Abstract
Objectives
To reduce the incidence of Urinary Tract Infection (UTI) in subacute SCI individuals admitted for tertiary inpatient rehabilitation.
Design
A quality improvement team was assembled to improve UTI prevention/diagnosis. To plan data collection, UTI-related factors were mapped in an Ishikawa (fishbone) driver diagram. Data including patient demographics, presence and frequency of signs and/or symptoms of UTI and antibiotic initiation from August to December 2015 were recorded. Sensitivity, Specificity, Positive and Negative Predictive Values (PPV, NPV), and Likelihood Ratios (LR) were calculated for each sign and symptom.
Setting
Tertiary SCI Rehabilitation
Results
Among 55 inpatients with subacute SCI who had signs/symptoms prompting urine culture and sensitivity (C&S), 32 (58.18%) were diagnosed with a UTI. The most frequent symptoms were foul smelling urine (41%), change in urine color (31%), and incontinence (25%), and the most common sign was fever (34%). Most UTIs (81%) occurred among individuals using Clean Intermittent Catheterization (CIC), with 46% of catheterizations performed by nurses. Foul smelling urine had the highest sensitivity (0.50, 95% CI: 0.31-0.69), and new incontinence had the highest specificity (0.88, 95% CI: 0.69-0.97) for UTI diagnosis. The highest PPV belonged to the cloudy urine (0.71, 95% CI: 0.42-0.92). The combination of cloudy and foul smelling urine increased the PPV to 78% (95% CI: (0.40-0.97).
Conclusions
The concurrent presence of cloudy and foul smelling urine is predicted of UTI diagnosis inpatients tertiary setting. SCI inpatients are susceptible to UTI when learning CIC technique from nurses.
Keywords: Rehabilitation, Urinary Tract Infection, Sensitivity, Specificity
Introduction
Urinary Tract Infections (UTI) are the most common secondary health complication following Spinal Cord Injury (SCI), a frequent cause of service interruptions during inpatient rehabilitation, and a major cause of morbidity among individuals living with chronic SCI.1–4 Incomplete voiding, increased intravesical pressure, using intermittent and/or indwelling catheters and an upper motor neuron bladder contribute to an increased risk of symptomatic UTI.5 UTIs increase direct and indirect costs for rehabilitation programs. The minimum additional cost of evaluating and treating a patient with hospital-acquired symptomatic UTI at the University of Michigan Health System (2009), as reported by Saint et al. was 676 USD.6
UTI refers to significant bacteriuria in a patient with symptoms or signs attributable to the urinary tract and no alternate source; whereas, asymptomatic bacteriuria refers to significant bacteriuria in a patient with no symptoms or signs attributable to the urinary tract.7 Three criteria are used to diagnose UTI in individuals with SCI: 1) significant bacteriuria; 2) pyuria (urine containing increased white blood cells); and 3) signs and symptoms.8 Frequent UTI in the SCI population are defined clinically as 3 or more infections per year, where both symptoms of infection and a positive urine culture are present.9 Although, UTI among the general population are typically manifested with key symptoms such as dysuria and frequency, typical manifestations of symptomatic UTI are usually absent in SCI patients with upper motor neuron neurogenic bladder dysfunction and impaired sensation. The diagnosis of UTI in SCI patients is often delayed or missed, because symptoms are often subtle.10,11. Signs and symptoms of UTI among individuals with SCI are non-specific, and generally described as having poor sensitivity and specificity.12 Mass et al. (2009) calculated the validity, accuracy and predictive value of urinary tract infection signs and symptoms among individuals with SCI who were on Intermittent Catheterization (CIC). They reported that cloudy urine had the highest accuracy (83.1%), and leukocytes in the urine had the highest sensitivity (82.8%) for the presence of UTI and fever had very high specificity (99%) but very low sensitivity (6.9%) for UTI diagnosis.12 Fever during a UTI suggests the presence of the inflammation, which may lead to severe sepsis13 or risk of developing an acquired immunodeficiency syndrome.14
The most prevalent risk factors for development of UTI in SCI patients is an indwelling catheter15 or prolonged time periods between catheterizations.16 Indwelling catheter users have a higher incidence of complications and hospitalization than individuals using CIC.17 Girard et al. reported a prevalence of 19.2% of UTI amongst individuals with SCI admitted to rehabilitation centres in France.18 The incidence of symptomatic UTI in men with SCI was reported to be 0.41/100 person-days with IC and 0.36/100 person-days among men using a condom catheter.19 This compares to 2.72/100 person/days for individuals with a chronic indwelling catheter.20 Early reports claimed that clean intermittent catheterization (CIC) greatly reduced rates of UTI among individuals with SCI; however, it is now clear that the majority of persons undergoing CIC develop recurrent or continuous bacteriuria in the chronic phase of injury.21 A randomized controlled clinical trial in North America reported the routine use of a ready-to-use, hydrophilic-coated intermittent catheter (SpeediCath) was associated with a delay of the onset of the first symptomatic UTI and a reduction in the incidence of UTI among patients with SCI during their initial hospital or rehabilitation unit stay.10
The purpose of this quality-improvement (QI) project was to reduce the incidence of UTI in individuals with subacute SCI admitted for tertiary inpatient rehabilitation centre. The specific aims were as follows:
To describe the frequency of individual urinary signs and/or symptoms among inpatients with SCI over a 5 month time period,
To report the sensitivity, specificity, predictive value and likelihood ratio of UTI signs and symptoms among SCI individuals during inpatient SCI rehab,
To develop strategies for reducing the frequency and severity of UTI from a health system perspective.
