pressure ulcers in this population.64,65 Thus, most clinicians typically consider surgical interventions as a last resort to reduce morbid obesity.27
Following bariatric surgery, combinations of physical exercise and diet therapy had the greatest effect in decreasing BW and BMI and remarkably improved body composition (increases in TLBM and reductions in TFM and WC), although the methodological quality of the five studies included in this review were evaluated as low. The advantage of diet therapy is that it is feasible to consider the special requirements and concerns of individuals in the SCI population. An anti-atherosclerotic diet, which basically lowers the amounts of saturated and trans fatty acids with a higher proportion of omega 3 fatty acids, limited salt, increased fiber and anti-oxidants intakes are recommended for these individuals, as cardiovascular diseases are the primary cause of morbidity and mortality among SCI out-patients.66 The findings of this review are in accordance with Espirito Santo et al. findings that body weight- support treadmill training increased muscular trophism,67 and reports from Nash et al. that a combination of circuit training and Mediterranean diet reduces dyslipidaemia (Total chol/HDL-C ratio or LDL-C levels).68
NMES and FES
Selected NMES and FES studies were difficult to interpret collectively because of controversial results, small sample size, short duration of follow-up and the variability in the intervention and outcome measure selection. In general a higher frequency of training each week had more favourable results. For example, the trials with five times per week intervention showed more significant results compared to trials with the interventions less than five times per week, regardless of the study duration. However, the feasibility of doing an exercise program five times per week is poor.69 Although NMES did not reduce BW or TFM, it increased regional lean mass and reduced regional fat mass. Similarly Gorgey et al. in a 2015 review reported that NMES or FES training can increase skeletal muscle size and soft tissue lean mass.70 One may argue that active voluntary aerobic exercises with calorie restrictions, improves body composition more efficiently than passive non-voluntary interventions such as FES or NMES, which have greater local rather than systemic effects.
Exercise therapy
Aerobic exercise results in weight loss and decreases in TFM in several ways: First, it causes loss of energy as heat during ATP synthesis in the mitochondria and ATP hydrolysis during muscular contraction and therefore, increases energy expenditure above the individuals’ basal energy expenditure. Secondly, basal metabolic rate (BMR) and thermic effect of food (TEF) increases with PhA. Thirdly, fat oxidation is increased during PhA, especially in endurance trained subjects, due to an increase in the oxidative capacity of muscles. Consequently, the ability to oxidize lipids, due to upregulation of the enzyme AMP-activated protein kinase in skeletal muscles increases; so maximal oxygen consumption and the proportion of fat to carbohydrate oxidation increases.71 Many hormonal and metabolic pathways are activated during exercise, including reductions in blood insulin levels or increases in Vo2 peak.72 If individuals sustain a low caloric diet, to maintain a low blood glucose level, insulin production by the pancreas will decrease and result in a rise in hormone sensitive lipoprotein lipase activity in fat tissues and a subsequent reduction of TFM. These processes may not occur to the same extent with passive interventions such as FES or NMES, which may explain why the lack of a substantial change in TFM although regional increase in the lean mass are reported where the stimulus was applied.
Passive modalities have also been shown to have some side effects. Bhambhani et al. in a study conducted in Alberta, reported FES exercise in SCI individuals elicits some degree of muscular deoxygenation;73 Villareal et al., in a clinical trial reported non-exercise-induced weight loss is associated with a decrease in bone mineral density (BMD) at clinically important sites of fracture, and suggested that exercise should be an important component of a weight loss program.74 This consideration is of importance in the SCI population where many patients have comorbid low bone mineral density and excess adiposity.
Active aerobic or resistance exercise such as wheelchair propulsion in individuals with paraplegic can cause shoulder pain or rotator cuff injury75 and carpal tunnel syndrome.76,77 So health care providers who prescribe exercises should educate patients regarding the correct way to perform exercise and monitor patients for side effects or advers consequences.78,79
Pharmacologic therapy
Pharmacologic treatment with anabolic agents - testosterone or oxandrolone- failed to show any significant change in BW, BMI, TLBM and TFM in SCI individuals. One RCT with alpha-lipoic acid showed a significant decrease in BW, BMI and WC but did not report data on TLBM and TFM.
