Abstract
Background
Different sleep‐disordered breathing (SDB) phenotypes, including coexisting obstructive and central sleep apnea (OSA‐CSA), have not yet been characterized in a large sample of patients with heart failure and reduced ejection fraction (HFrEF) receiving guideline‐based therapies. Therefore, the aim of the present study was to determine the proportion of OSA, CSA, and OSA‐CSA, as well as periodic breathing, in HFrEF patients with SDB.
Methods and Results
The German SchlaHF registry enrolled patients with HFrEF receiving guideline‐based therapies, who underwent portable SDB monitoring. Polysomnography (n=2365) was performed in patients with suspected SDB. Type of SDB (OSA, CSA, or OSA‐CSA), the occurrence of periodic breathing (proportion of Cheyne‐Stokes respiration ≥20%), and blood gases were determined in 1557 HFrEF patients with confirmed SDB. OSA, OSA‐CSA, and CSA were found in 29%, 40%, and 31% of patients, respectively; 41% showed periodic breathing. Characteristics differed significantly among SDB groups and in those with versus without periodic breathing. There was a relationship between greater proportions of CSA and the presence of periodic breathing. Risk factors for having CSA rather than OSA were male sex, older age, presence of atrial fibrillation, lower ejection fraction, and lower awake carbon dioxide pressure (pco 2). Periodic breathing was more likely in men, patients with atrial fibrillation, older patients, and as left ventricular ejection fraction and awake pco 2 decreased, and less likely as body mass index increased and minimum oxygen saturation decreased.
Conclusions
SchlaHF data show that there is wide interindividual variability in the SDB phenotype of HFrEF patients, suggesting that individualized management is appropriate.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01500759.
Keywords: heart failure, phenotypes, sleep apnea, sleep disorders
Subject Categories: Heart Failure, Risk Factors, Complications
Clinical Perspective
What Is New?
There are a number of different sleep‐disordered breathing phenotypes in patients with heart failure and reduced ejection fraction.
What Are the Clinical Implications?
A “one size fits all” approach to managing sleep‐disordered breathing in patients with heart failure and reduced ejection fraction is unlikely to maximize clinical outcomes for each patient, and an individualized approach to therapy after definition of the sleep apnea phenotype would be more appropriate.
Introduction
Heart failure (HF) is a relatively common condition, occurring in 1% to 2% of the adult population in Western countries.1, 2 There are a number of factors contributing to ongoing and projected increases in the prevalence of HF, including the aging population demographic and improved patient survival.3, 4 Despite advances in care, rates of hospitalization and readmission remain high,5 meaning that the economic and social burden of HF is likely to increase over time.
There is an increasing focus on treatment of comorbidities and optimization of risk factors in patients with HF.6 One such comorbidity is sleep‐disordered breathing (SDB), which is more common in HF patients than in the general population.7, 8 Data from the SchlaHF (Sleep‐Disordered Breathing in Heart Failure) registry showed that SDB in HF is highly prevalent, with nearly half of all studied patients with HF with reduced ejection fraction (HFrEF) having moderate to severe SDB, and identifying a number of risk factors for SDB in these patients, including increasing age and body mass index (BMI), decreasing left ventricular ejection fraction (LVEF), male sex, and the presence of atrial fibrillation.9
However, SDB can take a number of forms, including obstructive sleep apnea (OSA), central sleep apnea (CSA) and periodic breathing (Cheyne‐Stokes respiration, CSR). Many patients show a combination of different types of SDB breathing patterns that may change over the course of a night as well as over time.10 Although both OSA and CSA/CSR have been shown to be independent predictors of worse outcome in HF patients,11, 12, 13, 14, 15, 16 the different forms of SDB are likely to have different effects on the cardiovascular system.17 The findings of a post hoc analysis of the SERVE‐HF study provided some evidence that the impact of SDB and its treatment might be different in CSA and OSA, showing effect modification when the proportion of CSR at baseline was <20%.18 The results of a multistate model analysis of SERVE‐HF also showed that patients with poor ventricular function or a high proportion of CSR at baseline randomized to adaptive servo‐ventilation were at the highest risk of experiencing cardiovascular death, and that this occurred without a preceding hospital admission.9
Only a few studies to date have characterized different phenotypes for patients with HF and SDB.16, 19, 20, 21 Tkacova et al reported coexisting OSA and CSA in 12 of 65 patients with HFrEF.22 However, the reliability of these and other data was limited by the sample size, and studies were conducted in predominantly male patients not being treated with the most up‐to‐date HF therapies. In addition, periodic breathing was often not assessed.
Therefore, the aim of the present study was to determine the proportion of OSA, CSA and coexisting OSA‐CSA, as well as the proportion and extent of CSR, in a large population of optimally treated HFrEF patients with SDB referred to a sleep laboratory. In addition, the clinical phenotype of the different SDB populations and risk factors in each group were assessed.
