Abstract
Background
Thrombus aspiration is still being used in a substantial number of patients despite 2 large randomized clinical trials showing no favorable effect of routine thrombus aspiration during primary percutaneous coronary intervention in patients with ST‐segment–elevation myocardial infarction. The aim of this observational study was to evaluate the impact of thrombus aspiration on mortality, stent thrombosis, and stroke using all available data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
Methods and Results
We identified 42 829 consecutive patients registered in SCAAR between January 2005 and September 2014 who underwent percutaneous coronary intervention for ST‐segment–elevation myocardial infarction. Thrombus aspiration was used in 25% of the procedures. We used instrumental variable analysis with administrative healthcare region as the treatment‐preference instrumental variable to evaluate the effect of thrombus aspiration on mortality, stent thrombosis, and stroke. Thrombus aspiration was not associated with mortality at 30 days (risk reduction: −1.2; 95% confidence interval [CI], −5.4 to 3.0; P=0.57) and 1 year (risk reduction: −2.4; 95% CI, −7.6 to 3.0; P=0.37). Thrombus aspiration was associated with a lower risk of stent thrombosis both at 30 days (risk reduction: −2.7; 95% CI, −4.1 to −1.4; P<0.001) and 1 year (risk reduction: −3.5; 95% CI, −5.3 to −1.7; P<0.001). In‐hospital stroke and neurologic complications did not differ between groups (risk reduction: 0.1; 95% CI, −0.8 to 1.1; P=0.76).
Conclusions
Mortality was not different between the groups. Thrombus aspiration was associated with decreased risk of stent thrombosis. Our study provides important evidence for the external validity of previous randomized studies regarding mortality.
Keywords: myocardial infarction, thrombectomy, primary percutaneous coronary intervention
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Treatment
Clinical Perspective
What Is New?
This study is the largest prospective study of thrombus aspiration in primary percutaneous coronary intervention (PCI).
Thrombus aspiration was not associated with a reduction in mortality, confirming the results from the large randomized clinical trials TASTE (Thrombus Aspiration in ST‐Elevation Myocardial Infarction in Scandinavia) and TOTAL (Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with ST‐Segment–Elevation Myocardial Infarction Undergoing Primary PCI).
Thrombus aspiration was not associated with increased risk of in‐hospital stroke.
Thrombus aspiration was associated with decreased risk of stent thrombosis.
What Are the Clinical Implications?
Thrombus aspiration during primary PCI may reduce the risk of stent thrombosis even in the absence of reduction in mortality.
Our study does not show the increased risk of stroke reported in the TOTAL trial.
Future studies should investigate whether reduction in stent thrombosis after thrombus aspiration might be cost‐effective in primary PCI.
Thrombus aspiration during percutaneous coronary intervention (PCI) in patients with ST‐segment–elevation myocardial infarction (STEMI) is an intuitive and appealing method for reduction of thrombus burden and prevention of distal embolization. Initially, the results from several smaller studies reported that thrombus aspiration may improve survival in patients with STEMI due to improvement in coronary microcirculation and decreased infarct size.1, 2, 3, 4 However, the 2 large‐scale randomized controlled trials (RCTs) TASTE (Thrombus Aspiration in ST‐Elevation Myocardial Infarction in Scandinavia) and TOTAL (Trial of Routine Aspiration Thrombectomy With PCI Versus PCI Alone in Patients With STEMI Undergoing Primary PCI) did not confirm clinical benefits with routine thrombus aspiration in STEMI regarding short‐ and long‐term mortality.5, 6, 7 In TASTE, thrombus aspiration reduced risk of stent thrombosis and reinfarction, whereas in TOTAL, thrombus aspiration was associated with an increased risk of stroke.7
Information acquired from properly designed and conducted RCTs provides the foundation for the concept of evidence‐based medicine. However, an important limitation of RCTs is restricted external validity due to frequently present patient selection bias.8, 9, 10 High‐quality observational studies based on large‐scale registries and adequate statistical modeling provide valuable evidence for the external validity of RCTs; therefore, observational studies are a valuable complement to RCTs.10 This is particularly important for the areas in which significant clinical questions are not sufficiently studied or controversies and conflicts of opinions persist.
The aim of this study was to evaluate the effect of thrombus aspiration on mortality, stent thrombosis, and stroke or neurologic complications in a substantial number of STEMI patients, using information available in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
Methods
Databases and Patient Selection
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of the legal regulations about access to patient‐level data. We used data from the prospective SCAAR database. Established in 1992, SCAAR is a national registry that contains data about all coronary angiographies and PCIs performed in Sweden. According to the Swedish regulations, written informed consent from patients is not mandatory for registration of data. Each catheterization procedure is described with ≈50 angiographic and 200 PCI variables that include both demographic and procedure‐related data. The registry is sponsored by the Swedish Health Authorities and does not receive any funding from commercial interests. We included all consecutive patients who underwent PCI due to STEMI in Sweden between January 2005 and September 2014. We included all patients who were randomized in TASTE. More detailed information about the SCAAR organization and database has been published previously.11 The study was approved by the institutional review board at Gothenburg University.
Definitions and Outcomes
The patients were considered to have diabetes mellitus, hypertension, hyperlipidemia, previous myocardial infarction, or previous stroke if they had a diagnosis for the particular condition according to International Classification of Diseases (ICD) codes.5 Information about previous PCI, previous coronary artery bypass grafting, cardiogenic shock, and procedural details are entered directly into the database by interventional cardiologists.
End points
The primary end point was mortality at 30 days. Vital status and date of death were obtained from the Swedish National Population Registry through October 13, 2014. SCAAR was merged with the Swedish National Population Registry by the Epidemiologic Center of the Swedish National Board of Health and Welfare using Swedish personal identification numbers. Because use of personal identification numbers is mandatory, the death registry in Sweden has a high degree of completeness but is not reviewed or adjudicated to establish cardiac versus noncardiac causes of death. The secondary end points were mortality at 1 year, stent thrombosis at 30 days and 1 year, and reported in‐hospital neurological complications. Stent thrombosis was defined as an acute stent occlusion verified by coronary angiography. Neurological complication was defined as a new neurological deficit during PCI or during in‐hospital stay after index PCI. Neurological deficit was defined as receiving treatment for any of ICD codes I60 to I64.
