Abstract
Parabens are widely used as preservative substances in foods, pharmaceuticals, industrial products, and cosmetics. But several studies have cautioned that parabens have estrogenic or endocrine-disrupting properties. Drosophila melanogaster is an ideal model in vivo to detect the toxic effects of chemistry. The study was designed to assess the potential additive toxic effects of methylparaben (MP) and ethylparaben (EP) mixture (MP + EP) on lifespan and preadult development period in D. melanogaster. The data revealed that the MP + EP can reduce the longevity of flies compared with the control group, consistent with a significant reduction in malondialdehyde levels and an increase in superoxide dismutase activities. Furthermore, MP + EP may have a greater toxic effect on longevity of flies than separate using with the same concentration. Additionally, parabens had a nonmonotonic dose–response effect on D. melanogaster preadult development period, showing that MP + EP delayed preadult development period compared with control group while individual MP or EP significantly shortened (P < 0.01) at low concentration (300 mg/l). In conclusion, MP + EP had the potential additive toxicity on lifespan and preadult development period for D. melanogaster.
Keywords: methylparaben, ethylparaben, Drosophila melanogaster, lifespan, development period
Parabens, alkylesters of p-hydroxybenzoic acid, mainly including methylparaben (MP), ethylparaben (EP), and propylparaben (PP), have been widely used as preservatives in foods, pharmaceuticals, cosmetics, and industrial products because of their broad antimicrobial spectra with relatively low toxicity, good stability, and nonvolatility (Brausch and Rand 2011). MP and EP are regarded as the most common ingredient of personal care products, and the limits of detection in creams was up to 5–2,000 mg/l (Supp Table 1 [online only]). Parabens are present with low concentration in environmental samples such as rivers, air, dust, and so on. Human can be exposed to parabens by inhalation, oral intake, and dermal absorption. It is estimated that the average daily parabens exposure from personal care products (PCPs) is 0.833–2.4, 0.417 mg/kg bw/d for pharmaceutical products, and ∼0.77 × 10−4 mg/kg bw/d for food (Błędzka et al. 2014). Cosmetic Ingredient Review calculated an adult human dose of 1.2 mg/kg bw/d of multiple parabens and an infant dose of 0.3 mg/kg bw/d and compare this with a no observed adverse effect level for all parabens of 1,000 mg/kg bw/d (Andersen 2008). In European Union countries, the allowable content of parabens in cosmetic products is 0.4 % for single ester and 0.8 % for mixtures of all parabens (European Parliament and Council of European Union 2009). Parabens are also detected in human breast tissue (Harvey and Everett 2004), human milk (Schlumpf et al. 2010), human urine and serum (Carrasco-Correa et al. 2015) in recent years. Furthermore, parabens possess estrogenic activity (Routledge et al. 1998; Darbre and Harvey 2008; Lange et al. 2014) and antiandrogenic activity (Satoh et al. 2005; Chen et al. 2007), which were classified as potential endocrine-disrupting chemicals (EDCs) by the Endocrine Society (Diamanti-Kandarakis et al. 2009). Several studies showed that parabens can promote the proliferation of MCF-7 human breast cancer cells (Charles and Darbre 2013; Khanna et al. 2014), affect the male reproductive system in mice (Zhang et al. 2014), inhibit the mitochondrial respiratory capacities (Nakagawa and Moldéus 1998) and induce oxidative stress in the cell (Nishizawa et al. 2006; Popa et al. 2011; Shah and Verma 2011).
Nowadays, the ‘free radical theory of ageing is one of the most major theories providing a testable biological mechanism for aging process, which states that aging is a process of irreversible injuries associated with accumulation of oxidative damages induced by the most abundant free radicals in cells (Harman 1956). The free radicals are scavenged by a wide spectrum of enzymatic mechanisms such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase, to avoid oxidative damages in vivo (Guemouri et al. 1991). Lipid peroxidation is an autocatalytic process which may cause peroxidative tissue damage in inflammation, cancer, and aging (İnal et al. 2001). Malondialdehyde (MDA) is used as an index of lipid peroxidation (Draper and Hadley 1990).
Drosophila melanogaster as an invertebrate model organism, due to its short life span, well-defined genetics, ease rearing in the laboratory, has been used to test the effects of potential chemical pollutants on reproduction, endocrinology, and longevity (Rand 2010). Our laboratory has studied the endocrine-disrupting effect of individual paraben in D. melanogaster for many years. We found that individual MP had toxic effects on the fecundity and development of fruit flies, and lower concentration of MP had potential estrogenic activities (Gu et al. 2009). Individual EP not only affected longevity, fecundity, and the expression of apoptosis-related gene (reaper) (Ying 2012), but also influenced the expression of estrogen-related receptor, ecdysone receptor (EcR), and yolk protein receptor (Liu et al. 2014). Similarly individual PP also influenced the lifespan and induced the significant increase in MDA levels and a decrease of activities of SOD and CAT (Li et al. 2015).
