Abstract
Background
Cardiometabolic health among adult offspring of hypertensive disorders of pregnancy (HDP) is relatively unknown. We hypothesized that offspring of HDP would have abnormalities in the retinal microvasculature and cardiac structure by midadulthood.
Methods and Results
The Cardiovascular Risk in Young Finns Study included randomly selected children from 5 Finnish university cities. The mean age of participants was 40 years (range 34–49 years) at the time of retinal photography and cardiac assessment. Offspring born ≥37 weeks of gestation and appropriate for gestational age (n=1006) were included. Offspring of HDP had higher systolic blood pressure (β=4.68, P<0.001), body mass index (β=1.25, P=0.009), and waist circumference (β=0.25, P=0.042), compared with offspring of normotensive pregnancies. However, no differences in fasting glucose, insulin, lipid profile, carotid intima media thickness, or brachial artery flow‐mediated dilatation were shown. Retinal arteriolar diameters were narrower (β=−0.43, P=0.009) and longer (β=32.5, P=0.023) and the arteriolar length‐to‐diameter ratio was higher (β=2.32, P=0.006) among offspring of HDP, after adjustment for age and sex. Left atrial volume indexed to body surface area (β=1.34, P=0.040) was increased. Adjustment for the confounding effects of birth weight, body mass index, smoking and socioeconomic status, and the mediating effect of hypertension had little impact on the associations.
Conclusions
Abnormalities of the retinal microvasculature and cardiac structure are seen in offspring of HDP in midadulthood. These findings may need to be considered in future primary prevention strategies of cardiovascular disease among offspring of HDP.
Keywords: cardiac, health outcomes, microvascular dysfunction
Subject Categories: Epidemiology, Hypertension, Blood Pressure, Imaging
Clinical Perspective
What Is New?
Cardiometabolic health among adult offspring of hypertensive disorders of pregnancy, is relatively unknown.
This study has shown that abnormalities of the retinal microvasculature and cardiac structure are seen in offspring of hypertensive disorders of pregnancy in midadulthood.
What Are the Clinical Implications?
These findings may need to be considered in future primary prevention strategies of cardiovascular disease among offspring of hypertensive disorders of pregnancy.
Introduction
Hypertensive disorders of pregnancy ([HDP] including pre‐eclampsia and gestational hypertension) are common and associated with adverse outcomes among the offspring.1, 2 It is well established that offspring of women with HDP have higher blood pressure (BP) from childhood,3 with 1 recent study showing that BP trajectories remain consistently higher throughout adolescence.4 Evidence of early‐onset hypertension has recently been observed. In a study by Davis et al, 30% of 20‐year‐olds with hypertensive BP were born following a hypertensive pregnancy,5 and the lifetime risk of developing cardiovascular disease (CVD) was estimated to be 2.5 times higher, equivalent to a 40% greater risk of developing CVD.5
There is little evidence of structural damage from higher BP among offspring of HDP, as most studies have assessed a limited set of measures in children and young adults.6 In the general population, derangements in cardiac structure7, 8, 9, 10 and the retinal microvasculature11, 12 in both adults and children have been observed with elevated BP. These indicators of target organ damage including left ventricular (LV) hypertrophy, increased left atrial volume, and narrower retinal arteriolar diameters, have been associated with an increased risk of myocardial infarction and CVD mortality.13, 14, 15, 16 However, studies have not assessed these associations among offspring of HDP in midadulthood, where the risk of CVD is increased. These associations are of particular importance given the strong links with CVD events and the need for appropriate treatment at critical points in the disease process.
To address these gaps in knowledge between HDP and cardiometabolic health, we assessed the associations in the Cardiovascular Risk in Young Finns study, an adult cohort with in‐depth measures over 40 years. We hypothesized that offspring of HDP would have abnormalities in the retinal microvasculature and cardiac structure by midadulthood, and these associations would be independent of the mediating effect of current hypertension status.
Methods
The Cardiovascular Risk in Young Finns Study is an ongoing epidemiological study of atherosclerosis risk factors from childhood to adulthood. In 1980, children and adolescents aged 3 to 18 years were invited to participate (n=4320). The study was carried out in 5 Finnish university cities and their rural surroundings, with subjects chosen randomly from the national population register from these areas.17 Subjects and their parents completed a detailed questionnaire, including information on birth weight and preterm birth.18 Birth before 37 weeks' gestation was defined as preterm birth. Term births were classified as appropriate for gestational age when birth weight was in the 50th to 90th percentile for the population. HDP was defined as hypertension only during pregnancy. The current study included 1006 participants born at term (≥37 weeks) and appropriate for gestational size, who underwent retinal photography and cardiac assessment during the 2011–2012 clinic. In the current study, our final analysis included the participants who had information on retinal parameters or cardiac parameters, BP, medication used for BP measured in 2011, and data on HDP.