Material and Methods
We assembled a Quality Improvement team including physicians, nurses, administrators and a quality improvement officer, defined goals for the quality process, and used a Fishbone (Ishikawa) driver diagram to map out possible causes of UTI attributable to care processes among individuals with SCI (Fig. 1). In this diagram, those causes labeled with an “x” are targets for corrective action; those labeled with an “n” were not of primary concern, and the causes labeled with a “c” were constants that could not be altered. Two paper forms outlining known UTI signs and symptoms were developed with stakeholder input, first as “Baseline symptom form (Appendix A)”, and the second a “Culture result and antibiotic prescription form (Appendix B)”. Using these forms, data was gathered from three 20 bed units within the centre for each patient who complained of any UTI symptoms that prompted ordering of Routine and Microscopy (R&M) and/or urine Culture and Sensitivity (C&S) from 8/1/2015-12/31/2015. Data were collected and reviewed by (SMA, CC, MO) during the collection period.
Fig. 1.
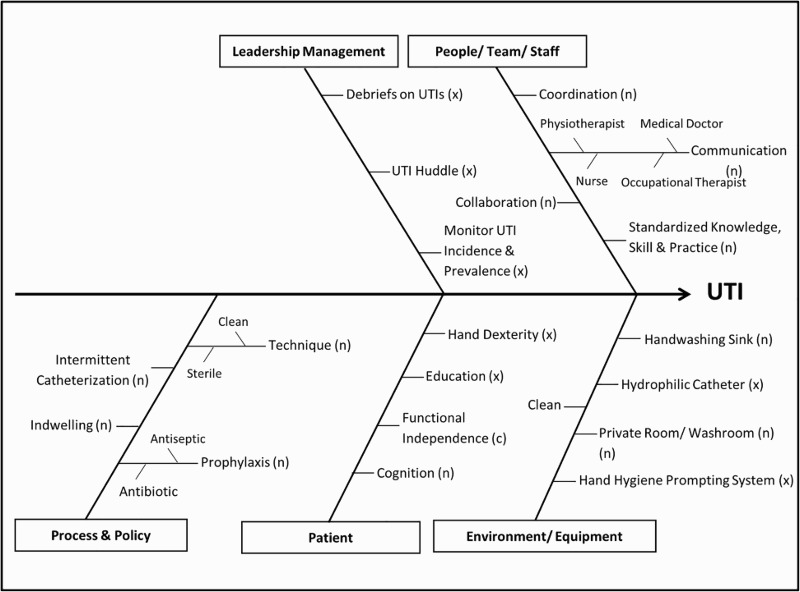
Urinary Tract Infection Fishbone Diagram.
The incidence of UTI was confirmed by a positive urine culture where the definition of a positive culture was modified for the mode of bladder management. In this regard, based on the lab report, for those whom were spontaneous voiding 105 colony forming units per milliliter (cfu/ml); and for those on Clean intermittent catheter (CIC); 102 cfu/ml, for clean-void specimens from catheter-free males; for those who use external condom collecting devices 104 cfu/ml; and, for specimens from indwelling catheters any detectable concentration of bacteria were considered as bacteriuria.22 The frequency of UTI signs and symptoms and UTI presence were calculated. Mean and standard deviation were used for the continuous variables. Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), Likelihood Ratio (LR) for UTI diagnosis with the corresponding 95% confidence intervals were calculated for each sign and symptoms, as well as a combinations of signs and symptoms. All data analyses were done using SPSS version 22 and R-3.2.3 package qcc.
