Abstract
Background
The knowledge of the impact of cardiovascular risk factors at different ages has mainly been based on different studies performed at different ages. This study aimed to investigate the change in impact of traditional cardiovascular risk factors over the aging process in subjects followed for 4 decades.
Methods and Results
In the ULSAM (Uppsala Longitudinal Study of Adult Men) study, 2322 men originally investigated in 1970 to 1974 have been followed regarding cardiovascular diseases until the end of 2013. This cohort has been investigated physically at ages 50, 60, 70, 77, and 82 years regarding body mass index, low‐density lipoprotein‐ and high‐density lipoprotein‐cholesterol, triglycerides, systolic blood pressure and diastolic blood pressure, fasting glucose, and smoking. These data were used to model the interactions between risk factors and age regarding incident myocardial infarction (n=540), ischemic stroke (n=343), or heart failure (n=397). Significant interactions were observed between age and the set of traditional risk factors regarding all 3 outcomes (P<0.05 for all). Generally, a decline in the rate ratios was seen with aging for most risk factors, being most pronounced for body mass index regarding myocardial infarction and for systolic blood pressure regarding ischemic stroke and heart failure. However, low‐density lipoprotein‐cholesterol was significantly related to incident myocardial infarction, whereas both body mass index and fasting glucose were significantly related to incident heart failure also at a high age.
Conclusions
Using a longitudinal design in middle‐aged men spanning 4 decades showed that the impact of traditional cardiovascular risk factors generally declined with aging. However, some of the risk factors remained significantly associated with incident cardiovascular disease also at old age.
Keywords: blood pressure, cardiovascular disease, lipids and cholesterol, obesity, risk factor
Subject Categories: Cardiovascular Disease, Risk Factors, Lipids and Cholesterol, Blood Pressure, Obesity
Clinical Perspective
What Is New?
The present study is the first to investigate changes in the strengths of classical risk factors for the major cardiovascular diseases myocardial infarction, ischemic stroke, and heart failure over a 40‐year follow‐up in a longitudinal study.
What Are the Clinical Implications?
Whereas the strengths of the risk factors were generally reduced by aging, low‐density lipoprotein‐cholesterol was significantly related to incident myocardial infarction, and both body mass index and fasting glucose were significantly related to incident heart failure also at a high age.
Despite efforts during recent years to identify new risk factors or biomarkers that can predict cardiovascular diseases, no major breakthrough has been made in the clinical setting to beat the traditional risk factors that have been known for decades: blood pressure, diabetes mellitus, low‐density lipoprotein (LDL)‐ and HDL, high‐density lipoprotein (HDL)‐cholesterol, smoking, and obesity. These traditional risk factors thus appear robust, and a deeper understanding of their usefulness is therefore desirable.
One question about their use regards aging. Most studies on the traditional risk factors have been performed in middle‐aged populations, typically followed for 10 years. When calculating risk scores based on the traditional risk factors, like the Europe‐based SCORE, no information is given for elderly subjects.1 Thus, it is evident that information on the usefulness of these traditional risk factors in the elderly is limited.
It is likely that the impact of a risk factor would decline by aging, given that there is a survival bias in the elderly: Many of those with high levels of risk factors at midlife would have experienced an event or died before being included in an investigation of risk factors in the elderly. And, indeed, the impact of a risk factor is usually lower than expected from studies in middle‐aged samples when elderly populations are investigated.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 However, using this approach, it is hard to compare the strength of a risk factor in younger versus elderly subjects.
In order to study the impact of aging on the strengths of risk factors in a longitudinal fashion, we have, in the present study, used a sample of men all aged 50 years at a baseline examination in the early 1970s who has, so far, been followed for 4 decades. We tested the interactions between age and the traditional risk factors regarding incident cases of 3 major cardiovascular diseases: myocardial infarction, ischemic stroke, and heart failure, with the hypothesis that the strength of most, but not all, traditional risk factors would decline by aging. The major question to be answered was which of the risk factors that still retained an important impact in the elderly.
Methods
The data, analytical methods, and study materials will be made available on request to other researchers for purposes of reproducing the results or replicating the procedure.
Study Sample
In 1970 to 1973, all men born in 1920 to 1924 and residing in the county of Uppsala were invited to a health survey (at age 50) aimed at identifying risk factors for cardiovascular disease; 82% of the invited men participated (n=2322). The design and selection criteria for the cohort have been described previously.12, 13 Reinvestigations with physical examinations of the cohort were performed at ages 60 (n=1836), 70 (n=1214), 77 (n=834), and 82 years (n=525).
In the present study, subjects with a history of myocardial infarction (MI), stroke, or heart failure at baseline at age 50 were excluded from further analysis (n=30).
Informed written consent was obtained and the Uppsala University Ethics Committee approved the study.
Baseline Examinations
The examination at age 50 has been described in detail previously.12, 13 Blood samples for fasting concentrations were drawn in the morning after an overnight fast. Cholesterol and triglyceride concentrations in serum and HDL were assayed by enzymatic techniques. LDL‐cholesterol was calculated by Friedewald's formula. Fasting blood glucose was determined by an oxidase method. Supine systolic blood pressure (SBP) and diastolic blood pressure were measured twice in the right arm after 10 minutes of rest, and means were calculated. The physical examinations at the reinvestigations were performed essentially in the same way as at age 50.
End Point Definitions
Date and cause of death were obtained from the Swedish Cause of Death Register. Date and cause of hospitalization were obtained from the Swedish Hospital Discharge Register in all individuals, not only those attending the re‐examinations. We evaluated 3 major cardiovascular diseases; acute MI (International Classification of Diseases [ICD‐9] code 410, ICD‐9 code 310, or ICD‐10 code I20), ischemic stroke (ICD‐8 codes 431, 433‐436, ICD‐9 code 431, 433‐436, ICD‐10 code I63‐I66), or heart failure using data from the Swedish Hospital Discharge Register. Combining data from the Swedish Cause of Death Registry and the Swedish Hospital discharge register is an efficient, validated alternative to revised hospital discharge notes and death certificates for both coronary heart disease and stroke.14 Previous studies suggest that the accuracy of the heart failure diagnosis in the Swedish hospital discharge register has lower validity15 when including all diagnosis positions. Therefore, we performed extensive medical chart review in order to promote the highest quality of the diagnosis of heart failure and to include as many correctly classified heart failure events as possible. In short, as a possible diagnosis of heart failure, we considered ICD heart failure codes 427.00, 427.10, 428.99 (ICD‐8), 428 (ICD‐9), and I50 (ICD‐10) and hypertensive heart disease with heart failure (I11.0 [ICD‐10]) from the Swedish Hospital Discharge Register. The medical records from all relevant hospitalizations for heart failure were reviewed by 1 experienced physician (L.L.), who, blinded to the baseline data, classified the cases as definite, questionable, or miscoded. Only the define cases were used in the following analyses. The classification relied on the definition proposed by the European Society of Cardiology.16
Statistical Analysis
Because the focus of this study was to investigate how the impact of risk factors of cardiovascular disease change over time, we used Poisson models with interactions between risk factors and age to account for the possibly time‐varying effects of risk factors. Although the Cox model, which is the most common method used to analyze time‐to‐event data, can be extended to accommodate time‐varying effects, the Poisson model allows for direct parametric modeling and estimation of the baseline rate which in this study was aging.