Forty‐five‐degree digital retinal images centered on the macula of each eye were captured using a Canon nonmydriatic retinal camera (Canon CR6‐45NM) fitted with a Canon 10D digital single lens reflex camera attachment. One observer, blinded to subject data, graded the retinal images. A semi‐automated grading system was used to capture a range of retinal geometric parameters. Measured parameters included the (1) arteriolar and venular diameters, (2) arteriolar‐to‐venular ratio, (3) arteriolar bifurcation angles, (4) length/diameter ratios of arteriolar segments and arteriolar/venular diameter ratios (these parameters provide measures of arteriolar narrowing that are relatively unaffected by differences in optical refraction), (5) arteriolar tortuosity (estimated as the actual length of the vessel divided by the straight line distance between bifurcations minus 1), and (6) arteriolar optimality ratio and optimality deviance. Optimality ratio is the ratio of sum of “daughter” arteriolar diameters divided by the “parent” arteriolar diameter corrected for asymmetry.19, 20 For a theoretically optimal bifurcation, the optimality ratio should be 0.79, and the optimality deviance was calculated as the absolute value of the optimality ratio minus 0.79. Reproducibility of this technique is high and the average absolute difference and SD between measurements of arteriolar diameter was 0.0±0.4 pixels, consistent with previous reports.20 The arteriolar and venular diameters were measured at a series of intensity cross‐sections normal to the vessel at 2‐pixel intervals along the entire length of the vessel segment. At each cross‐section, the vessel diameter was measured to subpixel accuracy using a sliding linear regression filter technique as described previously and an average was calculated for each vessel.21
Transthoracic echocardiograms were performed according to American and European guidelines.22, 23 Sonographers from each site were trained in cardiac echocardiography according to the study protocol. Transthoracic echocardiograms were performed with Acuson Sequoia 512 (Acuson, Mountain View, CA, USA) ultrasonography, using a 3.5‐MHz scanning frequency phased‐array transducer. Analysis of the echo images were performed by a single observer using the ComPACS 10.7.8 analysis program (MediMatic Solutions, Genova, Italy). Standard echocardiographic views were obtained in all participants (parasternal long and short axis, and apical 4‐chamber). Complete 2‐dimensional, M mode echocardiographic measurements, continuous and pulsed‐wave Doppler, and mitral annulus tissue Doppler velocities were performed. Left ventricular mass and chamber area/volumes were indexed to body surface area (BSA), as per the European Society of Cardiology guidelines. The formula used to calculate BSA was DuBois and DuBois ([weight] kg to the power of 0.425×[height] m to the power of 0.725×0.007184).24
Ultrasound measures of carotid intima media thickness (IMT) were undertaken in 2007.25 The measures were performed with a Sequoia‐512, ultrasound mainframe (Acuson, Mountain View, CA, USA). All measurements were performed by the same technician, blinded to participant details. The image, from the left common carotid artery, was focused on the posterior wall. To derive mean carotid IMT, at least 4 measurements were taken ≈10 mm proximal to the carotid bifurcation. Intra‐individual reproducibility was assessed resulting in an average absolute difference and SD between measurements of 0.05±0.04 mm.26 Brachial ultrasound studies were performed using Sequoia 512 ultrasound mainframes (Acuson) with 13.0 MHz linear array transducer. To assess brachial artery flow‐mediated dilatation (FMD), the left brachial artery diameter was measured both at rest and during reactive hyperemia. Increased flow was induced by inflation of a pneumatic tourniquet placed around the forearm to a pressure of 250 mm Hg for 4.5 minutes, followed by a release. Three measurements of arterial diameter were performed at end‐diastole at a fixed distance from an anatomic marker at rest and 40, 60, and 80 seconds after cuff release. The vessel diameter in scans after reactive hyperemia was expressed as the percentage relative to resting scan (100%). The greatest value between 40 and 80 seconds was used to derive the maximum FMD. The 3‐month‐between‐visit coefficient of variation was 3.2% for brachial artery diameter measurement and 26.0% for FMD measurement.25
Height and weight were measured, and body mass index kg/m2 (BMI) calculated. BP measurements were obtained using a random zero sphygmomanometer. Three measurements of BP were recorded and the mean value of these was presented. Hypertension was defined as systolic BP >140 mm Hg, diastolic BP >90 mm Hg, or taking antihypertensive medication. A self‐administered questionnaire was used to determine current health status (medication and medical conditions), smoking status, and employment. Participants who indicated they were regular smokers (smoke daily) were classified as smokers. Venous blood samples were drawn after an overnight fast. Serum total cholesterol and triglycerides were determined according to the Lipid Research Clinics Program, and high‐density lipoprotein cholesterol was analyzed following precipitation of apolipoprotein‐B‐containing lipoproteins with heparin‐manganese.27 The homeostatic model assessment index was calculated as insulin (μU/m)×(glucose [mmol/L]/22.5).28
The study complied with the Declaration of Helsinki, was approved by the local ethics committees, and all subjects gave written informed consent.
Statistical Methods
The data analysis was performed with Stata 12.0 IC (Stata Corp LP, College Station, TX, USA). Descriptive information for each variable was derived and distributions were assessed to determine normality. Data are presented as mean (SD), percentages, or median and (interquartile range). Univariate associations were assessed using ANOVA for metric variables and χ2 for categorical variables. The associations between HDP with CVD risk factors, retinal microvascular, and cardiac measures were analyzed using regression models. Interaction analysis between sex and offspring of hypertensive disorders of pregnancy were run for each outcome measure and none were significant; therefore data were not analyzed separately for males and females. Three models were developed. Model 1 regressed outcome variables (separately, each retinal microvascular and cardiac measure) on HDP adjusted for age and sex. Model 2 further adjusted for the potential confounding effects of birth weight, BMI, smoking, and socioeconomic status, and model 3 further adjusted for the possible mediating effect of current hypertension. Statistical significance was inferred as a 2‐sided probability at the 95% level of confidence. In order to provide a graphical illustration of the associations between vascular measures and systolic BP, quartiles were created after exclusion of those with hypertension: 1/83 to 108 mm Hg, 2/109 to 116 mm Hg, 3/117 to 126 mm Hg, and 4/>126 mm Hg and stratified by gestational status. Multivariate analysis of variance was used to assess the difference between and within groups.
Results
The characteristics of offspring participants, stratified by HDP status, are shown in Table 1. Offspring of HDP had a higher BMI and waist circumference, systolic and diastolic BP, and a higher percentage were on medication for hypertension, compared with offspring of normotensive pregnancies. There were no significant differences in sex, smoking, socioeconomic status, high‐density lipoprotein cholesterol, total cholesterol, apolipoprotein A1, apolipoprotein B, glucose, or insulin, between the groups.