Methods
Study Design
As described previously in the German SchlaHF registry, assessment of SDB using a 2‐channel respiratory monitor (ApneaLink, ResMed, Australia, Sydney) was being performed in 256 centers (NCT01500759) with sufficient infrastructure to participate.9, 23 Between February 2008 and January 2011, type of SDB (OSA, CSA or coexisting OSA and CSA [OSA‐CSA]) and the occurrence of periodic breathing (proportion of CSR ≥20%) were determined in 1557 HFrEF patients using in‐lab polysomnography (Figure 1). In addition, arteriocapillary blood gases, age, body mass index (BMI), sex, left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class, heart rhythm, nocturnal dyspnea symptoms, nocturia, apnea‐hypopnea index (AHI), HF etiology, and medication were documented. Oxygen desaturation index, average oxygen saturation (mean Spo 2), and minimum oxygen saturation (min Spo 2) were documented in 97% of patients. The SchlaHF registry received central ethics approval from the Freiburger Ethikkomission for Germany, and all participants provided written informed consent.
Figure 1.
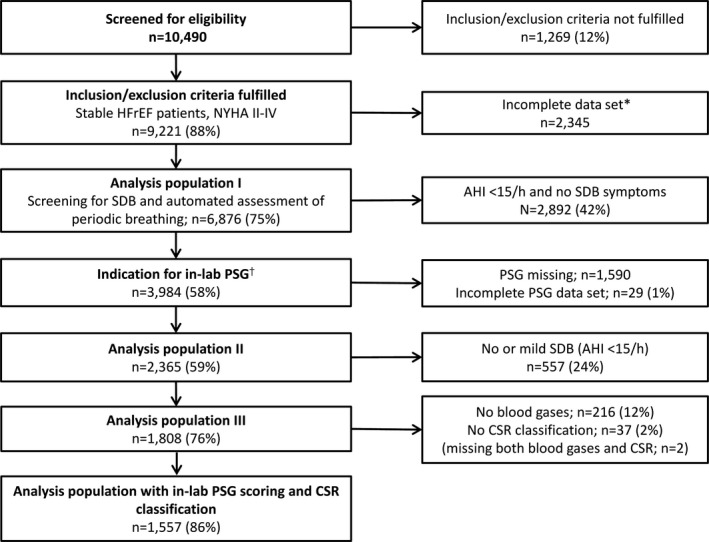
Patient populations and flow in the SchlaHF registry. AHI indicates apnea‐hypopnea index; CSR, Cheyne‐Stokes respiration; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; PSG, polysomnography; SDB, sleep‐disordered breathing. † SDB or typical clinical symptoms indicative of SDB. *Incomplete data included patients with no data on SDB screening (n=390), age (n=1), body mass index (BMI; n=1), left ventricular ejection fraction (LVEF; n=1946), NYHA class (n=1957), atrial fibrillation (n=1977), and etiology of HFrEF (patients could have more than 1 piece of data missing). The high number of missing values for BMI, LVEF, and NYHA was due to recording of these variables being introduced to the registry after a protocol change.
Participants
The inclusion criteria were as follows: chronic HF diagnosed according to the European Society of Cardiology (ESC) guidelines24 ≤12 weeks before enrollment; moderate to severe left ventricular systolic dysfunction (LVEF ≤45% by an imaging method such as echocardiography, radionuclide angiography, left ventriculography, or cardiac magnetic resonance imaging) documented <12 weeks before enrollment; NYHA class III or IV at the time of inclusion, or NYHA class II with ≥1 hospitalization for HF in the past 12 months; patient able to fully understand study information and give written informed consent. Patients were excluded if they had any of the following: current use of positive airway pressure therapy; life expectancy <1 year for diseases unrelated to chronic HF‐REF; cardiac surgery, percutaneous coronary intervention, myocardial infarction, or unstable angina within 6 months before randomization; cardiac resynchronization therapy implantation scheduled or performed within 6 months before randomization; transient ischemic attack or stroke within 3 months before enrollment; primary hemodynamically significant uncorrected valvular heart disease (obstructive or regurgitant) or any valvular disease expected to require surgery; acute myocarditis/pericarditis within 6 months before enrollment.
Assessment of SDB
Diagnosis of OSA or CSA With Polysomnography
As described previously,23 patients with positive SDB monitoring (AHI ≥15/h) and/or suspicious clinical symptoms were referred to a sleep laboratory, where polysomnography (PSG) was performed to make a definite diagnosis of SDB. A standardized qualification process was used to ensure comparability among centers in the scoring of respiratory events. Prior to participation, every center had to send at least 3 diagnostic respiratory scorings to a central lab that validated whether these scorings were performed according to a detailed operating manual for technical requirements of PSG and respiratory scoring. If quality was not acceptable, specific training was provided, followed by another quality assessment. A key element of the PSG scoring was the differentiation of apneas and hypopneas as central or obstructive using flattening of the inspiratory airflow curve, paradoxical breathing, arousal position, sleep stages, and breathing pattern at the end of the hypopnea.25, 26
PSG included brain activity (electroencephalogram), eye movement (electro‐oculogram), muscle activity or skeletal muscle activation (electromyogram), heart rhythm (ECG), breathing and respiratory effort measures during sleep, as well as the recording of body position. Flow measurement was performed by nasal cannula and thermistor during diagnostic procedures.