Recently, with the increasing probability of exposure, humans are likely exposed to mixtures of parabens and the most widely used preservative system consists of a combination of parabens (Steinberg 2010). So more research needs to be carried out to examine the potential additive toxicity of parabens mixtures. The tests for mixture toxicity of EDCs and/or parabens have been performed on aquatic organisms (Yamamoto et al. 2011), rats (Isling et al. 2014), and human cell (Evans et al. 2012), but rarely on fruit flies (Ayar and Uysal 2013). This study investigated the effect of the MP and EP mixture (MP + EP) on the lifespan and preadult development period in D. melanogaster with the attempt to elucidate the potential additive toxicity.
Materials and Methods
Strains Stocks and Culture
The wild Canton S (CS) strain was used in this study. The wild CS type flies were reared in the vials and maintained in an incubator. The incubator temperature and relative humidity were 25–26°C and 65–75%, respectively. The basal diet was prepared according to the standard formulation. In brief, 100 ml medium contained 30 g corn meal, 15 g glucose, 1 g yeast, 1.5 g agar, and 0.5 ml methylacetic acid. For the laying culture medium, it was prepared by adding 1 g active carbon to the basal diet per 100 ml and divided into a petri dish.
Treatment with Parabens
For all the analysis, the mixture of parabens by equal mass combination of MP and EP or individual paraben was dissolved in anhydrous ethanol (Tianjin Tianli Chemical Reagent Co., Ltd, TianJin, China) and added to basal diet to its final concentrations (300, 700, and 1,000 mg/l w/v). The same amount of ethanol, but without parabens, was maintained in control diet. Adult D. melanogaster or eggs were exposed to either the parabens-treatment or control diet (Fig. 1).
Fig. 1.
Summary of experimental procedure. For all the analysis, parabens were dissolved in ethanol and added to basal diet to its final concentrations (300, 700, and 1,000 mg/l). The same amount of ethanol was maintained in control. (a) Lifespan and antioxidation assay of flies. (b) Test of preadult development period
Lifespan Assay
Virgin male and female flies emerging from standard culture food were separated and divided into 4 groups (n = 200 each), and housed in 10 vials (20 flies per vial). The first group was maintained on the control diet, while the other groups were fed with the MP + EP diets (300, 700, and 1,000 mg/l) (Fig. 1a). Dead flies were counted every day and the remaining alive flies were transferred to a new vial containing the same diet every 2 d. The mean lifespan, maximum lifespan, and 50% survival time of flies were calculated as previous method (Liu et al. 2014). The experiments were repeated three times.
Antioxidation Assay
The fruit flies were cultured and treated as above mentioned approach and sacrificed at selected time points (days 10, 20, 30, 40, and 50) (Fig. 1a). The fruit flies (40 flies per sample) were homogenized in 0.40 ml of cold saline followed by centrifugation at a speed of 6,000 g for 10 min at 4°C. Then the supernatant transferred to new centrifugal tubes and was used for each test. SOD activity was quantified using a spectrophotometer (UNICAM, UV-300) according to a SOD assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). This assay is based on the inhibition of nitrite formation from hydroxyl ammonium in the presence of O2− generators mediated by SOD. The spectrophotometric absorbance was set at 550 nm and the results were expressed as units of SOD activity per mg protein. The protein contents were measured using commercial kit (Nanjing Jiancheng Bioengineering Company) by the coomassie brilliant blue method according to the manufacturer’s directions. Lipid peroxidation products were measured by commercial MDA kit (Nanjing Jiancheng Bioengineering Institute) using a spectrophotometer at 532 nm according to the manufacturer’s directions. The level of lipid peroxidation was calculated and expressed as mmol MDA per mg protein. Every experiment repeated three times.
Assay of Preadult Development Period
Within 24 hr newly ecloded flies were fed basal diet for 5 d, transferred to petri dish to lay eggs. One-hour egg collections were transferred to basal diet and parabens-treatment diets by inoculating needle in super-clean worktable (Suzhou Antai, Suzhou, China). Twenty eggs were reared in a vial (Fig. 1b). Eggs were allowed to hatch until eclosion. The number of pupation and eclosion was counted every day. In each replication 100 eggs were examined, with three replications.
Statistical Analysis
Statistical analyses were performed using SPSS package version 19.0 (IBM, Armonk, NY). Lifespan analyses were performed separately by sex and the significance of differences among samples was assessed using one-way ANOVA by least significant difference (LSD). Survival analysis was assessed by the Kaplan-Meier methods, and the differences between survival curves were analyzed using the log-rank test. The percentage of pupation (the ratio between number of pupation and number of egg examined), the percentage of adult eclosion (the ratio between number of eclosion and number of egg examined), larval period (time from egg to pupa), and pupal period (time from pupa to adult) were analyzed by LSD. Two-way ANOVA was performed to compare biochemical parameters and development period data for the Drosophila studies, and the association effects of each factor with the ANOVA model were analyzed by calculating their partial eta-squared (η2) values. Data were expressed as mean ± SD. Differences were considered significant when P < 0.05 or <0.01.