Table 1.
General Characteristic of the Offspring According to Maternal HDP Status
| Offspring of Women Without HDP | Offspring of Women With HDP | P Value | |
|---|---|---|---|
| N | 877 | 129 | |
| Age, y | 41.6 (5.0) | 39.7 (4.6) | <0.001 |
| Female, % | 51.2 | 48.8 | 0.185 |
| Birth weight, g | 3666 (402) | 3746 (458) | 0.039 |
| BMI, kg/m2 | 26.3 (5.0) | 27.3 (5.4) | 0.047 |
| BSA, m2 | 1.92 (0.22) | 1.97 (0.21) | 0.012 |
| Systolic BP, mmHg | 117 (14) | 121 (15) | 0.003 |
| Diastolic BP, mmHg | 74 (10) | 77 (11.0) | 0.002 |
| BP medication, % | 7.6 | 16.4 | 0.001 |
| HDL cholesterol, mmol/L | 1.34 (0.34) | 1.27 (0.3) | 0.065 |
| Total cholesterol, mmol/L | 5.12 (0.93) | 5.14 (0.98) | 0.742 |
| Apo A1, mmol/L | 1.59 (0.25) | 1.55 (0.22) | 0.113 |
| Apo B, mmol/L | 1.04 (0.28) | 1.06 (0.03) | 0.271 |
| Glucose, mmol/L | 5.4 (0.9) | 5.3 (0.66) | 0.935 |
| Diabetes mellitus, % | 3.2 | 1.6 | 0.306 |
| Insulin, mU/L | 9.2 (12.4) | 9.8 (9.3) | 0.616 |
| Regular smoking, % | 13 | 13 | 0.990 |
Apo indicates apolipoprotein; BMI, body mass index; BP, blood pressure; BSA, body surface area; HDL, high‐density lipoprotein; HDP, hypertensive disorders of pregnancy.
After adjustment for age and sex, offspring of HDP had higher systolic and diastolic BP, BMI, and waist circumference (Table 2). Further adjustment for confounding factors (model 2) had little impact on the regression coefficients for BMI and waist circumference and modestly reduced the regression coefficients for systolic and diastolic BP, although both remained statistically significant. After adjustment for HDP, the association with BMI and waist circumference were no longer significant.
Table 2.
Multiple Regression of HDP Status on Offspring CVD Risk Factors
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient HDP | P Value | Regression Coefficient HDP | P Value | Regression Coefficient HDP | P Value | |
| Outcome measures | ||||||
| Systolic BP, mmHg | 4.68 (2.27 to 7.09)a | <0.001a | 3.83 (1.53 to 6.13)a | 0.001a | b | |
| Diastolic BP, mmHg | 3.48 (1.63 to 5.32)a | <0.001a | 2.72 (0.98 to 4.46)a | 0.002a | b | |
| Heart rate, bpm | 0.49 (−1.30 to 2.28) | 0.595 | 0.21 (−1.56 to 1.98) | 0.817 | −0.46 (−2.22 to 1.29) | 0.603 |
| BMI, kg/m2 b | 1.25 (0.31 to 2.18)a | 0.009a | 1.20 (0.26 to 2.13)a | 0.012a | 0.59 (−0.32 to 1.50) | 0.203 |
| Waist circumference, cm | 0.25 (0.01 to 0.48)a | 0.042a | 0.24 (0.01 to 0.47)a | 0.049a | 0.09 (−0.14 to 0.32) | 0.456 |
| HDL cholesterol, mmol/L | −0.04 (−0.09 to 0.02) | 0.165 | −0.02 (−0.07 to 0.04) | 0.516 | −0.01 (−0.07 to 0.04) | 0.622 |
| Total cholesterol, mmol/L | 0.07 (−0.10 to 0.24) | 0.440 | 0.05 (−0.13 to 0.21) | 0.631 | 0.05 (−0.12 to 0.23) | 0.549 |
| Apo A1, mmol/L | −0.02 (−0.07 to 0.02) | 0.269 | −0.01 (−0.06 to 0.03) | 0.531 | −0.01 (−0.06 to 0.03) | 0.519 |
| Glucose, mmol/L | 0.01 (−0.15 to 0.17) | 0.857 | −0.04 (−0.19 to 0.11) | 0.608 | −0.08 (−0.23 to 0.08) | 0.316 |
| HOMA IR | 0.16 (−1.19 to 1.50) | 0.821 | −0.35 (−1.64 to 0.94) | 0.599 | −0.57 (−1.86 to 0.72) | 0.387 |
Model 1 adjusted for age and sex, Model 2 adjusted for age, sex, birth weight, BMI, smoking, and SES. Model 3 adjusted for age, sex, birth weight, BMI, smoking, SES, and hypertension. Apo indicates apolipoprotein; BMI, body mass index; BP, blood pressure; bpm, beats per minute; CVD, cardiovascular disease; HDL, high‐density lipoprotein; HDP, hypertensive disorders of pregnancy; HOMA IR, homeostasis model assessment insulin resistance; SES, socioeconomic status.
Where the risk factor is the primary variable of interest, it was not entered into the model as a covariate.
Where the risk factor is the primary variable of interest, it was not entered into the model as a covariate.
