Abstract
Background
Perceived risks of hyperkalemia and acute renal insufficiency may limit use of mineralocorticoid receptor antagonist (MRA) therapy in patients with heart failure, especially those with diabetes mellitus or chronic kidney disease.
Methods and Results
Using clinical registry data linked to Medicare claims, we analyzed patients hospitalized with heart failure between 2005 and 2013 with a history of diabetes mellitus or chronic kidney disease. We stratified patients by MRA use at discharge. We used inverse probability–weighted proportional hazards models to assess associations between MRA therapy and 30‐day, 1‐year, and 3‐year mortality, all‐cause readmission, and readmission for heart failure, hyperkalemia, and acute renal insufficiency. We performed interaction analyses for differential effects on 3‐year outcomes for reduced, borderline, and preserved ejection fraction. Of 16 848 patients, 12.3% received MRA therapy at discharge. Higher serum creatinine was associated with lower odds of MRA use (odds ratio, 0.66; 95% confidence interval, 0.61–0.71); serum potassium was not (odds ratio, 1.00; 95% confidence interval, 0.90–1.11). There was no mortality difference between groups. MRA therapy was associated with greater risks of readmission for hyperkalemia and acute renal insufficiency and lower risks of long‐term all‐cause readmission. Patients on MRA therapy with borderline or preserved ejection fraction had greater risks of readmission for hyperkalemia (P=0.02) and acute renal insufficiency (P<0.001); patients with reduced ejection fraction did not.
Conclusions
Among patients with heart failure and diabetes mellitus or chronic kidney disease, MRA use was associated with lower risk of all‐cause readmission despite greater risk of hyperkalemia and acute renal insufficiency.
Keywords: chronic kidney disease, diabetes mellitus, heart failure, outcomes research
Subject Categories: Heart Failure, Mortality/Survival, Quality and Outcomes
Clinical Perspective
What Is New?
Mineralocorticoid receptor antagonists are often underutilized in clinical practice, possibly because of concerns over risks of hyperkalemia and worsening renal function.
In high‐risk patients with heart failure and concomitant diabetes mellitus or renal insufficiency, mineralocorticoid receptor antagonist use was associated with lower risk of all‐cause hospitalization despite increased risk of hospitalization with hyperkalemia or acute renal insufficiency.
The increased risk of adverse events was mostly confined to patients with borderline or preserved ejection fraction.
What Are the Clinical Implications?
Because of the overall decrease in the risk of hospitalization for patients treated with mineralocorticoid receptor antagonist therapy, the benefits of therapy may outweigh the risks in a high‐risk population.
Introduction
The mineralocorticoid receptor antagonists (MRAs), spironolactone and eplerenone, are recommended for patients with symptomatic heart failure with ejection fraction of 35% or less.1 Although the role of MRAs in patients with ejection fraction greater than 35% is unclear,2, 3 they may benefit patients with comorbid hypertension, diabetes mellitus, or renal insufficiency. MRAs can be used for blood pressure management regardless of heart failure status, and addition of an MRA to angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker therapy reduces proteinuria in patients with chronic kidney disease and diabetic nephropathy and can delay progression of renal dysfunction.3, 4, 5, 6, 7, 8, 9, 10
Despite clinical trial evidence and guideline recommendations, MRA therapy in patients with heart failure with reduced ejection fraction is underused in clinical practice.11 Risks of hyperkalemia and worsening renal function often limit use of MRA therapy in patients with heart failure.12, 13, 14 Several factors increase hyperkalemia risk, including renal insufficiency and diabetes mellitus.15, 16, 17, 18, 19 Although the presence of renal insufficiency or diabetes mellitus increases the risk of adverse events with MRA therapy in heart failure, these drugs are potentially beneficial in these higher‐risk populations.
The landmark clinical trials of MRA therapy in heart failure have had conflicting results with respect to the benefit of therapy in high‐risk subgroups. The RALES (Randomized Aldactone Evaluation Study) trial of patients with reduced ejection fraction and severe symptoms showed a mortality benefit for spironolactone in patients with median creatinine of 1.2 mg/dL or greater.20 Subgroup analyses from EMPHASIS‐HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure), which included patients with reduced ejection fraction and mild symptoms, also showed a benefit for the primary end point of cardiovascular death or heart failure hospitalization in patients with an estimated glomerular filtration rate less than 60 mL/min per 1.73 m2 and in patients with a history of diabetes mellitus.21 However, in patients with left ventricular dysfunction after myocardial infarction, studied in the EPHESUS (Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study), for the outcomes of all‐cause mortality and cardiovascular death or hospitalization, the benefits of eplerenone in patients with serum creatinine of 1.1 mg/dL or greater and those with a history of diabetes mellitus were not statistically significant.22 Finally, in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial, for patients with preserved ejection fraction, spironolactone therapy did not significantly reduce the primary end point. There was no benefit for the subgroup of patients with an estimated glomerular filtration rate less than 60 mL/min per 1.73 m2 or the group of patients with a history of diabetes mellitus.2
Building on past work from registry analyses that examined outcomes and adverse events in patients with reduced ejection fraction who were prescribed an MRA at hospital discharge,23 we examined MRA use in patients with heart failure who were at greater risk for adverse events and outcomes. Our objective was to describe MRA initiation at discharge from a heart failure hospitalization and to evaluate associations between MRA therapy and short‐ and longer‐term outcomes in a registry‐based cohort of older patients with heart failure with concomitant diabetes mellitus or chronic kidney disease.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Data Sources
Data were from the American Heart Association's Get With the Guidelines‐Heart Failure registry linked to Medicare claims from the Centers for Medicare & Medicaid Services. The registry is an ongoing online registry for patients hospitalized with heart failure.24 Patients are eligible for inclusion in the registry if they are admitted or discharged with a diagnosis of heart failure (International Classification of Diseases, Ninth Revision, Clinical Modification ICD‐9‐CM codes 402.x1, 404.x1, 404.x3, and 428.x).
The Medicare data include 100% of Medicare Part A claims and associated denominator files from 2005 through 2013. Medicare Part A includes institutional claims from inpatient hospitalizations. Denominator files include information about demographic characteristics, Medicare eligibility and enrollment, and mortality. We linked the registry data to the Medicare data using indirect identifiers, as described and validated previously.25
Study Population
The study population included Medicare fee‐for‐service beneficiaries aged ≥65 years who were discharged from a registry hospitalization for heart failure between January 1, 2005, and December 31, 2013. To be eligible for this study, patients had to have a concomitant diagnosis of diabetes mellitus and/or chronic kidney disease before the index hospitalization, as recorded in the registry. In the medical history section of the registry, diabetes mellitus is recorded as “diabetes—insulin treated” or “diabetes—non‐insulin treated” and chronic kidney disease is recorded as “renal insufficiency‐chronic (serum creatinine >2.0).” Patients included in the analysis were required to be new users of MRA therapy, defined as no MRA therapy at admission. We excluded patients with a contraindication to aldosterone antagonists recorded in the registry. Only patients discharged to home were included. If the patient had multiple hospitalizations in the registry, we used the first hospitalization for the analysis.
Treatment
The treatment of interest was MRA therapy prescribed at discharge, as recorded in the registry. Dosage information was unavailable.