Statistical Analyses
Continuous variables are presented as mean±SD, and categorical variables are reported as frequencies. Normal distribution of variables was assessed by inspecting the distribution of values on histograms and by the Shapiro–Wilk test. Differences between the groups in continuous variables were tested with linear regression. Differences in categorical variables were tested by logistic regression.
Imputation protocol
We imputed missing data using the multiple imputation chain–equation method12 with 20 data sets. Calendar year, an indicator of missingness, and an event indicator were included and used as regular variables.13 Continuous variables were imputed by ordinary least squares multiple regression, binary variables were imputed using logistic regression, and categorical variables were imputed by multinomial logistic regression. The imputation procedure and subsequent analyses were performed according to the Rubin protocol14 under the assumption that missing data are missing at random. More information about the imputation protocol is provided in Data S1.
Statistical models
We used statistical modeling based on the instrumental variable to reduce bias due to unmeasured confounders. This method is a post hoc analytic technique based on statistical principles similar to those used in the analysis of randomized trials.15, 16, 17 To use instrumental variable analysis, one must identify a naturally varying phenomenon in the observed data that, like the act of randomization in an RCT, predicts the treatment that will be assigned to the individual patient. To become a valid instrument, a variable has to fulfill some necessary criteria. First, it has to be strongly associated with the received treatment. Second, it must not be associated either directly or indirectly with the outcome, except through the effect of the treatment itself. The variable with these statistical qualities is the instrumental variable, or instrument.
We used 6 administrative healthcare regions in Sweden based on geographic location as the treatment‐preference instruments. Geographic or administrative regions are frequently used as instruments because this type of variable usually fulfils the theoretical criteria for a valid instrument.18, 19, 20 In addition, we used a different instrument for sensitivity analysis based on of the “preference” for use of thrombus aspiration during primary PCI at the level of individual hospitals. To create this treatment‐preference instrument, we divided hospitals into quintiles based on the total number of procedures during the study period in which thrombus aspiration was used. Our primary model was based on instrumental variable 2‐stage least squares (2SLS) regression.17 The outcome (dependent) variables in the 2SLS regressions were mortality at 30 days and 1 year, stent thrombosis at 30 days and 1 year, and in‐hospital stroke or neurologic complications (Table 1). Our secondary models were based on unadjusted and propensity score–adjusted multilevel logistic regression. Because SCAAR is a hierarchical database with clustering of patients within hospitals and regions, we entered administrative healthcare regions and individual hospitals into the regression model as random‐effects variables. We used logistic regression to calculate the likelihood of undergoing thrombus aspiration and PCI or PCI alone to generate the propensity score for each patient. All variables in Table 2 except for frequency of direct stenting were entered into the logistic regression model. The calculated propensity score was entered into the model as quintiles of propensity score values based on complete case data and imputed data after multiple imputations of the missing data. We used the Durbin–Wu–Hausman specification test to evaluate the presence of residual confounding (endogeneity). The validity of the instrumental variable was tested with the Sargan test. To test for the strength of the instruments, we examined the partial F test from the first‐stage regression, which predicts treatment as a function of instrument and covariates. The partial F test has the null hypothesis that the coefficient for the effect of the instrument in the first‐stage regression model is zero.21 An F statistic >10 indicates that the instrument is not weak. Reported standard errors from instrumental variable 2SLS regression are robust and account for clustering of patients within hospitals using the sandwich estimator. We assessed trends in 30‐day and 1‐year mortality over the study period by including the calendar year in the logistic regression as a continuous variable in addition to age and sex.
Table 1.
Outcomes and Statistical Models
| Outcome | Primary Model | Secondary Models |
|---|---|---|
| Death within 30 d | IV‐2SLS | PS‐adjusted logistic regression |
| Death within 1 y | IV‐2SLS | PS‐adjusted logistic regression |
| Stroke or neurologic complications in hospital | IV‐2SLS | PS‐adjusted logistic regression |
| Stent thrombosis within 30 d | IV‐2SLS | PS‐adjusted logistic regression |
| Stent thrombosis within 1 y | IV‐2SLS | PS‐adjusted logistic regression |
Variables included in propensity score: age, sex, diabetes mellitus, smoking status, hypertension, hyperlipidemia, previous myocardial infarction, previous percutaneous coronary intervention, previous coronary artery bypass grafting, severity of coronary artery disease, completeness of revascularization, treatment with bivalirudin, treatment with unfractionated heparin/low molecular weight heparin, treatment with glycoprotein IIb/IIIa receptor inhibitor, treatment with aspirin, treatment with clopidogrel, treatment with ticagrelor, treatment with prasugrel, treatment with warfarin, treatment with thrombolysis and procedural success. IV‐2SLS indicates instrumental variable 2‐step least squares regression; PCI, percutaneous coronary intervention; PS, propensity score.
Table 2.