Follow‐up time for each individual was further split into 5‐year intervals in which we assumed that the rate was constant. Age was modeled with a 4‐degree of freedom restricted cubic spline with knots placed at the 5th, 35th, 65th, and 95th percentiles of the age distribution conditioned on the occurrence of an event in all models. Continuous risk factors (LDL and HDL cholesterol, SBP and diastolic blood pressure, body mass index [BMI], fasting blood glucose, and serum triglycerides) were modeled using restricted cubic splines with knots placed at the 10th, 50th, and 90th percentiles of each variable's marginal distribution. All these risk factors were included in the models together with current smoking. Interactions were restricted to include linear terms only. Nonlinear terms for the continuous risk factors were either kept or deleted based on a test of all nonlinear terms where the null hypothesis tested was that all regression coefficients corresponding to the nonlinear terms were equal to zero. Nonlinear terms for age were kept in all models. Also included in the models were offsets equal to the natural logarithm of follow‐up time for each individual.
These Poisson models were used to calculate:
Interactions between the total set of risk factors and age regarding the 3 outcomes.
Interactions between each separate risk factor and age regarding the 3 outcomes.
Three‐dimensional (3D) graphs illustrating the interplay between each separate risk factor and age regarding the 3 outcomes.
Two‐dimensional (2D) graphs and the corresponding rate ratios (RRs) for each separate risk factor regarding the 3 outcomes for a 5‐year follow‐up from the physical examinations performed at 50, 60, 70, 77, and 82 years.
Data on LDL‐ and HDL‐cholesterol, as well as triglycerides, were missing in a number of individuals at the examination at 60 years (68%, 88% and 88%, respectively). Those values were imputed using a hierarchical model in which each individual's trajectory over time was modeled. Imputations were then made using predicted values at 60 years from that model.
In a set of secondary analyses, we also included data on antihypertensive treatment, diabetes mellitus treatment, and lipid‐lowering treatment in the models.
We also added a new set of analyses using multiple imputation17 to create and analyze 20 multiply imputed data sets. Incomplete variables were imputed under fully conditional specification.18 Model parameters were estimated with the Poisson described above to each complete data set separately. The estimates and their SEs were then pooled using Rubin's rules.
All analyses were made using R (version 3.2.4; R Foundation for Statistical Computing, Vienna, Austria) and the lme4 and Epi packages.19, 20, 21
Results
Participant characteristics are presented in Table 1.
Table 1.
Risk Factors at Different Examinations
| Age at examination, y | 50 | 60 | 70 | 77 | 82 |
| n | 2322 | 1852 | 1221 | 838 | 526 |
| BMI, kg/m2 | 24.8 (22.9 –26.8) | 25.2 (23.3–27.3) | 26.0 (23.9–28.3) | 26.0 (24.0–28.2) | 26.1 (23.9–27.9) |
| Systolic blood pressure, mm Hg | 130 (120–140) | 140 (130–155) | 145 (133–160) | 150 (138–164) | 142 (131–158) |
| Diastolic blood pressure, mm Hg | 80 (75–90) | 90 (80–95) | 84 (78–90) | 80 (76–88) | 80 (73–88) |
| LDL‐cholesterol, mmol/L | 5.2 (4.4–6.0) | 4.2 (3.6–4.8) | 3.8 (3.3–4.5) | 3.4 (2.9–4.0) | 3.4 (2.8–3.9) |
| HDL‐cholesterol, mmol/L | 1.3 (1.1–1.6) | 1.1 (1.0–1.3) | 1.2 (1.0–1.5) | 1.3 (1.1–1.5) | 1.2 (1.0–1.3) |
| Fasting blood glucose, mmol/L | 5.4 (5.1–5.8) | 5.3 (5.0–5.8) | 5.4 (5.0–5.9) | 5.5 (5.1–6.1) | 5.6 (5.2–6.3) |
| Serum triglycerides, mmol/L | 1.7 (1.3–2.2) | 1.6 (1.2–2.1) | 1.3 (0.9–1.7) | 1.2 (0.9–1.7) | 1.3 (1.0–1.6) |
| Current smoker (n and %) | 1185 (51) | 584 (32) | 245 (21) | 61 (8) | 31 (6) |
| Antihypertensive medication (n and %) | 98 (4) | 351 (19) | 410 (34) | 347 (41) | 277 (53) |
| Diabetes mellitus medication (n and %) | 19 (1) | 42 (2) | 64 (5) | 70 (8) | 46 (9) |
| Lipid‐lowering medication (n and %) | 23 (1) | 126 (7) | 107 (9) | 134 (16) | 101 (19) |
Medians (or n and proportion for smoking) and interquartile ranges are given. BMI indicates body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Myocardial Infarction
Five hundred forty incident cases of MI occurred during a median total follow‐up of 29.6 years (range, 0.04–42.3, corresponding to 61 703 person‐years at risk [PYAR]) giving an incidence rate of 8.75/1000 PYAR.
A significant interaction was noted between age and the set of traditional risk factors regarding incident MI (P=0.0033). When the interactions between the individual risk factors and age regarding incident MI were calculated, only BMI and smoking showed P<0.05 (P=0.020 and P=0.026, respectively; Table S1). Interactions are graphically illustrated by 3D plots in Figure 1 and Figure S1. In those figures, the impact of the risk factors over time on incident MI is illustrated by the area in the 3D plot.
Figure 1.
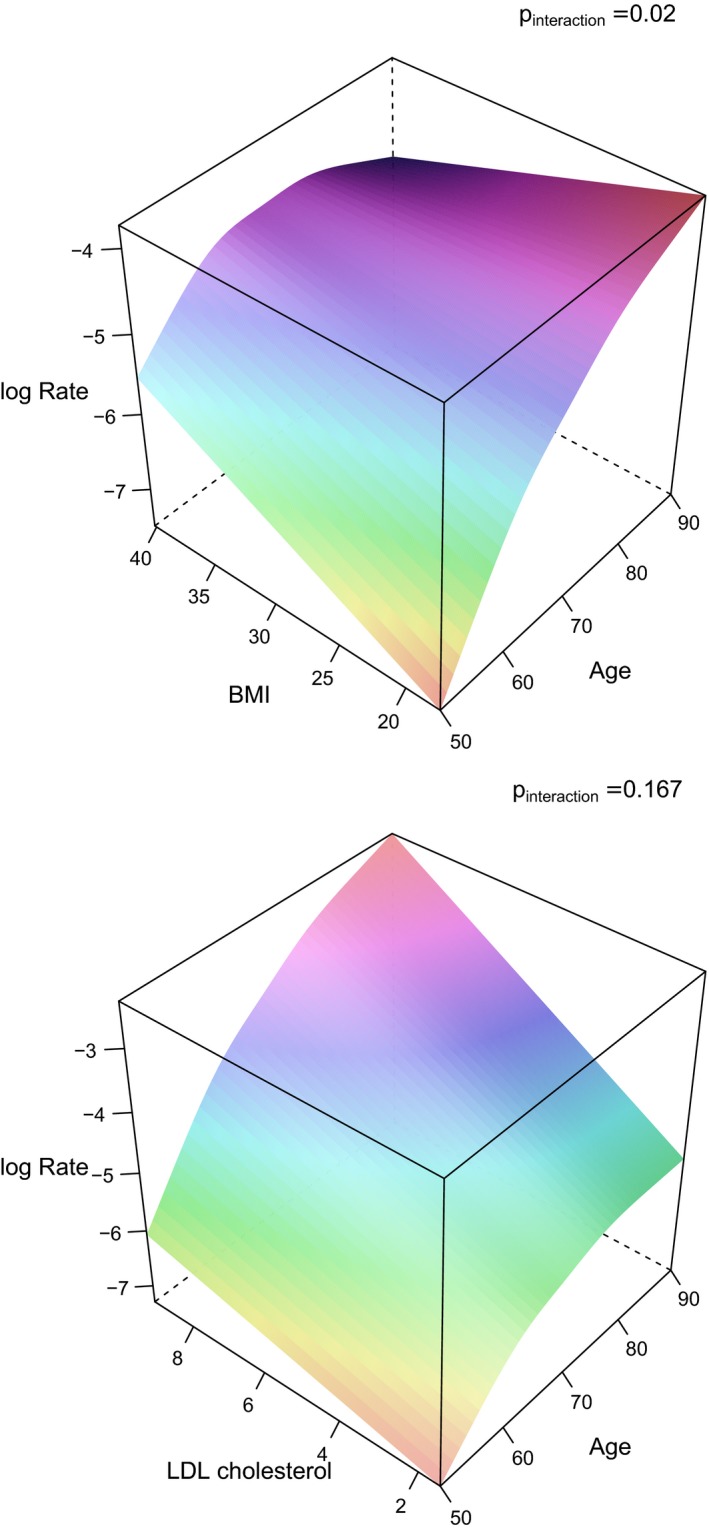
3D graphs illustrating the impact of BMI and age on the incident rate (log rate) of myocardial infarction (upper panel), and in a similar way LDL‐cholesterol (in the lower panel). The P values for the interaction term between those risk factors and age regarding myocardial infarction are given in the graphs. 3D indicates 3‐dimensional; BMI, body mass index; LDL, low‐density lipoprotein.