Outcomes
The primary outcome was all‐cause mortality at 30 days, 1 year, and 3 years. Other outcomes of interest included 30‐day, 1‐year, and 3‐year all‐cause readmission, heart failure readmission, and readmission with a diagnosis of hyperkalemia or acute renal insufficiency. We identified deaths based on death dates in the Medicare denominator files, and we calculated days to death from the index hospitalization discharge date. We identified all‐cause readmission using subsequent inpatient claims except those for transfers to or from another hospital and admissions for rehabilitation. We defined heart failure readmissions by a primary diagnosis of heart failure (ICD‐9‐CM diagnosis code 428.x, 402.x1, 404.x1, or 404.x3) on an inpatient claim. We defined hyperkalemia using ICD‐9‐CM diagnosis code 276.7 and acute renal insufficiency using ICD‐9‐CM diagnosis code 584.x on an inpatient claim.
Subgroups
We assigned patients in the study cohort to prespecified subgroups based on disease history and ejection fraction for interaction analyses, using registry indicator variables for history of diabetes mellitus and history of renal insufficiency. We also categorized patients as having ejection fraction of 35% or less or greater than 35%, because heart failure guidelines recommend MRA therapy in patients with reduced ejection fraction. We defined reduced ejection fraction as documentation of left ventricular ejection fraction of 35% or less or a qualitative assessment of moderate or severe left ventricular systolic dysfunction. We grouped together patients with heart failure with borderline and preserved ejection, defined as ejection fraction greater than 35% or a qualitative assessment of no or mild left ventricular systolic dysfunction. We excluded patients with no documentation of ejection fraction.
Covariates
Covariates in population comparisons and modeling included the following registry variables: age, sex, race, medical history (ie, anemia, atrial fibrillation, cerebrovascular accident or transient ischemic attack, chronic obstructive pulmonary disease, depression, diabetes mellitus, hyperlipidemia, hypertension, implantable cardioverter‐defibrillator, ischemic etiology of heart failure, pacemaker, peripheral vascular disease, renal insufficiency, and smoking in the past year), vital signs at admission (ie, systolic blood pressure, heart rate, and respiratory rate), laboratory tests at discharge (ie, creatinine, ejection fraction, potassium, sodium, and urea nitrogen), discharge medications (ie, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, β‐blocker, anticoagulant, digoxin, diuretic, and lipid‐lowering agent), and discharge year. If discharge laboratory test results were missing, we substituted admission laboratory test results.
Statistical Analysis
We describe baseline characteristics of the study population by treatment group, using frequencies with percentages for categorical variables and means with SDs for continuous variables. We tested for differences between groups using chi‐squared tests for categorical variables and Wilcoxon rank‐sum tests for continuous variables. We used logistic regression to assess unadjusted and adjusted associations between patient characteristics and MRA therapy at hospital discharge.
We describe observed event rates by treatment group. For mortality, we calculated cumulative incidence at 30 days, 1 year, and 3 years based on Kaplan–Meier estimates. We tested for mortality differences between groups using log‐rank tests. For other end points, we calculated cumulative incidence based on estimates from the cumulative incidence function, which accounts for the competing risk of death, a high risk in this population. We used Gray tests to test for differences between treatment groups on these outcomes.
We used an inverse probability‐weighted estimator—an extension of the propensity score—to assess differences in outcomes among treatment groups while accounting for confounding by observed covariates. We obtained the weights by fitting a treatment selection model as a logistic regression model with treatment as the dependent variable and the baseline characteristics described above as the independent variables. To evaluate the adequacy of the treatment selection model, we compared the baseline characteristics of each group after weighting. We used weighted chi‐squared tests to test for differences on categorical variables and weighted analysis of variance to test for differences on continuous variables. We also calculated standardized differences to assess balance after weighting. Balanced variables were those with a standardized difference of less than 10%.
We estimated the unadjusted relationship between MRA therapy and each outcome using Cox proportional hazards models in which the treatment indicator was the sole independent variable. Next, we estimated the adjusted relationship between treatment and each outcome using weighted proportional hazards regression models. Finally, we controlled for discharge medications in addition to the treatment indicator using weighted proportional hazards models. We used robust SEs to account for clustering of patients within hospitals.
In addition to estimating an overall treatment effect, we assessed differences between prespecified subgroups by testing the significance of interaction terms between treatment and subgroup variables. This analysis focused on 3‐year outcomes. Based on the interaction results, we repeated the main analyses for patients with reduced ejection fraction; we reevaluated the propensity model for these patients only, then estimated associations between treatment and outcomes in this subgroup using ejection fraction–specific weights.
In post hoc exploratory analyses, we used Cox proportional hazards models to examine associations between selected baseline characteristics and the outcomes of hospitalization for hyperkalemia or acute renal insufficiency among patients who were prescribed an MRA at hospital discharge.
Most variables had low rates of missingness. For variables with less than 5% missingness, we imputed continuous variables to the overall median value, dichotomous variables to “no,” and multichotomous variables to the most frequent categorical value. For variables with more than 5% missingness, we treated the missing value as a separate category.
We report 95% confidence intervals (CIs) and used α=0.05 to establish statistical significance of tests. All tests were 2‐sided. We used SAS software (version 9.4; SAS Institute Inc, Cary, NC) for all analyses. The institutional review board of the Duke University Health System approved the study. Informed consent was waived.
Results
Of 16 848 eligible patients, 2067 (12.3%) were prescribed MRA therapy at discharge. Table 1 shows the baseline characteristics of the study population. A higher proportion of patients on MRA therapy had diabetes mellitus (86.6% versus 82.2%), and a lower proportion had chronic kidney disease (27.8% versus 36.0%). In the overall study population, 6719 patients (39.9%) had reduced ejection fraction, and 10 129 (60.1%) had borderline or preserved ejection fraction. Patients receiving MRA therapy at discharge were more likely to have reduced ejection fraction at admission (60.5% versus 37%), and they had higher rates of angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker use (72.7% versus 62.6%) and β‐blocker use (87.6% versus 81.2%). After weighting by the inverse probability of treatment, baseline characteristics were similar between treatment groups (Table S1).
