Abstract
Background
It has never been possible to immediately evaluate heart rate variability (HRV) during exercise. We aimed to visualize the real‐time changes in the power spectrum of HRV during exercise and to investigate its relationship to the ventilatory threshold (VT).
Methods and Results
Thirty healthy subjects (29.1±5.7 years of age) and 35 consecutive patients (59.0±13.2 years of age) with myocardial infarctions underwent cardiopulmonary exercise tests with an RAMP protocol ergometer. The HRV was continuously assessed with power spectral analyses using the maximum entropy method and projected on a screen without delay. During exercise, a significant decrease in the high frequency (HF) was followed by a drastic shift in the power spectrum of the HRV with a periodic augmentation in the low frequency/HF (L/H) and steady low HF. When the HRV threshold (HRVT) was defined as conversion from a predominant high frequency (HF) to a predominant low frequency/HF (L/H), the VO 2 at the HRVT (HRVT‐VO 2) was substantially correlated with the VO 2 at the lactate threshold and VT) in the healthy subjects (r=0.853 and 0.921, respectively). The mean difference between each threshold (0.65 mL/kg per minute for lactate threshold and HRVT, 0.53 mL/kg per minute for VT and HRVT) was nonsignificant (P>0.05). Furthermore, the HRVT‐VO 2 was also correlated with the VT‐VO 2 in these myocardial infarction patients (r=0.867), and the mean difference was −0.72 mL/kg per minute and was nonsignificant (P>0.05).
Conclusions
A HRV analysis with our method enabled real‐time visualization of the changes in the power spectrum during exercise. This can provide additional information for detecting the VT.
Keywords: cardiopulmonary exercise testing, heart rate variability, myocardial infarction, real‐time visualization, ventilatory threshold
Subject Categories: Exercise Testing, Myocardial Infarction, Imaging, Diagnostic Testing
Clinical Perspective
What Is New?
A high‐frequency component (HF), low‐frequency component (LF), and ratio of the low‐frequency component and high‐frequency component (L/H) during incremental exercise were instantaneously visualized by a power spectral analysis of heart rate variability (HRV) using the maximum entropy method.
Furthermore, the conversion from a predominant HF to a predominant L/H, which was defined as the HRV threshold, was strongly correlated with the ventilatory threshold in a subset of patients with myocardial infarction as well as healthy subjects.
What Are the Clinical Implications?
By evaluation of the HRV during exercise, optimal exercise intensity can be determined without an expensive analyzer and expertise.
Real‐time assessment of HRV with ECG through a wireless data transfer system can offer a rigorous aerobic exercise in accordance with the day‐to‐day physical conditions of patients.
Exercise is of great importance in the prevention of cardiovascular diseases as well as appropriate nutrition and sleep. Previous reports showed that aerobic physical activity had a great effect on primary and secondary prevention of cardiovascular diseases.1, 2, 3 Although a cardiopulmonary test (CPX) is the only way to determine the ventilatory threshold (VT) noninvasively in clinical practice, assessment of the VT requires an expensive analyzer and expertise. Furthermore, it is incidentally difficult to confirm the VT because of the inconsistency among the several factors required for detecting the VT.4 An alternative method is needed to easily and precisely detect the VT without expensive analyzers and expertise.
Heart rate variability (HRV) is widely used to noninvasively evaluate cardiac autonomic nervous activity, and is associated with mortality and morbidity in patients with myocardial infarctions (MI) and left ventricular dysfunction.5, 6, 7 Power spectral analysis of the HRV mainly describes the high‐frequency component (HF; 0.15–0.4 Hz) and low‐frequency component (LF; 0.04–0.15 Hz). The HF reflects the cardiac parasympathetic nervous tone.8, 9 To date, although the power spectral analysis of the HRV using conventional methods such as the fast Fourier Transform method (FFT) or autoregressive method can evaluate the HF and LF in a steady state, it is difficult to assess it during high‐intensity exercise, where heart rates drastically change. A power spectral analysis of the HRV using the maximum entropy method (MEM) enables elucidation of the changes in the HF and LF from short time‐series data of the RR intervals.10, 11 However, real‐time changes in the power spectrum of the HRV during exercise are understudied.
This study aimed to investigate whether the power spectral analysis of the HRV every heartbeat using the MEM combined with recordings of the RR intervals at 1000 Hz could enable the visualization of the HF, LF, and the ratio of the LF to HF (L/H) during incremental exercise. In addition, we elucidated the relationship between the HRV and both the VT and VT threshold (lactate threshold [LT]) in healthy subjects and patients with MIs.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Sample and Ethical Approval
A total of 30 healthy volunteers were recruited by online public subscription, and 35 consecutive patients with MIs who underwent a CPX from February 2014 to December 2015 in the Keio University Hospital were enrolled in the present study. The healthy volunteers had a broad spectrum of aerobic capacities and fitness levels, but none were athletes. They had no comorbidities, such as hypertension, diabetes mellitus, or active lung diseases. The exclusion criteria of the MI patients included frequent ectopic beats, atrial fibrillation, 2‐ or 3‐degree‐conduction block, pulmonary hypertension, specific cardiomyopathies other than an ischemic pathogenesis, more than moderate primary valvular heart disease, <3 months after an acute MI, and respiratory quotient (RQ) <1.05 in the symptom‐limited peak exercise. Despite that the respiratory quotient values of each healthy subject were >1.10, 6 (17%) of the patients with MIs had an RQ of <1.10 because of fatigue or skeletal‐muscular complaints. The study protocol was approved by the Institutional Review Board of Keio University School of Medicine (permission numbers: 2014023, 20150319), and was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent.
Experimental Procedure
The CPXs were performed with the RAMP protocol ergometer in both healthy volunteers and patients with MIs, simultaneously visualizing the changes in the power spectrum of the HRV on another personal computer. In healthy subjects who provided written informed consent, the blood lactate concentrations were measured during the CPX. The VT, LT, and HRV threshold (HRVT) were assessed in our laboratory just after the CPX to investigate the relationship among them.