Quality Control Analyses of the data with the Pareto and Control Charts23 were used to visualize the main process aims. In the third month (October 2015) of the QI process and data gathering, a novel hand hygiene monitoring system, developed by Researchers at Toronto Rehabilitation Institute, was used to enhance hand hygiene performance. The system provided real-time prompting with discrete vibrations when hand hygiene was required but not completed. An electronic badge worn by each staff member communicated with electronic sensors at the entrance of every patient room and with all wall-mounted soap and alcohol gel dispensers to determine whether handwashing was completed when appropriate and prompted the wearer to wash if not completed. All three nursing units at rehabilitation centre participated in the hand hygiene Quality Improvement initiative. Every staff, student, and volunteer assigned to the nursing units was eligible to participate including nurses, physicians, allied health, administration, and facilities employees. 25 active in-patient rooms and 2 soiled utility rooms were instrumented with sensors. All wall-mounted soap (N=72) and alcohol gel dispensers (N=106) were instrumented.
Results
During the quality improvement process, among 55 inpatients that had urine sent for C&S, 32 (58.18%) patients were diagnosed with a UTI. Mean age of the patients was 46.30 (SD = ± 18.90) years with a range of 16 to 84 years. Table-1 shows the characteristics of patients with UTI as well as the primary signs and symptoms present prior to urine C&S. Among inpatients with urine C&S analyzed, 46 (84%) patients had more than one sign and/or symptom, the most prevalent symptom was foul smelling urine. The most common method of bladder management among patients with UTI signs and symptoms and urine sent for C & S was CIC (72.70%). Among SCI inpatients diagnosed with UTI, 46% had nurses performing all CICs.
Escherichia coli (E.coli) was the most frequent bacteria in urine culture as 53% of the patients had positive cultures for E.coli alone, while in 18.7%, E.coli was mixed with other organisms including Enterococcus, Group B Streptococcus, and Klebsiella Pneumoniae.
Table 2 shows the Sensitivity, Specificity, PPV, and NPV and their 95% Confidence Interval (CI) for UTI signs and symptoms among inpatients with SCI. All UTI signs and symptoms showed low sensitivity, among all of the symptoms, foul smelling urine had the highest sensitivity (0.50, 95% CI: 0.31 - 0.69), and new onset of incontinence showed the highest specificity (0.88, 95% CI: 0.69 - 0.97) for UTI diagnosis. The highest PPV belonged to the cloudy urine (0.71, 95% CI: 0.42 - 0.92). A combination of cloudy and foul smelling of urine increased the PPV to 78% (95% CI: (0.40 - 0.97). These symptoms together also had the highest specificity (0.92, 95% CI: 0.74 - 0.99). The presence of fever as a sign, had a high specificity (0.84, 95% CI: 0.64–0.95) and when combined with foul smelling urine, the specificity increased to 0.92 (95% CI: 0.74 - 0.99). Although none of the urine signs and symptoms showed a high LR, both positive and negative, the symptom of cloudy urine alone had the highest positive LR (2.08, 95% CI: 0.74 - 5.84), while the positive LR was 2.92 (CI: 0.66 -12.80) for the combination of cloudy and foul smelling urine.
Table 1.
Inpatient demographic characteristics, bladder management and primary signs and symptoms for those requiring Routine and Microscopy Culture and Sensitivity and those diagnosed with UTI
| Urine Order | UTI | |
|---|---|---|
| Age, Years (Mean ±SD) | 48.31 ± 18.5 | 46.30 ± 18.90 |
| Gender, n (%) | ||
| Male | 42 (76.40) | 23 (71.90) |
| Female | 13 (23.60) | 9 (28.10) |
| Bladder Management, n (%) | ||
| IC | 40(72.70) | 26 (81.30) |
| Indwelling | 7 (12.70) | 4 (12.50) |
| Voiding | 4(7.30) | 2 (6.30) |
| Condom Catheter | 1 (1.80) | - |
| Voiding & IC | 2 (3.60) | - |
| Ilio-conduit | 1 (1.80) | - |
| Primary Sign and symptom, n (%) | ||
| Change urine color | 14 (22.45) | 10 (31.25) |
| Foul smelling Urine | 23 (41.82) | 13 (40.63) |
| Sediment | 8 (14.55) | 5 (15.63) |
| Incontinence | 9 (16.36) | 8 (25.06) |
| Autonomic dysfunction | 3 (5.45) | - |
| Increased Spasticity | 10 (18.18) | 7 (21.88) |
| Hematuria | 7(12.73) | 4 (12.50) |
| Fever | 12 (21.82) | 11 (34.38) |
Table 2.