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | MRA Therapy | P Value | |
|---|---|---|---|
| Yes (n=2067) | No (n=14 781) | ||
| Age, mean (SD), y | 76.3 (7.4) | 77.8 (7.6) | <0.001 |
| Age group, n (%) | <0.001 | ||
| 65 to 79 y | 1386 (67.1) | 8534 (57.7) | |
| ≥80 y | 681 (32.9) | 6247 (42.3) | |
| Men, N (%) | 1156 (55.9) | 7369 (49.9) | <0.001 |
| Race | 0.19 | ||
| Black | 244 (11.8) | 1683 (11.4) | |
| White | 1504 (72.8) | 11 014 (74.5) | |
| Other/unknown | 319 (15.4) | 2084 (14.1) | |
| Disease state | |||
| Diabetes mellitus | 1791 (86.6) | 12 154 (82.2) | <0.001 |
| Chronic renal insufficiency | 574 (27.8) | 5326 (36.0) | <0.001 |
| Medical history | |||
| Anemia | 381 (18.4) | 3350 (22.7) | <0.001 |
| Atrial fibrillation | 762 (36.9) | 5231 (35.4) | 0.19 |
| Chronic obstructive pulmonary disease | 632 (30.6) | 4717 (31.9) | 0.22 |
| Depression | 201 (9.7) | 1432 (9.7) | 0.96 |
| Heart failure with ischemic etiology | <0.001 | ||
| No | 814 (39.4) | 6645 (45.0) | |
| Yes | 1129 (54.6) | 7204 (48.7) | |
| Missing | 124 (6.0) | 932 (6.3) | |
| Hyperlipidemia | 1227 (59.4) | 8478 (57.4) | 0.08 |
| Hypertension | 1678 (81.2) | 12 334 (83.4) | 0.01 |
| Implantable cardioverter‐defibrillator | 306 (14.8) | 1200 (8.1) | <0.001 |
| Pacemaker | 397 (19.2) | 2603 (17.6) | 0.08 |
| Peripheral vascular disease | 287 (13.9) | 2468 (16.7) | 0.001 |
| Smoker in the past y | 228 (11.0) | 1398 (9.5) | 0.02 |
| Vital signs at admission | |||
| Heart rate, mean (SD), bpm | 84.0 (19.1) | 81.8 (18.8) | <0.001 |
| Respiratory rate ≥30, N (%), breaths/min | 96 (4.6) | 922 (6.2) | 0.004 |
| Systolic blood pressure, mean (SD), mm Hg | 139.5 (28.4) | 145.9 (29.7) | <0.001 |
| Tests at admission/discharge | |||
| Reduced ejection fraction at admissiona | 1251 (60.5) | 5468 (37.0) | <0.001 |
| Serum creatinine, mean (SD), mg/dL | 1.5 (0.7) | 1.9 (1.3) | <0.001 |
| Serum potassium, mean (SD), mEq/L | 4.1 (0.5) | 4.1 (0.5) | 0.006 |
| Serum urea nitrogen, mean (SD), mg/dL | 31.9 (16.8) | 34.8 (18.0) | <0.001 |
| Medications at discharge | |||
| ACE inhibitor and/or ARB | 1502 (72.7) | 9247 (62.6) | <0.001 |
| Anticoagulant | 730 (35.3) | 4555 (30.8) | <0.001 |
| β‐blocker | 1810 (87.6) | 12 006 (81.2) | <0.001 |
| Digoxin | 504 (24.4) | 2235 (15.1) | <0.001 |
| Diuretic | 1790 (86.6) | 11 837 (80.1) | <0.001 |
| Lipid‐lowering agent | 1441 (69.7) | 9885 (66.9) | 0.01 |
| Discharge year | <0.001 | ||
| 2005 | 80 (3.9) | 499 (3.4) | |
| 2006 | 288 (13.9) | 2106 (14.2) | |
| 2007 | 221 (10.7) | 1898 (12.8) | |
| 2008 | 179 (8.7) | 1678 (11.4) | |
| 2009 | 203 (9.8) | 1710 (11.6) | |
| 2010 | 229 (11.1) | 1929 (13.1) | |
| 2011 | 291 (14.1) | 1868 (12.6) | |
| 2012 | 295 (14.3) | 1509 (10.2) | |
| 2013 | 281 (13.6) | 1584 (10.7) | |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist.
Reduced ejection fraction is defined as documentation of a left ventricular ejection fraction of 35% or less, or a qualitative assessment of moderate or severe left ventricular systolic dysfunction.
Among the patient characteristics associated with receipt of MRA therapy in the treatment selection model were age (adjusted odds ratio [OR], 0.98; 95% CI, 0.97–0.99) and sex (adjusted OR for women, 0.89; 95% CI, 0.81–0.99; Table 2). MRA prescription was not associated with a history of diabetes mellitus or renal insufficiency after adjustment. Reduced ejection fraction was strongly associated with MRA prescription (adjusted OR, 2.34; 95% CI, 2.11–2.59). Although discharge serum creatinine was associated with MRA prescription (adjusted OR, 0.66; 95% CI, 0.61–0.71), serum potassium was not (adjusted OR, 1.00; 95% CI, 0.90–1.11).
Table 2.
Associations Between Patient Characteristics and Prescription of MRA Therapy at Hospital Discharge
| Characteristic | Unadjusted OR (95% CI) | P Value | Adjusteda OR (95% CI) | P Value |
|---|---|---|---|---|
| Age, y | 0.97 (0.97–0.98) | <0.001 | 0.98 (0.97–0.99) | <0.001 |
| Women | 0.78 (0.71–0.86) | <0.001 | 0.89 (0.81–0.99) | 0.03 |
| Race | ||||
| Black | 1.06 (0.92–1.23) | 0.42 | 1.14 (0.98–1.33) | 0.09 |
| White | 1.00 [Reference] | 1.00 [Reference] | ||
| Other/unknown | 1.12 (0.98–1.28) | 0.08 | 0.97 (0.82–1.14) | 0.71 |
| Disease state | ||||
| Diabetes mellitus | 1.40 (1.23–1.60) | <0.001 | 1.11 (0.93–1.33) | 0.25 |
| Renal insufficiency | 0.68 (0.62–0.76) | <0.001 | 1.03 (0.89–1.20) | 0.68 |
| Medical history | ||||
| Anemia | 0.77 (0.69–0.87) | <0.001 | 0.96 (0.85–1.08) | 0.50 |
| Atrial fibrillation | 1.07 (0.97–1.17) | 0.19 | 1.01 (0.92–1.12) | 0.80 |
| Chronic obstructive pulmonary disease | 0.94 (0.85–1.04) | 0.22 | 0.91 (0.82–1.01) | 0.08 |
| Depression | 1.00 (0.86–1.17) | 0.96 | 1.00 (0.85–1.17) | 0.99 |
| Heart failure with ischemic etiology | ||||
| No | 1.00 [Reference] | 1.00 [Reference] | ||
| Yes | 1.28 (1.16–1.41) | <0.001 | 1.08 (0.97–1.19) | 0.16 |
| Missing | 1.09 (0.89–1.33) | 0.42 | 0.97 (0.79–1.20) | 0.78 |
| Hyperlipidemia | 1.09 (0.99–1.19) | 0.08 | 1.03 (0.93–1.14) | 0.61 |
| Hypertension | 0.86 (0.76–0.96) | 0.01 | 0.94 (0.83–1.06) | 0.32 |
| Implantable cardioverter‐defibrillator | 1.97 (1.72–2.25) | <0.001 | 1.37 (1.18–1.58) | <0.001 |
| Pacemaker | 1.11 (0.99–1.25) | 0.08 | 0.96 (0.85–1.09) | 0.50 |
| Peripheral vascular disease | 0.80 (0.71–0.92) | 0.001 | 0.80 (0.70–0.92) | 0.002 |
| Vital signs at admission | ||||
| Smoker in the past y | 1.19 (1.02–1.38) | 0.02 | 1.00 (0.86–1.18) | 0.96 |
| Heart rate | 1.01 (1.00–1.01) | <0.001 | 1.00 (1.00–1.01) | 0.007 |
| Respiratory rate ≥30 breaths/min | 0.73 (0.59–0.91) | 0.005 | 0.74 (0.59–0.93) | 0.01 |
| Tests at admission/discharge | ||||
| Systolic blood pressure | 0.99 (0.99–0.99) | <0.001 | 1.00 (0.99–1.00) | <0.001 |
| Reduced ejection fractionb | 2.61 (2.38–2.87) | <0.001 | 2.34 (2.11–2.59) | <0.001 |
| Serum creatinine | 0.66 (0.62–0.70) | <0.001 | 0.66 (0.61–0.71) | <0.001 |
| Serum potassium | 0.86 (0.78–0.95) | 0.002 | 1.00 (0.90–1.11) | 0.98 |
| Serum urea nitrogen | 0.99 (0.99–0.99) | <0.001 | 1.00 (1.00–1.00) | 0.87 |
| Discharge year | ||||
| 2005 | 0.90 (0.69–1.18) | 0.46 | 0.78 (0.58–1.05) | 0.10 |
| 2006 | 0.77 (0.65–0.92) | 0.004 | 0.68 (0.55–0.84) | <0.001 |
| 2007 | 0.66 (0.54–0.79) | <0.001 | 0.58 (0.47–0.73) | <0.001 |
| 2008 | 0.60 (0.49–0.73) | <0.001 | 0.52 (0.41–0.66) | <0.001 |
| 2009 | 0.67 (0.55–0.81) | <0.001 | 0.63 (0.50–0.78) | <0.001 |
| 2010 | 0.67 (0.56–0.81) | <0.001 | 0.63 (0.50–0.78) | <0.001 |
| 2011 | 0.88 (0.74–1.05) | 0.15 | 0.86 (0.69–1.05) | 0.14 |
| 2012 | 1.10 (0.92–1.32) | 0.28 | 1.05 (0.86–1.29) | 0.63 |
| 2013 | 1.00 [Reference] | 1.00 [Reference] | ||
CI indicates confidence interval; MRA, mineralocorticoid receptor antagonist; OR, odds ratio.