Medications for weight loss have not been very effective in the general population. Sibutramin was withdrawn from the market in 2010; Orlistat -a gastro intestinal lipase inhibitor- causes fat-soluble vitamin deficiencies (vitamin A, D, E and K) and gastrointestinal side effects such as diarrhea, steatorrhea, bloating, flatulence, fecal urgency and incontinence. These adverse GI symptoms will not be well tolerated in individuals with SCI. Phentermine, a non-adrenergic compound licensed for short term use in the US, can result in a racing heart and raise diastolic blood pressure, which is relatively contraindicated in the SCI population who are at risk of cardiovascular events such as autonomic dysreflexia, arterial and ventricular arrhythmia and coronary artery diseases. However, the food and drug administration (FDA) has recently approved the serotonin agonist lorcaserin and a combination of low dose phentermine /topiramate, raising the possibility of improving current paradigms for the treatment of obesity.80
Limitations
Some significant limitations should be taken into account when considering the clinical implications of this review. First, most studies suffered from a small sample size and a high risk of bias due to the study design (i.e., case reports versus gold standard clinical trials). For example the favorable results of the surgical interventions were obtained from case reports and not large prospective studies. Second, there was no consistency in outcome selection across the different obesity interventions. Third, there was variability in the choice of obesity biomarkers and thus a limited ability to pool data nor generalize the findings based on obesity severity. Future studies should prioritize decreases in visceral adipose tissue and increases in TFM (treatment of sarcopenic obesity) as the key outcome of importance. Understanding the role of the adipokines (adiponection, leptin, etc.) in predicting therapeutic responsiveness or lack of responsiveness is an important research priority for the field.
Conclusion
Although most of the studies selected for inclusion in this systematic review were low to moderate in terms of methodological quality, the greatest permanent weight reduction and BMI correction was produced by bariatric surgery, followed by a combination of long term physical exercise and diet therapy. Generally, NMES and pharmacologic treatment did not reduce weight or TFM, but resulted in increases in TLBM. Due to the link between adiposity and all-cause mortality, obesity is a legitimate therapeutic target. Based on feasibility and associated risk, trial of diet and exercise therapy is recommended prior to definitive bariatric surgery.
Acknowledgment
We would like to thank Maureen Pakosh, information specialist in Lyndhurst and Rumsey centers, Toronto rehab cites for great help and support.
Appendix 1: Search Strategy
exp Spinal Cord Injuries/
exp Spinal Cord Vascular Diseases/
exp Spinal Injuries/
exp Spinal Fractures/
exp Trauma, Nervous System/
Spinal Cord Compression/
exp Myelitis/
Epidural Abscess/
Syringomyelia/
exp Paraplegia/
(postoperative adj4 (spinal cord injur* or SCI)).af.
(spinal cord adj3 (contusion* or laceration or transaction* or trauma* or injur* or damage*)).tw.
(SCI or spinal cord injur*).tw.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
Overweight/
Obesity/ or Obesity, Morbid/ or Obesity, Abdominal/
((subcutaneous or intra-abdominal) adj3 fat).tw.
visceral fat.tw.
body mass index.tw.
waist circumference.tw.
android obesity.tw.
Adiposity/
Weight Gain/
exp Body Composition/
Waist-Hip Ratio/
Skinfold Thickness/
adiposity.tw.
weight gain.tw.
15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
14 and 29
prevention.tw.
treatment.tw.
exp Nutrition Therapy/
Physical Fitness/
“Physical Education and Training"/
exp Exercise Therapy/
exp Exercise/
Wheelchairs/
exp Ergometry/
Swimming/
swim*.tw.
strengthening program.mp.
(exercise adj4 (aerobic or resistance or therapy or physical or program or cardiopulmonary)).tw.
(train* adj3 (ergometry or flexibility or wheelchair*)).tw.
((wheelchair* or wheel-chair*) adj3 (exercise or ergometry or propulsion)).tw.
(pharmacotherapy adj3 (overweight or obesity or fitness)).tw.
(surgery adj3 (obesity or morbid obesityor overweight or fitness)).tw.
exp Bariatric Surgery/
exp Anti-Obesity Agents/
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49
30 and 50
Disclaimer statements
Contributors None.
Funding This study performed as a non-granted systematic review by Neural Engineering and Therapeutics team, Toronto Rehabilitation Institute.
Declaration of interest The authors declare no conflict of interest.
Conflicts of interest None.
Ethics approval None.