Sensitivity, Specificity, PPV, NPV, and LR I and their 95% Confidence Interval (CI) for UTI signs and symptoms
| Sensitivity | Specificity | PPVa | NPVb | |
|---|---|---|---|---|
| Fever | 0.27 (0.12 - 0.46) | 0.84 (0.64 - 0.95) | 0.67 (0.35 - 0.90) | 0.49 (0.33 - 0.65) |
| Hematuria | 0.10 (0.02 - 0.27) | 0.84 (0.64 - 0.95) | 0.43 (0.10 - 0.82) | 0.44 (0.29 - 0.59) |
| Cloudy Urine | 0.33 (0.17 - 0.53) | 0.84 (0.64 - 0.95) | 0.71 (0.42 - 0.92) | 0.51 (0.35 - 0.67) |
| Foul Smelling Urine | 0.50 (0.31 - 0.69) | 0.68 (0.46 - 0.85) | 0.65 (0.43 - 0.84) | 0.53 (0.35 - 0.71) |
| New onset of Incontinence | 0.20 (0.08 - 0.39) | 0.88 (0.69 - 0.97) | 0.67 (0.30 - 0.93) | 0.48 (0.33 - 0.63) |
| Increased Spasticity | 0.17 (0.06 - 0.35) | 0.80 (0.59 - 0.93) | 0.50 (0.19 - 0.81) | 0.44 (0.30 - 0.60) |
| WBCc in Urine | 0.13 (0.04, 0.31) | 0.84 (0.64, 0.95) | 0.50 (0.16, 0.84) | 0.45 (0.30, 0.60) |
| Fever & Foul-Smelling Urine | 0.10 (0.02 - 0.27) | 0.92 (0.74 - 0.99) | 0.60 (0.15 - 0.95) | 0.46 (0.32 - 0.61) |
| Cloudy & Foul-Smelling Urine | 0.23 (0.10 - 0.42) | 0.92 (0.74 - 0.99) | 0.78 (0.40 - 0.97) | 0.50 (0.35 - 0.65) |
aPositive Predictive value bNegative predictive value cWhite Blood Cell
The Pareto chart indicates that the highest frequency of UTI was in those on CIC for bladder management (Fig. 2), and also those whom have nursing administered CIC (Fig. 3). Figure 4, displays a control chart of the QI process from August 2015 through December 2015, illustrating the fluctuations in UTI incidence during the recording time period. Between October 28, 2015 and December 31, 2015, badges were worn by 206 unique users. 96,164 opportunities for hand washing were recorded during the same period and 77,597 hand washes were performed. The decrease in UTI incidence among inpatients with SCI in our centre may have been attributed to the installation of the hand hygiene prompting system.
Fig. 2.
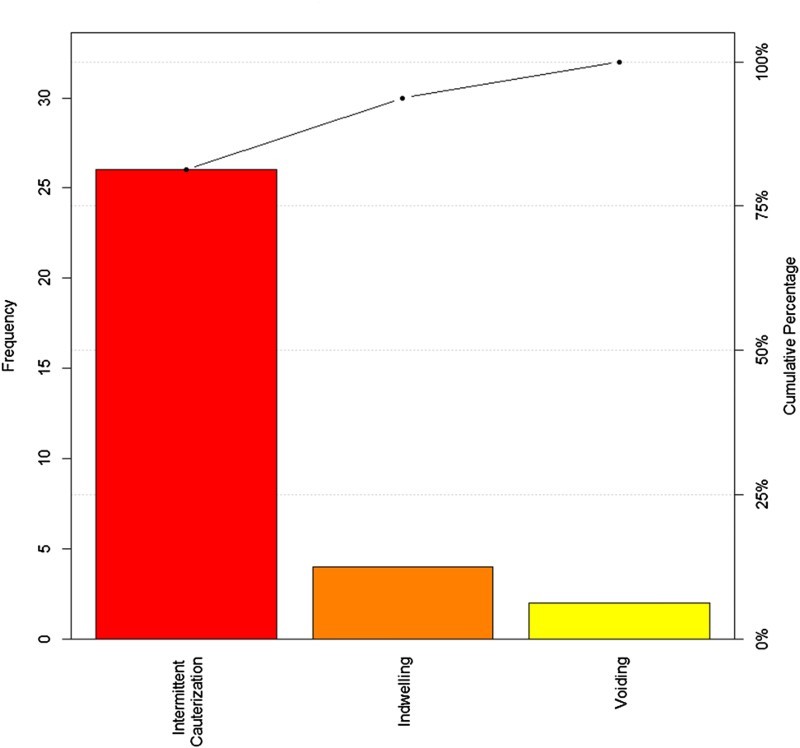
Frequency of Urinary Tract Infection by bladder management method.
Fig. 3.