Definitions for respiratory events were the same as those used in the SERVE‐HF study.18 Apnea was defined as >90% reduction from baseline in peak amplitude of the signal from nasal cannula and oral thermistor, lasting ≥10 seconds. Hypopnea was defined as a ≥30% reduction in flow and a ≥3% desaturation from baseline for ≥10 seconds or as a ≥50% reduction in flow from the preevent baseline for ≥10 seconds.
A central apnea was defined in the absence of thoracoabdominal excursions. If the central component of an apnea already satisfied the definition of a central apnea (ie, ≥10 seconds), 3 consecutive obstructive breaths were needed to classify that as an obstructive apnea. Just 1 or 2 obstructed breaths at the end of an apnea did not change the classification to a central event. Obstructive versus central hypopneas were determined based on the presence/absence of inspiratory flow limitation and/or paradoxical abdominal/thoracic movements on respiratory inductance plethysmography if available.25, 26
In contrast to SERVE‐HF,18 for this analysis the number of (central) apnea and hypopnea episodes per hour of sleep (AHI and cAHI [central apnea‐hypopnea index], respectively) was determined and reported for total sleep time. SDB was defined as an AHI ≥15 per hour of sleep. Patients with SDB were stratified into 3 groups according to the proportion of central apneas and hypopneas in relation to total apnea and hypopneas: OSA (defined as cAHI/AHI 0% to 19.9%), coexisting obstructive sleep apnea+central sleep apnea (OSA‐CSA, defined as cAHI/AHI ≥20.0% and <80%), and CSA (defined as cAHI/AHI ≥80.0%).
Polysomnographic Visual Assessment of CSR
CSR was defined as ≥3 episodes of continuous cycles of waxing and waning tidal volumes with periods of hyperventilation separated by central apneas or hypopneas. It was visually quantified by the presence or absence, and if present by the percentage of the recording time: <20%, ≥20% and <50%, ≥50%.18 A subgroup analysis of the SERVE‐HF trial indicated that adaptive servo‐ventilation use in patients with HFrEF and CSA without CSR was associated with a lower risk of the primary end point (all‐cause death or life‐saving cardiovascular intervention plus unplanned hospitalization for worsening chronic heart failure).18
Blood Gases
Blood gases were determined in arterialized earlobe capillary blood taken during the day when patients were at rest.
Statistical Analysis
Descriptive statistics were absolute and relative frequency or mean±SD (whichever was appropriate). Because prevalences varied considerably among centers (cardiology practices or departments), presumably reflecting differences in patient populations, all inferential statistics were calculated with centers as a random term.
A linear mixed model with percentage AHI/AHI as a continuous response variable was applied for the SDB severity analysis. A random effects logistic regression model with CSR ≥20% versus CSR <20% as the dichotomous response variable was applied to determine risk factors for CSR. Potential risk factors for both disorders analyzed were age, BMI, sex, LVEF, NYHA class, atrial fibrillation, ischemic origin, minimum Sao 2, and pco 2. To study sex differences, interaction terms with all determined risk factors were added to the model and selected by backward elimination based on likelihood ratio tests. Results are visualized by forest plots showing adjusted regression coefficients or odds ratios together with 95% confidence intervals. A P‐value of <0.05 was considered to be statistically significant. Statistical analysis was performed with STATA 14.1 (STATA Corporation, College Station, TX).
Results
A total of 9221 patients had stable HFrEF (NYHA class II‐IV) and were screened for SDB. Of these, 1557 had certified scoring of laboratory PSG results and CSR classification and were included in this analysis (Figure 1).
Patient Characteristics
Type of SDB: OSA, OSA‐CSA, and CSA
In this population of SchlaHF registry participants, the most common form of SDB was coexisting OSA‐CSA (624 patients, 40%); OSA and CSA were found in 452 (29%) and 481 (31%) patients, respectively. There were a number of statistically significant differences among patients with different types of SDB (Table 1). Patients with OSA were younger than those with OSA‐CSA, had a higher body mass index, LVEF, and NYHA class, were more likely to be female, less likely to have atrial fibrillation, be receiving ß‐blockers, diuretics, and digitalis, and had lower oxygen levels and higher carbon dioxide levels (Table 1). Other significant interpatient differences included a significantly higher proportion of men in the CSA versus OSA group, lower LVEF in those with CSA versus OSA‐CSA or OSA‐CSA versus OSA, and an increased prevalence of atrial fibrillation when the proportion of CSA was higher (Table 1). Baseline data by sex are shown in Table S1 and Table S2.
Table 1.