Data archiving There were no data to deposit.
ORCID
SM. Alaviniaa http://orcid.org/0000-0002-5503-9362
References
- 1.Buchholz AC, McGillivray CF, Pencharz PB.. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003 Feb;77(2):371–8. [DOI] [PubMed] [Google Scholar]
- 2.Rajan S, McNeely MJ, Warms C, Goldstein B.. Clinical assessment and management of obesity in individuals with spinal cord injury: a review. J Spinal Cord Med 2008;31(4):361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gater DR., Jr Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007 May;18(2):333–514. [DOI] [PubMed] [Google Scholar]
- 4.Rush EC, Goedecke JH, Jennings C, Micklesfield L, Dugas L, Lambert EV, et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes (Lond) 2007;31(8):1232–9. [DOI] [PubMed] [Google Scholar]
- 5.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 1998;68(4):899–917. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Global database on body mass index 2006.
- 7.Buchholz AC, Bugaresti JM.. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 2005;43(9):513–8. [DOI] [PubMed] [Google Scholar]
- 8.McDonald CM, Abresch-Meyer AL, Nelson MD, Widman LM.. Body mass index and body composition measures by dual x-ray absorptiometry in patients aged 10 to 21 years with spinal cord injury. J Spinal Cord Med 2007;30 Suppl 1:S97–S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laughton GE, Buchholz AC, Ginis KAM, Goy RE.. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury Spinal Cord 2009;47(10):757–62. [DOI] [PubMed] [Google Scholar]
- 10.Gorgey AS, Gater DR.. A preliminary report on the effects of the level of spinal cord injury on the association between central adiposity and metabolic profile 2011;3(5):440–6. [DOI] [PubMed] [Google Scholar]
- 11.Bauman WA, Cirnigliaro CM, Dengel DR, Liu J, LaFountaine MF, Kirshblum SC, et al Associations between visceral fat and metabolic parameters in spinal cord injury: JSCM Conference:2014;37 (4): 434–5. [Google Scholar]
- 12.Edwards LA, Bugaresti JM, Buchholz AC.. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr 2008;87(3):600–7. [DOI] [PubMed] [Google Scholar]
- 13.Onat A, Ugur M, Can G, Yuksel H, Hergenc G.. Visceral adipose tissue and body fat mass: predictive values for and role of gender in cardiometabolic risk among Turks. Nutrition. 2010 Apr;26(4):382–9. [DOI] [PubMed] [Google Scholar]
- 14.Inayama T, Higuchi Y, Tsunoda N, Uchiyama H, Sakuma H.. Associations between abdominal visceral fat and surrogate measures of obesity in Japanese men with spinal cord injury. Spinal Cord 2014;52(11):836–41. [DOI] [PubMed] [Google Scholar]
- 15.Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C.. Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med 2011;34(4):416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravensbergen HJC, Lear SA, Claydon VE.. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury: Journal of Neurotrauma 2014; 31 (3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchholz AC, McGillivray CF, Pencharz PB.. The use of bioelectric impedance analysis to measure fluid compartments in subjects with chronic paraplegia. Arch Phys Med Rehabil 2003;84(6):854–61. [DOI] [PubMed] [Google Scholar]
- 18.Cirnigliaro CM, La Fountaine MF, Emmons R, Kirshblum SC, Asselin P, Spungen AM, et al Prediction of limb lean tissue mass from bioimpedance spectroscopy in persons with chronic spinal cord injury. J Spinal Cord Med 2013;36(5):443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly TL, Wilson KE, Heymsfield SB.. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4(9):e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keil M, Totosy de Zepetnek JO, Brooke-Wavell K, Goosey-Tolfrey VL.. Measurement precision of body composition variables in elite wheelchair athletes, using dual-energy X-ray absorptiometry. European journal of sport science. 2016;16(1):65–71. [DOI] [PubMed] [Google Scholar]
- 21.Klebine P. Weight matters: getting started. PN 2005;59(1):22–3. [Google Scholar]
- 22.Mun EC, Blackburn GL, Matthews JB.. Current status of medical and surgical therapy for obesity. Gastroenterology 2001;120(3):669–81. [DOI] [PubMed] [Google Scholar]