Results
Effect of MP + EP on Longevity of Flies
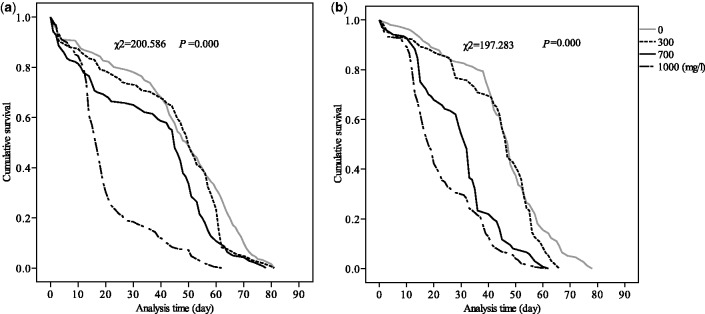
The results revealed that MP + EP groups had a lower mean and maximum lifespan than the control group not only for female [mean lifespan (F = 80.07; df = 3; P < 0.01), maximum lifespan (F = 8.07; = 3; P < 0.01)], but for male [mean lifespan (F = 128.51; df = 3; P < 0.01), maximum lifespan (F = 11.01; df = 3; P < 0.01)] (Table 1). Especially the flies in 1,000 mg/l MP + EP group decreased mean lifespan by 54.80% for female and 49.90% for male, and maximum lifespan by 35.00% for female and 31.10% for male (P < 0.01). Furthermore, 1,000 mg/l individual EP also significantly decreased mean lifespan by 24.62% for female and 27.29 % for male, and maximum lifespan by 16.04% for female and 12.61% for male (Table 2). For the 50% survival time, nearly no difference was observed between 300 mg/l MP + EP and control groups, but a significant decrease was obtained in the 700 and 1,000 mg/l MP + EP groups (P < 0.01). Additionally, MP + EP significantly reduced the flies survival rates for both female (χ2 = 200.59; df = 3; P < 0.01) and male (χ2 = 197.28; df = 3; P < 0.01) (Fig. 2), especially in the 700 mg/l female (χ2 = 87.61; df = 1; P < 0.01), male (χ2 = 77.68; df = 1; P < 0.01) and 1,000 mg/l female (χ2 = 95.71; df = 1; P < 0.01), male (χ2 = 138.94; df = 1; P < 0.01). The cumulative survival also significantly decreased in MP + EP groups compared with control group. For female, the survival function slowly declined before 45 d and then fell rapidly from 46 to 70 d in the control, but the survival function of 1,000 mg/l MP + EP group was slowly declined before 15 d, and dropped quickly from 15 to 25 d (Fig. 2a). For male, the survival curve slowly declined before 45 d, and then decreased more rapidly from 46 to 60 d in the control, but the survival curve of 700 and 1,000 mg/l MP + EP groups was reduced gently before 15 d, and fell quickly at 15–45 d (Fig. 2b). Furthermore, the turning drop point of 700 and 1,000 mg/l MP + EP groups was earlier than control group in all sexes. So MP + EP can reduce lifespan and survival rate of flies in both sexes.
Table 1.
Lifespan of flies fed with different concentrations of MP + EP and the control diets
| Gender | MP + EP (mg/l) | Mean lifespan (day)a | Change of mean lifespan (%)b | 50% survival (day)a | Maximum lifespan (day)a | Change of maximum lifespan (%)b |
|---|---|---|---|---|---|---|
| Female | 0 | 46.00 ± 2.95A | — | 49.00 ± 1.00A | 74.93 ± 1.62A | — |
| 300 | 43.56 ± 3.00A | −5.30 | 50.77 ± 1.55A | 73.45 ± 2.95A | −2.00 | |
| 700 | 37.31 ± 1.01B | −18.90 | 44.73 ± 0.40B | 69.00 ± 1.81A | −7.90 | |
| 1,000 | 20.81 ± 0.76C | −54.80 | 16.90 ± 0.53C | 48.70 ± 9.04B | −35.00 | |
| Male | 0 | 45.60 ± 2.68A | — | 47.33 ± 2.75A | 71.47 ± 0.75A | — |
| 300 | 43.46 ± 1.40A | −4.70 | 46.12 ± 0.70A | 62.70 ± 0.90B | −12.30 | |
| 700 | 29.94 ± 0.40B | −34.30 | 31.17 ± 1.04B | 56.40 ± 1.00C | −21.10 | |
| 1,000 | 22.86 ± 1.33C | −49.90 | 18.00 ± 1.45C | 49.23 ± 1.86D | −31.10 |
aThe data were given as means ± SD. Sharing the different capital letter do significantly differ from each other in same sex at P < 0.01.
bLifespan change (%) = (Reference lifespan-Treatment lifespan)/Reference lifespan × 100, control diet is used as reference.
Table 2.
Change of lifespan of flies fed with MP + EP and the individual EP
| Change of lifespan | Gender | 1,000 mg l−1 EPa | 2,000 mg l−1 EP a | 1,000 mg l−1 (MP + EP)b |
|---|---|---|---|---|
| Change of mean lifespan (%) | ♀ | −24.62 | −43.95 | −54.80 |
| ♂ | −27.29 | −47.30 | −49.90 | |
| Change of maximum lifespan (%) | ♀ | −16.04 | 0.33 | −35.00 |
| ♂ | −12.61 | −0.32 | −31.10 |
a Reference Liu et al. (2014).
b This study.