To interpret these risk factor versus age interactions in a more traditional way, the relationships between the risk factors and MI were calculated at the time of the different examination cycles (50, 60, 70, 77, and 82 years) with a follow‐up of 5 years from each examination cycle. These “2D data” are graphically given in Figure 2.
Figure 2.
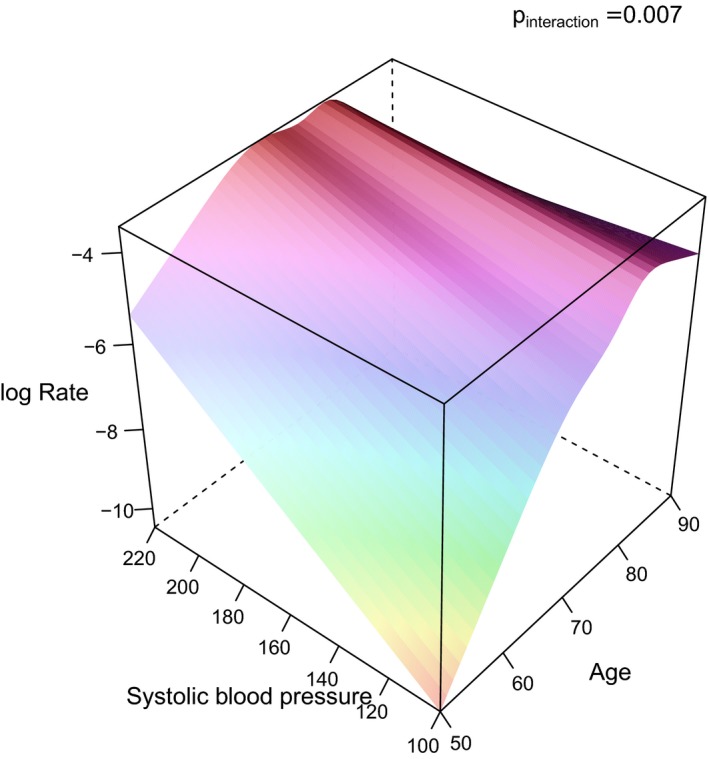
3D graph illustrating the impact of systolic blood pressure and age on the incident rate (log rate) of ischemic stroke. The P values for the interaction term between systolic blood pressure and age regarding ischemic stroke is given in the figure. 3D indicates 3‐dimensional.
When the RRs for the traditional risk factors were calculated from this “2D approach” separately for measurements performed at ages 50, 60, 70, 77, and 82 years, for BMI the RRs declined with increasing age and was only significantly (P<0.05) related to incident MI when measured at ages 50 and 60. RRs also declined by age for SBP and smoking, but in those cases a significant association was found at all examinations up to 77 years. On the contrary, the RRs for LDL‐cholesterol tended to increase with aging and was highly significant also at age 82. RRs for HDL‐cholesterol increased with age and was significant at ages 50, 60, and 70. A similar attenuation of the impact of fasting glucose was observed, being significant at ages 50, 60, 70, and 77. Serum triglycerides were not significant at any examination (details are given in Table 2).
Table 2.
RR, 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident MI Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years)
| Variable | RR | 95% CI Upper | 95% CI Lower | P Value |
|---|---|---|---|---|
| BMI | ||||
| BMI at age 50,y | 1.44 | 1.12 | 1.85 | 0.004 |
| BMI at age 60,y | 1.24 | 1.07 | 1.45 | 0.005 |
| BMI at age 70,y | 1.07 | 0.95 | 1.21 | 0.244 |
| BMI at age 77,y | 0.97 | 0.82 | 1.14 | 0.719 |
| BMI at age 82,y | 0.90 | 0.73 | 1.11 | 0.338 |
| LDL | ||||
| LDL at age 50,y | 1.27 | 1.01 | 1.61 | 0.044 |
| LDL at age 60,y | 1.39 | 1.19 | 1.61 | <0.001 |
| LDL at age 70,y | 1.51 | 1.32 | 1.73 | <0.001 |
| LDL at age 77,y | 1.60 | 1.33 | 1.93 | <0.001 |
| LDL at age 82,y | 1.67 | 1.33 | 2.11 | <0.001 |
| HDL | ||||
| HDL at age 50,y | 0.71 | 0.53 | 0.95 | 0.021 |
| HDL at age 60,y | 0.76 | 0.63 | 0.91 | 0.003 |
| HDL at age 70,y | 0.81 | 0.70 | 0.93 | 0.003 |
| HDL at age 77,y | 0.85 | 0.71 | 1.01 | 0.066 |
| HDL at age 82,y | 0.87 | 0.69 | 1.10 | 0.247 |
| SBP | ||||
| SBP at age 50,y | 1.45 | 0.99 | 2.11 | 0.055 |
| SBP at age 60,y | 1.35 | 1.07 | 1.71 | 0.012 |
| SBP at age 70,y | 1.26 | 1.08 | 1.48 | 0.004 |
| SBP at age 77,y | 1.20 | 0.99 | 1.47 | 0.069 |
| SBP at age 82,y | 1.16 | 0.90 | 1.51 | 0.253 |
| Triglycerides | ||||
| Triglycerides at age 50,y | 1.02 | 0.93 | 1.12 | 0.651 |
| Triglycerides at age 60,y | 1.03 | 0.97 | 1.09 | 0.374 |
| Triglycerides at age 70,y | 1.03 | 0.95 | 1.13 | 0.445 |
| Triglycerides at age 77,y | 1.04 | 0.92 | 1.17 | 0.546 |
| Triglycerides at age 82,y | 1.04 | 0.89 | 1.21 | 0.598 |
| Glucose | ||||
| Glucose at age 50,y | 1.15 | 1.04 | 1.27 | 0.005 |
| Glucose at age 60,y | 1.12 | 1.06 | 1.19 | <0.001 |
| Glucose at age 70,y | 1.09 | 1.05 | 1.14 | <0.001 |
| Glucose at age 77,y | 1.07 | 1.01 | 1.14 | 0.019 |
| Glucose at age 82,y | 1.06 | 0.98 | 1.14 | 0.158 |
| Smoking | ||||
| Smoking at age 50,y | 2.83 | 1.82 | 4.40 | <0.001 |
| Smoking at age 60,y | 2.19 | 1.69 | 2.84 | <0.001 |
| Smoking at age 70,y | 1.70 | 1.39 | 2.08 | <0.001 |
| Smoking at age 77,y | 1.42 | 1.06 | 1.89 | 0.017 |
| Smoking at age 82,y | 1.25 | 0.86 | 1.82 | 0.251 |
The RRs are based on an interquartile range change in each continuous risk factor during a 5‐year follow‐up from each examination. BMI indicates body mass index, CI, confidence interval; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein, MI, myocardial infarction; RR, rate ratios; SBP, systolic blood pressure.