Adjustment variables detailed in the Methods section.
Reduced ejection fraction is defined as documentation of a left ventricular ejection fraction of 35% or less, or a qualitative assessment of moderate or severe left ventricular systolic dysfunction.
Patients on MRA therapy had lower observed rates of 3‐year mortality (54.4% versus 57.5%), 30‐day heart failure readmission (7.9% versus 9.5%), and 1‐year (68.2% versus 72.2%) and 3‐year (84.9% versus 88.2%) all‐cause readmission (Table 3; Figure 1). At 30 days, patients on MRA therapy had higher rates of readmission for hyperkalemia and acute renal insufficiency (Table 3; Figure 2), though overall only 18 patients were admitted within 30 days for hyperkalemia as a primary diagnosis.
Table 3.
Observed Outcomes of the Study Population
| Outcome | MRA Therapy | P Value | |
|---|---|---|---|
| Yes (n=2067) | No (n=14 781) | ||
| Mortality | |||
| 30 d | 72 (3.5) | 515 (3.5) | 0.98 |
| 1 y | 521 (27.2) | 3887 (28.2) | 0.41 |
| 3 y | 896 (54.4) | 7034 (57.5) | 0.03 |
| Readmission | |||
| All causesa | |||
| 30 d | 465 (22.7) | 3531 (24.0) | 0.20 |
| 1 y | 1338 (68.2) | 10 194 (72.2) | <0.001 |
| 3 y | 1578 (84.9) | 11 939 (88.2) | <0.001 |
| Heart failurea | |||
| 30 d | 162 (7.9) | 1394 (9.5) | 0.02 |
| 1 y | 661 (34.1) | 4996 (35.6) | 0.09 |
| 3 y | 854 (48.0) | 6477 (49.2) | 0.09 |
| Hyperkalemia, primary diagnosisa | |||
| 30 d | ···b | ···b | <0.001 |
| 1 y | 22 (1.1) | 101 (0.7) | 0.05 |
| 3 y | 35 (2.1) | 176 (1.4) | 0.03 |
| Hyperkalemia, any diagnosisa | |||
| 30 d | 63 (3.1) | 258 (1.8) | <0.001 |
| 1 y | 200 (10.2) | 1227 (8.8) | 0.02 |
| 3 y | 275 (15.7) | 1928 (15.3) | 0.32 |
| Acute renal insufficiency, primary diagnosisa | |||
| 30 d | 40 (2.0) | 205 (1.4) | 0.05 |
| 1 y | 160 (8.2) | 985 (7.1) | 0.05 |
| 3 y | 234 (13.6) | 1633 (13.0) | 0.34 |
| Acute renal insufficiency, any diagnosisa | |||
| 30 d | 163 (7.9) | 1051 (7.2) | 0.19 |
| 1 y | 619 (31.8) | 4255 (30.5) | 0.18 |
| 3 y | 873 (49.5) | 6254 (48.5) | 0.27 |
MRA indicates mineralocorticoid receptor antagonist.
Death treated as a competing risk.
In accord with the privacy policy of the Centers for Medicare & Medicaid Services, data for cells containing 10 or fewer observations and data for cells that would allow for calculation of cells containing 10 or fewer observations are not reported.
Figure 1.
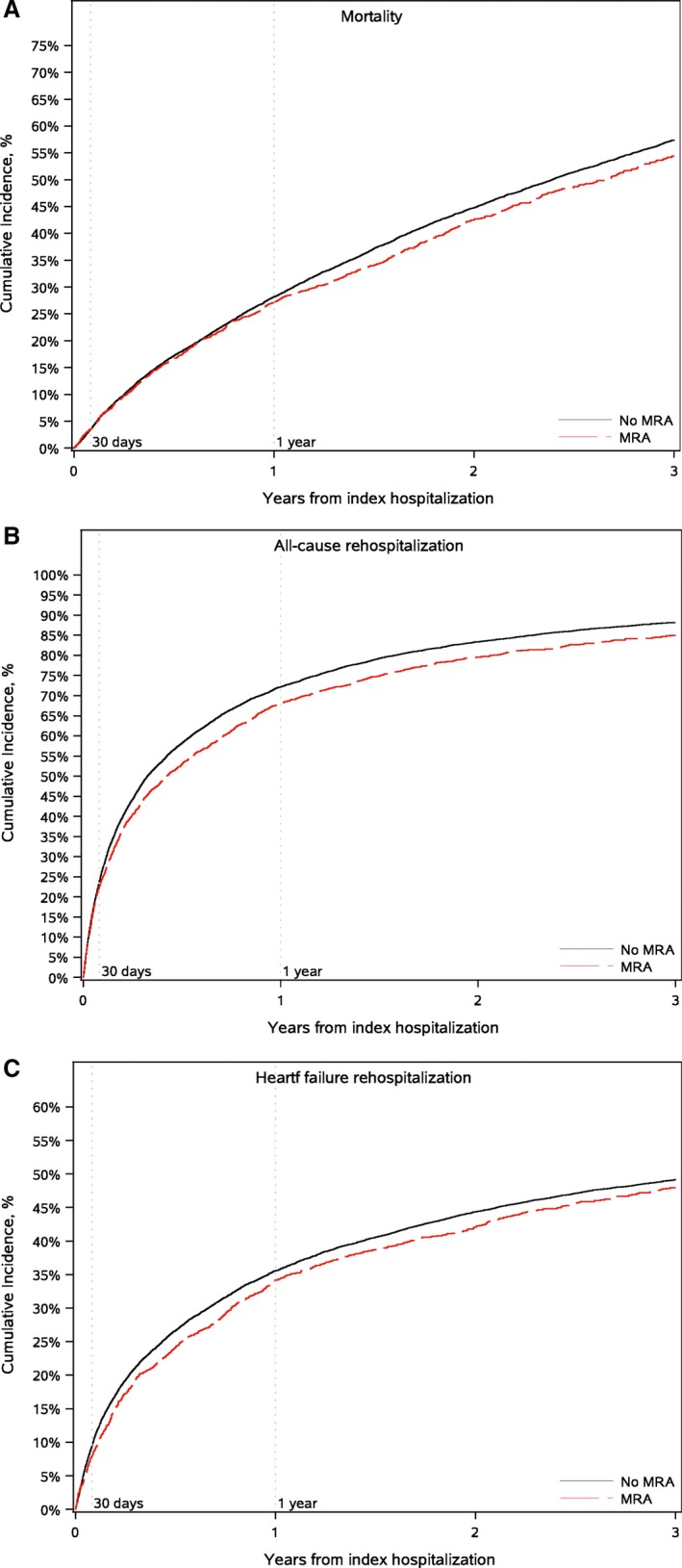
Cumulative incidence of mortality (A), all‐cause hospitalization (B), and heart failure hospitalization (C). MRA indicates mineralocorticoid receptor antagonist.