Fig. 2.
Kaplan-Meier cumulative survival function for different concentrations MP + EP (0, 300, 700, and 1,000 mg/l). Comparison of age-dependent survival curves of female (a) and male (b) flies indicated highly significant differences
SOD Activity
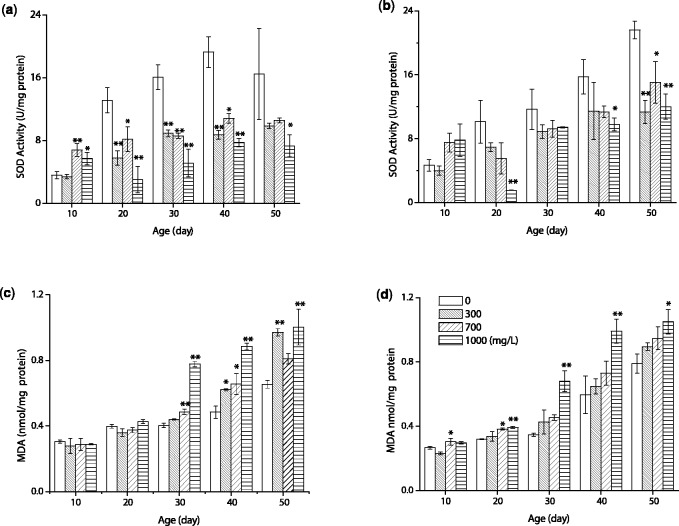
The research was designed to assess antioxidation of flies exposed to MP + EP diets. For female, the SOD activity in the control group gradually increased before 40 d and then decreased with aging, but the value of SOD activity in MP + EP groups was in a lower range (3.02 ± 1.68 to 10.80 ± 0.61 U/mg protein) (Fig. 3a). The activity of SOD in MP + EP groups was significantly decreased relative to those of the control group at day 20, 30, and 40 (P < 0.01 or < 0.05), only 1,000 mg/l MP + EP group was significantly decreased the activity of SOD at 50 d (P < 0.05). For male, the SOD activity in control and 300 mg/l MP + EP groups gradually increased from 10 to 50 d, while that of 700 and 1,000 mg/l MP + EP groups decreased at 20 d, and then increased (Fig. 3b). Moreover, MP + EP groups exhibited a decrease in SOD activity at 20–50 d, especially, in 1,000 mg/l group (P < 0.01 or < 0.05). Additionally, two-way ANOVA was performed to analyzed the interaction effects of age and concentration. The results showed that both age and concentration significantly affected SOD activity (P < 0.01). The age affected 81.30% for female and 89.60% for male of the total variance, concentration affected 86.10% for female and 70.00% for male, and the combined effect of the concentration and age was 73.30% for female and 72.10% for male (Table 3). In conclusion, MP + EP groups decreased the SOD activity of flies compared with control group at the same age.
Fig. 3.
The activity of antioxidant enzyme SOD and the MDA level in flies when fed with the diets containing 0 mg/l (control), 300, 700, and 1,000 mg/l MP + EP for 10, 20, 30, 40 and 50 d. (a) Activity of SOD for female. (b) Activity of SOD for male. (c) The MDA level in female. (d) The MDA level in male. Data are expressed as mean ± SD. * P < 0.05 and ** P < 0.01 compared with the value of control group at the same age
Table 3.
The result of two-way ANOVAfor the biochemical indexes parameters.
| Biochemical indexes | Parameters | df a |
F |
P |
Effects sizeb (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ||
| SOD | Age | 4 | 4 | 21.76 | 42.88 | 0.000 | 0.000 | 81.30 | 89.60 |
| Concentration | 3 | 3 | 41.27 | 15.55 | 0.000 | 0.000 | 86.10 | 70.00 | |
| Age × Concentration | 12 | 12 | 4.57 | 4.32 | 0.001 | 0.002 | 73.30 | 72.10 | |
| MDA | Age | 4 | 4 | 319.73 | 216.70 | 0.000 | 0.000 | 98.50 | 97.70 |
| Concentration | 3 | 3 | 70.88 | 33.11 | 0.000 | 0.000 | 91.40 | 83.20 | |
| Age × Concentration | 12 | 12 | 15.27 | 3.83 | 0.000 | 0.004 | 90.20 | 69.70 | |
aDegrees of freedom.
bEffect size is given in partial eta-squared (η2).
The Content of MDA
The MDA contents presented an increasing trend with age in all groups (Fig. 3c and d). For female, the MDA contents of each group were rather close among each group at day 10 and 20. However, MDA levels were significantly increased in MP + EP groups at day 30, 40, and 50 compared with control group (P < 0.01 or < 0.05) except for increasing of 300 mg/l group at day 30 and 700 mg/l group at day 50 (Fig. 3c). Similarly, MP + EP groups showed an increase in MDA levels compared with control group for male, especially 1,000 mg/l group at day 20, 30, 40 (P < 0.01) and 50 (P < 0.05) (Fig. 3d). According to the two-way ANOVA analysis, both exposure age and concentration significantly affected MDA contents (P < 0.01) and the combined effect of age and concentration was also significant (P < 0.01). The contribution of exposure age to the variance was 98.50% for female and 97.70% for male of the total variance, and that of exposure concentration was 91.40% for female and 83.20% for male. Their combined effect contributed 90.20% for female and 69.70% for male (Table 3). In summary, MP + EP can increase MDA contents compared with control group at the same age, especially in 1,000 mg/l group for all sexes.