Table 3 shows the effect of HDP status on offspring vascular outcomes. Retinal arteriolar diameters were narrower (β=−0.43, P=0.009) and longer (β=32.5, P=0.023), the arteriolar length‐to‐diameter ratio was higher (β=2.32, P=0.006), and the arteriolar‐to‐venular ratio decreased among offspring of hypertensive pregnancies, after adjustment for age and sex. Adjustment for the confounding effects of birth weight, BMI, smoking, and socioeconomic status, and the mediating effect of hypertension had little impact on the associations with retinal architecture measures. No association was observed for FMD or IMT. The analysis for FMD was re‐run using diameter after hyperemia as the outcome and baseline as the covariate. It made little difference to the outcome. The associations between FMD and offspring of hypertensive disorders of pregnancy was not significant (β=1.01 [−0.02 to 0.04] P=0.482), after adjustment for age and sex. Adjustment for the confounding effects of birth weight, BMI, smoking, and socioeconomic status (β=0.004 [−0.02 to 0.03] P=0.750), and the mediating effect of hypertension (β=0.01 [−0.02 to 0.03] P=0.661), had little impact on the association.
Table 3.
Association Between HDP Status and Offspring Vascular Outcomes
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient O‐HDP | P Value | Regression Coefficient O‐HDP | P Value | Regression Coefficient O‐HDP | P Value | |
| Outcome retinal microvasculature measures | ||||||
| Arteriolar diameter (pixels) | −0.43 (−0.76 to −0.11) | 0.009 | −0.39 (−0.72 to −0.07) | 0.017 | −0.36 (−0.69 to 0.04) | 0.030 |
| Venular diameter (pixels) | −0.11 (−0.61 to 0.39) | 0.674 | −0.11 (−0.61 to 0.40) | 0.675 | −0.11 (−0.62 to 0.39) | 0.657 |
| Arteriolar tortuosity (102) | −0.01 (−0.03 to −0.00) | 0.073 | −0.01 (0.03 to 0.00) | 0.062 | 0.02 (0.03 to −0.00) | 0.039 |
| Venular tortuosity (103) | −0.05 (−0.13 to 0.04) | 0.279 | −0.05 (−0.14 to 0.03) | 0.222 | −0.05 (−0.14 to −0.03) | 0.199 |
| Arteriolar length (pixels) | 32.5 (4.60 to 60.45) | 0.023 | 34.5 (6.51 to 62.53) | 0.016 | 32.5 (4.29 to 60.74) | 0.024 |
| Venular length (pixels) | 6.23 (−6.47 to 18.93) | 0.336 | 5.64 (−7.09 to 18.38) | 0.385 | 5.32 (−7.52 to 18.16) | 0.416 |
| Arteriolar length/diameter ratio | 2.32 (0.66 to 3.98) | 0.006 | 2.40 (0.74 to 4.05) | 0.005 | 2.23 (0.56 to 3.90) | 0.009 |
| Venular length/diameter ratio | 0.35 (−0.41 to 1.11) | 0.368 | 0.32 (−0.44 to 1.08) | 0.410 | 0.31 (−0.45 to 1.08) | 0.422 |
| Optimality ratio | −2.05 (−4.99 to 0.90) | 0.173 | −1.92 (−4.89 to 1.04) | 0.204 | −2.34 (−0.32 to 0.65) | 0.125 |
| Arteriolar‐to‐venular ratio | −0.03 (−0.05 to 0.001) | 0.027 | −0.03 (−0.05 to 0.01) | 0.027 | −0.02 (−0.05 to −0.01) | 0.047 |
| Outcome vascular measures | ||||||
| FMD, mma | −0.07 (−1.03 to 0.89) | 0.885 | −0.35 (−1.30 to 0.60) | 0.465 | −0.34 (−1.30 to 0.61) | 0.480 |
| IMT, mma | 0.005 (−0.012 to 0.024) | 0.528 | 0.004 (−0.014 to 0.023) | 0.650 | 0.003 (−0.016 to 0.021) | 0.789 |
Model 1 adjusted for age and sex, Model 2 adjusted for age, sex, birth weight, body mass index (BMI), smoking, and socioeconomic status (SES). Model 3 adjusted for age, sex, birth weight, BMI, smoking, SES, and hypertension. FMD indicates flow‐mediated dilatation; HDP, hypertensive disorders of pregnancy; IMT, intermedia thickness; O‐HDP, offspring of hypertensive disorders of pregnancy.
Measured in 2007.
Associations between HDP status and offspring cardiac measures are shown in Table 4. Left atrial volume indexed to BSA (β=1.34, P=0.040) and peak A wave (β=2.56, P=0.016) were higher and the ratio of early diastolic mitral annulus velocity to mitral annulus velocity associated with atrial contraction (e′/a′ ratio; β=−0.06, P=0.005) was lower among offspring of HDP after adjustment for age and sex (Table 4). Adjustment for the confounding effects of birth weight, BMI, smoking, and socioeconomic status (model 2) reduced the association with left atrial (LA) volume indexed to BSA, and peak A wave. Further adjustment for the mediating effect of hypertension had little impact on the regression coefficients for LA volume indexed to BSA and revealed an association with LV end‐systolic area indexed to BSA. The associations with transmitral Doppler A wave and e′/a′ ratio was no longer significant after adjustment for the mediating effect of current hypertensive status.
Table 4.