Exercise Testing Protocol
All the subjects were examined under standard conditions between 2 and 6 pm. CPX was performed within 1 to 3 hours after a meal and any caffeinated beverages were restricted 3 hours before exercise. There were no subjects who smoke. On the day of CPX, the subjects avoided heavy physical activity before CPX. The height and body weight were measured using a stadiometer (TANITA Corporation, Japan) upon arrival at the laboratory. An incremental cycle exercise was carried out in a quiet room maintained at a constant temperature (22–24°C). The subjects performed the test in the upright position on an electronically braked ergometer (Strength Ergo 8; Mitsubishi Electric Engineering Company, Japan). At first, the subjects rested for 2 minutes on the ergometer until the heart rate (HR) and respiratory condition slowed down. Following a 2‐minute rest, the subjects performed a 2‐minute warm‐up pedaling at 0 W, and then exercised with a progressive intensity until the subjects could no longer maintain the pedaling rate (volitional exhaustion). Every 1 minute, the intensity was increased by 20‐W increments for healthy men, and 15‐W increments for healthy women and MI patients. The pedaling frequency was set at 60 rev/min. The incremental exercise testing time ranged from 10 to 20 minutes, depending on the exercise capacities of each subject. After the exercise tests were terminated, the subjects were instructed to stop pedaling and to stay on the ergometer for 3 minutes (recovery phase). No attempt was made to control the breathing frequency during or after the test. A 12‐lead ECG continuously recorded the HR. The blood pressure was measured every minute with an indirect automatic manometer.
Respiratory Gas Analysis
The expired gas flows were measured using a breath‐by‐breath automated system (Vmax; Nihon Koden, Tokyo, Japan). The respiratory gas exchange, including the ventilation, oxygen uptake (VO2), and carbon dioxide production, were monitored continuously and measured using a 10‐second average. This system was subjected to a 3‐way calibration process, involving a flow volume sensor, gas analyzer, and delay time calibration.
The peak VO2 was calculated as the average oxygen consumption during the last 30 seconds of exercise. The ventilation/carbon dioxide (ventilator efficiency) slope (ventilation–carbon dioxide production slope) was based on data from the onset of exercise to the respiratory compensation point, and it was obtained by a linear regression analysis of the data acquired throughout the entire period of exercise.12, 13 The resting HR was defined as the average of the HRs in the sitting position for 2 minutes before the exercise.
HRV Measurement
The ECG data were stored with a sampling rate of 1000 Hz by an LRR‐03 (Crosswell, Yokohama, Japan). A Reflex Meijin (Crosswell, Yokohama, Japan) was used to automatically measure the RR intervals (beat‐to‐beat fluctuations in the HR) of the subjects at 1000 Hz during the CPX. The data of the RR intervals were instantaneously stored for real‐time analyses. Based on the data of the RR intervals, the power spectral densities were computed continuously by the MEM analyzing the RR intervals for 30 seconds using the Reflex Meijin. After storing the data of the RR intervals for the first 30 seconds at rest, the power continued to be quantified as an HF and LF component with updating the data every heartbeat. With the continuous analyses of every heartbeat, the power spectrum, such as the HF, LF, and L/H, was projected on the screen without delay during the CPX.
LT Determination
In a total of 21 healthy subjects who provided written informed consent, the blood lactate concentrations were measured during incremental exercise. The blood lactate values were obtained via auricular pricking and squeezing the ear lobe gently to obtain a capillary blood sample every 2 minutes during the CPX. The samples were analyzed immediately for the whole blood lactate concentration (mmol/L) using a standard enzymatic method on a lactate analyzer (Lactate Pro; ARKRAY, Japan).
The LT was determined through graphical plots. A visual interpretation was independently made of each subject by 2 experienced researchers to locate the first rise from baseline. If the independent determinations of the stage at LT differed between the 2 researchers, a third researcher adjudicated the difference by independently determining VT. The 3 researchers then jointly agreed on the LT point.
VT Determination
The VT was determined classically through the procedures described by Gaskill et al (ie, the ventilatory equivalent, excess carbon dioxide, and modified V‐slope methods).4 First, 2 of 3 experienced researchers independently and randomly evaluated the VT of each subject through the 3 methods. The researchers used all 3 methods to assess concurrent breakpoint and to eliminate false breakpoint. Second, if the VO2 values determined by the independent researchers were within 3%, then the VO2 values for the 2 investigators were averaged. Third, if the VO2 values determined by the independent evaluators were not within 3% of one another, a third researcher then independently determined VO2. The third VO2 value was then compared with those of the initial investigators. If the adjudicated VO2 value was within 3% of either of the initial investigators, then 2 VO2 values were averaged.
HRVT Determination
The HRVT was identified as follows: coefficient of component variance (CCV) L/H elevated to a value of >0.1, following a decrease to a value of <5 ms2 in the HF continuously for 60 seconds. The CCV L/H was defined as an index in which the L/H was divided by the square root of the RR interval.14 The CCV L/H was used to minimize any considerable variation in the L/H because of the heart rate.
We validated the relationships between each VO2 at the LT, VT, and several HRVTs (HRVT and HRVT‐1, 2, 3, and 4) to establish which HRVT was the best threshold for clinically predicting the VT or LT. Each HRVT was defined as follows. HRVT‐1 was defined as an index in which the CCV L/H elevated to a value of >0.1, following by a decrease to a value of <5 in the HF continuously for 8 heartbeats. HRVT‐2 was defined as an index in which the CCV L/H elevated to a value of >0.35, following by a decrease to a value of <5 in the HF continuously for 8 heartbeats. HRVT‐3 was defined as an index in which the CCV L/H elevated to a value of >0.35, following by a decrease to a value of <10 in the HF continuously for 8 heartbeats. HRVT‐4 was defined as an index in which the CCV L/H elevated to a value of >0.35, following by a decrease to a value of <1 in the HF continuously for 8 heartbeats.