Effect of Parabens on the Preadult Development Period
One-way ANOVA analysis showed MP + EP delayed larval period, especially in 700 mg/l (P < 0.05) and 1,000 mg/l groups (P < 0.01). Furthermore, 1,000 mg/l individual MP or EP groups also delayed larval period (P < 0.01), but 300 and 700 mg/l individual MP (P < 0.01) as well as 300 mg/l individual EP accelerated larval period (P < 0.01) (Table 4). More importantly, comparing with different experimental groups at same concentration, the larval period in 300 mg/l individual MP or EP groups was significantly shorter (P < 0.01) than control, but the larval period in same concentration MP + EP groups was longer (Table 4). In other words, MP + EP had a more severe effect on pre-adult development period compared with individual MP or EP at the same concentration. In addition, there was no impact on the pupal period, and percentage of pupa and eclosion for parabens. According to two-way ANOVA analysis, the exposure type of parabens, concentration, and their combined effects all significantly altered growth rate in fly (P < 0.01). The contribution of the exposure type of parabens to the variance of growth rate was 86.10%, and that of exposure concentration was 96.80%. Their combined effect contributed 76.90% (Table 5). Hence, parabens had a nonmonotonic dose–response effect on preadult development period for D. melanogaster and the larval period was more sensitive.
Table 4.
Effect of different concentrations of parabens on Drosophila pre-adult development period
| Treatment | Concentration (mg/l) | Larval period (day) | Pupal period (day) | Percentage of pupa (%) | Percentage of eclosion (%) |
|---|---|---|---|---|---|
| Control | 0 | 5.78 ± 0.15 | 3.93 ± 0.24 | 75.37 | 97.43 |
| MP + EP | 300 | 6.94 ± 0.12 | 3.69 ± 0.27 | 80.00 | 96.95 |
| 700 | 7.90 ± 0.27* | 4.20 ± 0.17 | 65.00 | 96.20 | |
| 1,000 | 10.06 ± 1.16** | 3.59 ± 0.21 | 73.00 | 85.40 | |
| MP | 300 | 5.18 ± 0.04** | 3.72 ± 0.20 | 74.50 | 99.50 |
| 700 | 5.09 ± 0.15** | 3.68 ± 0.12 | 66.50 | 99.50 | |
| 1,000 | 8.61 ± 0.06** | 3.66 ± 0.12 | 71.00 | 97.50 | |
| EP | 300 | 4.88 ± 0.06** | 3.72 ± 0.06 | 75.00 | 95.20 |
| 700 | 6.54 ± 0.02** | 4.03 ± 0.03 | 70.00 | 98.85 | |
| 1,000 | 8.90 ± 0.25** | 3.54 ± 0.47 | 75.50 | 94.95 |
*P < 0.05 and **P < 0.01 compared with the value of control group.
Table 5.
The result of two-way analyses of variance for the larval period parameters
| Parameters | df a | F | P | Effects sizeb (%) |
|---|---|---|---|---|
| Parabens | 2 | 37.04 | 0.000 | 86.10 |
| Concentrations | 3 | 121.45 | 0.000 | 96.80 |
| Parabens × Concentrations | 6 | 6.65 | 0.003 | 76.90 |
aDegrees of freedom.
bEffect size is given in partial eta-squared (η2).
Discussion
In this study, we found that MP + EP could reduce the mean lifespan, maximum lifespan and 50% survival time of flies when compared with that of the control group (Table 1). More importantly, the mean lifespan in 1,000 mg/l MP + EP group was about a half of that in control group, while the mean lifespan in 1,000 mg/l individual EP was nearly three quarters of that in control group (Table 2). It indicated that MP + EP may present greater toxic effect on longevity of flies than individual paraben at the same concentration. Furthermore, our results suggested that parabens could impair antioxidant capacity (Fig. 3). Parabens may increase levels of oxidative damages on macromolecules and gradual disruption of organelle function to promote senescence. The same conclusions about the relationship between parabens exposure and oxidative stress biomarkers have been made in rodents (Popa et al. 2011; Shah and Verma 2011), pregnant women and infant (Kang et al. 2013). In addition, the shortening lifespan in parabens-treatment groups may be associated with endocrinic disruption. Flies that are heterozygous for mutations in the EcR presented an increase in lifespan and the ability to avoid various stresses (Simon et al. 2003; Tricoire et al. 2009). Juvenile hormone (JH) can regulate the lifespan of flies by mechanisms independent of direct physiological trade-offs with egg production (Yamamoto et al. 2013). It was also proven that hormones and mutations in genes involved in endocrine signaling, in particular, the insulin/insulin-like growth factor-1 (IGF-1) signaling (IIS) pathway, can affect D. melanogaster longevity (Gáliková et al. 2011). Parabens may disrupt endocrine signaling pathways and abnormal hormone production, and further affect the lifespan.