Association Between HDP Status and Offspring Cardiac Measures
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient O‐HDP | P Value | Regression Coefficient O‐HDP | P Value | Regression Coefficient O‐HDP | P Value | |
| LV structural measures | ||||||
| LV diameter, diastole, cm | 0.02 (−0.06 to 0.11) | 0.591 | −0.02 (−0.10 to 0.06) | 0.568 | −0.02 (−0.10 to 0.06) | 0.654 |
| LV diameter, systole, cm | −0.02 (−0.09 to 0.06) | 0.701 | −0.03 (−0.11 to 0.05) | 0.413 | −0.03 (−0.10 to 0.05) | 0.506 |
| LV ejection fraction, % | −0.18 (−0.84 to 0.47) | 0.581 | −0.15 (−0.81 to 0.51) | 0.651 | 0.10 (−0.76 to 0.57) | 0.777 |
| LV mass index, g/m2 | −0.48 (−2.91 to 1.94) | 0.696 | −0.83 (−3.25 to 1.59) | 0.500 | −0.81 (−3.24 to 1.62) | 0.514 |
| Relative wall thickness | 0.01 (−0.02 to 0.40) | 0.477 | 0.01 (−0.02 to 0.04) | 0.476 | 0.01 (−0.02 to 0.04) | 0.475 |
| LV area end‐systole indexed BSA, mL/m2 | 0.29 (−0.02 to 0.61) | 0.069 | 0.29 (−0.03 to 0.61) | 0.078 | 0.33 (0.01 to 0.65) | 0.044 |
| LA volume indexed BSA, mL/m2 | 1.34 (0.06 to 2.61) | 0.040 | 1.14 (−0.13 to 2.41) | 0.079 | 1.29 (0.01 to 2.57) | 0.049 |
| Transmitral Doppler | ||||||
| E wave, cm/s | 1.66 (−0.55 to 3.87) | 0.140 | 1.64 (−0.56 to 3.85) | 0.144 | 1.77 (−0.46 to 3.99) | 0.119 |
| A wave, cm/s | 2.56 (0.49 to 4.64) | 0.016 | 2.08 (0.09 to 4.07) | 0.041 | 1.55 (−0.43 to 3.54) | 0.125 |
| E/A ratio | −0.05 (−0.13 to 0.02) | 0.137 | 0.04 (−0.11 to 0.03) | 0.267 | −0.02 (−0.09 to 0.05) | 0.545 |
| Tissue Doppler | ||||||
| Systolic velocity (s′), cm/s | 0.38 (−0.27 to 1.04) | 0.246 | 0.44 (−0.21 to 1.10) | 0.186 | 0.49 (−0.17 to 1.15) | 0.148 |
| Early diastolic velocity (e′), cm/s | −0.26 (−0.76 to 0.24) | 0.311 | −0.15 (−0.64 to 0.35) | 0.555 | 0.03 (−0.46 to 0.52) | 0.909 |
| Late diastolic velocity (a′), cm/s | 0.54 (−0.05 to 1.13) | 0.074 | 0.44 (−0.14 to 1.03) | 0.138 | 0.42 (−0.17 to 1.01) | 0.165 |
| Mean E/e′ ratio | 0.17 (−0.01 to 0.35) | 0.068 | 0.13 (−0.05 to 0.31) | 0.158 | 0.08 (−0.10 to 0.26) | 0.367 |
| Mean e′/a′ ratio | −0.06 (−0.11 to −0.02) | 0.005 | −0.05 (−0.09 to −0.01) | 0.025 | −0.03 (−0.08 to 0.01) | 0.110 |
Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, birth weight, body mass index (BMI), smoking, and socioeconomic status (SES). Model 3 adjusted for age, sex, birth weight, BMI, smoking, SES, and hypertension. BSA indicates body surface area; HDP, hypertensive disorders of pregnancy; LA, left atria; LV, left ventricular; O‐HDP, offspring of hypertensive disorders of pregnancy.
The association between arteriolar diameter and offspring of HDP after adjustment for age, sex, birth weight, BMI, smoking, socioeconomic status, and the mediating effect of systolic BP was slightly weaker (β=−0.28 [−0.60 to 0.04] P=0.082) than when adjusted for hypertensive status. A similar pattern was evident for arteriolar length (β=30.40 [2.35–58.50] P=0.034), arteriolar length‐to‐diameter ratio (β=2.04 [0.38–3.69] P=0.016), arteriolar‐to‐venular ratio (β=−0.02 [−0.05 to 0.001] P=0.067), LV area end‐systole indexed to BSA (β=0.26 [−0.06 to 0.58] P=0.115), and LA volume indexed to BSA (β=0.99 [−0.29 to 0.2.27] P=0.0.128).
Figure 1 shows the age‐ and sex‐adjusted mean and 95% confidence interval for retinal microvascular measures by quartiles of systolic BP. Those on current treatment for hypertension were excluded from this analysis. For arteriolar diameter, there was a negative association with systolic BP among both groups and in each quartile of systolic BP, offspring of HDP had narrower diameters. A similar pattern of association was observed for arteriolar length and arteriolar length/diameter ratio, except the offspring of HDP had longer arterioles and a higher length/diameter ratio compared with those of a normotensive pregnancy. Figure 2 shows the age‐ and sex‐adjusted mean and 95% confidence interval for cardiac measures by quartiles of systolic BP. Both LV end‐systolic area and LA volume indexed to BSA values were higher in each quartile of systolic BP among offspring of hypertensive pregnancies. No association was observed for transmitral A wave, where the association was mediated by current systolic BP.
Figure 1.