Patient Characteristics
| PCI+TA (n=10 660) | Missing (%) | PCI Only (n=32 169) | Missing (%) | P Value | P Value PS | |
|---|---|---|---|---|---|---|
| Age, y, mean±SD | 66±12 | 0 | 67±12 | 0 | <0.001 | 0.12 |
| Male sex, % | 74 | 0 | 70 | 0 | <0.001 | 0.41 |
| Diabetes mellitus, % | 13 | 1.7 | 15 | 1.8 | <0.001 | 0.63 |
| Hypertension, % | 39 | 3.9 | 42 | 4.0 | <0.001 | 0.43 |
| Hyperlipidemia, % | 20 | 5.1 | 21 | 5.1 | <0.01 | 0.77 |
| Smoking status, % | 11 | 11 | ||||
| Current smoker | 29 | 27 | <0.01 | 0.59 | ||
| Previous smoker | 26 | 25 | 0.33 | 0.99 | ||
| Previous MI, % | 12 | 3 | 14 | 3 | <0.001 | 0.47 |
| Previous PCI, % | 9 | 0.1 | 9 | 0.1 | 0.34 | 0.96 |
| Previous CABG, % | 3 | 0.1 | 3 | 0.1 | 0.64 | 0.89 |
| Extent of CAD, % | 0.5 | 0.4 | ||||
| 1 vessel | 54 | 47 | <0.001 | 0.35 | ||
| 2 vessels | 26 | 29 | <0.001 | 0.78 | ||
| 3 vessels | 15 | 18 | <0.001 | 0.45 | ||
| Left main | 4.0 | 5.2 | <0.001 | 0.68 | ||
| Puncture site, % | 0.0 | 0.0 | <0.001 | 0.83 | ||
| Femoral | 51 | 58 | ||||
| Radial | 49 | 42 | ||||
| Drug‐eluting stent, % | 34 | 0 | 35 | 0.01 | 0.25 | |
| Antithrombotic treatment, % | ||||||
| ASA | 96 | 0.0 | 96 | 0.1 | 0.54 | 0.84 |
| Clopidogrel | 72 | 0.1 | 72 | 0.1 | 0.60 | 0.36 |
| Ticagrelor | 23 | 10 | 20 | 5 | <0.001 | 0.51 |
| Prasugrel | 8.3 | 5.9 | 5.2 | 8.6 | <0.001 | 0.15 |
| GP2b3a receptor inhibitor | 43 | 0.1 | 46 | 0.1 | <0.001 | <0.01 |
| UH/LMWH | 90 | 0.1 | 89 | 0.3 | 0.05 | 0.65 |
| Bivalirudin | 54 | 2.4 | 41 | 3.0 | <0.001 | 0.13 |
| Thrombolysis | 2.6 | 0.1 | 3.4 | 0.2 | <0.001 | 0.42 |
| Warfarin | 2.1 | 0.1 | 2.1 | 0.2 | 0.94 | 0.66 |
| Cardiogenic shock, % | 3.6 | 1.5 | 3.9 | 1.8 | 0.26 | 0.94 |
| Complete revascularization, % | 61 | 0.7 | 54 | 0.8 | <0.001 | 0.37 |
| Success, % | 96 | 0.0 | 95 | 0.0 | <0.001 | 0.49 |
| Direct stenting, % | 38 | 0.0 | 23 | 0.1 | <0.001 | NA |
ASA indicates acetylsalicylic acid; CABG, coronary artery bypass grafting; CAD, coronary artery disease; GP2b3a, glycoprotein IIb/IIIa; MI, myocardial infarction; PCI, percutaneous coronary intervention; PS, propensity score; TA, thrombus aspiration; UH/LMWH, unfractionated heparin/low molecular weight heparin.
Postestimation diagnostics
Goodness of fit (calibration) for the models was assessed with the Hosmer–Lemeshow test. Multicollinearity between the variables in the model was assessed by calculation of the variance inflation factor. All statistical analyses were performed using Stata software (version 14.1; StataCorp). Instrumental variable 2SLS regression models were completed using the IVREG2 module.22 All tests were 2‐tailed, and P<0.05 was considered statistically significant. Because of multiple analyses, P<0.05 was expected to occur accidentally in 1 of 20 analyses. The validity of instrumental variables was examined by calculation of the standardized difference of variables that reflects known patient characteristics and procedural details in treated and untreated patients stratified by administrative healthcare region (primary instrument) and quintiles of hospitals' total usage of thrombus aspiration during the study period (secondary instrument). We used logistic regression to evaluate the predictive power of instruments for treatment with thrombus aspiration and for primary and secondary outcomes.
Results
Patient Characteristics and Treatments
During the study period between January 2005 and September 2014, we identified 45 151 patients who underwent primary PCI (Figure 1). We excluded patients without Swedish personal identification numbers (n=2242), patients with missing follow‐up times for any of the primary or secondary end points (n=2159), and patients with missing information about treatment with thrombus aspiration (n=163). After exclusion of these patients, 42 829 patients (29% female) were included in the study, of which 10 660 (26% female) were treated with thrombus aspiration. There were 3804 primary PCIs in Sweden in 2005, after which the number increased and remained steady at ≈4500 procedures per year between 2006 and 2014. The proportion of female patients who were treated with primary PCI during the study period remained constant at 29%. General patient characteristics are presented in Table 2. Patient characteristics stratified by region are presented in Table 3. Patients treated with thrombus aspiration were on average younger; more likely to be male; and less likely to have treatment for hypertension, hyperlipidemia, or diabetes mellitus. They were more likely to be active smokers and were more often treated with ticagrelor, prasugrel, and bivalirudin but were less likely to be treated with GP2b/3a (glycoprotein IIb/IIIa) receptor inhibitor or thrombolysis before or during PCI. Patients who were not treated with thrombus aspiration were less likely to have a radial access site or to have 1‐vessel disease. They were more often completely revascularized at index PCI but were less likely to receive a drug‐eluting stent. Stents were more often placed without prior balloon dilatation of the lesion (direct stenting) in patients treated with thrombus aspiration. The frequency of thrombus aspiration varied among administrative healthcare regions from 18% to 35% (Table 3).
Figure 1.
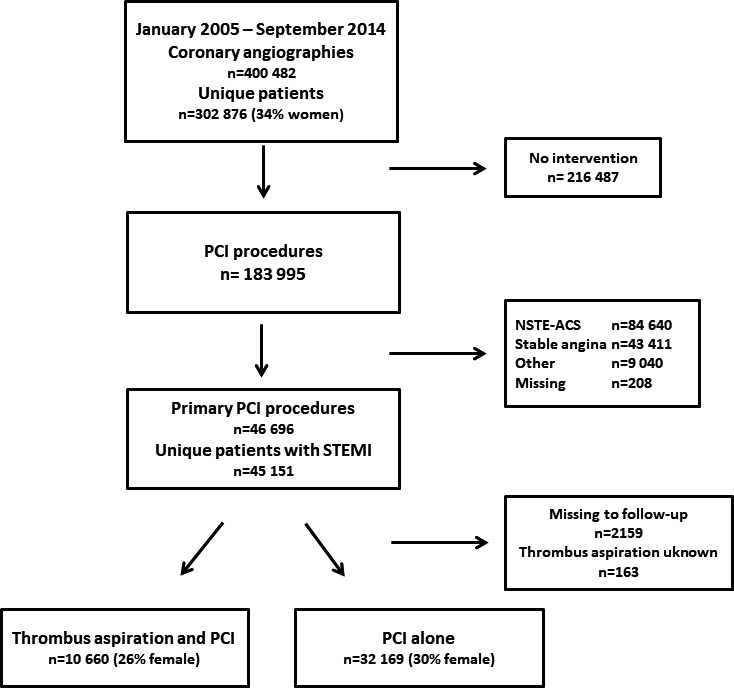
Flow chart of patient selection in the Swedish Coronary Angiography and Angioplasty Registry. NSTE‐ACS indicates non–ST‐segment–elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Table 3.