Drosophila belongs to holometabolous insects which includes a transition from an immature juvenile to a sexually mature adult coordinated by two hormones, 20-hydroxyecdysone and JH (Jindra et al. 2013; Gautam et al. 2014). In Drosophila, a high titer of ecdysone, relative to the titers produced during the instar transformations, at the end of the third-instar larval stage initiates the onset of pupariation/metamorphosis (Thummel 2001). Our results revealed that parabens had a negative impact on preadult development period of flies and showed a nonmonotonic dose–response. Parabens, as the potential EDCs, can interfere with hormone synthesis. Moreover, we found EP can cause the disordered expression of EcR in D. melanogaster (Liu et al. 2014). In addition, IIS can regulate ecdysone biosynthesis in the prothoracic glands (Walkiewicz and Stern 2009; Koyama et al. 2014). Parabens may disturb the modification of the hormone synthesis metabolism and hormone action to alter metamorphosis of fruit flies. When compared with the control group, 300 and 700 mg/l individual MP and 300 mg/l individual EP could accelerate development rate (P < 0.01) (Table 4). The reason might be that individual MP or EP at low concentration display ‘hormesis’ effects by controlling the hormone synthesis and producing biological benefits in organisms (Radak et al. 2008). Similarly, bisphenol A, one of EDCs, can also accelerate larval growth in the low dose group (Weiner et al. 2014). Additionally, the larval period was delayed in 300 mg/l MP + EP group, and shortened in the same concentration of individual MP or EP groups (P < 0.01). 700 mg/l individual MP accelerated the development rate (P < 0.01), but individual EP prolonged at the same concentration (P < 0.05) (Table 4). Therefore, we deduced MP + EP may have a greater toxicity than individual and the order of the toxic was MP + EP > EP > MP on D. melanogaster.
In summary, D. melanogaster is a promising organism to study chemical pollutants toxicity on endocrinology and longevity with the accurate sensitivity and operability. The results revealed that parabens had an adverse effect on lifespan and preadult development period in D. melanogaster, especially in combining use. However, the study only focused on the additive toxicity of two kinds of parabens and ignored the toxicity on fertility and the mechanism of parabens-induced endocrine disruption in flies. Further studies may be necessary to investigate the molecular mechanisms of parabens toxicity on flies. In particular, we should focus on the additive effects of multiple parabens to reevaluate their safe margin.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
This work was supported by the Natural Science Foundation of Shaanxi Province, China (2012JQ3012) and the Fundamental Research Funds for the Central Universities (GK201402026).
References Cited
- Alcudia-León M.C., Lucena R., Cárdenas S., Valcárcel M. 2013. Determination of parabens in waters by magnetically confined hydrophobic nanoparticle microextraction coupled to gas chromatography/mass spectrometry. Microchem. J. 110: 643–648. [Google Scholar]
- Andersen F. A. 2008. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int. J. Toxicol. 27 (Suppl 4): 1–82. [DOI] [PubMed] [Google Scholar]
- Ayar A., Uysal H. 2013. Genotoxic and safety assessment of 2 parabens in somaticcells of in vivo Drosophila melanogaster. Turk. J. Biol. 37: 683–688. [Google Scholar]
- Błędzka D., Gromadzińska J., Wąsowicz W. 2014. Parabens. From environmental studies to human health. Environ. Int. 67: 27–42. [DOI] [PubMed] [Google Scholar]
- Brausch J. M., Rand G. M. 2011. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82: 1518–1532. [DOI] [PubMed] [Google Scholar]
- Carrasco-Correa E. J., Vela-Soria F., Ballesteros O., Ramis-Ramos G., Herrero-Martínez J. M. 2015. Sensitive determination of parabens in human urine and serum using methacrylate monoliths and reversed-phase capillary liquid chromatography-mass spectrometry. J. Chromatogr. A 1379: 65–73. [DOI] [PubMed] [Google Scholar]
- Celeiro M., Lamas J. P., Garcia-Jares C., Llompart M. 2015. Pressurized liquid extraction-gas chromatography-mass spectrometry analysis of fragrance allergens, musks, phthalates and preservatives in baby wipes. J. Chromatogr. A 1384: 9–21 [DOI] [PubMed] [Google Scholar]
- Charles A. K., Darbre P. D. 2013. Combinations of parabens at concentrations measured in human breast tissue can increase proliferation of MCF-7 human breast cancer cells. J. Appl. Toxicol. 33: 390–398. [DOI] [PubMed] [Google Scholar]
- Chen J., Ahn K. C., Gee N. A., Gee S. J., Hammock B. D., Lasley B. L. 2007. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl. Pharmacol. 221: 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Wang C. C., Chen Y. L., Wu S. M. 2012. Large volume sample stacking with EOF and sweeping in CE for determination of common preservatives in cosmetic products by chemometric experimental design. Electrophoresis 33: 1443–1448. [DOI] [PubMed] [Google Scholar]