Patient Demographic Data and Characteristics at Baseline Based on Type of Sleep‐Disordered Breathing
| OSA | OSA‐CSA | CSA | |
|---|---|---|---|
| N (%) | 452 (29) | 624 (40) | 481 (31) |
| Age, y | 66±11a | 69±10 | 69±10b |
| Female, n (%) | 94 (21)a | 67 (11) | 43 (9)b |
| Body mass index, kg/m2 | 31±6a | 29±5c | 28±4b |
| LVEF, % | 35±8a | 34±8c | 32±8b |
| NYHA class III+IV, n (%) | 334 (74)a | 422 (68)c | 356 (74) |
| Ischemic cardiomyopathy, n (%) | 226 (50) | 343 (55) | 279 (58)b |
| Atrial fibrillation, n (%) | 103 (23)a | 203 (33) | 174 (36)b |
| Medication, n (%) | |||
| ACE inhibitors and/or ARBs | 394 (87) | 572 (92) | 431 (90) |
| β‐Blockers | 391 (87)a | 571 (92) | 436 (91)b |
| Diuretics | 349 (77)a | 519 (83) | 398 (83)b |
| Digitalis | 64 (14)a | 126 (20) | 101 (21)b |
| Aldosterone antagonists | 195 (43) | 282 (45) | 237 (49) |
| Polysomnography | |||
| AHI, per h | 37±19 | 36±16 | 38±15b |
| cAHI, per h | 2±3a | 20±11c | 35±14b |
| AI, per h | 19±19a | 21±16 | 24±18b |
| cAHI/AHI, % | 6±6a | 54±17c | 92±6b |
| Mean Sao 2, %d | 92±3a | 93±2 | 93±3b |
| Minimum Sao 2, %d | 78±9a | 80±8c | 81±7b |
| Time with Sao 2<90%, mine | 62±78 | 51±69c | 50±70b |
| Awake pco 2, mm Hg | 39±5a | 37±4c | 37±5b |
Values are mean±SD or number of patients (%). P‐values from ANOVA for metric variables and logistic regression for dichotomous variables; groupwise comparisons only in case of global significance (P<0.05). ACE indicates angiotensin‐converting enzyme; AHI, apnea‐hypopnea index; AI, apnea index; ARB, angiotensin receptor blocker; cAHI, central AHI; CSA, central sleep apnea (defined as cAHI/AHI ≥80.0%); LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OSA, obstructive sleep apnea (defined as cAHI/AHI 0‐19.9%); OSA‐CSA, obstructive sleep apnea+central sleep apnea (defined as cAHI/AHI ≥20.0% and <80%); pco 2, partial pressure of carbon dioxide; Sao 2, oxygen saturation.
P<0.05 OSA vs OSA‐CSA.
P<0.05 CSA vs OSA.
P<0.05 OSA‐CSA vs CSA.
Values missing for 1 patient with OSA.
Values missing for 4 patients with OSA, 4 with OSA‐CSA, and 4 with CSA.
Type of Breathing Pattern: CSR
The majority of patients (59%) showed <20% CSR on PSG; the proportions of patients with ≥20% to <50% CSR and ≥50% CSR on PSG were similar (22% and 19%, respectively). Patients with less periodic breathing were significantly younger, significantly more likely to be female, had a significantly higher LVEF, and were significantly less likely to have atrial fibrillation in comparison to patients with CSR ≥50% (Table 2). Patients with ≥50% CSR on PSG had a significantly higher AHI, cAHI, and apnea index than patients with lower proportions of CSR on PSG, and also spend more time with oxygen saturation at <90%; use of cardiac medication did not vary by the proportion of CSR on PSG (Table 2).
Table 2.
Baseline Characteristics in Patients With and Without Periodic Breathing
| CSR‐PSG | |||
|---|---|---|---|
| <20% | ≥20% to <50% | ≥50% | |
| N (%) | 915 (59) | 349 (22) | 293 (19) |
| Age, y | 67±11a | 69±10 | 70±9b |
| Female, n (%) | 154 (17)a | 33 (9) | 17 (6)b |
| Body mass index, kg/m2 | 30±5a | 28±4 | 29±5b |
| LVEF, % | 34±8a | 33±8 | 33±8b |
| NYHA class III+IV, n (%) | 656 (72) | 249 (71) | 207 (71) |
| Ischemic cardiomyopathy, n (%) | 484 (53) | 193 (55) | 171 (58) |
| Atrial fibrillation, n (%) | 250 (27)a | 126 (36) | 104 (35)b |
| Medication, n (%) | |||
| ACE inhibitors and/or ARBs | 817 (89) | 313 (90) | 267 (91) |
| β‐Blockers | 805 (88) | 320 (92)b | 273 (93) |
| Diuretics | 733 (80) | 296 (85) | 237 (81) |
| Digitalis | 156 (17) | 74 (21) | 61 (21) |
| Aldosterone antagonists | 413 (45) | 172 (49) | 129 (44) |
| Polysomnography | |||
| AHI, per h | 35±18a | 36±14c | 43±14b |
| cAHI, per h | 11±13a | 26±14c | 35±13b |
| AI, per h | 19±17a | 20±15c | 30±17b |
| cAHI/AHI, % | 34±33a | 73±22c | 82±15b |
| Mean Sao 2, %d | 93±3 | 93±2 | 92±3 |
| Minimum Sao 2, %d | 79±8a | 81±6c | 79±9 |
| Time with Sao 2<90%, mine | 52±72 | 49±64c | 68±79b |
| Awake pco 2, mm Hg | 38±5a | 37±4 | 36±5b |
Values are mean±SD or number of patients (%). P‐values from ANOVA for metric variables and logistic regression for dichotomous variables; groupwise comparisons only in case of global significance (P<0.05). ACE indicates angiotensin‐converting enzyme; AHI, apnea‐hypopnea index; AI, apnea index; ARB, angiotensin receptor blocker; cAHI, central AHI; CSR‐PSG, Cheyne‐Stokes respiration on polysomnography; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; pco 2, partial pressure of carbon dioxide; Sao 2, oxygen saturation.