- 23.Cheskin LJ, Poddar KH.. Obesity Management. In: Modern Nutrition in Health and Disease. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. p 786–99. [Google Scholar]
- 24.Butte NF, Caballero B.. Energy Needs: Assessment and Requirements. In: Modern Nutrition in Health and Disease. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. p 88–100. [Google Scholar]
- 25.Buchholz AC, Pencharz PB.. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care 2004;7(6):635–9. [DOI] [PubMed] [Google Scholar]
- 26.Jacob JJ, Isaac R.. Behavioral therapy for management of obesity. Indian journal of endocrinology and metabolism 2012;16(1):28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NIH conference Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1999;115(12):956–61. [PubMed] [Google Scholar]
- 28.Tymitz K, Magnuson T, Schweitzer M.. Bariatric Surgery. In: Modern Nutrition in Health and Disease. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. p 800–7. [Google Scholar]
- 29.Downs SH, Black N.. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of epidemiology and community health 1998;52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DI, Lee H, Lee BS, Kim J, Jeon JY.. Effects of a 6-week indoor hand-bike exercise program on health and fitness levels in people with spinal cord injury: A randomized controlled trial study. Archives of Physical Medicine and Rehabilitation 2015;96(11):2033–40. [DOI] [PubMed] [Google Scholar]
- 31.Astorino TA, Harness ET.. Effect of intense activity-based therapy on body composition in persons with spinal cord injury: ACRM. Conference: 2015; 96 (10) :e39–e40. [Google Scholar]
- 32.Chen Y, Henson S, Jackson AB, Richards JS.. Obesity intervention in persons with spinal cord injury. Spinal Cord 2006;44(2):82–91. [DOI] [PubMed] [Google Scholar]
- 33.Gorla JI, Costa ESAA, Borges M, Tanhoffer RA, Godoy PS, Calegari DR, et al Impact of wheelchair rugby on body composition of subjects with tetraplegia: A pilot study. Arch Phys Med Rehabil 2016;97(1):92–6. [DOI] [PubMed] [Google Scholar]
- 34.Neto FR, Lopes GH.. Body composition modifications in people with chronic spinal cord injury after supervised physical activity. J Spinal Cord Med 2011;34(6):586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radomski M, Finkelstein M, Hagel S, Masemer S, Theis J, Thompson M.. A pilot wellness and weight management program for individuals with spinal cord injury: Participants’ goals and outcomes: Topics in Spinal Cord Injury Rehabilitation 2011; 17 (2):59–69. [Google Scholar]
- 36.Dolbow DR, Miller J, Harnish C, Poarch H, Gorgey A, Gater DR.. Arm crank exercise increases VO2 peak and reduces body fat mass in older adult with chronic paraplegia. Clinical Kinesiology 2010. Winter;64(4):51–5. [Google Scholar]
- 37.Giangregorio L, Craven C, Kapadia N, Richards K, Popovic MR.. A randomized controlled trial of functional electrical stimulation therapy for walking versus a conventional exercise program in patients with chronic incomplete spinal cord injury: effects on body composition. 5th National Spinal Cord Injury Conference: ‘Translating Neural Engineering and Novel Therapies. J SCM 2012;35(5):438–9. [Google Scholar]
- 38.Gorgey AS, Mather KJ, Cupp HR, Gater DR.. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 2012;44(1):165–74. [DOI] [PubMed] [Google Scholar]
- 39.Carty A, McCormack K, Coughlan GF, Crowe L, Caulfield B.. Alterations in body composition and spasticity following subtetanic neuromuscular electrical stimulation training in spinal cord injury. J Rehabil Res Dev 2013;50(2):193–202. [DOI] [PubMed] [Google Scholar]
- 40.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22. [DOI] [PubMed] [Google Scholar]
- 41.Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H.. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol 1997;273(3 Pt 2):R1072–R9. [DOI] [PubMed] [Google Scholar]
- 42.Kim DI, Park DS, Lee BS, Jeon JY.. A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body composition in people with spinal cord injury: a pilot study. [Erratum appears in Spinal Cord 2014 Oct;52(10):785]. Spinal Cord 2014;52(8):621–4. [DOI] [PubMed] [Google Scholar]