Figure 2.
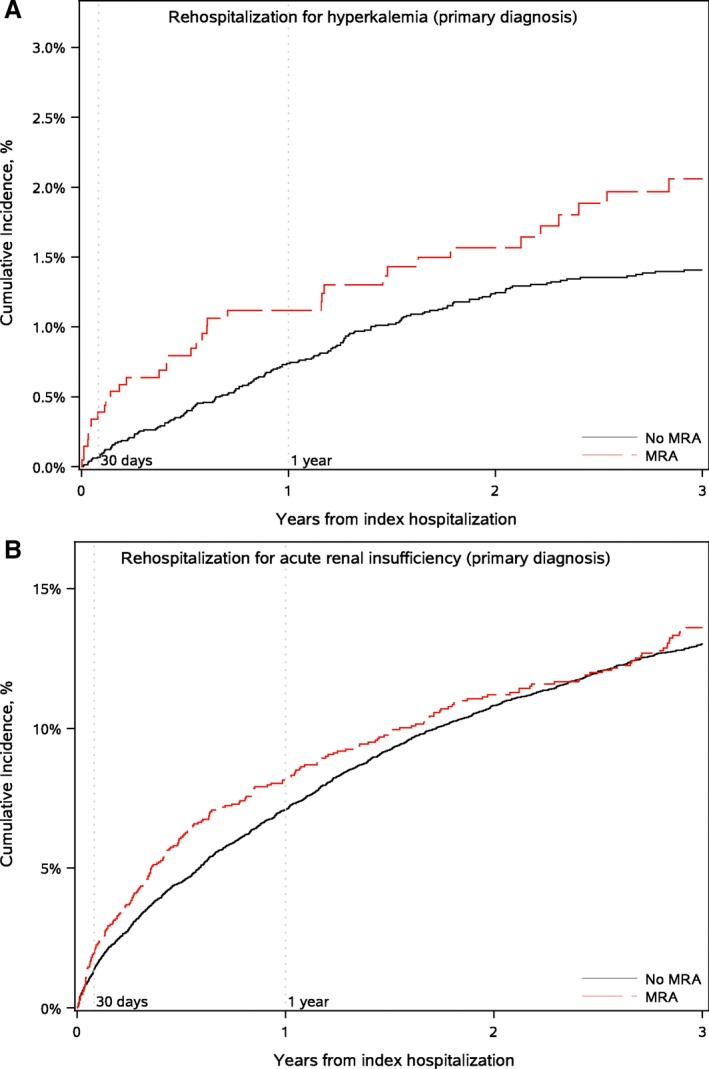
Cumulative incidence of hospitalization for hyperkalemia as the primary diagnosis (A) and acute renal insufficiency as the primary diagnosis (B). MRA indicates mineralocorticoid receptor antagonist.
After inverse probability weighting, MRA use was not associated with 30‐day, 1‐year, or 3‐year mortality (Table 4) or with 30‐day all‐cause readmission (hazard ratio [HR], 0.97; 95% CI, 0.87–1.09). MRA use was associated with lower risk of readmission at 1 year (HR, 0.92; 95% CI, 0.87–0.98) and 3 years (HR, 0.93; 95% CI, 0.89–0.98). These relationships remained after additional adjustment for discharge medications.
Table 4.
Associations Between MRA Therapy and Outcomes
| Events | Unadjusteda | Weighteda | Weighted and Adjusteda , b | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Mortality | ||||||
| 30 d | 1.01 (0.78–1.30) | 0.96 | 0.94 (0.68–1.30) | 0.69 | 0.97 (0.70–1.34) | 0.84 |
| 1 y | 0.96 (0.90–1.04) | 0.31 | 0.99 (0.91–1.09) | 0.89 | 1.00 (0.91–1.09) | >0.99 |
| 3 y | 0.93 (0.87–0.99) | 0.02 | 1.02 (0.94–1.09) | 0.68 | 1.02 (0.95–1.10) | 0.58 |
| Readmission | ||||||
| All causes | ||||||
| 30 d | 0.94 (0.86–1.03) | 0.20 | 0.97 (0.87–1.09) | 0.63 | 0.98 (0.87–1.10) | 0.73 |
| 1 y | 0.89 (0.84–0.94) | <0.001 | 0.92 (0.87–0.98) | 0.01 | 0.93 (0.87–0.98) | 0.01 |
| 3 y | 0.88 (0.84–0.92) | <0.001 | 0.93 (0.89–0.98) | 0.006 | 0.94 (0.89–0.98) | 0.01 |
| Heart failure | ||||||
| 30 d | 0.83 (0.72–0.96) | 0.01 | 0.83 (0.69–0.99) | 0.04 | 0.83 (0.69–1.00) | 0.045 |
| 1 y | 0.93 (0.86–1.00) | 0.05 | 0.93 (0.84–1.03) | 0.15 | 0.92 (0.83–1.02) | 0.11 |
| 3 y | 0.92 (0.87–0.98) | 0.01 | 0.94 (0.87–1.02) | 0.14 | 0.93 (0.85–1.01) | 0.09 |
| Hyperkalemia, any diagnosis | ||||||
| 30 d | 1.77 (1.29–2.41) | <0.001 | 2.08 (1.49–2.90) | <0.001 | 2.11 (1.51–2.94) | <0.001 |
| 1 y | 1.19 (1.02–1.38) | 0.03 | 1.42 (1.18–1.71) | <0.001 | 1.44 (1.20–1.74) | <0.001 |
| 3 y | 1.04 (0.91–1.21) | 0.55 | 1.28 (1.09–1.51) | 0.002 | 1.30 (1.11–1.53) | 0.001 |
| Acute renal insufficiency, any diagnosis | ||||||
| 30 d | 1.12 (0.97–1.30) | 0.13 | 1.31 (1.09–1.56) | 0.003 | 1.31 (1.10–1.57) | 0.003 |
| 1 y | 1.05 (0.96–1.14) | 0.26 | 1.17 (1.06–1.28) | 0.001 | 1.16 (1.06–1.27) | 0.002 |
| 3 y | 1.01 (0.93–1.10) | 0.78 | 1.14 (1.05–1.25) | 0.004 | 1.14 (1.04–1.25) | 0.005 |
| Hyperkalemia, primary diagnosis | ||||||
| 30 d | ···c | ···c | ···c | |||
| 1 y | 1.57 (0.97–2.54) | 0.06 | 2.67 (1.54–4.62) | <0.001 | 2.90 (1.67–5.02) | <0.001 |
| 3 y | 1.45 (0.97–2.17) | 0.07 | 2.20 (1.42–3.41) | <0.001 | 2.34 (1.49–3.67) | <0.001 |
| Acute renal insufficiency, primary diagnosis | ||||||
| 30 d | 1.40 (1.03–1.90) | 0.03 | 1.65 (1.16–2.34) | 0.005 | 1.62 (1.13–2.31) | 0.008 |
| 1 y | 1.17 (1.02–1.35) | 0.03 | 1.32 (1.11–1.56) | 0.002 | 1.30 (1.10–1.54) | 0.003 |
| 3 y | 1.04 (0.91–1.19) | 0.52 | 1.18 (1.02–1.38) | 0.03 | 1.17 (1.01–1.36) | 0.04 |
CI indicates confidence interval; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist.