Statistical Analyses
The results are represented as the mean±SD or median with an interquartile range for continuous variables and as percentages for categorical variables, as appropriate. Multiple comparisons of the HF, LF, L/H, and coefficient of the variation in the RR intervals involving each period of the incremental exercise were made using a mixed‐effect model with the restricted maximum likelihood analyses. The null hypothesis indicated that the mean difference was equal to zero. The relationships among the studied methods of the LT, VT, and HRVT were investigated by the Pearson correlation coefficient test. In addition, the Bland and Altman technique was applied to verify the similarities among the different methods (LT, VT, and HRVT).15 This comparison was a graphical representation of the difference between the methods and the average of these methods. All probability values were 2‐tailed, and P values of <0.05 were considered to be statistically significant. All statistical analyses were performed with SPSS version 24.0 software (SPSS Inc., Chicago, IL).
Results
Study Subjects
The baseline characteristics of the healthy subjects are summarized in Table 1. The healthy subjects were predominantly male (70%), with an average age of 29.1±5.7 years. Table 2 demonstrates the patient background in the MI patients. The MI patients were predominantly male (80%), with an average age and LVEF of 59.0±13.2 years and 53.3±9.1%, respectively. Thirty‐four (97%) patients were taking β‐blockers.
Table 1.
Subject Background in the Healthy Volunteers (n=30)
| Characteristics | |
|---|---|
| Demographic and anthropometric data | |
| Age, y | 29.1±5.7 |
| Male, n (%) | 21 (70) |
| Height, cm | 169.9±8.6 |
| Weight, kg | 61.9±11.9 |
| BMI, kg/m2 | 21.3±2.5 |
| Rest | Warm‐Up | VT | Peak | |
|---|---|---|---|---|
| Cardiopulmonary test data | ||||
| HR, bpm | 78±10 | 87±11 | 127±14 | 181±9 |
| SBP, mm Hg | 125±14 | 134±14 | 161±26 | 184±23 |
| DBP, mm Hg | 77±13 | 73±13 | 76±14 | 82±16 |
| BR, /min | 17±4 | 22±3 | 26±4 | 46±10 |
| VO2, mL/kg per minute | 4.7±0.7 | 8.1±1.5 | 23.3±5.3 | 41.7±8.1 |
| WR, watt | ··· | 0 | 101±39 | 201±62 |
| RQ | ··· | ··· | 0.94±0.05 | 1.25±0.08 |
| VE‐VCO2 slope | 23.9±2.4 | |||
The values are the mean±SD or numbers (percentages). BMI indicates body mass index; BR, breathing rate; DBP, diastolic blood pressure; HR, heart rate; RQ, respiratory quotient; SBP, systolic blood pressure; VE‐VCO2, ventilation–carbon dioxide production; VO2, oxygen uptake; VT, ventilatory threshold; WR, work rate.
Table 2.
Subject Background of the Patients With MIs (n=35)
| Characteristics | |||
|---|---|---|---|
| Demographic and anthropometric data | Medication | ||
| Age, y | 59.0±13.2 | β‐Blocker, n (%) | 34 (97) |
| Male, n (%) | 28 (80) | ACEI or ARB, n (%) | 20 (57) |
| Height, cm | 165.1±7.2 | Antiplatelet drug | 30 (100) |
| Weight, kg | 67.6±9.7 | Statin, n (%) | 32 (91) |
| BMI, kg/m2 | 24.7±2.7 | Laboratory data | |
| Hypertension, n (%) | 16 (46) | eGFR, mL/min per 1.73 m2 | 64.0±16.0 |
| Diabetes mellitus, n (%) | 9 (26) | HbA1c, % | 6.0±1.0 |
| Dyslipidemia, n (%) | 24 (69) | BNP, pg/mLa | 32.7 (15.1–61.2) |
| Echocardiography data | |||
| LVEF, % | 53.3±9.1 | ||
| Rest | Warm‐Up | VT | Peak | |
|---|---|---|---|---|
| Cardiopulmonary test data | ||||
| HR, bpm | 74±11 | 79±12 | 103±14 | 134±19 |
| SBP, mm Hg | 122±22 | 135±21 | 155±22 | 176±28 |
| DBP, mm Hg | 74±11 | 78±11 | 83±10 | 90±14 |
| BR, /min | 16±3 | 22±4 | 25±4 | 38±7 |
| VO2, mL/kg per minute | 3.7±0.7 | 7.1±1.4 | 15.8±4.1 | 23.7±7.4 |
| WR, watt | ··· | 0 | 66±20 | 114±34 |
| RQ | ··· | ··· | 0.95±0.05 | 1.19±0.08 |
| VE‐VCO2 slope | 27.2±4.4 | |||
The values are the mean±SD, or numbers (percentages). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; BR, breathing rate; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HR, heart rate; LVEF, left ventricular ejection fraction; MIs, myocardial infarctions; RQ, respiratory quotient; SBP, systolic blood pressure; VE‐VCO2, ventilation–carbon dioxide production; VO2, oxygen uptake; VT, ventilatory threshold; WR, work rate.
The BNP is expressed as the median and interquartile range.
Real‐Time Visualization of the HRV
The power spectrum of the HRV during the CPX, such as the HF, LF, and L/H, was completely visualized in both the healthy subjects and MI patients. Figure 1B and Video S1 show the power spectrum density during exercise. The HF (yellow area in Video S1) decreased drastically with a peak shift linked with the breathing rate (Table 1) to a high frequency after starting the exercise and disappeared after the VT. Based on these raw data, the changes in the HF, LF, L/H, and HR during the CPX in healthy men were projected on the screen in real time (Figure 1A and Video S2). After starting the exercise, a continuous increase in the HR and decrease in the HF were observed without a change in the L/H. Then a drastic shift in the power spectrum of the HRV occurred with a periodic augmentation in the L/H and steady low HF until the recovery phase. Similar trends were observed in all healthy subjects (Figure 2) and the HF significantly decreased after the VT, compared with that before and the L/H became significantly elevated (Table 3). We identified the conversion from the dominant HF to the dominant L/H as the HRVT, which was easily detected by our method in all healthy subjects.