Utilization of Thrombus Aspiration, Outcomes, and Patient Characteristics by Administrative Region
| Region 1 | M | Region 2 | M | Region 3 | M | Region 4 | M | Region 5 | M | Region 6 | M | Stand. Diff. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 6623 | 11 115 | 5654 | 8835 | 7715 | 2885 | |||||||
| Thrombus aspiration, % | 35 | 25 | 20 | 28 | 18 | 23 | |||||||
| Mortality | |||||||||||||
| Died within 30 d, % | 6.5 | 6.1 | 6.5 | 6.0 | 6.5 | 6.5 | 0.01 | ||||||
| Died within 1 y, % | 8.7 | 8.9 | 9.6 | 9.0 | 8.9 | 8.6 | 0.02 | ||||||
| Stent thrombosis at 30 d, % | 0.5 | 0.6 | 1.0 | 0.7 | 1.1 | 0.5 | 0.05 | ||||||
| Stent thrombosis at 1 y, % | 0.8 | 0.8 | 1.2 | 1.0 | 1.4 | 0.7 | 0.04 | ||||||
| In‐hospital stroke or neurologic complication, % | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 0.01 | ||||||
| Age, y, mean±SD | 65±12 | 0 | 68±12 | 0 | 68±12 | 0 | 67±12 | 0 | 67±12 | 0 | 67±12 | 0 | 0.09 |
| Sex male, % | 74 | 0 | 70 | 0 | 69 | 0 | 70 | 0 | 72 | 0 | 73 | 0 | 0.06 |
| Diabetes mellitus, % | 14 | 3.0 | 15 | 1.6 | 16 | 1.7 | 14 | 1.4 | 13 | 2.0 | 15 | 0.6 | 0.03 |
| Hypertension, % | 41 | 5.5 | 44 | 3.4 | 43 | 2.7 | 42 | 3.7 | 40 | 4.8 | 51 | 3.2 | 0.04 |
| Hyperlipidemia, % | 20 | 7.7 | 24 | 4.6 | 23 | 2.9 | 21 | 4.9 | 20 | 6.1 | 27 | 4.0 | 0.02 |
| Smoking status, % | 19 | 8 | 5 | 6 | 17 | 16 | |||||||
| Current smoker | 32 | 30 | 29 | 34 | 30 | 29 | 0.02 | ||||||
| Previous smoker | 23 | 29 | 30 | 32 | 28 | 28 | 0.03 | ||||||
| Previous MI, % | 13 | 4.3 | 15 | 3.9 | 15 | 3.2 | 14 | 2.9 | 12 | 2.6 | 14 | 2.8 | 0.06 |
| Previous PCI, % | 8 | 0.2 | 11 | 0.1 | 9 | 0.2 | 8 | 0.2 | 8 | 0.1 | 9 | 0 | 0.01 |
| Previous CABG, % | 3 | 0.1 | 3 | 0.1 | 3 | 0.2 | 3 | 0.1 | 4 | 0.1 | 4 | 0 | 0.01 |
| Extent of CAD, % | 1.0 | 0.3 | 0.6 | 0.6 | 0.1 | 0.3 | |||||||
| 1 vessel | 53 | 55 | 45 | 44 | 46 | 45 | 0.11 | ||||||
| 2 vessels | 27 | 26 | 30 | 28 | 30 | 29 | 0.04 | ||||||
| 3 vessels | 15 | 14 | 21 | 21 | 18 | 21 | 0.08 | ||||||
| Left main | 4 | 4 | 5 | 6 | 6 | 4 | 0.05 | ||||||
| Puncture site, % | 0 | 0 | 0.1 | 0 | 0 | 0 | 0.26 | ||||||
| Radial | 23 | 76 | 31 | 31 | 41 | 43 | |||||||
| Femoral | 77 | 24 | 69 | 69 | 59 | 57 | |||||||
| Drug‐eluting stent, % | 34 | 0 | 49 | 0 | 33 | 0 | 32 | 0 | 25 | 0 | 37 | 0 | 0.08 |
| Antithrombotic treatment, % | |||||||||||||
| ASA | 98 | 0.1 | 96 | 0.1 | 92 | 0.2 | 97 | 0.2 | 98 | 0.1 | 98 | 0.1 | 0.01 |
| Clopidogrel | 86 | 0.1 | 71 | 0.1 | 54 | 0.2 | 71 | 0.2 | 80 | 0.1 | 59 | 0.1 | 0.17 |
| Ticagrelor | 24 | 5.9 | 20 | 6.5 | 14 | 5.9 | 26 | 5.9 | 22 | 7.0 | 11 | 6.6 | 0.21 |
| Prasugrel | 0.3 | 5.1 | 5.7 | 7.4 | 0.2 | 5.8 | 7.5 | 6.5 | 0.3 | 6.7 | 42 | 7.9 | 0.14 |
| Warfarin | 2.1 | 0.1 | 1.8 | 0.2 | 2.9 | 0.2 | 2.0 | 0.3 | 2.2 | 0.1 | 2.0 | 0 | 0.02 |
| GP2b3a | 60 | 0 | 43 | 0.1 | 61 | 0 | 38 | 0.2 | 37 | 0.1 | 32 | 0 | 0.19 |
| UH/LMWH | 91 | 0.1 | 95 | 0.2 | 97 | 0.2 | 88 | 0.2 | 81 | 0.6 | 80 | 0.4 | 0.01 |
| Bivalirudin | 32 | 3.1 | 44 | 2.5 | 18 | 2.8 | 54 | 3.3 | 61 | 3.0 | 54 | 2.1 | 0.34 |
| Thrombolysis | 2.0 | 0.1 | 2.1 | 0.2 | 1.5 | 0.2 | 0.3 | 0.3 | 1.6 | 0.1 | 26 | 0 | 0.04 |
| Cardiogenic shock, % | 4.5 | 0.8 | 3.7 | 1.8 | 3.9 | 5.8 | 2.9 | 0.9 | 4.9 | 1.0 | 2.3 | 0.6 | 0.01 |
| Complete revascularization, % | 55 | 0.5 | 67 | 0.7 | 53 | 2.0 | 51 | 1.0 | 50 | 0.3 | 53 | 0.3 | 0.12 |
| Success, % | 95 | 0 | 95 | 0 | 93 | 0 | 96 | 0 | 95 | 0 | 96 | 0 | 0.05 |
ASA indicates acetylsalicylic acid; CABG, coronary artery bypass grafting; CAD, coronary artery disease; GP2b3a, glycoprotein IIb/IIIa receptor inhibitor; M, missing data; MI, myocardial infarction; PCI, percutaneous coronary intervention; Stand. Diff, standardized differences for treated vs untreated with thrombus aspiration; UH/LMWH, unfractionated heparin/low molecular weight heparin.