In the secondary analyses, also including antihypertensive treatment, diabetes mellitus treatment, and lipid‐lowering treatment in the models, the results were essentially the same as in the primary analyses not including treatments, except that the RRs were reduced for SBP at ages 50, 60 and 70 and now were significant only at age 70 (see Table S2 for details).
Ischemic Stroke
Three hundred forty‐three incident cases of ischemic stroke occurred during a median total follow‐up of 30.6 years (range, 0.04–43.3, corresponding to 64 074 PYAR) giving an incidence rate of 5.35/1000 PYAR.
A significant interaction was observed between age and the set of traditional risk factors regarding incident ischemic stroke (P=0.025). When the interactions between the individual risk factors and age regarding incident ischemic stroke were calculated, only SBP showed P<0.05 (P=0.0067; Table S3). These interactions are graphically illustrated by 3D plots for each risk factor in Figure 3 and Figure S2. As with MI, the 2D equivalent graphs at ages 50, 60, 70, 77, and 82 years are given in Figure 2.
Figure 3.
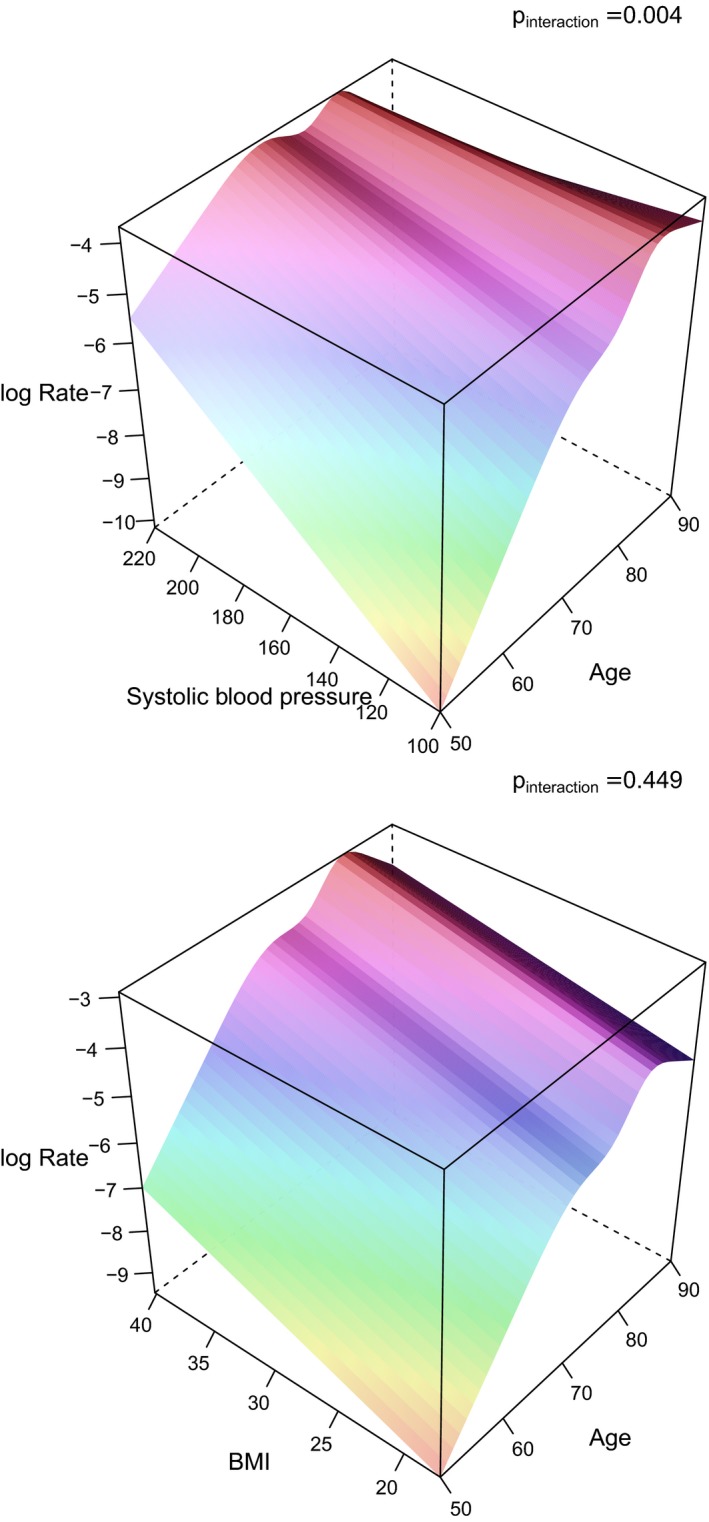
3D graphs illustrating the impact of systolic blood pressure and age on the incident rate (log rate) of heart failure (upper panel), and in a similar way BMI (in the lower panel). The P values for the interaction term between those risk factors and age regarding myocardial infarction are given in the graphs. 3D indicates 3‐dimensional; BMI, body mass index.
When RRs for the traditional risk factors were calculated separately for measurements performed at ages 50, 60, 70, 77, and 82 years, RRs for SBP, fasting glucose, and smoking declined by aging, being significant for SBP at all ages up to 77 years, whereas glucose and smoking were only significant at ages 50 and 60 (details are given in Table 3). The impact of both HDL‐ and LDL‐cholesterol, on the other hand, tended to increase over time and was significantly related to incident ischemic stroke at ages 77 and 82. The interaction term between HDL‐ and LDL‐cholesterol and age was, however, not significant. BMI and serum triglycerides were not significantly associated with incident ischemic stroke at any age.
Table 3.