Figure 1.
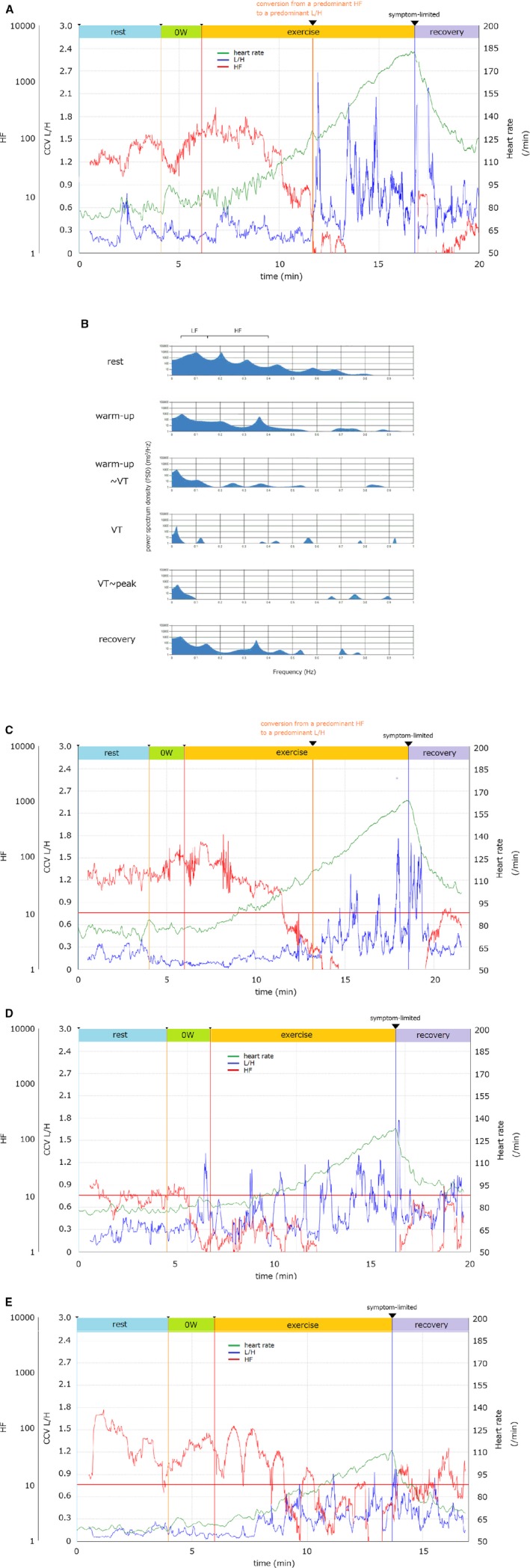
Quantitative imaging of the power spectrum of heart rate variability during incremental exercise. A, C, D, and E, Representative graphs of the high‐frequency component (HF; red), coefficient of component variance ratio of the low‐frequency component to the HF (CCV L/H; blue) and heart rate (HR; green) with an RAMP (15 W/min) protocol ergometer are shown in the healthy volunteer (A) and patients with myocardial infarctions ([C]; a normal value of the HF, [D] a very low value of the HF, [E] a vacillated pattern of the HF). A power spectral analysis of RR intervals measured at 1000 Hz, quantified the power spectral densities of the HF and LF using the maximum entropy method. With updating the data every heartbeat, the HF and LF were visualized as these graphs without a delay during the CPX. B, The power spectrum density (PSD) in a healthy volunteer at every particular point during exercise: rest, warm‐up, warm‐up—ventilator threshold (VT), VT, VT—peak, recovery. The vertical and horizontal lines show the power spectrum density and frequency, respectively. CCV indicates coefficient of component variance; CPX, cardiopulmonary test; HF, high‐frequency component; L/H, the ratio of the LF to HF; 0W, warm‐up; VT, ventilatory threshold.
Figure 2.
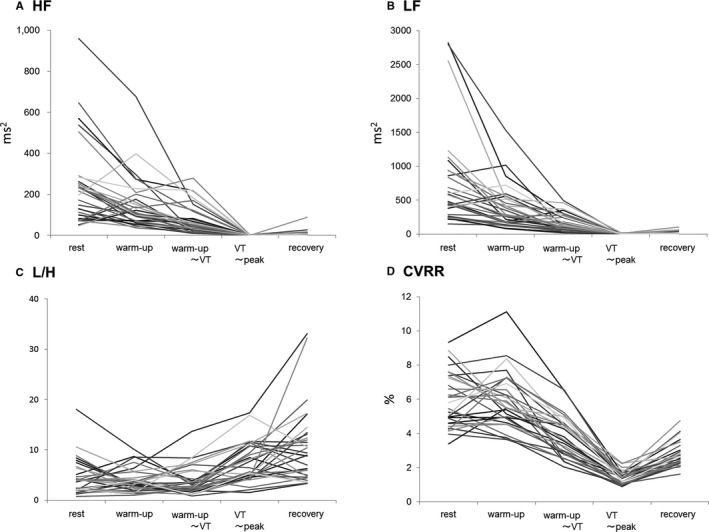
Changes in the power spectrum of the heart rate variability during incremental exercise. Individual patient data during the cardiopulmonary test for (A) the high‐frequency component (HF), (B) low‐frequency component (LF), (C) ratio of the LF and HF (L/H), and (D) coefficient of variation of the RR intervals (CVRR). VT indicates ventilatory threshold.
Table 3.