Primary Outcome
Mortality at 30 days
During the study period, age‐ and sex‐adjusted mortality decreased significantly at 30 days (odds ratio: 0.97; 95% confidence interval [CI], 0.96–0.98; P<0.001) and 1 year (odds ratio: 0.98; 95% CI, 0.96–0.99; P<0.001). This positive trend was similar for men and women. Mortality at 30 days (6.1% versus 6.3%) was similar in both groups (Figure 2A). The primary analysis—2SLS regression with administrative healthcare region as the treatment preference instrumental variable—showed no difference in 30‐day mortality between groups (risk reduction: −1.2; 95% CI, −5.4 to 3.0; P=0.57). Sensitivity analysis based on the alternative instrumental variable (ie, hospital preference for thrombus aspiration) showed similar results (risk reduction: 3.8; 95% CI, −4.1 to 4.8; P=0.86). The secondary analysis based on unadjusted multilevel logistic regression showed no difference in 30‐day mortality; however, when we adjusted for quintiles of propensity score, we found an association between thrombus aspiration and increased risk of mortality. This was true both for the models based on the complete‐case analyses and the analyses after multiple imputation of missing data (Table 4).
Figure 2.
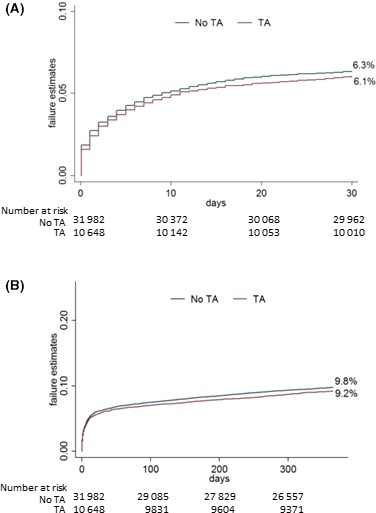
Kaplan–Meier curves. A, Thirty‐day mortality. B, One‐year mortality. TA indicates thrombus aspiration.
Table 4.
Primary and Secondary Outcomes
| IV‐2SLS Regressiona | Unadjusted Multilevel Logistic Regressionb | Complete Case PS‐Adjusted Multilevel Logistic Regressionb | PS‐Adjusted Multilevel Logistic Regression After Multiple Imputationb | |
|---|---|---|---|---|
| Death within 30 d |
−1.2 (−5.4 to 3.0) P=0.57 |
0.95 (0.87–1.05) P=0.32 |
1.21 (1.07–1.37) P<0.01 |
1.16 (1.06–1.28) P<0.01 |
| Death within 1 y |
−2.4 (−7.6 to 3.0) P=0.37 |
0.93 (0.86–1.01) P=0.07 |
1.16 (1.05–1.28) P<0.01 |
1.12 (1.04–1.22) P<0.01 |
| Stroke or neurologic complication in hospital |
0.1 (−0.8 to 1.1) P=0.76 |
1.05 (0.70–1.58) P=0.80 |
1.31 (0.81–2.12) P=0.27 |
1.30 (0.86–1.95) P=0.22 |
| Stent thrombosis within 30 d |
−2.7 (−4.1 to −1.4) P<0.001 |
0.69 (0.50–0.95) P=0.02 |
0.74 (0.50–1.08) P=0.13 |
0.75 (0.54–1.03) P=0.08 |
| Stent thrombosis within 1 y |
−3.5 (−5.3 to −1.7) P<0.001 |
0.74 (0.58–0.95) P=0.02 |
0.75 (0.55–1.02) P=0.06 |
0.79 (0.61–1.01) P=0.06 |
| Stent thrombosis within 1 y landmarkc |
−0.01 (−0.03 to 0.05) P=0.59 |
1.05 (0.73–1.51) P=0.79 |
1.15 (0.76–1.74) P=0.49 |
1.15 (0.79–1.67) P=0.46 |
CI indicates confidence interval; IV‐2SLS, instrumental variable 2‐stage least squares; PS, propensity score.
Risk reduction per 100 patients (95% confidence interval).
Odds ratio (95% confidence interval).
Landmark analysis after exclusion of patients with stent thrombosis within 30 days.
Secondary Outcomes
Mortality at 1 year
The majority of patients (90%) were alive after ≥1 year of follow‐up. The probability of death at 1 year was 9.8% in patients treated with PCI alone and 9.2% in patients treated with thrombus aspiration (Figure 2B). The primary model based on instrumental variable analysis showed no difference in mortality at 1 year between groups (risk reduction: −2.4; 95% CI, −7.6 to 3.0; P=0.37). The secondary analysis based on unadjusted multilevel logistic regression with administrative region treated as random effect showed a trend for higher mortality at 30 days and became significant after propensity score adjustment. This was true both for the models based on the complete‐case analyses and the analyses with imputed data (Table 4). Sensitivity analysis based on the alternative instrumental variable showed similar results (risk reduction: 1.1; 95% CI, −4.3 to 6.5; P=0.69).