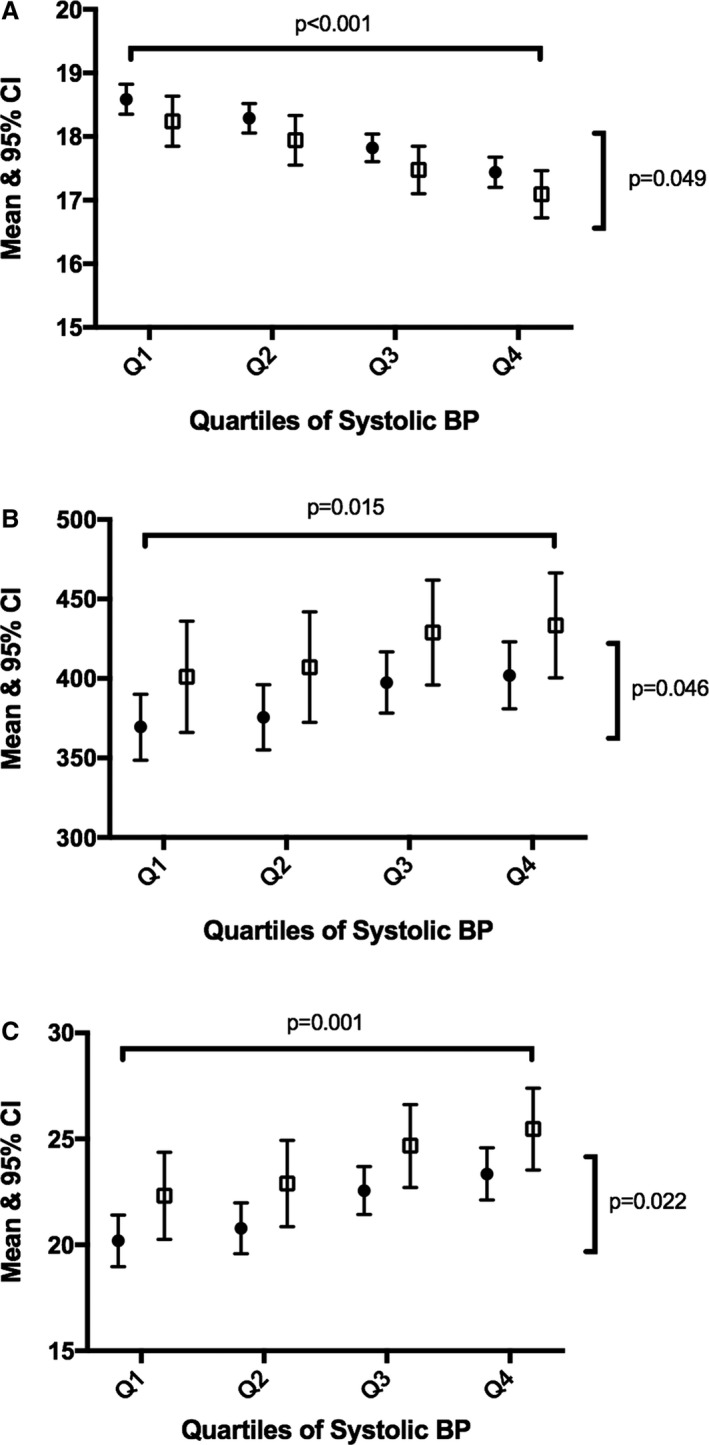
Association between quartiles of systolic BP and retinal microvascular measures by HDP status. A, Retinal arteriolar diameter. B, Retinal arteriolar length. C, Retinal arteriolar length/diameter ratio. BP indicates blood pressure; CI, confidence interval; HDP, hypertensive disorders of pregnancy.
Figure 2.

Association between quartiles of systolic BP and cardiac measures by HDP. A, Left atrium volume indexed to BSA. B, LV area end‐systole. C, Transmitral Doppler A wave. BP indicates blood pressure; BSA, body surface area; CI, confidence interval; HDP, hypertensive disorders of pregnancy; LV, left ventricular.
Discussion
This is the first study to comprehensively examine the cardiometabolic effects of HDP among adult offspring. The study showed that offspring of women with HDP had higher BMI and waist circumference, systolic and diastolic BP, as well as evidence of adverse changes in the retinal microvasculature and cardiac structure. However, no evidence of an association with glucose, insulin, lipid profile, IMT, or FMD was found. Our study indicates that HDP are not only associated with higher BP among offspring, but also with abnormalities of the retinal microvascular and to a lesser degree cardiac structure by midadulthood, independent of risk factors and adjustment for the mediating effect of current hypertensive status.
Previous studies have assessed vascular markers in offspring of HDP (including FMD and pulse wave velocity) with mixed results; however, this is the first study to assess measures of the retinal microvasculature and cardiac structure in midadulthood using measures that are very closely associated with BP. We have shown that adult offspring of HDP have a worse retinal microvascular profile and some early alterations in their cardiac profile, compared with offspring from a normotensive pregnancy (Figures 1 and 2). Although not previously shown among offspring of HDP, the ALSPAC (Avon Longitudinal Study of Parents and Children) did speculate that associations with a wide range of vascular outcomes may emerge in adulthood.6 Although hypertension is the preferred measure in the current study, as on treatment BP may not capture BP load, the models were run for the measures showing a significant association after adjustment for hypertension. Although the associations were weakened, the same pattern of association was observed.