Changes in Each Variable of the HRV Analysis Among Healthy Subjects (n=30)
| Rest | Warm‐Up | Warm‐Up—VT | VT—Peak | Recovery | P Value | |
|---|---|---|---|---|---|---|
| HF, ms2 | 235.5±171.6 | 162.1±159.4 | 93.5±119.5 | 0.9±0.8 | 6.9±16.6 | <0.001 |
| LF, ms2 | 739.2±649.0 | 445.4±535.9 | 170.5±130.6 | 4.2±4.3 | 18.3±24.6 | <0.001 |
| L/H | 5.2±4.7 | 4.1±2.9 | 4.0±2.9 | 7.4±3.8 | 10.4±7.8 | <0.001 |
| CVRR, % | 6.0±1.6 | 5.9±1.7 | 3.9±1.2 | 1.4±0.4 | 2.7±0.7 | <0.001 |
The data were compared using a mixed‐effect model. CVRR indicates coefficient of variation of RR intervals; HF, high‐frequency component; HRV, heart rate variability; L/H, ratio of LF and HF; LF, low‐frequency component; VT, ventilatory threshold.
In MI patients, the changes in the HF, L/H, and HR were similar to that in the healthy subjects (Figure 1C), and the HF had also shown a significant decrease after the VT, compared with that before with a significant increase in the L/H (Table 4). However, some cases had a very low HF at rest with a periodic augmentation in the L/H and steady low HF just after starting the exercise until the recovery state (Figure 1D), and a vacillated pattern of the HF during the exercise period (Figure 1E).
Table 4.
Change in Each Variable of the HRV Analysis Among MI Subjects (n=35)
| Rest | Warm‐Up | Warm‐Up—VT | VT—Peak | Recovery | P Value | |
|---|---|---|---|---|---|---|
| HF, ms2 | 60.3±57.6 | 54.5±62.1 | 28.7±32.7 | 3.3±6.9 | 23.1±37.1 | <0.001 |
| LF, ms2 | 115.9±145.1 | 105.4±134.8 | 47.3±66.9 | 7.6±10.8 | 45.3±85.2 | <0.001 |
| L/H | 3.8±4.6 | 4.5±5.1 | 5.0±4.8 | 6.1±3.6 | 6.8±4.5 | 0.004 |
| CVRR, % | 2.5±0.9 | 2.9±1.0 | 2.1±0.7 | 1.7±0.4 | 2.9±0.9 | <0.001 |
The data were compared using a mixed‐effect model. CVRR indicates coefficient of variation of RR intervals; HF, high‐frequency component; HRV, heart rate variability; L/H, ratio of LF and HF; LF, low‐frequency component; MI, myocardial infarction; VT, ventilator threshold.
Relationship Between the LT, VT, and HRVT
First, a total of 30 healthy subjects were evaluated to investigate the relationship between the HRVT and VT or LT. The relationship between the LT, VT, and HRVT or the other HRVTs (HRVT‐1, 2, 3, 4) showed that the HRVT was the best threshold for clinically predicting the VT (Table 5). The relationship between the LT, VT, and HRVT is shown in Figure 3, which describes the strong relationships between each threshold (r=0.907 for LT and VT; r=0.853 for LT and HRVT; and r=0.921 for VT and HRVT; for all P<0.001) in the healthy subjects (Figure 3A, 3C, and 3E). The Bland‐Altman plot revealed that the mean difference between each threshold (−0.26 mL/kg per minute for LT and VT, 0.65 mL/kg per minute for LT and HRVT, 0.53 mL/kg per minute for VT and HRVT) was nonsignificant (P>0.05). These findings clarified that there was no bias between the mean values, which displayed strong agreements between the LT, VT, and HRVT. Further, when the power spectrum density in the HF was defined as 0.15 to 1.0 Hz, the relationship also exhibited a good correlation between the HRVT and VT (r=0.831; P<0.001), and the mean difference was 2.10 mL/kg per minute (Figure 4) and was nonsignificant (P>0.05).
Table 5.
Relationships Between Each LT, VT, and HRVT in Healthy Subjects (n=30)
| VO2 (mL/kg per min) | Correlation Coefficient | ||
|---|---|---|---|
| LT | VT | ||
| LTa | 23.7±3.7 | ··· | ··· |
| VT | 23.3±5.3 | 0.907b | ··· |
| HRVT: HF <5, CCV L/H >0.1, for 60 s | 23.3±5.7 | 0.853b | 0.921b |
| HRVT‐1: HF <5, CCV L/H >0.1 | 22.4±5.1 | 0.801b | 0.868b |
| HRVT‐2: HF <5, CCV L/H >0.35 | 21.9±5.5 | 0.612b | 0.729b |
| HRVT‐3: HF <10, CCV L/H >0.35 | 21.0±5.9 | 0.617b | 0.735b |
| HRVT‐4: HF <1, CCV L/H >0.35 | 26.9±6.6 | 0.601b | 0.722b |
Each HRVT was defined as follows. HRVT: CCV L/H elevated to a value of >0.1, following by a decrease to a value of <5 ms2 in the HF continuously for 60 s; HRVT‐1: CCV L/H elevated to a value of >0.1, following by a decrease to a value of <5 ms2 in the HF continuously for 8 heartbeats; HRVT‐2: CCV L/H elevated to a value of >0.35, following by a decrease to a value of <5 ms2 in the HF continuously for 8 heartbeats; HRVT‐3: CCV L/H elevated to a value of >0.35, following by a decrease to a value of <10 ms2 in the HF continuously for 8 heartbeats; HRVT‐4: CCV L/H elevated to a value of >0.35, following by a decrease to a value of <1 ms2 in the HF continuously for 8 heartbeats. CCV indicates coefficient of component variance; HF, high‐frequency component; HRVT, heart rate variability threshold; L/H, the ratio of low‐frequency component to high‐frequency component; LT, lactate threshold; VO2, oxygen uptake; VT, ventilatory threshold.
The data of LT were available in 21 healthy volunteers.
Significant correlation P<0.001.
Figure 3.