Stent thrombosis
The incidence of stent thrombosis at 30 days after primary PCI in all patients was 0.6% (n=255). At 30 days, stent thrombosis developed in 49 patients (0.47%) in the thrombus aspiration group and 206 patients (0.67%) in the PCI‐alone group (Figure 3A). The unadjusted risk estimate was higher in the PCI‐alone group (P=0.037). The primary model with instrumental variable analysis showed a significant association between thrombus aspiration and reduced risk of stent thrombosis both at 30 days (risk reduction: −2.7; 95% CI, −4.1 to −1.4; P<0.001) and 1 year (risk reduction: −3.5; 95% CI, −5.3 to −1.7; P<0.001; Figure 3B). However, landmark analysis after 30 days showed no difference between groups (risk reduction: −3.3; 95% CI, −1.3 to 0.7; P=0.54; Figure 3C). We found no evidence of treatment heterogeneity. The interaction test was not significant for patients aged <65 years (P=0.95) or with female sex (P=0.39), diabetes mellitus (0.29), treated vessel disease (P=0.29), or cardiogenic shock (P=0.39). The frequency of thrombus aspiration varied annually. There was a negative correlation between the annual number of reported cases of stent thrombosis and the annual number of thrombus aspirations performed during primary PCI. The frequency of thrombus aspiration at 30 days was highest in 2010 (37.3%), 2011 (40.6%) and 2012 (38.6%) and lowest in 2005 (17.5), 2006 (9.8), 2007 (10.1%) and 2008 (21.2%) (Fig. 4A). The frequency of stent thrombosis at 30 days was lowest in 2010 (0.42%), 2011 (0.48%) and 2012 (0.34%) and highest in 2007 (0.74%), 2008 (0.89%) and 2009 (0.95%) (Fig. 4B).
Figure 3.
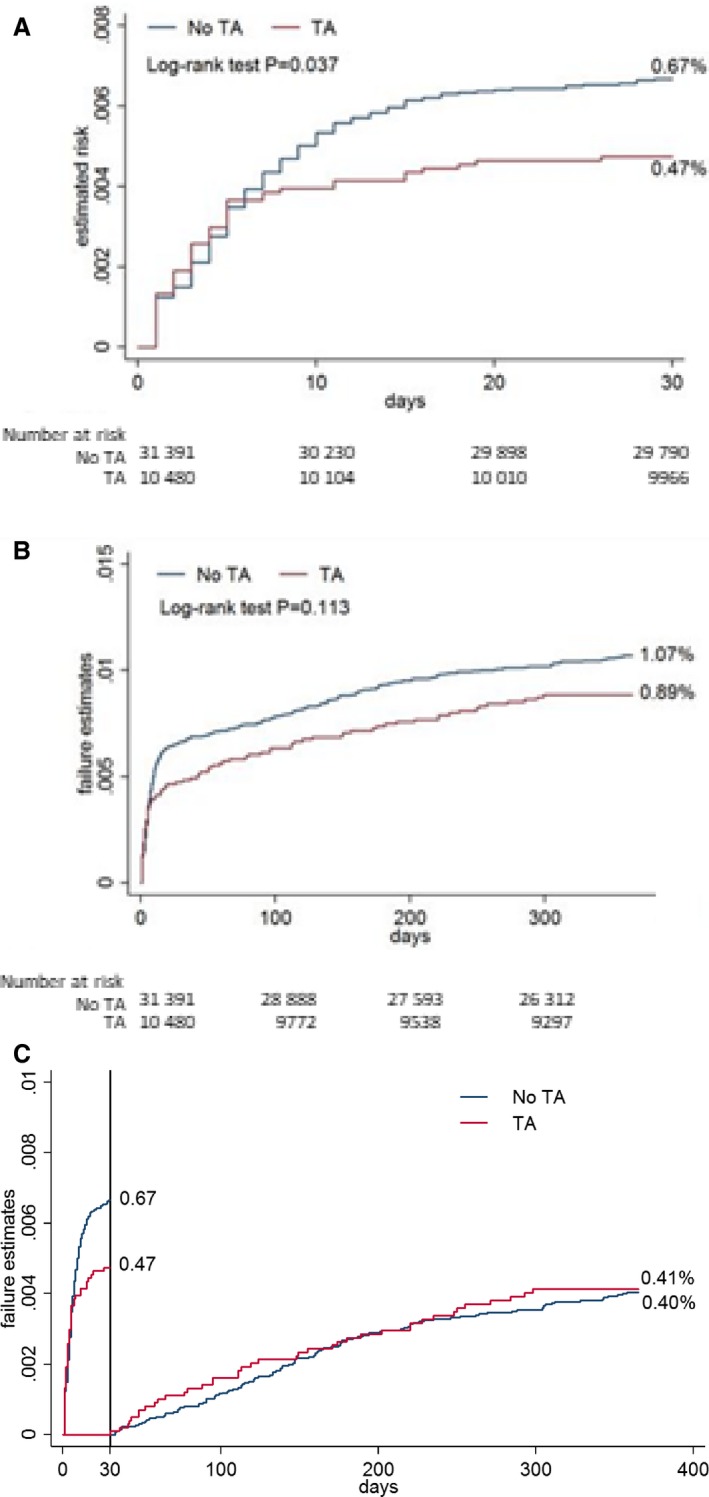
Kaplan–Meier curves for stent thrombosis. A, Stent thrombosis at 30 days. B, Stent thrombosis at 1 year. C, Landmark analysis for stent thrombosis at 30 days. TA indicates thrombus aspiration.
Figure 4.

Annual rate of thrombus aspiration during primary PCI in Sweden A). Annual rate of stent thrombosis after primary PCI in Sweden B). PCI indicates percutaneous coronary intervention.
In‐hospital neurologic complication
There were 122 (0.3%) in‐hospital neurologic complications, of which 90 occurred in the PCI‐only group (0.3%) and 32 in the thrombus aspiration group (0.3%). Unadjusted and adjusted analyses showed no differences in risk of stroke or neurologic complications between groups (Table 4).
Data Analysis and Postestimation Diagnostics
Most variables had missing information; however, information was missing for >5% patients in only 4 variables: smoking status, hyperlipidemia, treatment with ticagrelor, and treatment with prasugrel (Table 2). Postestimation analysis for the logistic regression models, including propensity score estimation, by the Hosmer–Lemeshow test showed the adequate goodness of fit for the models (P>0.05). Squared covariate terms had no explanatory power in any models (link test, P>0.05). Balancing properties of the calculated propensity scores were evaluated using multivariate linear and logistic regression. After adjustment with quintiles of propensity score as a covariate in the regression model, there was no statistical difference in the baseline characteristics between groups except for treatment with GP2b/3a receptor inhibitors (Table 2). Average variance inflation factor was <5.0 for all models, indicating a lack of multicollinearity between variables. Comparison of patient characteristics after stratification based on administrative healthcare region supported the assumption that this instrumental variable fulfills the criteria for the valid instrument (Table 3). After stratification, all variables had a standardized difference of the mean below or close to 0.25, which is an accepted cutoff level for balance in patient characteristics23, 24 (Table 3). The Durbin–Wu–Hausman test for endogeneity was statistically significant at P<0.001 for all dependent variables modeled with 2SLS regression.