Proportional hazards assumptions were assessed for 3‐y weighted models: It was violated for hyperkalemia readmission, any diagnosis (P=0.02).
Adjusted for discharge medications.
In accord with the privacy policy of the Centers for Medicare & Medicaid Services, data for cells containing 10 or fewer observations and data for cells that would allow for calculation of cells containing 10 or fewer observations are not reported.
In the weighted analyses, MRA use was associated with a greater risk of 30‐day readmission with a diagnosis of hyperkalemia (HR, 2.08; 95% CI, 1.49–2.90) and acute renal insufficiency (HR, 1.31; 95% CI, 1.09–1.56), as well as at 1 and 3 years. MRA use was also associated with greater risk of readmission with a primary diagnosis of hyperkalemia at 1 year (OR, 2.67; 95% CI, 1.54–4.62) and 3 years (OR, 2.20; 95% CI, 1.42–3.41) and acute renal insufficiency at 30 days (OR, 1.65; 95% CI, 1.16–2.34), 1 year (OR, 1.32; 95% CI, 1.11–1.56), and 3 years (OR, 1.18; 95% CI, 1.02–1.38). These relationships remained after additional adjustment for discharge medications.
In the interaction analysis of reduced ejection fraction versus borderline or preserved ejection fraction, there were significant interactions for readmission for acute renal insufficiency as the primary (P=0.01) or any diagnosis (P<0.001), and for hyperkalemia in any diagnosis position (P=0.02; Table 5). Although MRA therapy was not associated with readmission for acute renal insufficiency at 3 years for patients with reduced ejection fraction (HR, 0.94; 95% CI, 0.75–1.18), it was associated with readmission for renal insufficiency among patients with borderline or preserved ejection fraction (HR. 1.34; 95% CI, 1.11–1.62). There were no significant interactions by ejection fraction for mortality, all‐cause or heart failure readmission, or hyperkalemia in the primary diagnosis position. There were no significant interactions by disease history (ie, renal insufficiency or diabetes mellitus).
Table 5.
Subgroup‐Specific Treatment Effects at 3 Years, Based on the Weighted Model
| Readmission Event | Hazard Ratio (95% CI) | P Value for Interaction | |
|---|---|---|---|
| Ejection Fraction >35% | Ejection Fraction ≤35% | ||
| All‐cause rehospitalization | 0.96 (0.90–1.03) | 0.89 (0.83–0.95) | 0.13 |
| Mortality | 1.04 (0.93–1.16) | 0.99 (0.89–1.08) | 0.47 |
| Heart failure rehospitalization | 0.99 (0.88–1.12) | 0.88 (0.79–0.97) | 0.13 |
| Hyperkalemia, any diagnosis | 1.44 (1.17–1.78) | 1.04 (0.86–1.25) | 0.02 |
| Hyperkalemia, primary diagnosis | 2.31 (1.29–4.15) | 1.96 (1.07–3.60) | 0.71 |
| Acute renal insufficiency, any diagnosis | 1.29 (1.15–1.44) | 0.94 (0.82–1.07) | <0.001 |
| Acute renal insufficiency, primary diagnosis | 1.34 (1.11–1.62) | 0.94 (0.75–1.18) | 0.01 |
CI indicates confidence interval.
To further explore the role of subtype of heart failure, we restricted inverse probability weighting to patients with reduced ejection fraction (Table 6). MRA therapy was associated with lower risk of all‐cause readmission at 3 years (HR, 0.91; 95% CI, 0.85–0.98), but not at 30 days or 1 year. This association remained after adjustment for discharge medications (HR, 0.93; 95% CI, 0.86–1.00). MRA therapy was not associated with greater risk of readmission with hyperkalemia. Unlike the overall analyses, there were no short‐term or long‐term associations between MRA therapy and hospitalization for acute renal insufficiency in the analysis restricted to patients with reduced ejection fraction.
Table 6.
Associations Between MRA Therapy and Outcomes Among Patients With Heart Failure With Reduced Ejection Fraction
| Outcome | Unadjusted | Weighted | Weighted and Adjusteda | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Mortality | ||||||
| 30 d | 0.99 (0.73–1.35) | 0.97 | 1.08 (0.76–1.54) | 0.66 | 1.15 (0.80–1.65) | 0.46 |
| 1 y | 0.86 (0.77–0.96) | 0.007 | 1.03 (0.87–1.23) | 0.71 | 1.07 (0.91–1.26) | 0.40 |
| 3 y | 0.84 (0.77–0.92) | <0.001 | 1.06 (0.95–1.18) | 0.31 | 1.10 (0.99–1.22) | 0.07 |
| Readmission | ||||||
| All causes | ||||||
| 30 d | 0.87 (0.76–0.99) | 0.04 | 0.89 (0.76–1.05) | 0.16 | 0.91 (0.77–1.07) | 0.25 |
| 1 y | 0.85 (0.79–0.92) | <0.001 | 0.92 (0.85–1.00) | 0.06 | 0.94 (0.86–1.02) | 0.12 |
| 3 y | 0.83 (0.78–0.89) | <0.001 | 0.91 (0.85–0.98) | 0.01 | 0.93 (0.86–1.00) | 0.04 |
| Heart failure | ||||||
| 30 d | 0.79 (0.65–0.97) | 0.03 | 0.84 (0.66–1.06) | 0.14 | 0.87 (0.68–1.10) | 0.24 |
| 1 y | 0.84 (0.75–0.93) | <0.001 | 0.92 (0.80–1.06) | 0.25 | 0.94 (0.82–1.07) | 0.34 |
| 3 y | 0.81 (0.74–0.89) | <0.001 | 0.92 (0.82–1.03) | 0.13 | 0.93 (0.83–1.04) | 0.19 |
| Hyperkalemia, any diagnosis | ||||||
| 30 d | 1.36 (0.85–2.18) | 0.20 | 1.37 (0.83–2.27) | 0.22 | 1.41 (0.84–2.35) | 0.20 |
| 1 y | 1.08 (0.88–1.34) | 0.46 | 1.13 (0.89–1.42) | 0.31 | 1.13 (0.89–1.42) | 0.31 |
| 3 y | 0.94 (0.78–1.14) | 0.55 | 1.08 (0.89–1.31) | 0.43 | 1.09 (0.90–1.32) | 0.38 |
| Acute renal insufficiency, any diagnosis | ||||||
| 30 d | 0.85 (0.66–1.11) | 0.23 | 0.92 (0.68–1.26) | 0.61 | 0.95 (0.70–1.29) | 0.76 |
| 1 y | 0.91 (0.80–1.02) | 0.10 | 0.98 (0.86–1.13) | 0.81 | 1.00 (0.88–1.15) | 0.96 |
| 3 y | 0.88 (0.79–0.99) | 0.03 | 0.97 (0.85–1.10) | 0.62 | 0.98 (0.87–1.11) | 0.80 |
| Hyperkalemia, primary diagnosis | ||||||
| 30 d | ···b | ···b | ···b | |||
| 1 y | 1.44 (0.68–3.01) | 0.34 | 1.89 (0.86–4.16) | 0.11 | 1.81 (0.83–3.94) | 0.13 |
| 3 y | 1.60 (0.86–2.96) | 0.14 | 2.01 (1.08–3.73) | 0.03 | 2.00 (1.08–3.72) | 0.03 |
| Acute renal insufficiency, primary diagnosis | ||||||
| 30 d | 1.02 (0.61–1.69) | 0.95 | 1.25 (0.71–2.23) | 0.44 | 1.28 (0.72–2.28) | 0.39 |
| 1 y | 0.99 (0.81–1.22) | 0.96 | 1.13 (0.89–1.45) | 0.31 | 1.16 (0.91–1.47) | 0.23 |
| 3 y | 0.89 (0.73–1.07) | 0.22 | 0.98 (0.78–1.23) | 0.86 | 1.00 (0.80–1.24) | 0.99 |
CI indicates confidence interval; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist.