- Darbre P. D., Harvey P. W. 2008. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 28: 561–578. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30: 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper H. H., Hadley M. 1990. Malondialdehyde determination as index of lipid Peroxidation. Method. Enzymol. 186: 421–431. [DOI] [PubMed] [Google Scholar]
- European Parliament and Council of European Union. 2009. Regulation (EC) no 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L342: 59–209. [Google Scholar]
- Evans R. M., Scholze M., Kortenkamp A. 2012. Additive mixture effects of estrogenic chemicals in human cell-based assays can be influenced by inclusion of chemicals with differing effect profiles. Plos One 7, e43606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh M. A., Khosrowshahi E. M., Khorram P. 2013. Simultaneous derivatization and air-assisted liquid-liquid microextraction of some parabens in personal care products and their determination by GC with flame ionization detection. J. Sep. Sci. 36: 3571–3578. [DOI] [PubMed] [Google Scholar]
- Ferreira A.M.C., Möder M., Laespada M.E.F. 2011a. GC-MS determination of parabens, triclosan and methyl triclosan in water by in situ derivatisation and stir-bar sorptive extraction. Anal. Bioanal. Chem. 399, 945–953. [DOI] [PubMed] [Google Scholar]
- Ferreira A.M.C., Möder M., Laespada M. F. 2011b. Stir bar sorptive extraction of parabens, triclosan and methyl triclosan from soil, sediment and sludge with in situ derivatization and determination by gas chromatography–mass spectrometry. J. Chromatogr. A 1218: 3837–3844. [DOI] [PubMed] [Google Scholar]
- Gáliková M., Klepsatel P., Senti G., Flatt T. 2011. Steroid hormone regulation of C. elegans and Drosophila aging and life history. Exp. Gerontol. 46: 141–147. [DOI] [PubMed] [Google Scholar]
- Gautam N. K., Verma P., Tapadia M. G. 2014. Ecdysone regulates morphogenesis and function of malpighian tubules in Drosophila melanogaster through EcR-B2 isoform. Dev. Biol. 398: 163–176 [DOI] [PubMed] [Google Scholar]
- Gu W., Xie D. J., Hou X. W. 2009. Toxicity and estrogen effects of methylparaben on Drosophila melanogaster. Food Sci. 30: 252–254. (in Chinese) [Google Scholar]
- Guemouri L., Artur Y., Herbeth B., Jeandel C., Cuny G., Siest G. 1991. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin. Chem. 37: 1932–1937. [PubMed] [Google Scholar]
- Harman D. 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11: 298–300. [DOI] [PubMed] [Google Scholar]
- Harvey P. W., Everett D. J. 2004. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J. Appl. Toxicol. 24: 1–4. [DOI] [PubMed] [Google Scholar]
- İnal M. E., Kanbak G., Sunal E. 2001. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta 305: 75–80. [DOI] [PubMed] [Google Scholar]
- Isling L. K., Boberg J., Jacobsen P. R., Mandrup K. R., Axelstad M., Christiansen S., Vinggaard A. M., Taxvig C., Kortenkamp A., Hass U. 2014. Late-life effects on rat reproductive system after developmental exposure to mixtures of endocrine disrupters. Reproduction 147: 465–476. [DOI] [PubMed] [Google Scholar]
- Jain R., Mudiam M.K.R., Chauhan A., Ch R., Murthy R. C., Khan H. A. 2013. Simultaneous derivatisation and preconcentration of parabens in food and other matrices by isobutyl chloroformate and dispersive liquid–liquid microextraction followed by gas chromatographic analysis. Food Chem. 141: 436–443. [DOI] [PubMed] [Google Scholar]
- Jindra M., Palli S. R., Riddiford L. M. 2013. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58: 181–204. [DOI] [PubMed] [Google Scholar]
- Kang S., Kim S., Park J., Kim H. J., Lee J., Choi G., Choi S., Kim S., Kim S. Y., Moon H. B., et al. 2013. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 461-462: 214–221. [DOI] [PubMed] [Google Scholar]
- Khanna S., Dash P. R., Darbre P. D. 2014. Exposure to parabens at the concentration of maximal proliferative response increases migratory and invasive activity of human breast cancer cells in vitro. J. Appl. Toxicol. 34: 1051–1059. [DOI] [PubMed] [Google Scholar]
- Koyama T., Rodrigues M. A., Athanasiadis A., Shingleton A. W., Mirth C. K. 2014. Nutritional control of body size through FoxO-Ultraspiracle mediated ecdysone biosynthesis. eLife 3, e03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Kuch B., Metzger J. W. 2014. Estrogenic activity of constituents of underarm deodorants determined by E-Screen assay. Chemosphere 108: 101–106 [DOI] [PubMed] [Google Scholar]
- Li Y., Hou X., Zhang M., Gu W. 2015. Effects of propylparaben on fecundity and lifespan in Drosophila melanogaster. Toxico. Enviro. Chem. 96: 1064–1074. [Google Scholar]