Discussion
We investigated the effect of thrombus aspiration on mortality, stent thrombosis, and stroke or neurologic complications in 45 151 patients with STEMI in the prospective SCAAR database, of whom 10 660 were treated with thrombus aspiration. We found that treatment with thrombus aspiration was not associated with risk of mortality and in‐hospital stroke or neurologic complications and that thrombus aspiration was associated with decreased risk of stent thrombosis.
The large number of prospectively followed STEMI patients in SCAAR provided a unique opportunity to evaluate the association between thrombus aspiration and important clinical outcomes in unselected patients from everyday clinical practice. Before TASTE and TOTAL, meta‐analyses of smaller studies showed diverging results. Although some studies reported improved survival with thrombus aspiration, others did not.4, 25, 26, 27 Our data are in agreement with the results from TASTE and TOTAL, which showed that routine thrombus aspiration does not decrease mortality. The present study thus constitutes important evidence for the external validity of these 2 trials regarding mortality. The STEMI cohort from SCAAR is by far the largest cohort of consecutive STEMI patients in whom thrombus aspiration has been evaluated; the cohort consists of more patients than those in all other RCTs combined to date. An important criticism of TASTE regards the presence of selection bias. Although ≈60% of all patients with STEMI were included in the study—this is the largest percentage of patients with STEMI included in a single RCT within the field of interventional cardiology—the number of excluded patients was still high (≈40%). This may have caused limited external validity. Indeed, the excluded patients were substantially different from the patients included in the study, as shown by almost 3 times higher mortality in the nonrandomized patients. Similar criticism has been forwarded against TOTAL, in which mortality at 180 days was ≈3.5%—fairly low compared with that observed in an unselected STEMI population. Given these circumstances, the strength and importance of our study are grounded on the fact that our data are derived from all consecutive STEMI patients and all PCI centers in Sweden over a period of 9 years. We used a statistical method based on treatment‐preference instrumental variable analysis with 2SLS regression.15, 17, 28 This method allows adjustment for measured and unmeasured confounders. If the central methodological assumptions are fulfilled (ie, a valid instrument that reflects a naturally occurring randomization process), observational studies based on the instrumental variable method mimic the act of randomization in an RCT. Compared with the results based on the propensity score–adjusted logistic regression, the results from the instrumental variable–based statistical modeling have shown risk estimates more in line with those from TASTE and TOTAL.
Stent thrombosis is a feared complication during and after primary PCI because it is associated with high mortality.29 Any treatment strategy that decreases this complication holds high value for clinical practice. In TASTE, thrombus aspiration decreased the risk of stent thrombosis by more than half at 30 days5 but not at 1 year.6 Because our study mirrors the results from TASTE regarding the short‐ and long‐term risk of stent thrombosis, it strengthens the evidence for the protective role of thrombus aspiration against acute and subacute stent thrombosis. More indirect evidence in this study supports the association between thrombus aspiration and decreased risk of stent thrombosis. Routine thrombus aspiration in STEMI was introduced on a larger scale in Sweden after the report from TAPAS (Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction Study), which showed increased survival in the thrombus aspiration group.1 In subsequent years, the number of procedures with thrombus aspiration increased steadily in our country. But after the publication of TASTE, the use of thrombus aspiration in Sweden decreased substantially. During this period of varying practice, we observed a negative correlation between the yearly incidence of stent thrombosis, on one side, and the use of thrombus aspiration, on the other side. The incidence of stent thrombosis was higher in Sweden during the years when treatment with thrombus aspiration was lower.
The possibility that thrombus aspiration increases the risk of stroke is an important consideration that needs to be studied in detail to properly evaluate the clinical importance and cost‐effectiveness of this treatment strategy. Similar to TASTE, our study did not show an increase risk of stroke or neurologic complication, as was reported in TOTAL. The reason for the discrepancy may be explained by the play of chance given the low number of events in TOTAL. With only 49 stroke events, the statistical power in this study was limited. Furthermore, the latest meta‐analyses in which data from TASTE and TOTAL were included have not shown a statistically significantly increased risk of stroke within 30 days.30, 31 Current European Society of Cardiology guidelines for primary PCI do not recommend routine thrombus aspiration. However, in cases of large residual thrombus burden after opening the vessel, the guidelines suggests that thrombus aspiration may be considered.32 Our study supports this recommendation.
Several limitations need to be discussed. First, this study is observational, and as such, it provides only associative evidence, not causative, because we cannot exclude selection bias and residual confounding. In contrast, the observational nature of our study provides real‐world data from a large cohort of patients. Second, we do not have data on cause‐specific mortality. Third, a proportion of patients had missing data. Exclusion of patients from the analysis who had missing data might have produced biased results; however, results from the multiple imputation models were congruent with the data from the complete‐case analysis. Last, we performed many analyses using several different statistical models. This increases the risk of associations occurring by chance.
In conclusion, our study provides important evidence of the external validity of TASTE and TOTAL regarding mortality. Thrombus aspiration during primary PCI may decrease the risk of stent thrombosis, and future studies should determine whether this treatment may be cost‐effective for prevention of stent thrombosis even in the absence of a survival benefit.
Sources of Funding
This work was supported by AFA Insurance (AFA 110115); Swedish Heart and Lung Foundation (HLF 20120670); Swedish Scientific Research Council (VR 2008‐2487); ALF Västra Götaland; and University of Gothenburg, Sweden (ALFGBG 141131).
Disclosures
None.
Oskar Angerås, Inger Haraldsson, Björn Redfors, Ole Fröbert, Petur Petursson, Per Albertsson, Dan Ioanes, Jacob Odenstedt, Hans Olsson, Nils Witt, Andreas Ruck, Jonas Millgård, Johan Nilsson, Jonas Persson, Måns Söderbom, Hans Wedel have no disclosures to report.
David Erlinge has fees for lectures and advisory boards from AstraZeneca and Medicines Company.
Stefan James has received institutional grants from Medtronic, Vascular Solutions, Terumo, Boston Scientific, Abbot Vascular, and personal lecture fees from Boston Scientific, Medtronic, Abbot and Biotronic.
Truls Råmunddal has received proctoring honoraria from Boston Scientific and consultant honoraria Abbott.