Adjusted for discharge medications.
In accord with the privacy policy of the Centers for Medicare & Medicaid Services, data for cells containing 10 or fewer observations and data for cells that would allow for calculation of cells containing 10 or fewer observations are not reported.
In post hoc analyses restricted to patients who were prescribed MRA at discharge, we further explored associations between baseline characteristics and readmission for hyperkalemia or acute renal insufficiency by heart failure subtype. Among patients on MRA therapy with preserved ejection fraction, women had a greater 3‐year risk of hospitalization for hyperkalemia (adjusted HR, 1.84; 95% CI, 1.28–2.64) and for acute renal insufficiency (adjusted HR, 1.28; 95% CI, 1.05–1.56; Table S2). Serum potassium was positively associated with increased risk of hyperkalemia (adjusted HR, 1.53; 95% CI, 1.02–2.30). Among patients with reduced ejection fraction, women had a greater risk of hospitalization for hyperkalemia (adjusted HR, 1.47; 95% CI, 1.10–1.95; Table S3), but not acute renal insufficiency (adjusted HR, 0.95; 95% CI, 0.76–1.19). Serum potassium was not associated with a greater risk of hyperkalemia in patients with reduced ejection fraction (adjusted HR, 1.33; 95% CI, 0.93–1.90).
Discussion
In this large, retrospective study of patients hospitalized with heart failure and concomitant diabetes mellitus and/or chronic kidney disease, prescription of MRA therapy at discharge was not associated with a lower risk of mortality. MRA therapy was associated with lower long‐term risk of all‐cause readmission, but with greater short‐term and long‐term risks of readmission with acute renal insufficiency and hyperkalemia. The risk of acute renal insufficiency was limited to patients with borderline or preserved ejection fraction.
Two previous studies using data from the Get With the Guidelines‐Heart Failure registry examined MRA therapy in heart failure and risks of adverse events.11, 23 Both studies focused on patients with reduced ejection fraction. Our work expands on previous work by including patients with reduced, borderline, and preserved ejection fraction; focusing on high‐risk patients with diabetes mellitus and/or chronic kidney disease; and using more‐recent data.
In our study, 12% of patients were prescribed MRA therapy at discharge, and most of these patients had reduced ejection fraction. Using data from 2005 through 2007, Albert et al11 likewise found that MRA therapy was markedly underused in appropriate patients, though rates of inappropriate and potentially inappropriate use were low. Patient characteristics associated with MRA therapy have not changed substantially, with age, systolic blood pressure, and presence of an implantable cardioverter‐defibrillator strongly associated with prescription of MRA therapy. However, in the previous study, history of renal insufficiency was associated with a lower likelihood of receiving MRA therapy, whereas we found no such association.
We also found no association between MRA therapy and lower risk of mortality. Using data from 2005 through 2010, Hernandez et al23 found that MRA therapy was not associated with lower risks of death or cardiovascular readmission overall, but was associated with a lower risk of heart failure readmission among patients with reduced ejection fraction. We found lower risks of heart failure readmission at 30 days and all‐cause readmission at 1 and 3 years. The beneficial long‐term association between MRA therapy and all‐cause readmission was independent of ejection fraction and the presence of diabetes mellitus and chronic kidney disease. Although the appropriateness of MRA therapy in patients with preserved ejection fraction is uncertain on the basis of clinical trial data, our findings suggest a benefit in high‐risk patients. Further study of MRA therapy in patients with heart failure and borderline or preserved ejection fraction is warranted.2, 26
Although the benefits of MRA therapy are well known, adverse effects have also been documented. Past work showed an greater risk of 30‐day and 1‐year admission with hyperkalemia in patients with reduced ejection fraction who were treated with an MRA, compared with patients not receiving an MRA; however, there were few hospitalizations with a primary diagnosis of hyperkalemia.23 Similarly, in our study patients at high risk for adverse events with MRA therapy, the risk of 30‐day, 1‐year, or 3‐year hospitalization for a primary or other diagnosis of hyperkalemia was higher for patients on MRA therapy. In the stratified analyses, however, the association between MRA therapy and increased risk for hospitalization with hyperkalemia in any diagnosis position was limited to patients with borderline or preserved ejection fraction. Ejection fraction type did not significantly alter the positive association between MRA therapy and 3‐year risk of hyperkalemia as a primary diagnosis; however, the absolute incidence of hyperkalemia as a primary diagnosis was very low in both groups even at 3 years. Among patients discharged on MRA therapy, women had a greater 3‐year risk of hospitalization with a diagnosis of hyperkalemia in any diagnosis position compared with men, regardless of ejection fraction subtype. Notably, higher baseline serum potassium was associated with a greater risk of hyperkalemia among patients with borderline or preserved ejection fraction, but not among patients with reduced ejection fraction.
Similar to the risk of hyperkalemia, the risk of 30‐day, 1‐year, or 3‐year hospitalization for a diagnosis of acute renal insufficiency was higher for patients on MRA therapy; however, this risk was limited to patients with borderline or preserved ejection fraction. In post hoc analyses restricted to patients discharged on MRA, the risk of 3‐year hospitalization for acute renal insufficiency in any diagnosis position was greater among women with borderline or preserved ejection fraction, but not among women with reduced ejection fraction. Further research is needed to investigate the mechanisms of increased risk in certain populations.
Despite the greater risk of hospitalization for hyperkalemia and acute kidney injury, there was an overall decrease in the risk of hospitalization for patients treated with MRA therapy, suggesting the benefits of therapy may outweigh the risks in this high‐risk population. Past analyses from landmark clinical trials of MRA therapy in heart failure had similar conclusions, with sustained benefit of MRA therapy despite increased risk of adverse events.12, 19, 27, 28, 29 Moreover, past work has shown that MRA therapy is beneficial even at higher serum potassium levels, up to a serum potassium level of 5.5 mmol/L.19 Maximizing the beneficial effects of MRA therapy in heart failure will depend on minimizing risks of adverse events, particularly in patients at highest risk for adverse events. Novel therapeutic agents, such as potassium binders, may protect against hyperkalemia in patients with heart failure who are at risk of hyperkalemia with MRA use, though additional studies are needed.30 Furthermore, development of more‐selective MRA therapies may achieve the benefits of MRA therapy with fewer adverse events.31, 32 In addition, appropriate patient selection and laboratory monitoring during therapy may decrease the risk of adverse events.33, 34, 35 These considerations warrant further investigation.