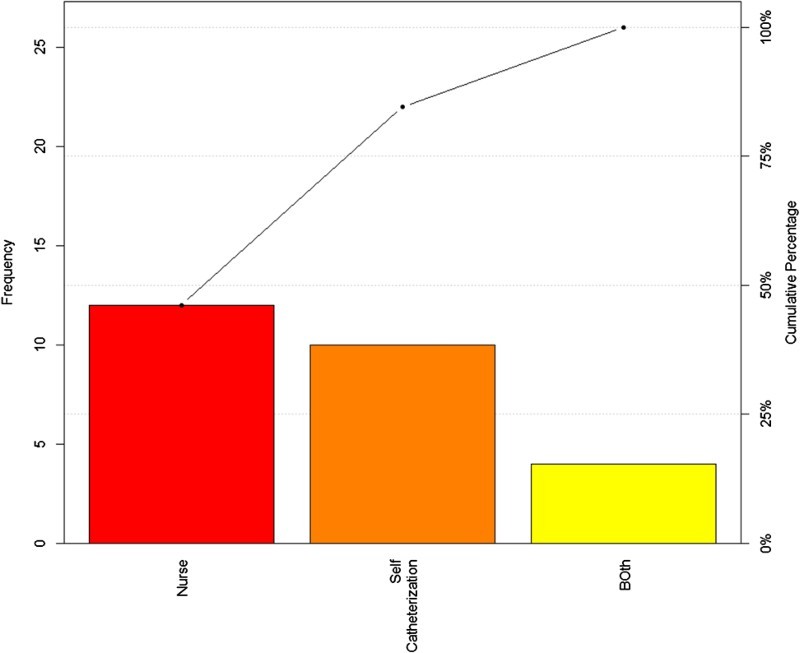
Intermittent Catheterization in Patient with UTI.
Fig. 4.
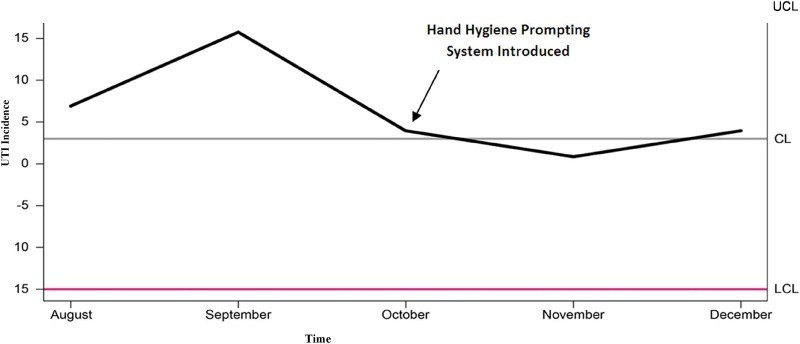
Control Chart of UTI incidence from August to November 2015, Mean= 3, SD= 5.98, CL= Control Limit, UCL= Upper Control Limit, LCL= Lower Control Limit, UTI= Urinary Tract Infection.
Discussion
The Predictive Value of Signs and Symptoms
There is a paucity of published papers that address the sensitivity, specificity, PPV, NPV of urine signs and symptoms among individuals with subacute SCI in tertiary rehabilitation settings. The sub-acute or Intermediate Phase of spinal cord injury begins 2 weeks after, up until 6 months post injury; this phase is characterized by the continued maturation of the glial scar and by regenerative axonal sprouting.24 In this study, cloudy or foul smelling urine and the onset of new incontinence were associated with a high likelihood of UTI diagnosis among inpatients with SCI. The recent onset of incontinence alone showed the highest specificity for UTI diagnosis, and when present is sufficient to strongly suspect the diagnosis of UTI. Massa et al. (2009) reported fever as having the highest specificity (99%) with low sensitivity (6.9%).12 While in our study, fever showed a high specificity (84%), it did not have the highest specificity. We found a higher sensitivity for fever in our setting (27%). The difference in study populations may in part explain the observed differences as the Massa study population consisted of individuals with SCI on CIC, while we included all SCI inpatients. As localized symptoms are often lacking or absent among individuals with subacute SCI, it is crucial to evaluate all alternative sources of infection before attributing a fever to a UTI. A combination of fever and foul smelling urine, as well as a combination of cloudy and foul-smelling urine increased UTI specificity to 92%. The combination of the cloudy and foul smelling urine also resulted in a higher PPV (78%, 95% CI: 0.40 - 0.97). Therefore, in individuals with subacute SCI the presence of fever and foul-smelling urine or cloudy and foul-smelling urine should prompt antibiotic initiation prior to the C&S result, due to their high PPV for UTI. Our findings are in contrast to Deresinski and Perkash who studied the value of symptom evaluation in predicting the presence of bladder bacteriuria in males with SCI who were catheter-free but using condom drainage.25 They concluded that the reported symptoms were not particularly useful in predicting urine culture results because of their non-specific nature and the absence of bacteriuria. Nurse monitoring and reporting of UTI signs and symptoms may in part explain the observed differences in these studies.