RR, 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Ischemic Stroke Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years)
| Variable | RR | 95% CI Upper | 95% CI Lower | P Value |
|---|---|---|---|---|
| BMI | ||||
| BMI at age 50,y | 1.25 | 0.78 | 2.00 | 0.350 |
| BMI at age 60,y | 1.13 | 0.85 | 1.51 | 0.407 |
| BMI at age 70,y | 1.02 | 0.87 | 1.20 | 0.789 |
| BMI at age 77,y | 0.95 | 0.80 | 1.14 | 0.590 |
| BMI at age 82,y | 0.91 | 0.71 | 1.15 | 0.420 |
| LDL | ||||
| LDL at age 50,y | 1.10 | 0.65 | 1.83 | 0.728 |
| LDL at age 60,y | 1.17 | 0.85 | 1.62 | 0.342 |
| LDL at age 70,y | 1.25 | 1.03 | 1.51 | 0.022 |
| LDL at age 77,y | 1.31 | 1.06 | 1.61 | 0.011 |
| LDL at age 82,y | 1.35 | 1.03 | 1.78 | 0.031 |
| HDL | ||||
| HDL at age 50,y | 1.13 | 0.72 | 1.78 | 0.593 |
| HDL at age 60,y | 1.00 | 0.76 | 1.33 | 0.976 |
| HDL at age 70,y | 0.89 | 0.75 | 1.06 | 0.182 |
| HDL at age 77,y | 0.82 | 0.67 | 1.00 | 0.049 |
| HDL at age 82,y | 0.77 | 0.59 | 1.01 | 0.056 |
| SBP | ||||
| SBP at age 50,y | 3.18 | 1.79 | 5.65 | <0.001 |
| SBP at age 60,y | 2.28 | 1.60 | 3.26 | <0.001 |
| SBP at age 70,y | 1.64 | 1.35 | 2.00 | <0.001 |
| SBP at age 77,y | 1.30 | 1.05 | 1.61 | 0.016 |
| SBP at age 82,y | 1.10 | 0.82 | 1.47 | 0.515 |
| Triglycerides | ||||
| Triglycerides at age 50,y | 0.92 | 0.64 | 1.32 | 0.640 |
| Triglycerides at age 60,y | 0.93 | 0.75 | 1.17 | 0.549 |
| Triglycerides at age 70,y | 0.95 | 0.83 | 1.09 | 0.455 |
| Triglycerides at age 77,y | 0.96 | 0.83 | 1.12 | 0.627 |
| Triglycerides at age 82,y | 0.97 | 0.79 | 1.19 | 0.781 |
| Glucose | ||||
| Glucose at age 50,y | 1.15 | 0.98 | 1.34 | 0.088 |
| Glucose at age 60,y | 1.12 | 1.02 | 1.23 | 0.022 |
| Glucose at age 70,y | 1.08 | 1.03 | 1.14 | 0.002 |
| Glucose at age 77,y | 1.06 | 0.99 | 1.14 | 0.074 |
| Glucose at age 82,y | 1.05 | 0.95 | 1.15 | 0.325 |
| Smoking | ||||
| Smoking at age 50,y | 2.77 | 1.26 | 6.11 | 0.011 |
| Smoking at age 60,y | 1.85 | 1.16 | 2.95 | 0.009 |
| Smoking at age 70,y | 1.24 | 0.94 | 1.63 | 0.126 |
| Smoking at age 77,y | 0.93 | 0.65 | 1.34 | 0.709 |
| Smoking at age 82,y | 0.76 | 0.46 | 1.26 | 0.292 |
The RRs are based on an interquartile range change in each continuous risk factor during a 5‐year follow‐up from each examination. BMI indicates body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
In the secondary analyses, also including antihypertensive treatment, diabetes mellitus treatment, and lipid‐lowering treatment in the models, the results were essentially the same as in the primary analyses not including treatments, except that RRs were reduced for glucose and now were not statistically significant (see Table S4 for details).
Heart Failure
Three hundred ninety‐seven incident cases of heart failure occurred during a median total follow‐up of 31.0 years (range, 0.04–43.3, corresponding to 64 573 PYAR), giving an incidence rate of 6.14/1000 PYAR.
A significant interaction was observed between age and the set of 7 traditional risk factors regarding incident heart failure (P=0.0007). When interactions between the individual risk factors and age regarding incident ischemic stroke were calculated, only SBP and HDL‐cholesterol showed P<0.05 (P=0.0045 and P=0.016, respectively; Table S5). These interactions are graphically illustrated by 3D plots for each risk factor in Figure 4 and Figure S3. As with the other 2 outcomes, the 2D equivalent graphs at ages 50, 60, 70, 77, and 82 years are given in Figure 2.
Figure 4.
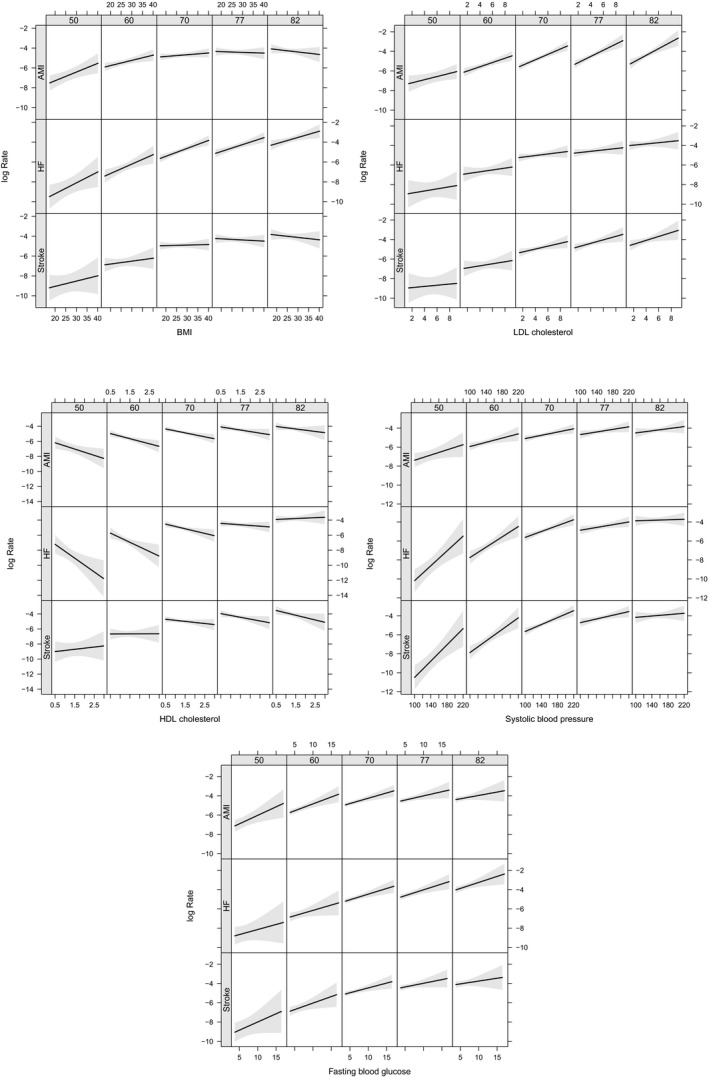
2D graphs showing the relationships between (A) body mass index (BMI), (B) LDL and (C) HDL‐cholesterol, (D) systolic blood pressure (SBP), (E) fasting glucose and log incidence rate of myocardial infarction (AMI), heart failure (HF) and ischemic stroke at ages 50, 60, 70, 77 and 82 years. The grey areas represent the 95% confidence intervals. 2D indicates 2‐dimensional; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
When the RRs for the traditional risk factors were calculated separately for measurements performed at ages 50, 60, 70, 77, and 82 years, the RRs for BMI showed only a minor decline by aging and were significant at all examination cycles. Also, the RRs for fasting glucose were constant over time, and were significant at the older ages. RRs for SBP and smoking showed a pronounced decline with age and were no longer significant at age 82. RRs for HDL‐cholesterol increased with aging, being significant only at ages 50, 60, and 70. Serum triglycerides were not significant at any examination (details are given in Table 4).
Table 4.