- Liu T., Li Y., Zhao X., Zhang M., Gu W. 2014. Ethylparaben affects lifespan, fecundity, and the expression levels of ERR, EcR and YPR in Drosophila melanogaster. J. Insect Physiol. 71: 1–7. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Moldéus P. 1998. Mechanism of p-hydroxybenzoate ester-induced mitochondrial dysfunction and cytotoxicity in isolated rat hepatocytes. Biochem. Pharmacol. 55: 1907–1914. [DOI] [PubMed] [Google Scholar]
- Nishizawa C., Takeshita K., Ueda J. I., Nakanishi I., Suzuki K. T., Ozawa T. 2006. Reaction of para-hydroxybenzoic acid esters with individualt oxygen in the presence of glutathione produces glutathione conjugates of hydroquinone, potent inducers of oxidative stress. Free Rad. Res. 40: 233–240. [DOI] [PubMed] [Google Scholar]
- Popa D. S., Kiss B., Vlase L., Pop A., Iepure R., Păltinean R., Loghin F. 2011. Study of oxidative stress induction after exposure to bisphenol A and methylparaben in rats. Farmacia 59: 539–49. [Google Scholar]
- Radak Z., Chung H. Y., Koltai E., Taylor A. W., Goto S. 2008. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 7: 34–42. [DOI] [PubMed] [Google Scholar]
- Ramírez N., Borrull F., Marcé R. M. 2012. Simultaneous determination of parabens and synthetic musks in water by stir-bar sorptive extraction and thermal desorption-gas chromatography-mass spectrometry. J. Sep. Sci. 35: 580–588. [DOI] [PubMed] [Google Scholar]
- Ramírez N., Marcé R. M., Borrull F. 2011. Determination of parabens in house dust by pressurised hot water extraction followed by stir bar sorptive extraction and thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 1218: 6226–6231. [DOI] [PubMed] [Google Scholar]
- Rand M. D. 2010. Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicol. Teratol. 32: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge E. J., Parker J., Odum J., Ashby J., Sumpter J. P. 1998. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharm. 153: 12–19. [DOI] [PubMed] [Google Scholar]
- Satoh K., Nonaka R., Ohyama K.I., Nagai F. 2005. Androgenic and antiandrogenic effects of alkylphenols and parabens assessed using the reporter gene assay with stably transfected CHO-K1 cells (AR-EcoScreen System). J. Health Sci. 51: 557–568. [Google Scholar]
- Schlumpf M., Kypke K., Wittassek M., Angerer J., Mascher H., Mascher D., Vökt C., Birchler M., Lichtensteiger W. 2010. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere 81: 1171–1183. [DOI] [PubMed] [Google Scholar]
- Shah K. H., Verma R. J. 2011. Butyl p-hydroxybenzoic acid induces oxidative stress in mice liver-an in vivo study. Acta Pol. Pharm. 68: 875–879. [PubMed] [Google Scholar]
- Simon A. F., Shih C., Mack A., Benzer S. 2003. Steroid control of longevity in Drosophila melanogaster. Science 299: 1407–1410. [DOI] [PubMed] [Google Scholar]
- Steinberg D. C. 2010. 2010 frequency of preservative use. Cosmetics and Toiletries 125: 46–51. [Google Scholar]
- Thummel C. S. 2001. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev. Cell 1: 453–465. [DOI] [PubMed] [Google Scholar]
- Tricoire H., Battisti V., Trannoy S., Lasbleiz C., Pret A. M., Monnier V. 2009. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 130: 547–552. [DOI] [PubMed] [Google Scholar]
- Walkiewicz M. A., Stern M. 2009. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. Plos One 4, e5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Ding X., Li Y., Ang U. 2012. Simultaneous determination of eleven preservatives in cosmetics by micellar electrokinetic chromatography. J. AOAC Int. 95: 1069–1073. [DOI] [PubMed] [Google Scholar]
- Weiner A. K., Ramirez A., Zintel T., Rose R. W., Wolff E., Parker A. L., Bennett K., Johndreau K., Rachfalski C., Zhou J., et al. 2014. Bisphenol A affects larval growth and advances the onset of metamorphosis in Drosophila melanogaster. Ecotox. Environ. Safe. 101: 7–13. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Tamura I., Hirata Y., Kato J., Kagota K., Katsuki S., Yamamoto A., Kagami Y., Tatarazako N. 2011. Aquatic toxicity and ecological risk assessment of seven parabens: individual and additive approach. Sci. Total Environ. 410–411: 102–11. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Bai H., Dolezal A. G., Amdam G., Tatar M. 2013. Juvenile hormone regulation of Drosophila aging. BMC Biol. 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye N., Shi P., Li J., Wang Q. 2013. Application of graphene as solid phase extraction absorbent for the determination of parabens in cosmetic products by capillary electrophoresis. Anal. Lett. 46: 1991–2000. [Google Scholar]
- Ying Q. Q. 2012. The Effect of ethylparaben on longevity and the expression of reaper in Drosophila melanogaster. M.S. thesis, Shaanxi Normal University, China. (in Chinese) [Google Scholar]
- Zhang L., Dong L., Ding S., Qiao P., Wang C., Zhang M., Zhang L., Du Y., Li Y., Tang N., Chang B. 2014. Effects of n-butylparaben on steroidogenesis and spermatogenesis through changed E 2 levels in male rat offspring. Environ. Toxicol. Phar. 37: 705–717. [DOI] [PubMed] [Google Scholar]