There are 2 possible mechanisms as to why HDP is associated with the microvascular and cardiac abnormalities. HDP may cause abnormalities in microvascular and the cardiac system or HDP may be a marker/consequence of abnormalities in the microvascular/cardiac system. We are unable to distinguish between these and existing evidence is limited and inconclusive.29 The implications of these findings independent of hypertensive status are 2‐fold. A single measure of BP may be inadequate to capture the lifetime load of BP, and target organ damage better reflects lifetime BP load, which may be higher in HDP offspring. Alternatively, using arteriolar narrowing as an example, it may be a causal mechanism of elevated BP that precedes hypertension. From a hemodynamic perspective, narrow arterioles (if widespread) would result in increased systemic vascular resistance, a characteristic feature of hypertension. There is some evidence that arteriolar narrowing is a risk factor for development of hypertension.30 A recent meta‐analysis showed that each 20‐μm decrease in retinal caliber was associated with 1.12 mm Hg increase in systolic BP over 5 years. The study findings were consistent with the hypothesis that generalized microvascular dysfunction precedes the onset of hypertension.31 In addition, LA volume is strongly associated with elevated BP and obesity. It is also an indicator of increased filling pressure over time, in a similar way that hemoglobin A1c is a measure of blood glucose over time. It is also a powerful predictor of CVD outcomes.16 Although change in LA volume indexed to BSA was observed in the present study, other evidence of abnormalities of diastolic function was limited and further evaluation at a later time point is warranted, particularly given a study in mice has shown that gestational hypertension can lead to LV hypertrophy in offspring, with further injections of isoproterenol (used to induce cardiac stress) resulting in LV diastolic dysfunction (increased E/A ratio and E/e′) and interstitial myocardial fibrosis.32
ALSPAC assessed offspring FMD, pulse wave velocity, and brachial artery diameter (age 9 to 12 years) and showed no association with gestational hypertension. Similarly, vascular outcomes in subgroups born preterm were the same as offspring born at term.6 Consistent with the findings of ALSPAC, this study assessed FMD at age 40 years and found no association between HDP and FMD despite higher BP in offspring of HDP. FMD has previously been observed to predict incident hypertension over an ≈8‐year follow‐up period in older individuals (average age 60 years) in the Framingham Heart Study Offspring cohort.33 Whether these differences are attributable to differences in ages between studies or some other factor is unknown.
Other studies, from more selected, smaller samples have shown evidence of an association with pulmonary pressure, carotid IMT, and FMD. A study by Lazdam et al assessed a sample of 71 participants born preterm and showed that offspring of a hypertensive pregnancy had increased carotid IMT and 30% lower FMD at age 24 years.34 The present study excluded those born preterm and small for gestational age from the main analysis, as it has been demonstrated that being born small for gestational age and in particular preterm birth are associated with changes in the retinal microvascular,35 cardiac structure, IMT, and FMD.18 So although impaired fetal growth and preterm birth contribute to the mechanisms of early vascular changes, they are not the primary mechanistic link responsible for the observed abnormalities among offspring of HDP in the present study. In a small study of 138 women living at high altitude, pulmonary pressure was 30% higher and FMD 30% lower in offspring of mothers with preeclampsia. The study went further to assess whether dysfunction was related to preeclampsia or to a genetic abnormality that predisposes the mother to preeclampsia and the offspring to vascular alterations. They assessed the vascular function of offspring of mothers with preeclampsia; the offspring were born following a normotensive pregnancy. The study showed no impairment in FMD or pulmonary artery pressure in this group, suggesting that preeclampsia might induce epigenetic alterations leading to vascular dysfunction in the offspring. Epigenetic modifications are thought to play a central role in the programming of adult disease.36 Other possible mechanisms include shared genetics, which may better explain the isolated association with maternal and offspring elevated BP shown in the larger cohort studies,3 or direct intrauterine effects related to placental dysfunction in association with HDP.
The present study has several limitations. We were only able to assess HDP and not the subcategories of long‐term hypertension in pregnancy, preeclampsia/eclampsia, and gestational hypertension separately, which would provide a greater understanding of the differences between the conditions. Limited information on the mother's prepregnancy characteristic was available. These may have provided additional information on the maternal risk factor profile and their contributions to adverse outcomes. This was an observational study, with loss to follow‐up over many years,37 and while loss to follow‐up may compromise the representativeness of the sample, it is unlikely that it would account for the associations observed.
Conclusions
Abnormalities of the retinal microvasculature and cardiac structure are seen in offspring of HDP. These findings may need to be considered in future primary prevention strategies of cardiovascular disease among offspring of HDP.
Sources of Funding
The Young Finns Study has been financially supported by the Academy of Finland: grants 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117797 (Gendi), 41071 (Skidi), and 286284, the Social Insurance Institution of Finland, Kuopio, Tampere and Turku University Hospital Medical Funds, Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation of Cardiovascular Research and Finnish Cultural Foundation, Tampere Tuberculosis Foundation, and Emil Aaltonen Foundation. Hughes received support from a Biomedical Research Centre Award to University College London Hospital and from the British Heart Foundation (CS/15/6/31468, PG/12/29/29497).
Disclosures
None.
Acknowledgments
We would like to thank Sumangali Wijetunge, Simon Thom, and Nish Chaturvedi for their valued contribution to grading of the retinal images.