Our study has limitations. First, as in all observational studies, unmeasured confounders may have influenced the results. Second, the population was limited to Medicare fee‐for‐service beneficiaries, so the results may not be generalizable to other populations. Also, patients were recently discharged from an acute heart failure hospitalization, so the results may not apply to stable outpatients with heart failure. Furthermore, hospital participation in the registry is voluntary, and the practices of participating hospitals may not reflect practices at hospitals that do not participate. Third, we were limited by the data available. For the Get With the Guidelines‐Heart Failure registry, we were limited by the fields available in the registry and the completeness of each field, and for outcome data, we were limited to Medicare claims data. We only examined whether MRA therapy was prescribed at discharge, but we did not have information about doses prescribed. Furthermore, we did not analyze outpatient medication initiation, discontinuation, or adherence, though this has been reported in a past Get With the Guidelines‐Heart Failure study, which found that eligible patients who were not prescribed an MRA at discharge were less likely to initiate it in the outpatient setting.36 In addition, we did not have laboratory data with which to further explore the outcomes of hospitalizations for hyperkalemia or acute renal insufficency, so we were not able to comment on the severity of these adverse events. Finally, the results of our subgroup analyses must be interpreted with caution. For patients without quantitative assessment of ejection fraction, we used qualitative assessments, which may decrease the precision of these categories. Furthermore, we were unable to differentiate patients with recovered ejection fraction.
Conclusion
In conclusion, among older patients with heart failure and concomitant diabetes mellitus or renal insufficiency, MRA use was associated with lower risk of all‐cause hospitalization despite increased risk of hospitalization with hyperkalemia or acute renal insufficiency. The increased risk of adverse events was mostly confined to patients with borderline or preserved ejection fraction. MRAs may be safe in a selected group of patients with heart failure and concomitant diabetes mellitus or renal insufficiency.
Sources of Funding
This project was supported, in part, by grant number U19HS021092 from the Agency for Healthcare Research and Quality. The Get With the Guidelines‐Heart Failure program is provided by the American Heart Association; is sponsored, in part, by Amgen Cardiovascular; and has been funded previously through support from Medtronic, GlaxoSmithKline, Ortho‐McNeil, and the AHA Pharmaceutical Roundtable.
Disclosures
Dr Sharma reported receiving a European Society of Cardiology young investigator award and a Canadian Cardiovascular Society–Bayer Resident Vascular Award; and receiving research support from Takeda and Roche Diagnostics. Dr Fonarow reported having consultant relationships with Amgen, Medtronic, Novartis, and St Jude Medical/Abbott. Dr Hernandez reported receiving research support from AstraZeneca, Luitpold, Merck, and Novartis; and having consultant relationships with Amgen, Bayer, Boston Scientific, and Novartis.
Supporting information
Table S1. Baseline Characteristics of the Study Population by Study Group After Application of Inverse Probability Weights
Table S2. Associations Between Baseline Characteristics and 3‐Year Outcomes Among Patients With Heart Failure With Preserved Ejection Fraction
Table S3. Associations Between Baseline Characteristics and 3‐Year Outcomes Among Patients With Heart Failure With Reduced Ejection Fraction
Acknowledgments
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the article. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
(J Am Heart Assoc. 2017;6:e006540 DOI: 10.1161/JAHA.117.006540.)29275368
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 2. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 3. Pfeffer MA, Braunwald E. Treatment of heart failure with preserved ejection fraction: reflections on its treatment with an aldosterone antagonist. JAMA Cardiol. 2016;1:7–8. [DOI] [PubMed] [Google Scholar]
- 4. Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double‐masked, cross‐over study. Diabetes Care. 2005;28:2106–2612. [DOI] [PubMed] [Google Scholar]
- 5. Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51:199–211. [DOI] [PubMed] [Google Scholar]
- 6. Chrysostomou A, Becker G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med. 2001;345:925–926. [DOI] [PubMed] [Google Scholar]
- 7. Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–951. [DOI] [PubMed] [Google Scholar]
- 8. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 9. Bianchi S, Bigazzi R, Campese VM. Long‐term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. [DOI] [PubMed] [Google Scholar]
- 10. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:Cd007004. [DOI] [PubMed] [Google Scholar]
- 11. Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–1665. [DOI] [PubMed] [Google Scholar]
- 12. Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, Pitt B. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS‐HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013;62:1585–1593. [DOI] [PubMed] [Google Scholar]
- 13. Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–214. [DOI] [PubMed] [Google Scholar]
- 14. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 15. Ramadan FH, Masoodi N, El‐Solh AA. Clinical factors associated with hyperkalemia in patients with congestive heart failure. J Clin Pharm Ther. 2005;30:233–239. [DOI] [PubMed] [Google Scholar]
- 16. Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life‐threatening hyperkalemia during combined therapy with angiotensin‐converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med. 2001;110:438–441. [DOI] [PubMed] [Google Scholar]
- 17. Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. [DOI] [PubMed] [Google Scholar]
- 18. Jarman PR, Mather HM. Diabetes may be independent risk factor for hyperkalaemia. BMJ. 2003;327:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD. Incidence, predictors, and outcomes related to hypo‐ and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7:573–579. [DOI] [PubMed] [Google Scholar]
- 20. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 21. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.21073363 [Google Scholar]
- 22. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Qualls LG, Peterson ED, Fonarow GC, Curtis LH. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. [DOI] [PubMed] [Google Scholar]
- 24. Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O'Connor CM, Yancy CW, Young J. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design. Am Heart J. 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 25. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kristensen SL, Køber L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, Zile MR, Granger CB, Wikstrand J, Komajda M, Carson PE, Pfeffer MA, Swedberg K, Wedel H, Yusuf S, McMurray JJ. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation. 2015;131:43–53. [DOI] [PubMed] [Google Scholar]
- 27. Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol. 2012;60:2082–2089. [DOI] [PubMed] [Google Scholar]
- 28. Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R. Serum potassium and clinical outcomes in the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation. 2008;118:1643–1650. [DOI] [PubMed] [Google Scholar]
- 29. Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF). Circ Heart Fail. 2014;7:51–58. [DOI] [PubMed] [Google Scholar]
- 30. Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double‐blind, placebo‐controlled study in patients with chronic heart failure (the PEARL‐HF) trial. Eur Heart J. 2011;32:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non‐steroidal mineralocorticoid receptor antagonist BAY 94‐8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double‐blind trial. Eur Heart J. 2013;34:2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp‐Kirschbaum N, Ruilope LM. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–894. [DOI] [PubMed] [Google Scholar]
- 33. Cooper LB, Hammill BG, Peterson ED, Pitt B, Maciejewski ML, Curtis LH, Hernandez AF. Consistency of laboratory monitoring during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. JAMA. 2015;314:1973–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen LA, Shetterly SM, Peterson PN, Gurwitz JH, Smith DH, Brand DW, Fairclough DL, Rumsfeld JS, Masoudi FA, Magid DJ. Guideline concordance of testing for hyperkalemia and kidney dysfunction during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. Circ Heart Fail. 2014;7:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei L, Struthers AD, Fahey T, Watson AD, Macdonald TM. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768. [DOI] [PubMed] [Google Scholar]
- 36. Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Setoguchi S, Hernandez AF, Fonarow GC. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of the Study Population by Study Group After Application of Inverse Probability Weights
Table S2. Associations Between Baseline Characteristics and 3‐Year Outcomes Among Patients With Heart Failure With Preserved Ejection Fraction
Table S3. Associations Between Baseline Characteristics and 3‐Year Outcomes Among Patients With Heart Failure With Reduced Ejection Fraction