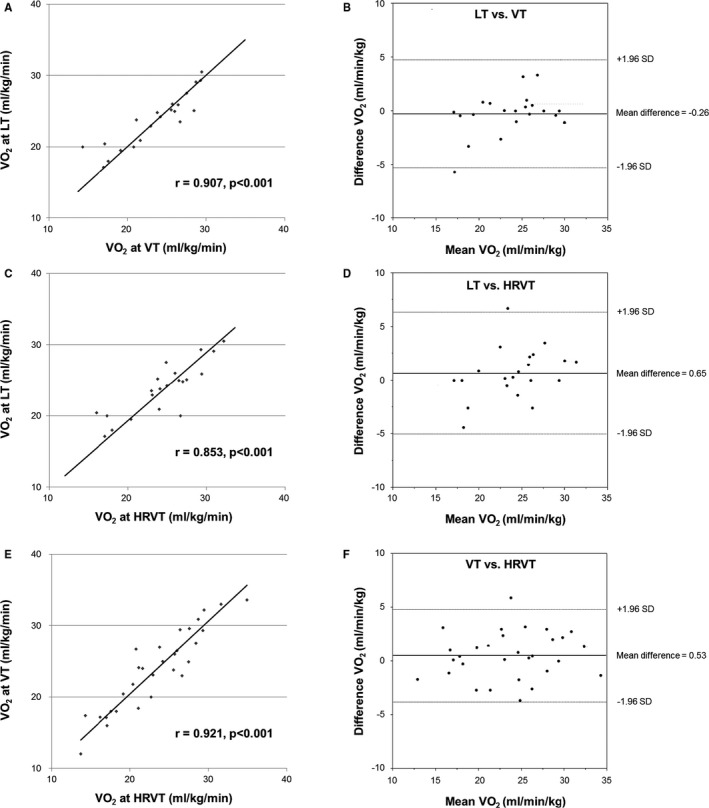
Validity testing of the oxygen uptake (VO 2) at the HRVT, LT, and VT in healthy subjects (n=30). The graphs in the left panel show the relationship between each VO 2 at the heart rate variability threshold (HRVT), ventilator threshold (VT), and lactate threshold (LT) (A, C, and E). The graphs in the right panel show the Bland‐Altman plots (B, D, and F), which indicate the respective differences between each VO 2 at the HRVT, LT, and VT (y‐axis) for each individual against the mean of the VO 2 at the HRVT, LT, and VT (x‐axis). The dark lines in each Bland‐Altman plot represent a ±1.96 SD.
Figure 4.
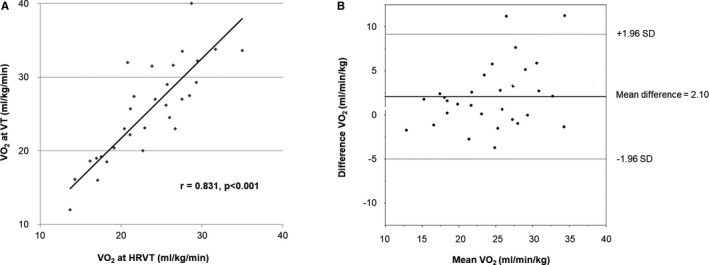
Validity testing of the VO 2 at the HRVT and VT; HF 0.15 to 1.0 Hz (n=30). The graphs in the left panel show the relationship between each VO 2 at the heart rate variability threshold (HRVT) and ventilator threshold (VT) when the HF was defined as 0.15 to 0.4 Hz (A). The graphs in the right panel show the Bland‐Altman plots (B), which indicate the respective differences between each VO 2 at the HRVT and VT (y‐axis) for each individual against the mean of the VO 2 at the HRVT and VT (x‐axis). The dark lines in each Bland‐Altman plot represent a ±1.96 SD. AT indicates ; HF, high‐frequency component; VO 2, oxygen uptake.
Secondly, among the MI patients, the HRVT was not able to be clarified automatically in only 3 patients because they had a vacillated pattern of the HF during the exercise period (Figure 1E). In the remaining MI patients, the relationship between the VT and HRVT was also assessed. Similarly, a good correlation was observed between the VT and HRVT (r=0.867) (Figure 5A). The Bland‐Altman plot described a strong agreement in the MI patients (Figure 5B).
Figure 5.
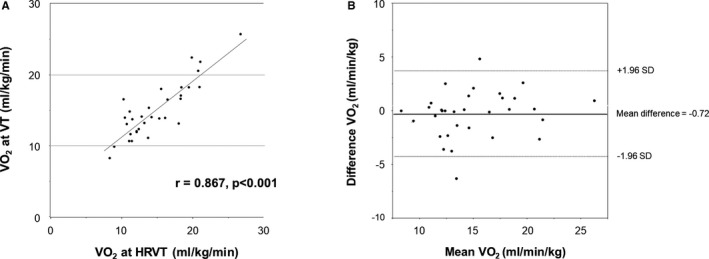
Validity testing of the VO 2 at the HRVT, LT, and VT in patients with MI (n=32). The graphs in the left panel show the relationship between each VO 2 at the heart rate variability threshold (HRVT) and ventilator threshold (VT) (A). The graphs in the right panel show the Bland‐Altman plots (B), which indicate the respective differences between each VO 2 at the HRVT and VT (y‐axis) for each individual against the mean of the VO 2 at the HRVT and VT (x‐axis). The dark lines in each Bland‐Altman plot represent a ±1.96 SD. LT indicates lactate threshold; MI, myocardial infarction; VO 2, oxygen uptake.
Discussion
In the present study, we demonstrated that the HF, LF, and L/H during incremental exercise were instantaneously visualized by a power spectral analysis of the HRV using the MEM. Furthermore, the conversion from a predominant HF to a predominant L/H, which was defined as the HRVT, was strongly correlated with both the LT and VT in a subset of MI patients as well as healthy subjects. The real‐time HRV assessment could be applicable for the detection of the VT.