RR, 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Heart Failure Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 Years)
| Variable | IRR | 95% CI Upper | 95% CI Lower | P Value |
|---|---|---|---|---|
| BMI | ||||
| BMI at age 50,y | 1.58 | 1.07 | 2.35 | 0.023 |
| BMI at age 60,y | 1.49 | 1.16 | 1.91 | 0.002 |
| BMI at age 70,y | 1.40 | 1.22 | 1.61 | <0.001 |
| BMI at age 77,y | 1.34 | 1.16 | 1.55 | <0.001 |
| BMI at age 82,y | 1.30 | 1.07 | 1.57 | 0.008 |
| LDL | ||||
| LDL at age 50,y | 1.18 | 0.74 | 1.86 | 0.492 |
| LDL at age 60,y | 1.15 | 0.86 | 1.55 | 0.350 |
| LDL at age 70,y | 1.13 | 0.94 | 1.35 | 0.186 |
| LDL at age 77,y | 1.11 | 0.92 | 1.34 | 0.264 |
| LDL at age 82,y | 1.10 | 0.87 | 1.40 | 0.433 |
| HDL | ||||
| HDL at age 50,y | 0.47 | 0.28 | 0.80 | 0.006 |
| HDL at age 60,y | 0.61 | 0.43 | 0.85 | 0.004 |
| HDL at age 70,y | 0.78 | 0.64 | 0.94 | 0.009 |
| HDL at age 77,y | 0.93 | 0.79 | 1.09 | 0.355 |
| HDL at age 82,y | 1.05 | 0.85 | 1.30 | 0.663 |
| SBP | ||||
| SBP at age 50,y | 2.87 | 1.65 | 4.99 | <0.001 |
| SBP at age 60,y | 2.09 | 1.47 | 2.97 | <0.001 |
| SBP at age 70,y | 1.52 | 1.25 | 1.85 | <0.001 |
| SBP at age 77,y | 1.22 | 1.01 | 1.47 | 0.044 |
| SBP at age 82,y | 1.04 | 0.81 | 1.33 | 0.778 |
| Triglycerides | ||||
| Triglycerides at age 50,y | 1.05 | 0.91 | 1.21 | 0.536 |
| Triglycerides at age 60,y | 1.02 | 0.92 | 1.12 | 0.716 |
| Triglycerides at age 70,y | 0.99 | 0.90 | 1.09 | 0.843 |
| Triglycerides at age 77,y | 0.97 | 0.85 | 1.10 | 0.654 |
| Triglycerides at age 82 | 0.96 | 0.82 | 1.12 | 0.592 |
| Glucose | ||||
| Glucose at age 50,y | 1.09 | 0.94 | 1.26 | 0.259 |
| Glucose at age 60,y | 1.09 | 1.00 | 1.20 | 0.051 |
| Glucose at age 70,y | 1.10 | 1.05 | 1.15 | <0.001 |
| Glucose at age 77,y | 1.10 | 1.05 | 1.16 | <0.001 |
| Glucose at age 82,y | 1.11 | 1.03 | 1.19 | 0.008 |
| Smoking | ||||
| Smoking at age 50,y | 1.74 | 0.84 | 3.60 | 0.138 |
| Smoking at age 60,y | 1.58 | 1.01 | 2.47 | 0.043 |
| Smoking at age 70,y | 1.44 | 1.12 | 1.87 | 0.005 |
| Smoking at age 77,y | 1.36 | 1.00 | 1.84 | 0.050 |
| Smoking at age 82,y | 1.29 | 0.85 | 1.96 | 0.223 |
The RRs are based on an interquartile range change in each continuous risk factor during a 5‐year follow‐up from each examination. BMI indicates body mass index CI, confidence interval;; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RR, rate ratios; SBP, systolic blood pressure.
In the secondary analyses, also including antihypertensive treatment, diabetes mellitus treatment, and lipid‐lowering treatment in the models, the results were essentially the same as in the primary analyses not including treatments, except that the RRs were reduced for SBP at ages 50, 60, 70 and 70 and now were significant only at ages 50, 60, and 70 (see Table S6 for details). Also, the RRs for glucose were slightly reduced and no longer significant at age 82 (P<0.059).
In the additional analyses using multiple imputation, only minor changes in the results were observed, although confidence intervals for some contrasts were slightly wider. Because the results did not change to any major degree and did not affect the conclusions of the study, these new calculations are added in a Table S7.
Diastolic blood pressure did not have a significant relationship with any of the 3 cardiovascular outcomes studied and was therefore not included in the tables or figures.
Serum Triglycerides
Because it is well known that serum triglycerides have a larger measurement variation than the closely related HDL‐cholesterol and BMI, the impact of serum triglycerides is often attenuated in models including also HDL‐cholesterol and BMI. We therefore analyzed how serum triglycerides were related to the 3 cardiovascular outcomes in models only including serum triglycerides and age. Table S8 shows that RRs for serum triglycerides were highly significant at all ages regarding MI. Regarding heart failure, a slight attenuation in RR with aging was observed with significant RRs at all ages except age 82. Serum triglycerides were not related to incident ischemic stroke at any age.
Discussion
The present study showed, as expected, that most risk factors measured at middle age lost in power during the aging process regarding associations with incident cardiovascular disease. However, some exceptions from this general rule were noted: LDL‐cholesterol was significantly related to incident MI, whereas BMI and fasting glucose were related to incident heart failure also in the elderly.
The main reason for the general decline in the power of the risk factors over time is likely to be attributed to the fact that individuals with the highest values of the risk factors at midlife will experience an event at an early age, and therefore mainly low‐risk individuals will remain at risk as the cohort becomes older. Thus, every cohort will consist of “survivors” when the follow‐up increases, and in this group of survivors the impact of risk factors will be diminished.
Comparisons With the Literature
The knowledge that the impact of many risk factors decline with age is not new. However, this knowledge is mainly based on different studies performed in elderly subjects,2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and very few longitudinal studies exist.
Abbot et al investigated the impact of aging on the strength of risk factors for coronary heart disease in the Honolulu Heart Program with 26 years of follow‐up in a cohort of males aged 45 to 68 years at baseline.22 Using 6‐year follow‐up periods from 3 physical examinations, they showed that the impact of blood pressure, smoking, and BMI declined significantly with aging, serum total cholesterol showed a nonsignificant decline, whereas the impact of diabetes mellitus was fairly constant over time.
The same researchers also published a similar analysis regarding ischemic stroke in the same sample and found that the impact of blood pressure and smoking declined by aging, whereas diabetes mellitus continued to be a risk factor at all ages.23
The present and the 2 articles by Abbot et al share many similarities in that they deal only with men, have follow‐up periods of 5 to 6 years from each physical examination, and were conducted in similar calendar periods. The present study has a longer follow‐up period and is standardized for age at baseline, which makes the evaluation of the impact of the aging process easier to interpret.
We have previously compared risk factors regarding MI and stroke between ages 50 and 70 in the present cohort,12, 13 and this study adds information of the impact of risk factors up to age 82 years. Furthermore, no other study has studied the impact of aging on risk factors for heart failure in a longitudinal fashion.
BMI as a Risk Factor
BMI is an interesting risk factor, given that it repeatedly has been shown that obesity at middle age is an important risk factor for cardiovascular disease,24 whereas a high BMI seems to be protective in subjects who have experienced a cardiovascular event, like MI or heart failure. This has been termed the “obesity paradox.”25 In the present study, the impact of BMI during aging was very much dependent on the studied outcome. For MI, the hazard ratio for BMI declined rapidly from age 50 and was not of importance from age 70, despite the fact that BMI actually increased in the cohort during this time period. The decline in the impact of BMI as a risk factor for MI with aging in this population is probably 1 explanation for the obesity paradox, given that the majority of MI cases occur later than age 70, but other mechanisms are likely to contribute as well.
Contrary to the rapid decline in the impact of BMI on incident MI, a much less pronounced decline was observed regarding heart failure, and in this case BMI was an important risk factor also at age 82. It is well documented that lean heart failure patients have a worse prognosis than obese and overweight patients.26 However, an age‐related decline of the impact of BMI cannot be responsible for this obesity paradox, and other pathogenetic mechanisms in the state of heart failure must be suspected.
Lipids as Risk Factors
LDL‐cholesterol declined with age in the cohort. This is possibly both attributed to a survival effect as well as an awareness to decrease fat intake in the population in the 1970s and 1980s. Despite this decline in LDL‐cholesterol levels, LDL‐cholesterol remained a strong risk factor for MI over the entire follow‐up period and actually became significantly related to ischemic stroke at older ages. However, a beneficial use of statin therapy to lower LDL‐cholesterol in the elderly is a matter of debate, especially in primary prevention.27, 28, 29
Recent genetic Mendelian randomization studies have highlighted the role of serum triglycerides as a causal risk factor for coronary heart disease.30 In the present analysis with all risk factors in the model, serum triglyceride levels were not an important independent cardiovascular risk factor. This is probably because serum triglycerides are closely related to both HDL‐cholesterol and BMI, 2 risk factors that are measured more precisely than serum triglycerides, which show a high day‐to‐day variation also in the fasting state. A variable with a high coefficient of variation in the measurements are usually attenuated in the multiple models.31 A secondary analysis in the present study using only serum triglyceride in the models showed a major impact of this lipid measurement regarding MI at all ages.