Strategies for Reducing UTI Incidence
i. Ensure all patients and clinicians are vigilant in looking out for UTI signs and symptoms
Up to 58.2% of patients who reported any signs and/or symptoms related to the urinary tract or other symptoms related to the SCI such as autonomic dysreflexia and increased spasticity, were diagnosed with UTI during inpatient rehabilitation. Catheter-associated UTI is the most common nosocomial infection, accounting for 1 million cases each year in US hospitals and nursing homes, and about one-half of all hospital-acquired infections originate in the urinary tract, in association with urinary catheters or other drainage devices.26 UTI in these patients may be preventable through good catheter management.27 UTI is an important cause of septicemia in individuals with SCI that causes bacteremia in 2-4% of patients and has been associated with a case fatality rate three times higher than that observed in non-bacteriuric patients.28 In the past century, renal failure was the primary cause of death in the SCI population29; interim improvements in antibiotics, early removal of indwelling catheters, and CIC initiation, and screening for renal disease have markedly decreased renal complications.30,31
ii. Nursing competence in Intermittent Catheterizations-
We found that the time when individuals with SCI were learning CIC and were being assisted by nurses was associated with a higher likelihood of UTI. A high level of knowledge and skills are required to effectively and safely manage patients with neurogenic bladder requiring CIC.32 It is essential that nurses have the necessary expertise to provide optimal care and minimize the problems associated with routine CIC.33 Education of nursing staff, including registered nurses and nursing assistants was a priority recommendation to standardize the CIC method locally. Hands-on instruction was provided for nurses regarding hand washing and aseptic techniques for catheter management including catheter insertion, catheter care, catheter immobilization, and urine collection and disposal technique. The training should be integrated into the nursing orientation for new hires, and an annual assessment of competence conducted. Frequent Hand Hygiene-
Hand hygiene is an essential component of UTI reduction. Excellent evidence exists that alcohol hand rubs effectively reduce the transmission of potential pathogens from health care workers’ hands to patients.34 For hands that are not visibly soiled, alcohol hand rubs are more effective than hand washing with plain or antimicrobial soap.35 However, in institutional settings, the preferred method for cleaning visibly soiled hands is to wash with warm water and antimicrobial soap.
iii. Hydrophilic Catheters
Hydrophilic catheters have a polymer coating that binds to the surface of the catheter. When the polymer coating is submersed in water, it absorbs and binds the water to the catheter. The catheter surface then becomes smooth and slippery. The hydrophilic coating is designed to reduce friction as the catheter is inserted into the urethra, and maintains lubrication throughout the length of the urethra to reduce urethral damage. It is hypothesized that because hydrophilic catheters do not require manual lubrication, they are less likely to cause infection. Most hydrophilic catheters are prepackaged in sterile water, or there is a pouch of sterile water that is broken and released into the catheter package when the catheter is ready for use.36 Two studies among individuals with acute SCI showed a significant reduction in the incidence of symptomatic UTI with routine use of hydrophilic catheters.5,37 Two problems arise with using hydrophilic catheters; first, good hand function is required, therefore individuals with tetraplegia and poor to moderate hand function, are not able to independently use this catheter. Second, the cost of hydrophilic catheters is about twice the cost a standard catheter in the community setting.36
Several limitations are worthy of discussion prior to generalization the study results. First, the calculated PPV, NPV, and LRs reported apply to the tertiary inpatient setting for individuals with subacute SCI and not to the outpatient setting where, nonspecific symptoms and chronic bacteriuria are common and confound UTI diagnosis. Finally, the hand washing prompting system is a prototype in our centre, which may become commercially available in the future but is not widely available.
Conclusion
The enclosed findings emphasize the importance of assessing urine appearance and odour to confirm suspicion of UTI, before sending a urine for R&M/C&S. The concurrent presence of cloudy and foul smelling urine is important for early diagnosis of UTI among inpatients with subacute SCI. Optimizing bladder drainage strategies that reduce dependence on indwelling catheters, training in CIC techniques and effort to improve hand hygiene may contribute to reduce UTI occurrence.