Although many reports have examined the HRV during exercise by using a power spectral analysis,16, 17, 18, 19, 20 they have described that an HRV analysis by conventional methods (eg, fast Fourier Transform and autoregressive method) was not possible to estimate the precise values of the HF, LF, and total power at higher intensities (above the VT) because of the short RR intervals and decrease in the HRV total power.16, 21, 22, 23 Those techniques have some weaknesses and limitations, which need stationary data and cannot be applied to the duration of drastic changes in the HR during exercise. Furthermore, the fast Fourier Transform has a poor resolution because of the effect of the window shape and width of the spectrum. The fast Fourier Transform is insufficient for estimating the precise power spectral density from short time‐series data. The autoregressive method presents a highly smoothed spectrum because of the small value of the lag, which is considered to be possibly misleading.
On the other hand, the MEM does not depend on stationary data.24, 25 It enables the assessment of the HRV based on an analysis of short‐term RR intervals.10, 24 As the lower threshold of the LF component on the HRV analysis using the MEM is 0.04 Hz, the time length of the shortest time‐series data required for the assessment of the LF component is ≈25 seconds. We developed a new system continuously updating the 30‐second‐series data of the RR intervals every heartbeat to acquire the dynamic change in the power spectrum density. Further, automatic recordings and measurements of the RR intervals at 1000 Hz enabled an analysis of a precise power spectrum density. Taken together, the development of the data collection, measurement, and analysis enabled the real‐time assessment of a low HF of <1 and L/H even during high‐intensity exercise.
Although several studies have demonstrated that an assessment of the HRV was related to the VT,26, 27, 28 this is the first study to clarify that continuous monitoring of the HRV aided in clinically detecting the VT without delay during CPX. In this study, we compared several indexes of the conversion from the dominant HF to the dominant L/H. No HF re‐elevations to a value of >5 continuing for 60 seconds were added to the definition of the HRVT to predict the VT more precisely because the HF fluctuated around the VT level in certain cases. It is often difficult to determine the VT mainly because of the inconsistency among the several factors required for detecting the VT, such as the ventilation/VO2 or carbon dioxide production/VO2 slope, and oscillations in the minute ventilation. In such cases, the HRVT could help detect the VT. Furthermore, as the HRVT can predict the VT with only ECG monitoring, optimal intensity exercise (in the level of aerobic exercise) can be performed even if they have no expensive analyzer and expertise. Real‐time assessments of the HRV with ECG through a wireless data transfer system can offer a rigorous aerobic exercise in accordance with the day‐to‐day physical conditions of patients. This innovative system might improve the persistence of cardiac rehabilitation in outpatients by expanding this safer rehabilitation program from hospitals to other institutions, such as commercial fitness clubs that are close to patients' homes.
Limitations
This study had some limitations. First, the HRV indicates the variability of the RR interval, not a quantitative index of the determination of the cardiac autonomic nervous activity during exercise. Therefore, we need to cautiously estimate the relationship between the HF and parasympathetic nervous activity during exercise. Secondly, the applicability of the HF (0.15–0.4 Hz) is limited by the requirement of a fixed breathing rate, such as 15 breaths/min.29 It is also known that the amplitude of the HF declines as the breathing rate increases,30 and the HF spectrum peak shifts toward a high frequency when the breathing rate is increased.31 As in the previous studies, the HF decreased drastically with a peak shift to a high frequency after starting the exercise (Figure 1A, yellow area in Video S1). These findings suggested that the HF was underestimated after starting the exercise, particularly in the vicinity of the VT where the breathing rate was around 24/min (Tables 1 and 2). However, the relationship also showed a good correlation between the HRVT and VT when the HF spectrum peak was defined as 0.15 to 1.0 Hz, suggesting that the HF spectrum peak shift may have had little effect on the assessment of the HF during exercise. Thirdly, among the patients with MIs, there were patients with low levels of the HF before and during exercise or a fluctuating HF during exercise. Further, no conversion from a dominant HF to a dominant L/H was recognized automatically in the patients with a fluctuating HF. Several factors that may explain the low or fluctuating HF could potentially exist in patients with MIs: comorbidities (such as hypertension or diabetes mellitus), use and/or dose of β‐blockers, LVEF, and the infarction area (eg, left anterior descending coronary artery, left circumflex coronary artery, and right coronary artery). Further research is warranted to investigate the relevance of the HF to the patient background.
Conclusions
This was the first study to show a real‐time evaluation of the power spectrum of the HRV during the CPX in MI patients as well as healthy subjects. Given the difficult situation of deciding the VT, the power spectrum assessment with an HRV analysis could be helpful for improving the detection of the VT.
Disclosures
None.
Supporting information
Video S1. The power spectrum density (PSD) of the heart rate variability during incremental exercise. The graph in the upper panel shows the RR interval during the CPX. The graph in the lower panel presents the power spectrum density (PSD). The yellow area shows the high‐frequency component (HF). The HF decreased drastically with a peak shift to a high frequency after starting the exercise and disappeared after the VT. The vertical and horizontal lines show the PSD and frequency, respectively.
Video S2. Quantitative movie imaging of the power spectrum of the heart rate variability during incremental exercise. A representative movie of the high‐frequency component (HF; green), coefficient of component variance ratio of the low‐frequency component to the HF (CCV L/H; yellow) and heart rate (HR; red) during a cardiopulmonary test (CPX) with an RAMP (15 W/min) protocol ergometer are shown in the same healthy volunteers as Figure 1. The yellow, red, and blue lines show the start of the exercise, start of the load, and finish of the load, respectively. The power spectral analysis of the RR intervals measured at 1000 Hz quantified the power spectral densities of the HF and LF using the maximum entropy method. With updating the data every heartbeat, the HF and LF were visualized as these graphs without a delay during the CPX.
Acknowledgments
The authors thank Chieko Fujii, Atsushi Abe, C. Yoshida, and K. Takeuchi for their technical assistance. The authors also thank the patients and volunteers for their willing participation in this study. We are grateful to John Martin for his linguistic advice.