(J Am Heart Assoc. 2018;7:e006284 DOI: 10.1161/JAHA.117.006284.)29306901
References
- 1. Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, Catalano PM, Morris CD. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28. [DOI] [PubMed] [Google Scholar]
- 2. Vogel JP, Lee AC, Souza JP. Maternal morbidity and preterm birth in 22 low‐ and middle‐income countries: a secondary analysis of the WHO Global Survey dataset. BMC Pregnancy Childbirth. 2014;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fraser A, Nelson SM, Macdonald‐Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staley JR, Bradley J, Silverwood RJ, Howe LD, Tilling K, Lawlor DA, Macdonald‐Wallis C. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422 DOI: 10.1161/JAHA.114.001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20‐year prospective follow‐up birth cohort. BMJ Open. 2015;5:e008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawlor DA, Macdonald‐Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, Sattar N, Deanfield J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniels SR, Witt SA, Glascock B, Khoury PR, Kimball TR. Left atrial size in children with hypertension: the influence of obesity, blood pressure, and left ventricular mass. J Pediatr. 2002;141:186–190. [DOI] [PubMed] [Google Scholar]
- 8. Schieken RM, Clarke WR, Lauer RM. Left ventricular hypertrophy in children with blood pressures in the upper quintile of the distribution. The Muscatine Study. Hypertension. 1981;3:669–675. [DOI] [PubMed] [Google Scholar]
- 9. Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African‐American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41:955–960. [DOI] [PubMed] [Google Scholar]
- 10. Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, Devereux RB. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47:2267–2273. [DOI] [PubMed] [Google Scholar]
- 11. Hughes AD, Wong TY, Witt N, Evans R, Thom SA, Klein BE, Chaturvedi N, Klein R. Determinants of retinal microvascular architecture in normal subjects. Microcirculation. 2009;16:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheung N, Saw SM, Islam FM, Rogers SL, Shankar A, de Haseth K, Mitchell P, Wong TY. BMI and retinal vascular caliber in children. Obesity (Silver Spring). 2007;15:209–215. [DOI] [PubMed] [Google Scholar]
- 13. Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med. 2006;166:2388–2394. [DOI] [PubMed] [Google Scholar]
- 14. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. [see comment]. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 15. Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M‐mode echocardiographic predictors of six‐ to seven‐year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87:1051–1057. [DOI] [PubMed] [Google Scholar]
- 16. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 17. Raitakari OT, Juonala M, Ronnemaa T, Keltikangas‐Jarvinen L, Rasanen L, Pietikainen M, Hutri‐Kahonen N, Taittonen L, Jokinen E, Marniemi J, Jula A, Telama R, Kahonen M, Lehtimaki T, Akerblom HK, Viikari JS. Cohort profile: the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–1226. [DOI] [PubMed] [Google Scholar]
- 18. Skilton MR, Viikari JS, Juonala M, Laitinen T, Lehtimaki T, Taittonen L, Kahonen M, Celermajer DS, Raitakari OT. Fetal growth and preterm birth influence cardiovascular risk factors and arterial health in young adults: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2011;31:2975–2981. [DOI] [PubMed] [Google Scholar]
- 19. Tapp RJ, Williams C, Witt N, Chaturvedi N, Evans R, Thom SA, Hughes AD, Ness A. Impact of size at birth on the microvasculature: the Avon Longitudinal Study of Parents and Children. Pediatrics. 2007;120:e1225–e1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, McNamara M, Thom SA, Klein R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47:975–981. [DOI] [PubMed] [Google Scholar]
- 21. Chapman N, Mohamudally A, Cerutti A, Stanton A, Sayer AA, Cooper C, Barker D, Rauf A, Evans J, Wormald R, Sever P, Hughes A, Thom S. Retinal vascular network architecture in low‐birth‐weight men. J Hypertens. 1997;15:1449–1453. [DOI] [PubMed] [Google Scholar]
- 22. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 23. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 24. Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady‐state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27:2879–2888. [DOI] [PubMed] [Google Scholar]
- 25. Juonala M, Viikari JS, Ronnemaa T, Marniemi J, Jula A, Loo BM, Raitakari OT. Associations of dyslipidemias from childhood to adulthood with carotid intima‐media thickness, elasticity, and brachial flow‐mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2008;28:1012–1017. [DOI] [PubMed] [Google Scholar]
- 26. Kestila P, Magnussen CG, Viikari JS, Kahonen M, Hutri‐Kahonen N, Taittonen L, Jula A, Loo BM, Pietikainen M, Jokinen E, Lehtimaki T, Kivimaki M, Juonala M, Raitakari OT. Socioeconomic status, cardiovascular risk factors, and subclinical atherosclerosis in young adults: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2012;32:815–821. [DOI] [PubMed] [Google Scholar]
- 27. Juonala M, Magnussen CG, Venn A, Gall S, Kahonen M, Laitinen T, Taittonen L, Lehtimaki T, Jokinen E, Sun C, Viikari JS, Dwyer T, Raitakari OT. Parental smoking in childhood and brachial artery flow‐mediated dilatation in young adults: the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health study. Arterioscler Thromb Vasc Biol. 2012;32:1024–1031. [DOI] [PubMed] [Google Scholar]
- 28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 29. Scantlebury DC, Hayes SN, Garovic VD. Pre‐eclampsia and maternal placental syndromes: an indicator or cause of long‐term cardiovascular disease? Heart. 2012;98:1109–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, Klein R, Klein BB, Cotch MF, Wang JJ, Mitchell P, Shaw JE, Takamasa K, Sharrett AR, Wong TY. Retinal vascular caliber and the development of hypertension: a meta‐analysis of individual participant data. J Hypertens. 2014;32:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armstrong DW, Tse MY, Wong PG, Ventura NM, Meens JA, Johri AM, Matangi MF, Pang SC. Gestational hypertension and the developmental origins of cardiac hypertrophy and diastolic dysfunction. Mol Cell Biochem. 2014;391:201–209. [DOI] [PubMed] [Google Scholar]
- 33. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, Kharbanda R, Alp N, Kelly B, Leeson P. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. [DOI] [PubMed] [Google Scholar]
- 35. Hussain SM, Kahonen M, Raitakari OT, Skilton MR, Witt N, Chaturvedi N, Hutri‐Kahonen N, Lehtimaki T, Vaahtoranta‐Lehtonen H, Juonala M, Wijetunge S, Hughes AD, McG Thom SA, Metha A, Tapp RJ. Impact of fetal growth and preterm birth on the retinal microvasculature in mid‐adulthood. Microcirculation. 2015;22:285–293. [DOI] [PubMed] [Google Scholar]
- 36. Julian CG, Pedersen BS, Salmon CS, Yang IV, Gonzales M, Vargas E, Moore LG, Schwartz DA. Unique DNA methylation patterns in offspring of hypertensive pregnancy. Clin Transl Sci. 2015;8:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Juonala M, Viikari JS, Raitakari OT. Main findings from the prospective Cardiovascular Risk in Young Finns Study. Curr Opin Lipidol. 2013;24:57–64. [DOI] [PubMed] [Google Scholar]