P<0.05 <20% CSR‐PSG vs ≥20% to <50% CSR‐PSG.
P<0.05 ≥50% CSR‐PSG vs <20% CSR‐PSG.
P<0.05 ≥20% to <50% CSR‐PSG vs ≥50% CSR‐PSG.
Values missing for 1 patient with OSA.
Values missing for 6 patients with OSA, 1 patient with OSA‐CSA, and 5 patients with CSA.
Influence of Sex
OSA was markedly more common in female heart failure patients compared with male patients with HFrEF, whereas men were more likely to have OSA‐CSA or CSA (Figure 2). There were no important differences between male and female patients in the proportion of periodic breathing within different types of SDB (Figure 3).
Figure 2.
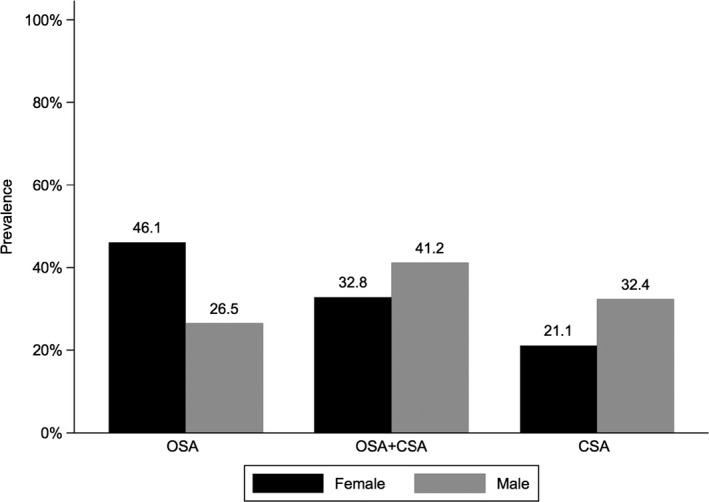
Prevalence of obstructive and central sleep apnea by sex. CSA indicates central sleep apnea (defined as cAHI/AHI ≥80%); OSA, obstructive sleep apnea (defined as cAHI/AHI 0% to 19.9%); OSA+CSA, obstructive sleep apnea+central sleep apnea (defined as cAHI/AHI 20% to 79.9%). AHI indicates apnea‐hypopnea index; cAHI, central AHI.
Figure 3.
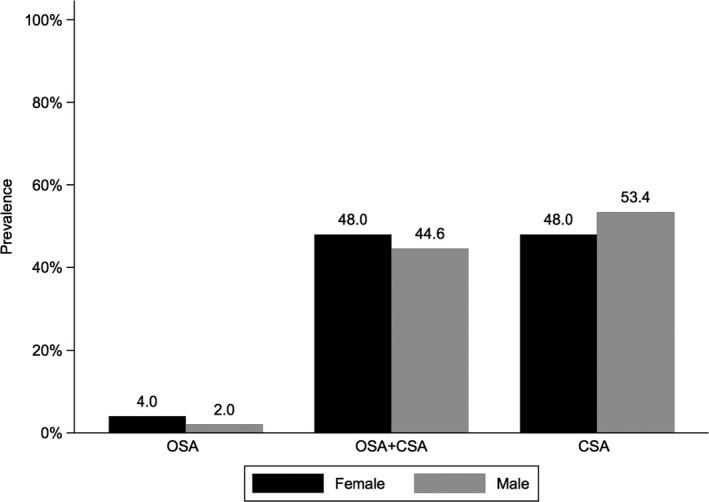
Prevalence of periodic breathing (defined as Cheyne‐Stokes respiration [CSR] ≥20%) according to sleep apnea type. CSA indicates central sleep apnea (defined as cAHI/AHI ≥80.0%); OSA, obstructive sleep apnea (defined as cAHI/AHI 0% to 19.9%); OSA+CSA, obstructive sleep apnea+central sleep apnea (defined as cAHI/AHI 20% to 79.9%). AHI indicates apnea‐hypopnea index; cAHI, central AHI.