- 43.Liu CW, Chen SC, Chen CH, Chen TW, Chen JJJ, Lin CS, et al Effects of functional electrical stimulation on peak torque and body composition in patients with incomplete spinal cord injury. Kaohsiung J Med Sci 2007;23(5):232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skold C, Lonn L, Harms-Ringdahl K, Hultling C, Levi R, Nash M, et al Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehabil Med 2002;34(1):25–32. [DOI] [PubMed] [Google Scholar]
- 45.Dolbow DR, Gorgey AS, Gater DR, Moore JR.. Body composition changes after 12 months of FES cycling: case report of a 60-year-old female with paraplegia. Functional Electrical Stimulation. Spinal Cord 2014;52:S3–S4. [DOI] [PubMed] [Google Scholar]
- 46.Dolbow DR, Gorgey AS, Moore JR, Gater DR.. Report of practicability of a 6-month home-based functional electrical stimulation cycling program in an individual with tetraplegia. J Spinal Cord Med 2012;35(3):182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorgey AS, Caudill C, Khalil RE.. Effects of once weekly NMES training on knee extensors fatigue and body composition in a person with spinal cord injury. J Spinal Cord Med 2016;39(1):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, et al A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 2011;43(8):574–9. [DOI] [PubMed] [Google Scholar]
- 49.Halstead LS, Groah SL, Libin A, Hamm LF, Priestley L.. The effects of an anabolic agent on body composition and pulmonary function in tetraplegia: a pilot study.[Erratum appears in Spinal Cord. 2010 Feb;48(2):180]. Spinal Cord 2010;48(1):55–9. [DOI] [PubMed] [Google Scholar]
- 50.Mohammadi V, Khalili M, Eghtesadi S, Dehghani S, Jazayeri S, Aghababaee SK, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: A clinical trial: Spinal Cord 2015;53(8): 621–624. [DOI] [PubMed] [Google Scholar]
- 51.Alaedeen DI, Jasper J.. Gastric bypass surgery in a paraplegic morbidly obese patient. Obes Surg 2006 Aug;16(8):1107–8. [DOI] [PubMed] [Google Scholar]
- 52.Gros Herguido N, Pereira Cunill JL, Barranco Moreno A, Socas Macias M, Morales-Conde S, Garcia-Luna PP.. [Bariatrica paraplegia patient and morbid obesity. New challenge in bariatric surgery]. Nutr Hosp 2014;29(6):1447–9. [DOI] [PubMed] [Google Scholar]
- 53.Lutrzykowski M. Bariatric surgery in morbidly obese patients in wheelchairs. Obes Surg 2008;18(12):1647–8. [DOI] [PubMed] [Google Scholar]
- 54.Wong S, Forbes A, Armitage F, Barnes T, Pounds-Cornish E, Appleton S, et al Morbid obesity after spinal cord injury: A bariatric surgery case study: Topics in Spinal Cord Injury Rehabilitation. Conference: 2013; 19 (1): 45. [Google Scholar]
- 55.Kailasam VK, Decastro C, Macaluso C, Kleiman A.. Postbariatric Surgery Neuropathic Pain (PBSNP): Case Report, Literature Review, and Treatment Options: Pain Medicine (United States) 2015;16(2): 374–82. [DOI] [PubMed] [Google Scholar]
- 56.Yarandi SS, Griffith DP, Sharma R, Mohan A, Zhao VM, Ziegler TR.. Optic neuropathy, myelopathy, anemia, and neutropenia caused by acquired copper deficiency after gastric bypass surgery. J Clin Gastroenterol 2014;48(10):862–5. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura KM, Haglind EGC, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ, et al. Fracture risk following bariatric surgery: A population-based study: Osteoporosis International 2014; 25 (1):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landais AF. Rare neurologic complication of bariatric surgery: Acute motor axonal neuropathy (AMAN), a severe motor axonal form of the Guillain Barre syndrome: Surgery for Obesity and Related Diseases 2014;10 (6):e85–e87. [DOI] [PubMed] [Google Scholar]
- 59.John JK, Al-Hashel JY, Rady A, Periasamy V.. Neurologic complications after bariatric surgery: Journal of Neurology. Conference:2014;. 261: S256. [Google Scholar]
- 60.Arsalane A, Herman D, Bazelly B.. Left strangulated diaphragmatic hernia: An unusual complication of gastric bypass. [French] L’anneau gastrique: Cause inhabituelle de hernie diaphragmatique gauche etranglee. Rev Pneumol Clin 2005;61(6):374–7. [DOI] [PubMed] [Google Scholar]