HDL‐cholesterol has been debated as being causally related to MI using genetic studies in recent years.32, 33 In the present study, HDL‐cholesterol seems to be protective mainly at middle age.
Smoking as Risk Factor
Smoking was the risk factor showing the greatest decline in prevalence over time, changing from above 50% to 6% during the follow‐up period. This decline in smoking prevalence was paralleled with a decline in its impact as a risk factor on cardiovascular disease. The survival effect might well play a role here, but the rapid decline in smoking is probably mainly attributed to an increased awareness in the general population on the hazard of smoking in the 1970s and 1980s, resulting in a voluntary smoking cessation in the majority of subjects.
Blood Pressure as Risk Factor
The risk factor showing the most pronounced decline in impact over time in terms of reduction in RR was SBP. This was observed despite that the levels of SBP increased with aging. This phenomenon has previously been described by others.22, 23
Statistical Method
The Cox proportional hazards model is the most widely used regression model for time‐to‐event data. In its most natural form, the model assumes that the regression coefficients are constant over time and analyses are conducted on a single time scale. Although the Cox model can easily be extended to allow for time‐varying coefficients, the Poisson modeling approach using time‐split data not only allows for analysis on multiple time scales simultaneously, but also yields a simpler way of understanding the time‐varying effects by treating the time, on an arbitrary scale, as a variable in the model. The time‐varying coefficients are then simply interactions between the covariates and the time scale(s). In this study, we investigated the age dependency on several risk factors, which, in the Poisson model, reduces to the coefficients for the risk factors' interactions with attained age.
Although the change in risk by aging is best described by the areas in the 3D plots, we also calculated RRs over 5‐year intervals from each physical examination to make the data easier to compare in a traditional way. Five‐year follow‐up intervals allowed us to evaluate all individuals from the 82‐year examination and were comparable to those used in the Honolulu Heart Program.22, 23
Several lipid measurements were missing at the 60‐year examination and were therefore imputed. However, because the regression models used measured data from all other examinations, the impact of the missing data at age 60 is likely to be minor.
In the main analyses, we did not include treatments versus hypertension, diabetes mellitus, or lipids, but given that such treatment might affect the results, we also performed secondary analyses with information on those medications included in the models. In general, the results were not principally changed, but the RRs for SBP and, to some extent, also for glucose were reduced. However, the main conclusion of the study, namely that LDL‐cholesterol was significantly related to incident MI, whereas BMI and fasting glucose were related to incident heart failure also in the elderly, was still valid after adjustment for medications.
Strengths and Limitations
Among the strengths is the longitudinal design with a long follow‐up period in a sample of individuals with the same age at baseline, which allowed us to study the change in risk factor importance over 4 decades. The fact that we only investigated white males makes the data less generalizable. It remains to be seen whether the same results are found in women.
During a follow‐up period of many decades, such as in the present study, it is likely that changes in lifestyle have occurred in the society that might have an impact on the results. In the present study, the male cohort showed a high prevalence of smoking and high LDL‐cholesterol levels and rather low BMI at middle age in the 1970s. At the age of 70, these 2 major risk factors had declined to the levels usually observed today in that age group.34 Thus, the male, rather thin, smoking, high LDL‐cholesterol “phenotype” being common in the present sample at age 50 is not so prevalent in industrialized societies currently. Therefore, the results in the present study from ages 50 and 60 years might not be applicable to middle‐aged males presently.
Conclusions
Using a longitudinal design in middle‐aged men spanning 4 decades showed that the impact of traditional cardiovascular risk factors generally declined with aging. However, some of the risk factors, such as LDL‐cholesterol for MI, and BMI and fasting glucose regarding heart failure, remained significantly associated with incident cardiovascular disease also at old age.
Sources of Funding
This work was funded by the Swedish Heart‐Lung Foundation and the Swedish Research Council.
Disclosures
None.
Supporting information
Table S1. anova Table for the Total Model Regarding Risk of Myocardial Infarction
Table S2. Rate Ratios (RR), 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Myocardial Infarction (MI), Adjusted for Medication Use, Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years). The RRs are based on an interquartile range (IQR) change in each continuous risk factor during a 5‐year follow‐up from each examination
Table S3. anova Table for the Total Model Regarding Risk of Ischemic Stroke
Table S4. Rate Ratios (RR), 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Ischemic Stroke, Adjusted for Medication Use, Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years). The RRs are based on an interquartile range (IQR) change in each continuous risk factor during a 5‐year follow‐up from each examination
Table S5. anova Table for the Total Model Regarding Risk of Heart Failure
Table S6. LDL (Low‐Density Lipoprotein), Rate Ratios (RR), 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Heart Failure, Adjusted for Medication Use, Given When the Risk Factors were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years). The RRs are based on an interquartile range (IQR) change in each continuous risk factor during a 5‐year follow‐up from each examination
Table S7. Risk Ratios (RR) and 95% CI (lower and upper) Are Given for Relationships Between Different Risk Factors and Incident Myocardial Infarction, Ischemic Stroke, and Heart Failure at Ages 50, 60, 70, 77 and 82 Years When Recalculated Using Multiple Imputation for Missing Variables
Table S8. RR (and 95% CI) and P Values for the Associations Between Serum Triglycerides and Incident Myocardial Infarction, Ischemic Stroke and Heart Failure in Models Including Serum Triglycerides as the Only Risk Factor (together with age) at Ages 50, 60, 70, 77, and 82 Years. The RR is based on an interquartile range (IQR) change in serum triglycerides
Figure S1. 3D graphs illustrating the impact of the different risk factors and age on the log incident rate of myocardial infarction. The P values for the interaction term between those risk factors and age regarding myocardial infarction are given in the figures.
Figure S2. 3D graphs illustrating the impact of the different risk factors and age on the log incident rate of ischemic stroke. The P values for the interaction term between those risk factors and age regarding myocardial infarction are given in the figures.
Figure S3. 3D graphs illustrating the impact of the different risk factors and age on the log incident rate of heart failure. The P values for the interaction term between those risk factors and age regarding heart failure are given in the figures.