Different SDB Phenotypes
There was a clear relationship between greater proportions of central apneas and the presence of periodic breathing. Periodic breathing was seen infrequently in patients with OSA (both male and female) and was much higher (≈50%) in patients with OSA‐CSA or CSA, irrespective of sex (Figure 3).
Clinical Risk Factors for Different SDB Phenotypes in HFrEF Patients With SDB
Central Sleep Apnea
The likelihood of having CSA (higher cAHI/AHI) rather than OSA (lower cAHI/AHI) was significantly higher in male versus female heart failure patients and those with versus without atrial fibrillation (Figure 4). In addition, there was a significant increase in the risk for higher cAHI/AHI as age increased and as awake pco 2 and LVEF decreased. Conversely, the risk of CSA decreased significantly as BMI increased and minimum oxygen saturation decreased (Figure 4). None of the interaction terms with sex was statistically significant.
Figure 4.
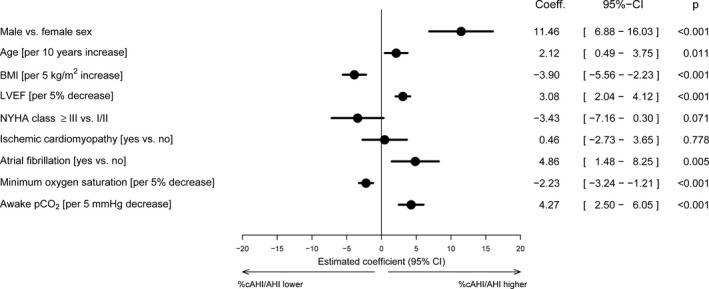
Forest plot of risk factors for central sleep apnea (higher cAHI/AHI percentage) and obstructive sleep apnea (lower cAHI/AHI percentage). AHI indicates apnea‐hypopnea index; BMI, body mass index; cAHI, central AHI; CI, confidence interval; LVEF, left ventricular ejection fraction; min, minimum; NYHA, New York Heart Association; pco 2, partial pressure of carbon dioxide; Sao 2, oxygen saturation.
Cheyne‐Stokes Respiration
Male sex was significantly associated with the presence of periodic breathing, as were increasing age, the presence of atrial fibrillation, worsening left ventricular function, and decreasing awake pco 2 (Figure 5). The risk of having periodic breathing decreased significantly as BMI increased (Figure 5). Again, none of the interaction terms with sex was statistically significant.
Figure 5.
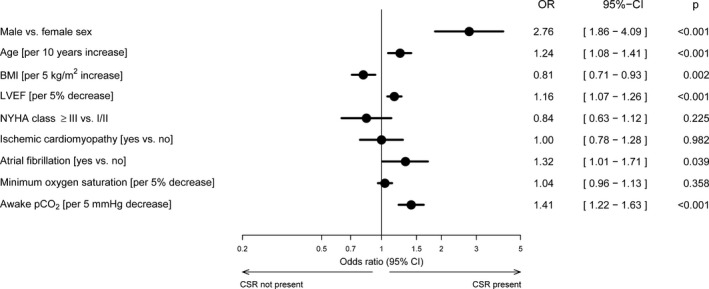
Forest plot of risk factors for periodic breathing (defined as Cheyne‐Stokes respiration [CSR] ≥20%). AHI indicates apnea‐hypopnea index; BMI, body mass index; cAHI, central AHI; CI, confidence interval; LVEF, left ventricular ejection fraction; min, minimum; NYHA, New York Heart Association; pco 2, partial pressure of carbon dioxide; Sao 2, oxygen saturation.
Discussion
This analysis of the SchlaHF registry has identified several major novel findings. The first is that a large proportion (40%) of a population with chronic HFrEF and SDB present with coexisting OSA and CSA. Clearly predominant OSA or CSA (≥80% obstructive or central events, respectively) was diagnosed in 29% and 31% of HFrEF patients with SDB. Second, although the proportion of patients with coexisting OSA and CSA was greater than the frequency of predominant OSA or CSA in men, the most common type of SDB in women was predominant OSA. Third, male sex, older age, lower BMI and LVEF, atrial fibrillation, and lower pco 2 were identified as significant and independent clinical predictors for the presence of predominant CSA rather than OSA in patients with chronic HFrEF and SDB. Furthermore, periodic breathing (CSR for >20% of recording time) was present in 41% of HFrEF patients with SDB. Although only 3% of HFrEF patients with OSA had underlying CSR, a significant proportion of those with CSA (71%) had periodic breathing. Across all types of SDB, the frequency of periodic breathing was similar in men and women. Thus, significant and independent clinical predictors for the presence of periodic breathing were male sex, older age, lower BMI and LVEF, atrial fibrillation, and lower pco 2.
The finding that the most common form of SDB in our population of HFrEF patients was coexisting OSA and CSA is new—there are few published data reporting the rate of coexisting OSA and CSA in HF patients.22 In terms of OSA in HFrEF patients, our rate of 29% is higher than values reported previously, which range from 11% to 26%.16, 19, 20 Conversely, our CSA prevalence rate of 31% was somewhat lower than in some studies (37% to 40%)19, 20 but higher than in another (26%).16 None of these other studies reported data on the prevalence of coexisting OSA‐CSA.