- 61.Blam OG, Vaccaro AR, Vanichkachorn JS, Albert TJ, Hilibrand AS, Minnich JM, et al Risk factors for surgical site infection in the patient with spinal injury. Spine 2003;28(13):1475–80. [DOI] [PubMed] [Google Scholar]
- 62.Ribeiro SML, Da Silva RC, De Castro IA, Tirapegui J.. Assessment of nutritional status of active handicapped individuals. Nutrition Research 2005;25(3):239–49. [Google Scholar]
- 63.Perret C, Stoffel-Kurt N.. Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J Spinal Cord Med 2011;34(6):569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doley J. Nutrition management of pressure ulcers. Nutr Clin Pract 2010;25(1):50–60. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Devivo MJ, Jackson AB.. Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil 2005;86(6):1208–13. [DOI] [PubMed] [Google Scholar]
- 66.Feasel S, Groah SL.. The impact of diet on cardiovascular disease risk in individuals with spinal cord injury. Topics in Spinal Cord Injury Rehabilitation 2009;14(3):58–68. [Google Scholar]
- 67.Espírito Santo CC, Swarowsky A, Recchia TL, Lopes APF, Ilha J.. Is body weight-support treadmill training effective in increasing muscle trophism after traumatic spinal cord injury? A systematic review. Spinal Cord 2015;53(3):176–81. [DOI] [PubMed] [Google Scholar]
- 68.Nash MS1, Jacobs PL, Mendez AJ, Goldberg RB. Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia. JSCM. 2001;24(1):2–9. [DOI] [PubMed] [Google Scholar]
- 69.Scelza WM1, Kalpakjian CZ, Zemper ED, Tate DG. Perceived barriers to exercise in people with spinal cord injury. Am J Phys Med Rehabil. 2005;84(8):576–83. [DOI] [PubMed] [Google Scholar]
- 70.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Gater DR.. The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury - part II: Journal of Spinal Cord Medicine 201538 (1):23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finelli C, Gioia S, La Sala N.. Physical activity: an important adaptative mechanism for body-weight control. ISRN obesity 2012. 2012:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Johnston TE, Smith BT, Mulcahey MJ, Betz RR, Lauer RT.. A randomized controlled trial on the effects of cycling with and without electrical stimulation on cardiorespiratory and vascular health in children with spinal cord injury. Arch Phys Med Rehabil 2009;90(8):1379–88. [DOI] [PubMed] [Google Scholar]
- 73.Bhambhani Y, Tuchak C, Burnham R, Jeon J, Maikala R.. Quadriceps muscle deoxygenation during functional electrical stimulation in adults with spinal cord injury Spinal Cord. 2000;38(10):630–8. [DOI] [PubMed] [Google Scholar]
- 74.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, et al Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: A randomized controlled trial. Arch Intern Med 2006;166(22):2502–10. [DOI] [PubMed] [Google Scholar]
- 75.Dyson-Hudson TA, Kirshblum SC.. Shoulder pain in chronic spinal cord injury, Part I: Epidemiology, etiology, and pathomechanics. J Spinal Cord Med 2004;27(1):4–17. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Boninger ML, Leath JD, Fitzgerald SG, Dyson-Hudson TA, Chang MW.. Carpal tunnel syndrome in manual wheelchair users with spinal cord injury: a cross-sectional multicenter study. Am J Phys Med Rehabil 2009;88(12):1007–16. [DOI] [PubMed] [Google Scholar]
- 77.Gellman H, Sie I, Waters RL.. Late complications of the weight-bearing upper extremity in the paraplegic patient. Clin Orthop 1988;233:132–5. [PubMed] [Google Scholar]
- 78.Dyson-Hudson TA, Sisto SA, Bond Q, Emmons R, Kirshblum SC.. Arm Crank Ergometry and Shoulder Pain in Persons with Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation 2007;88(12):1727–9. [DOI] [PubMed] [Google Scholar]
- 79.Giner-Pascual M, Alcanyis-Alberola M, Millan Gonzalez L, Aguilar-Rodriguez M, Querol F.. Shoulder pain in cases of spinal injury: influence of the position of the wheelchair seat. Int J Rehabil Res 2011;34(4):282–9. [DOI] [PubMed] [Google Scholar]
- 80.Manning S, Pucci A, Finer N. Pharmacotherapy for obesity: novel agents and paradigms. Therapeutic advances in chronic disease 2014 May;5(3):135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