(J Am Heart Assoc. 2018;7:e006612 DOI: 10.1161/JAHA.117.006612.)29307865
References
- 1. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. [DOI] [PubMed] [Google Scholar]
- 2. Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP. Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10‐year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol. 2012;60:1521–1528. [DOI] [PubMed] [Google Scholar]
- 4. Gaskill SE, Ruby BC, Walker AJ, Sanchez OA, Serfass RC, Leon AS. Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sports Exerc. 2001;33:1841–1848. [DOI] [PubMed] [Google Scholar]
- 5. La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short‐term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. [DOI] [PubMed] [Google Scholar]
- 6. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 7. Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. [DOI] [PubMed] [Google Scholar]
- 8. Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–H153. [DOI] [PubMed] [Google Scholar]
- 9. Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho‐vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki S, Sumi K, Matsubara M. Cardiac autonomic control immediately after exercise in female distance runners. J Physiol Anthropol. 2008;27:325–332. [DOI] [PubMed] [Google Scholar]
- 11. Kohara K, Igase M, Maguchi M, Fukuoka T, Kitami Y, Hiwada K. Autonomic nervous function in essential hypertension in the elderly. Evaluation by power spectral analysis of heart rate variability. Am J Hypertens. 1996;9:1084–1089. [DOI] [PubMed] [Google Scholar]
- 12. Clark AL, Poole‐Wilson PA, Coats AJ. Relation between ventilation and carbon dioxide production in patients with chronic heart failure. J Am Coll Cardiol. 1992;20:1326–1332. [DOI] [PubMed] [Google Scholar]
- 13. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J. 2000;21:154–161. [DOI] [PubMed] [Google Scholar]
- 14. Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am J Cardiol. 1991;67:199–204. [DOI] [PubMed] [Google Scholar]
- 15. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 16. Pichon AP, de Bisschop C, Roulaud M, Denjean A, Papelier Y. Spectral analysis of heart rate variability during exercise in trained subjects. Med Sci Sports Exerc. 2004;36:1702–1708. [DOI] [PubMed] [Google Scholar]
- 17. Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, Colucci WS. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256:H132–H141. [DOI] [PubMed] [Google Scholar]
- 18. Perini R, Orizio C, Baselli G, Cerutti S, Veicsteinas A. The influence of exercise intensity on the power spectrum of heart rate variability. Eur J Appl Physiol. 1990;61:143–148. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto Y, Hughson RL, Peterson JC. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol (1985). 1991;71:1136–1142. [DOI] [PubMed] [Google Scholar]
- 20. Tulppo MP, Makikallio TH, Seppanen T, Laukkanen RT, Huikuri HV. Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol. 1998;274:H424–H429. [DOI] [PubMed] [Google Scholar]
- 21. Warren JH, Jaffe RS, Wraa CE, Stebbins CL. Effect of autonomic blockade on power spectrum of heart rate variability during exercise. Am J Physiol. 1997;273:R495–R502. [DOI] [PubMed] [Google Scholar]
- 22. Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol. 2003;90:317–325. [DOI] [PubMed] [Google Scholar]
- 23. Tulppo MP, Makikallio TH, Takala TE, Seppanen T, Huikuri HV. Quantitative beat‐to‐beat analysis of heart rate dynamics during exercise. Am J Physiol. 1996;271:H244–H252. [DOI] [PubMed] [Google Scholar]
- 24. Sumi K, Suzuki S, Matsubara M, Ando Y, Kobayashi F. Heart rate variability during high‐intensity field exercise in female distance runners. Scand J Med Sci Sports. 2006;16:314–320. [DOI] [PubMed] [Google Scholar]
- 25. Macor F, Fagard R, Amery A. Power spectral analysis of RR interval and blood pressure short‐term variability at rest and during dynamic exercise: comparison between cyclists and controls. Int J Sports Med. 1996;17:175–181. [DOI] [PubMed] [Google Scholar]
- 26. Sales MM, Campbell CS, Morais PK, Ernesto C, Soares‐Caldeira LF, Russo P, Motta DF, Moreira SR, Nakamura FY, Simoes HG. Noninvasive method to estimate anaerobic threshold in individuals with type 2 diabetes. Diabetol Metab Syndr. 2011;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karapetian GK, Engels HJ, Gretebeck RJ. Use of heart rate variability to estimate LT and VT. Int J Sports Med. 2008;29:652–657. [DOI] [PubMed] [Google Scholar]
- 28. Cottin F, Lepretre PM, Lopes P, Papelier Y, Medigue C, Billat V. Assessment of ventilatory thresholds from heart rate variability in well‐trained subjects during cycling. Int J Sports Med. 2006;27:959–967. [DOI] [PubMed] [Google Scholar]
- 29. Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975;39:801–805. [DOI] [PubMed] [Google Scholar]
- 30. Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R‐R interval power spectra is largely ignored. J Appl Physiol (1985). 1993;75:2310–2317. [DOI] [PubMed] [Google Scholar]
- 31. Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, Coffeng R, Scheinin H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol. 2001;21:365–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. The power spectrum density (PSD) of the heart rate variability during incremental exercise. The graph in the upper panel shows the RR interval during the CPX. The graph in the lower panel presents the power spectrum density (PSD). The yellow area shows the high‐frequency component (HF). The HF decreased drastically with a peak shift to a high frequency after starting the exercise and disappeared after the VT. The vertical and horizontal lines show the PSD and frequency, respectively.
Video S2. Quantitative movie imaging of the power spectrum of the heart rate variability during incremental exercise. A representative movie of the high‐frequency component (HF; green), coefficient of component variance ratio of the low‐frequency component to the HF (CCV L/H; yellow) and heart rate (HR; red) during a cardiopulmonary test (CPX) with an RAMP (15 W/min) protocol ergometer are shown in the same healthy volunteers as Figure 1. The yellow, red, and blue lines show the start of the exercise, start of the load, and finish of the load, respectively. The power spectral analysis of the RR intervals measured at 1000 Hz quantified the power spectral densities of the HF and LF using the maximum entropy method. With updating the data every heartbeat, the HF and LF were visualized as these graphs without a delay during the CPX.