(J Am Heart Assoc. 2018;7:e007061 DOI: 10.1161/JAHA.117.007061.)29306895
References
- 1. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 2. Coope J, Warrender TS, McPherson K. The prognostic significance of blood pressure in the elderly. J Hum Hypertens. 1988;2:79–88. [PubMed] [Google Scholar]
- 3. Hakim AA, Curb JD, Burchfiel CM, Rodriguez BL, Sharp DS, Yano K, Abbott RD. Screening for coronary heart disease in elderly men based on current and past cholesterol levels. J Clin Epidemiol. 1999;52:1257–1265. [DOI] [PubMed] [Google Scholar]
- 4. Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, Tsukahara R, Ostfeld AM, Berkman LF. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all‐cause mortality in persons older than 70 years. JAMA. 1994;272:1335–1340. [PubMed] [Google Scholar]
- 5. Langer RD, Ganiats TG, Barrett‐Connor E. Paradoxical survival of elderly men with high blood pressure. BMJ. 1989;298:1356–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattila K, Haavisto M, Rajala S, Heikinheimo R. Blood pressure and five year survival in the very old. Br Med J (Clin Res Ed). 1988;296:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pekkanen J, Nissinen A, Vartiainen E, Salonen JT, Punsar S, Karvonen MJ. Changes in serum cholesterol level and mortality: a 30‐year follow‐up. The Finnish Cohorts of the Seven Countries Study. Am J Epidemiol. 1994;139:155–165. [DOI] [PubMed] [Google Scholar]
- 8. Siegel D, Kuller L, Lazarus NB, Black D, Feigal D, Hughes G, Schoenberger JA, Hulley SB. Predictors of cardiovascular events and mortality in the Systolic Hypertension in the Elderly Program pilot project. Am J Epidemiol. 1987;126:385–399. [DOI] [PubMed] [Google Scholar]
- 9. Staessen J, Bulpitt C, Clement D, De Leeuw P, Fagard R, Fletcher A, Forette F, Leonetti G, Nissinen A, O'Malley K. Relation between mortality and treated blood pressure in elderly patients with hypertension: report of the European Working Party on High Blood Pressure in the Elderly. BMJ. 1989;298:1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor JO, Cornoni‐Huntley J, Curb JD, Manton KG, Ostfeld AM, Scherr P, Wallace RB. Blood pressure and mortality risk in the elderly. Am J Epidemiol. 1991;134:489–501. [DOI] [PubMed] [Google Scholar]
- 11. Weijenberg MP, Feskens EJ, Kromhout D. Total and high density lipoprotein cholesterol as risk factors for coronary heart disease in elderly men during 5 years of follow‐up. The Zutphen Elderly Study. Am J Epidemiol. 1996;143:151–158. [DOI] [PubMed] [Google Scholar]
- 12. Möller CS, Häggström J, Zethelius B, Wiberg B, Sundström J, Lind L. Age and follow‐up time affect the prognostic value of the ECG and conventional cardiovascular risk factors for stroke in adult men. J Epidemiol Community Health. 2007;61:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Möller CS, Zethelius B, Sundström J, Lind L. Impact of follow‐up time and re‐measurement of the electrocardiogram and conventional cardiovascular risk factors on their predictive value for myocardial infarction. J Intern Med. 2006;260:22–30. [DOI] [PubMed] [Google Scholar]
- 14. Merlo J, Lindblad U, Pessah‐Rasmussen H, Hedblad B, Rastam J, Isacsson SO, Janzon L, Rastam L. Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two Swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol. 2000;16:235–243. [DOI] [PubMed] [Google Scholar]
- 15. Ingelsson E, Ärnlov J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. [DOI] [PubMed] [Google Scholar]
- 16. The Task Force on Heart Failure of the European Society of Cardiology . Guidelines for the Diagnosis of Heart Failure. Eur Heart J. 1995;16:741–751. [PubMed] [Google Scholar]
- 17. Rubin DB. Multiple Imputation of Non‐Response in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 18. van Buuren S, Groothuis‐Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 19. Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed‐effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 20. Epi: A Package for Statistical Analysis in Epidemiology. R package version 2.12 [computer program]; Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 21. R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 22. Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Yano K, Schatz IJ, Ross GW, Petrovitch H. Age‐related changes in risk factor effects on the incidence of coronary heart disease. Ann Epidemiol. 2002;12:173–181. [DOI] [PubMed] [Google Scholar]
- 23. Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Popper JS, Ross GW, Petrovitch H. Age‐related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol. 2003;56:479–486. [DOI] [PubMed] [Google Scholar]
- 24. Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–381. [DOI] [PubMed] [Google Scholar]
- 25. Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and prevalence of cardiovascular diseases and prognosis‐the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. [DOI] [PubMed] [Google Scholar]
- 26. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. [DOI] [PubMed] [Google Scholar]
- 27. Pedro‐Botet J, Climent E, Chillarón JJ, Toro R, Benaiges D, Flores‐Le Roux JA. Statins for primary cardiovascular prevention in the elderly. J Geriatr Cardiol. 2015;12:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone NJ, Turin A, Spitz JA, Valle CW, Kazmi S. Statin therapy across the lifespan: evidence in major age groups. Expert Rev Cardiovasc Ther. 2016;14:341–366. [DOI] [PubMed] [Google Scholar]
- 29. Teng M, Lin L, Zhao YJ, Khoo AL, Davis BR, Yong QW, Yeo TC, Lim BP. Statins for primary prevention of cardiovascular disease in elderly patients: systematic review and meta‐analysis. Drugs Aging. 2015;32:649–661. [DOI] [PubMed] [Google Scholar]
- 30. Triglyceride Coronary Disease Genetics C, Emerging Risk Factors C , Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, Lawlor D, Reilly MP, Hingorani AD, Talmud PJ, Danesh J. Triglyceride‐mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Austin MA. Plasma triglyceride as a risk factor for coronary heart disease. The epidemiologic evidence and beyond. Am J Epidemiol. 1989;129:249–259. [DOI] [PubMed] [Google Scholar]
- 32. Burgess S, Freitag DF, Khan H, Gorman DN, Thompson SG. Using multivariable Mendelian randomization to disentangle the causal effects of lipid fractions. PLoS One. 2014;9:e108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Voight BF, Peloso GM, Orho‐Melander M, Frikke‐Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton‐Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland‐van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland‐Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg‐Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium‐dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–2375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. anova Table for the Total Model Regarding Risk of Myocardial Infarction
Table S2. Rate Ratios (RR), 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Myocardial Infarction (MI), Adjusted for Medication Use, Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years). The RRs are based on an interquartile range (IQR) change in each continuous risk factor during a 5‐year follow‐up from each examination
Table S3. anova Table for the Total Model Regarding Risk of Ischemic Stroke
Table S4. Rate Ratios (RR), 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Ischemic Stroke, Adjusted for Medication Use, Given When the Risk Factors Were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years). The RRs are based on an interquartile range (IQR) change in each continuous risk factor during a 5‐year follow‐up from each examination
Table S5. anova Table for the Total Model Regarding Risk of Heart Failure
Table S6. LDL (Low‐Density Lipoprotein), Rate Ratios (RR), 95% CI, and P Values for the Associations Between Traditional Risk Factors and Incident Heart Failure, Adjusted for Medication Use, Given When the Risk Factors were Measured at 5 Examination Cycles (50, 60, 70, 77, and 82 years). The RRs are based on an interquartile range (IQR) change in each continuous risk factor during a 5‐year follow‐up from each examination
Table S7. Risk Ratios (RR) and 95% CI (lower and upper) Are Given for Relationships Between Different Risk Factors and Incident Myocardial Infarction, Ischemic Stroke, and Heart Failure at Ages 50, 60, 70, 77 and 82 Years When Recalculated Using Multiple Imputation for Missing Variables
Table S8. RR (and 95% CI) and P Values for the Associations Between Serum Triglycerides and Incident Myocardial Infarction, Ischemic Stroke and Heart Failure in Models Including Serum Triglycerides as the Only Risk Factor (together with age) at Ages 50, 60, 70, 77, and 82 Years. The RR is based on an interquartile range (IQR) change in serum triglycerides
Figure S1. 3D graphs illustrating the impact of the different risk factors and age on the log incident rate of myocardial infarction. The P values for the interaction term between those risk factors and age regarding myocardial infarction are given in the figures.
Figure S2. 3D graphs illustrating the impact of the different risk factors and age on the log incident rate of ischemic stroke. The P values for the interaction term between those risk factors and age regarding myocardial infarction are given in the figures.
Figure S3. 3D graphs illustrating the impact of the different risk factors and age on the log incident rate of heart failure. The P values for the interaction term between those risk factors and age regarding heart failure are given in the figures.