Elmir Omerovic has received institutional grants from Abbot Vascular and Astra Zeneca, advisory board from Boston Scientific and Bayer.
Supporting information
Appendix S1. Participating Centers in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
Data S1. Description of the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) and SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies).
(J Am Heart Assoc. 2018;7:e007680 DOI: 10.1161/JAHA.117.007680.)29317403
References
- 1. Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. [DOI] [PubMed] [Google Scholar]
- 2. Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1‐year follow‐up study. Lancet. 2008;371:1915–1920. [DOI] [PubMed] [Google Scholar]
- 3. Sardella G, Mancone M, Bucciarelli‐Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G, Fedele F. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct‐related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–315. [DOI] [PubMed] [Google Scholar]
- 4. De Luca G, Navarese EP, Suryapranata H. A meta‐analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166:606–612. [DOI] [PubMed] [Google Scholar]
- 5. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK; Trial T . Thrombus aspiration during ST‐segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 6. Lagerqvist B, Frobert O, Olivecrona GK, Gudnason T, Maeng M, Alstrom P, Andersson J, Calais F, Carlsson J, Collste O, Gotberg M, Hardhammar P, Ioanes D, Kallryd A, Linder R, Lundin A, Odenstedt J, Omerovic E, Puskar V, Todt T, Zelleroth E, Ostlund O, James SK. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–1120. [DOI] [PubMed] [Google Scholar]
- 7. Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, Kedev S, Thabane L, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemelä K, Steg PG, Bernat I, Xu Y, Cantor WJ, Overgaard CB, Naber CK, Cheema AN, Welsh RC, Bertrand OF, Avezum A, Bhindi R, Pancholy S, Rao SV, Natarajan MK, ten Berg JM, Shestakovska O, Gao P, Widimsky P, Džavík V. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauer MS, D'Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med. 2013;369:1579–1581. [DOI] [PubMed] [Google Scholar]
- 9. Steg PG, Lopez‐Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA Jr, Himbert D, Allegrone J, Van de Werf F. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. [DOI] [PubMed] [Google Scholar]
- 10. Ho PM, Peterson PN, Masoudi FA. Evaluating the evidence: is there a rigid hierarchy? Circulation. 2008;118:1675–1684. [DOI] [PubMed] [Google Scholar]
- 11. Ramunddal T, Hoebers LP, Henriques JP, Dworeck C, Angeras O, Odenstedt J, Ioanes D, Olivecrona G, Harnek J, Jensen U, Aasa M, Albertsson P, Wedel H, Omerovic E. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535–1544. [DOI] [PubMed] [Google Scholar]
- 12. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 13. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubin DB. Inference and missing data. Biometrika. 1976;63:581–590. [Google Scholar]
- 15. Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res. 1998;33:1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 16. Mcclellan M, Mcneil BJ, Newhouse JP. Does more intensive treatment of acute myocardial‐infarction in the elderly reduce mortality—analysis using instrumental variables. JAMA. 1994;272:859–866. [PubMed] [Google Scholar]
- 17. Rassen JA, Schneeweiss S, Glynn RJ, Mittleman MA, Brookhart MA. Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol. 2009;169:273–284. [DOI] [PubMed] [Google Scholar]
- 18. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol. 2001;19:1064–1070. [DOI] [PubMed] [Google Scholar]
- 21. Bound J, Jaeger DA, Baker RM. Problems with instrumental variables estimation when the correlation between the instruments and the endogeneous explanatory variable is weak. J Am Stat Assoc. 1995;90:443–450. [Google Scholar]
- 22. Baum CF, Schaffer ME, Stillman S. IVREG2: Stata module to extended instrumental variables/2SLS, GMM and AC/HAC, LIML and k‐class regression. 2002.
- 23. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 24. Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. [Google Scholar]
- 25. Burzotta F, De Vita M, Gu YL, Isshiki T, Lefevre T, Kaltoft A, Dudek D, Sardella G, Orrego PS, Antoniucci D, De Luca L, Biondi‐Zoccai GG, Crea F, Zijlstra F. Clinical impact of thrombectomy in acute ST‐elevation myocardial infarction: an individual patient‐data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–2203. [DOI] [PubMed] [Google Scholar]
- 26. Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta‐analysis of randomized trials. Eur Heart J. 2008;29:2989–3001. [DOI] [PubMed] [Google Scholar]
- 27. Mongeon FP, Belisle P, Joseph L, Eisenberg MJ, Rinfret S. Adjunctive thrombectomy for acute myocardial infarction: a Bayesian meta‐analysis. Circ Cardiovasc Interv. 2010;3:6–16. [DOI] [PubMed] [Google Scholar]
- 28. Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiology. 2006;17:260–267. [DOI] [PubMed] [Google Scholar]
- 29. Brener SJ, Kirtane AJ, Stuckey TD, Witzenbichler B, Rinaldi MJ, Neumann F‐J, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL, Mehran R, Parvataneni R, Brodie BR, Stone GW. The impact of timing of ischemic and hemorrhagic events on mortality after percutaneous coronary intervention: the ADAPT‐DES Study. JACC Cardiovasc Interv. 2016;9:1450–1457. [DOI] [PubMed] [Google Scholar]
- 30. Ghatak A, Singh V, Shantha GP, Badheka A, Patel N, Alfonso CE, Biswas M, Pancholy SB, Grines C, O'Neill WW, de Marchena E, Cohen MG. Aspiration thrombectomy in patients undergoing primary angioplasty for ST elevation myocardial infarction: an updated meta‐analysis. J Interv Cardiol. 2015;28:503–513. [DOI] [PubMed] [Google Scholar]
- 31. Jolly SS, James S, Džavík V, Cairns JA, Mahmoud KD, Zijlstra F, Yusuf S, Olivecrona GK, Renlund H, Gao P, Lagerqvist B, Alazzoni A, Kedev S, Stankovic G, Meeks B, Frøbert O. Thrombus aspiration in ST‐segment‐elevation myocardial infarction: an individual patient meta‐analysis: Thrombectomy Trialists Collaboration. Circulation. 2017;135:143–152. [DOI] [PubMed] [Google Scholar]
- 32. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;ehx393, https://doi.org/10.1093/eurheartj/ehx393. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Participating Centers in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
Data S1. Description of the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) and SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies).