Acknowledgement
The authors would like to express their appreciation to Tess Devji, the process improvement lead at Lyndhurst centre who assisted in the implementation of the aforementioned quality improvement processes. The authors acknowledge the assistance of the nurses and physicians at Toronto Rehabilitation, Lyndhurst Centre, Toronto, Ontario, Canada. This quality improvement process was embedded in SCI-HIGH project funded by the Rick Hansen Institute (Grant #G2015-33). M Alavinia receives salary support from the Toronto Rehabilitation Institute Foundation. The authors would also like to acknowledge the contribution of the research team that developed and implemented the electronic hand hygiene monitoring system: Dr. Geoff Fernie (Principal Investigator), Steven Pong and Pamela Holliday.


Disclaimer statements
Contributors None.
Funding None.
Declaration of interest: None.
Conflicts of interest None.
Ethics approval None.
ORCID
Mohammad Alavinia http://orcid.org/0000-0002-5503-9362
Beverley Catharine Craven http://orcid.org/0000-0001-8234-6803
References
- 1.Charlifue SW, Weitzenkamp DA, Whiteneck GG.. Longitudinal outcomes in spinal cord injury: aging, secondary conditions, and well-being. Arch Phys Med Rehabil. 1999;80(11):1429–34. doi: 10.1016/S0003-9993(99)90254-X [DOI] [PubMed] [Google Scholar]
- 2.Evans CT, LaVela SL, Weaver FM, Priebe M, Sandford P, Niemiec P, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234–42. doi: 10.1086/527509 [DOI] [PubMed] [Google Scholar]
- 3.Adriaansen JJ, Post MW, de Groot S, van Asbeck FW, Stolwijk-Swuste JM, Tepper M, et al. Secondary health conditions in persons with spinal cord injury: a longitudinal study from one to five years post-discharge. J Rehabil Med. 2013;45(10):1016–22. doi: 10.2340/16501977-1207 [DOI] [PubMed] [Google Scholar]
- 4.Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT.. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil. 2000;79(6):526–35. doi: 10.1097/00002060-200011000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Cardenas DD, Moore KN, Dannels-McClure A, Scelza WM, Graves DE, Brooks M, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PM & R : the journal of injury, function, and rehabilitation. 2011;3(5):408–17. doi: 10.1016/j.pmrj.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 6.Saint S, Meddings JA, Calfee D, Kowalski CP, Krein SL.. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 200;150(12):877–84. doi: 10.7326/0003-4819-150-12-200906160-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50(5):625–63. doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 8.Bladder Management for Adults with Spinal Cord Injury: A Clinical Practice Guideline for Health-Care Providers Consortium for Spinal Cord Medicine. J Spinal Cord Med. 2006;29(5): 527–73. [PMC free article] [PubMed] [Google Scholar]
- 9.Hill TC, Baverstock R, Carlson KV, Estey EP, Gray GJ, Hill DC, et al. Best practices for the treatment and prevention of urinary tract infection in the spinal cord injured population: The Alberta context. Canadian Urological Association journal. 2013;7(3–4):122–30. doi: 10.5489/cuaj.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenas DD, Hooton TM.. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76(3):272–80. doi: 10.1016/S0003-9993(95)80615-6 [DOI] [PubMed] [Google Scholar]
- 11.Garcia Leoni ME, Esclarin De Ruz A.. Management of urinary tract infection in patients with spinal cord injuries. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2003;9(8):780–5. doi: 10.1046/j.1469-0691.2003.00643.x [DOI] [PubMed] [Google Scholar]
- 12.Massa LM, Hoffman JM, Cardenas DD.. Validity, accuracy, and predictive value of urinary tract infection signs and symptoms in individuals with spinal cord injury on intermittent catheterization. J Spinal Cord Med. 2009;32(5):568–73. doi: 10.1080/10790268.2009.11754562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinson AG, Philbrick JT, Lindbeck GH, Schorling JB.. Fever in the clinical diagnosis of acute pyelonephritis. Am J Emerg Med. 1997;15(2):148–51. doi: 10.1016/S0735-6757(97)90087-5 [DOI] [PubMed] [Google Scholar]
- 14.Brommer B, Engel O, Kopp MA, Watzlawick R, Muller S, Pruss H, et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain: a journal of neurology. 2016;139(Pt 3):692–707. doi: 10.1093/brain/awv375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biering-Sorensen F. Urinary tract infection in individuals with spinal cord lesion. Current opinion in urology. 2002;12(1):45–9. doi: 10.1097/00042307-200201000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Disease-a-Month. 2003;49(2):53–70. doi: 10.1067/mda.2003.7 [DOI] [PubMed] [Google Scholar]