Although OSA‐CSA was the most common type of SDB overall, there were significant variations by sex, with predominant OSA being the most common type of SDB in women (46.1%) and predominant CSA being relatively less common (21.1%); the most common type of SDB in men was OSA‐CSA (41.2%), similar to the overall result. Although there is growing interest in sex differences in SDB,27 women are often underrepresented in clinical trials. In fact, some previous studies of SDB in HF patients have included only male subjects,19, 20 making comparisons with the current data impossible. In 1 study of HFrEF patients with moderate to severe SDB, the prevalence of both OSA and CSA was significantly higher (by about 2.5‐fold) in men than in women. The rate of CSA in our study was about 1.5 times higher in men compared with women, but the opposite was the case for OSA, where the rate in women was 1.7 times higher than in men.
In terms of risk factors for predominant CSA, we identified male sex, older age, lower BMI and LVEF, atrial fibrillation, and lower pco 2 as significant and independent clinical predictors for the presence of predominant CSA (versus predominant OSA) in our large population of patients with chronic HFrEF and SDB. These are largely consistent with existing data on CSA risk factors, which have been reported to include atrial fibrillation, ventricular arrhythmias, lower LVEF, low pco 2, older age, and male sex.16, 19, 20 In our study, OSA was more common when BMI was high and NYHA class and oxygen saturation were lower. Interestingly, this analysis did not identify male sex and age as risk factors for OSA, in contrast to what has been reported previously.16
In the SchlaHF registry population, almost half (41%) had CSR for >20% of the PSG recording time (defined as periodic breathing). This subgroup of HFrEF patients with SDB was shown to be at higher risk of experiencing a primary end‐point event during treatment with adaptive servo‐ventilation in a subgroup analysis of the SERVE‐HF trial.18 Therefore, these patients might represent 1 specific SDB phenotype in HF, with specific data available on which to define treatment options. In contrast to the sex differences we identified in the different types of SDB, the frequency of periodic breathing in the SchlaHF population was similar in men and women. This is a novel finding because sex comparisons were not possible in previous studies.19, 20
This is the first large study to look at different SDB phenotypes in patients with HF. Its strengths include the inclusion of a large population of male and female patients being treated with up‐to‐date, guideline‐based medical and device therapy. Therefore, the results should be widely applicable to patients with HFrEF, but they cannot be generalized to those with HF and preserved ejection fraction, which were not included in the SchlaHF registry. Other limitations include its design as a registry analysis, meaning that data were not prospectively collected, and there is therefore a potential for bias. Of the HFrEF patients with an indication to undergo PSG, only 59% ultimately underwent PSG for a variety of reasons, such as long waiting periods in German sleep laboratories and possible insufficient subjective priority for a sleep study as defined by the referring cardiologist and the patient. In addition, there may be limitations on the amount of data available (eg, echocardiographic data other than LVEF), and information on confounding factors may be lacking.
Conclusions
These data from a large number of patients enrolled in the SchlaHF registry show that there is marked interindividual variability in the SDB phenotype of HFrEF patients, with many showing coexisting OSA‐CSA. Taken together with data from recent large, randomized clinical trials,18, 28 our findings suggest that all HF patients with SDB are not alike, and therefore, management strategies need to be tailored on an individual basis in order to optimize patient outcomes.
Sources of Funding
The SchlaHF registry was funded by ResMed Ltd, Sydney, Australia and ResMed Germany Inc, Martinsried, Germany. ResMed also providing funding for English language editing assistance by an independent medical writer (Nicola Ryan).
Disclosures
Arzt received grant support from ResMed (Martinsried, Germany), the ResMed Foundation (San Diego, CA), and Philips Respironics (Murrysville, PA). Arzt was the holder of an endowed professorship from the Free State of Bavaria at the University of Regensburg that was donated by ResMed (Martinsried, Germany) and Philips Respironics (Murrysville, PA); Arzt has previously received lecture fees from Philips Respironics (Murrysville, PA) and ResMed (Martinsried, Germany). Teschler received grant support from ResMed (Sydney, Australia), the ResMed Foundation (San Diego, CA), and Linde (Munich, Germany). Teschler has previously received lecture fees from AstraZeneca, Novartis, Linde, Boehringer Ingelheim, Berlin Chemie, and ResMed Germany. Oldenburg has acted as a consultant for ResMed, LivaNova, Novartis, and Boehringer Ingelheim, received a research grant from ResMed, and has received lecture honoraria from ResMed, Respicardia, LivaNova, Novartis, and Boehringer Ingelheim. Erdmann and Wegscheider received consulting fees or honoraria and travel grants from ResMed. Wegscheider and Graml are employees of ResMed, Germany. Suling has no conflicts of interest to declare.