- 17.Wilde MH, Brasch J, Getliffe K, Brown KA, McMahon JM, Smith JA, et al. Study on the use of long-term urinary catheters in community-dwelling individuals. J Wound Ostomy Continence Nurs. 2010;37(3):301–10. doi: 10.1097/WON.0b013e3181d73ac4 [DOI] [PubMed] [Google Scholar]
- 18.Girard R, Mazoyer MA, Plauchu MM, Rode G.. High prevalence of nosocomial infections in rehabilitation units accounted for by urinary tract infections in patients with spinal cord injury. J. Hosp. Infect. 2006;62(4):473–9. doi: 10.1016/j.jhin.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 19.Nicolle LE. Urinary tract infections in special populations: diabetes, renal transplant, HIV infection, and spinal cord injury. Infect Dis Clin North Am. 2014;28(1):91–104. doi: 10.1016/j.idc.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 20.De Ruz A Esclarin, Leoni E Garcia, Cabrera R Herruzo. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol. 2000;164(4):1285–9. doi: 10.1016/S0022-5347(05)67157-1 [DOI] [PubMed] [Google Scholar]
- 21.Gribble MJ, Puterman ML.. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141–52. doi: 10.1016/0002-9343(93)90254-M [DOI] [PubMed] [Google Scholar]
- 22.Vigil HR, Hickling DR.. Urinary tract infection in the neurogenic bladder. Translational andrology and urology. 2016;5(1):72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagasse RS, Steinberg ES, Katz RI, Saubermann AJ.. Defining quality of perioperative care by statistical process control of adverse outcomes. Anesthesiology. 1995;82(5):1181–8. doi: 10.1097/00000542-199505000-00013 [DOI] [PubMed] [Google Scholar]
- 24.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG.. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurgical focus. 2008;25(5):E2. doi: 10.3171/FOC.2008.25.11.E2 [DOI] [PubMed] [Google Scholar]
- 25.Deresinski SC, Perkash I.. Urinary tract infections in male spinal cord injured patients. Part one: Bacteriologic diagnosis. J Am Paraplegia Soc. 1985;8(1):4–6. [DOI] [PubMed] [Google Scholar]
- 26.Tambyah PA, Maki DG.. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients Arch Intern Med. 2000;160(5):678–82. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Arguello LY, O'Horo JC, Farrell A, Blakney R, Sohail MR, Evans CT, et al. Infections in the spinal cord-injured population: a systematic review. Spinal cord. 2016;55(6):1–9. [DOI] [PubMed] [Google Scholar]
- 28.Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91(3B):65S–71S. doi: 10.1016/0002-9343(91)90345-X [DOI] [PubMed] [Google Scholar]
- 29.Geisler WO, Jousse AT, Wynne-Jones M, Breithaupt D.. Survival in traumatic spinal cord injury. Paraplegia. 1983;21(6):364–73. doi: 10.1038/sc.1983.60 [DOI] [PubMed] [Google Scholar]
- 30.Bauman WA, Spungen AM, Raza M, Rothstein J, Zhang RL, Zhong YG, et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med. 1992;59(2):163–8. [PubMed] [Google Scholar]
- 31.Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia. 1992;30(9):617–30. doi: 10.1038/sc.1992.124 [DOI] [PubMed] [Google Scholar]
- 32.Parker LJ. Urinary catheter management: minimizing the risk of infection. Br J Nurs. 1999;8(9):563–6, 8, 70 passim. doi: 10.12968/bjon.1999.8.9.6616 [DOI] [PubMed] [Google Scholar]
- 33.Godfrey H, Evans A.. Catheterization and urinary tract infections: microbiology. Br J Nurs. 2000;9(11):682–4, 6, 8–90. doi: 10.12968/bjon.2000.9.11.6256 [DOI] [PubMed] [Google Scholar]
- 34.WHO WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. WHO Guidelines Approved by the Guidelines Review Committee; Geneva: 2009. [PubMed] [Google Scholar]
- 35.Kac G, Podglajen I, Gueneret M, Vaupre S, Bissery A, Meyer G.. Microbiological evaluation of two hand hygiene procedures achieved by healthcare workers during routine patient care: a randomized study. J Hosp Infect. 2005;60(1):32–9. doi: 10.1016/j.jhin.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 36.Health Quality Ontario Hydrophilic catheters: an evidence-based analysis. Ontario health technology assessment series. 2006;6(9):1–31. [PMC free article] [PubMed] [Google Scholar]
- 37.De Ridder DJ, Everaert K, Fernandez LG, Valero JV, Duran AB, Abrisqueta ML, et al. Intermittent catheterisation with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: a prospective randomised parallel comparative trial. Eur Urol. 2005;48(6):991–5. doi: 10.1016/j.eururo.2005.07.018 [DOI] [PubMed] [Google Scholar]