Supporting information
Table S1. Patient Demographic Data and Characteristics at Baseline Based on Type of Sleep‐Disordered Breathing and Sex
Table S2. Patient Demographic Data and Characteristics at Baseline for Patients With Versus Without In‐Lab PSG and CSR Classification (Included Versus Excluded From the Current Analysis)
Appendix S1. SchlaHF investigators (All From Germany).
Acknowledgments
Medical writing assistance was provided by Nicola Ryan, independent medical writer, funded by ResMed. Role of the Sponsor: The SchlaHF steering committee members (including Woehrle, Arzt, Oldenburg, Erdmann, and Teschler) had full control of the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
(J Am Heart Assoc. 2017;6:e005899 DOi: 10.1161/JAHA.116.005899.)29187390
References
- 1. Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. [DOI] [PubMed] [Google Scholar]
- 2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Resource utilization among congestive heart failure study. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. [DOI] [PubMed] [Google Scholar]
- 4. Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2012 update: a report from the American Heart association. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 7. Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep‐disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. [DOI] [PubMed] [Google Scholar]
- 8. Schulz R, Blau A, Borgel J, Duchna HW, Fietze I, Koper I, Prenzel R, Schadlich S, Schmitt J, Tasci S, Andreas S. Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201–1205. [DOI] [PubMed] [Google Scholar]
- 9. Arzt M, Woehrle H, Oldenburg O, Graml A, Suling A, Erdmann E, Teschler H, Wegscheider K; SchlaHF Investigators . Prevalence and predictors of sleep‐disordered breathing in patients with stable chronic heart failure: the SchlaHF registry. JACC Heart Fail. 2016;4:116–125. [DOI] [PubMed] [Google Scholar]
- 10. Ryan CM, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson KA, Belenkie I, Pfeifer M, Fleetham J, Hanly PJ, Smilovitch M, Arzt M, Bradley TD; CANPAP Investigators . Shift in sleep apnoea type in heart failure patients in the CANPAP trial. Eur Respir J. 2010;35:592–597. [DOI] [PubMed] [Google Scholar]
- 11. Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O. Cheyne‐Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter‐defibrillator therapies in patients with congestive heart failure. Eur Heart J. 2011;32:61–74. [DOI] [PubMed] [Google Scholar]
- 12. Hanly PJ, Zuberi‐Khokhar NS. Increased mortality associated with Cheyne‐Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. [DOI] [PubMed] [Google Scholar]
- 13. Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. [DOI] [PubMed] [Google Scholar]
- 14. Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne‐Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, Ruttanaumpawan P, Tomlinson G, Bradley TD. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. [DOI] [PubMed] [Google Scholar]
- 16. Yumino D, Wang H, Floras JS, Newton GE, Mak S, Ruttanaumpawan P, Parker JD, Bradley TD. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15:279–285. [DOI] [PubMed] [Google Scholar]
- 17. Yumino D, Kasai T, Kimmerly D, Amirthalingam V, Floras JS, Bradley TD. Differing effects of obstructive and central sleep apneas on stroke volume in patients with heart failure. Am J Respir Crit Care Med. 2013;187:433–438. [DOI] [PubMed] [Google Scholar]
- 18. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015; 373:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106:21–28. [DOI] [PubMed] [Google Scholar]
- 20. Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. [DOI] [PubMed] [Google Scholar]
- 21. Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne‐Stokes respiration. Circulation. 2000;102:61–66. [DOI] [PubMed] [Google Scholar]
- 22. Tkacova R, Niroumand M, Lorenzi‐Filho G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure: role of pco 2 and circulatory delay. Circulation. 2001;103:238–243. [DOI] [PubMed] [Google Scholar]
- 23. Woehrle H, Oldenburg O, Arzt M, Graml A, Erdmann E, Teschler H, Wegscheider K; SCHLA‐HF Investigators . Determining the prevalence and predictors of sleep disordered breathing in patients with chronic heart failure: rationale and design of the SCHLA‐HF registry. BMC Cardiovasc Disord. 2014;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez‐Sendon JL, Nieminen MS, Pierard L, Remme WJ. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. [DOI] [PubMed] [Google Scholar]
- 25. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP; Davidson Ward SL; Tangredi MM; American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Randerath WJ, Treml M, Priegnitz C, Stieglitz S, Hagmeyer L, Morgenstern C. Evaluation of a noninvasive algorithm for differentiation of obstructive and central hypopneas. Sleep. 2013;36:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Won C, Guilleminault C. Gender differences in sleep disordered breathing: implications for therapy. Expert Rev Respir Med. 2015;9:221–231. [DOI] [PubMed] [Google Scholar]
- 28. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi‐Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Demographic Data and Characteristics at Baseline Based on Type of Sleep‐Disordered Breathing and Sex
Table S2. Patient Demographic Data and Characteristics at Baseline for Patients With Versus Without In‐Lab PSG and CSR Classification (Included Versus Excluded From the Current Analysis)
Appendix S1. SchlaHF investigators (All From Germany).


