Abstract
Background
There is a heightened interest in plant‐based diets for cardiovascular disease prevention. Although plant protein is thought to mediate such prevention through modifying blood lipids, the effect of plant protein in specific substitution for animal protein on blood lipids remains unclear. To assess the effect of this substitution on established lipid targets for cardiovascular risk reduction, we conducted a systematic review and meta‐analysis of randomized controlled trials using the Grading of Recommendations Assessment, Development, and Evaluation system.
Methods and Results
MEDLINE, EMBASE, and the Cochrane Registry were searched through September 9, 2017. We included randomized controlled trials of ≥3 weeks comparing the effect of plant protein in substitution for animal protein on low‐density lipoprotein cholesterol, non–high‐density lipoprotein cholesterol, and apolipoprotein B. Two independent reviewers extracted relevant data and assessed risk of bias. Data were pooled by the generic inverse variance method and expressed as mean differences with 95% confidence intervals. Heterogeneity was assessed (Cochran Q statistic) and quantified (I2 statistic). The overall quality (certainty) of the evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation system. One‐hundred twelve randomized controlled trials met the eligibility criteria. Plant protein in substitution for animal protein decreased low‐density lipoprotein cholesterol by 0.16 mmol/L (95% confidence interval, −0.20 to −0.12 mmol/L; P<0.00001; I2=55%; moderate‐quality evidence), non–high‐density lipoprotein cholesterol by 0.18 mmol/L (95% confidence interval, −0.22 to −0.14 mmol/L; P<0.00001; I2=52%; moderate‐quality evidence), and apolipoprotein B by 0.05 g/L (95% confidence interval, −0.06 to −0.03 g/L; P<0.00001; I2=30%; moderate‐quality evidence).
Conclusions
Substitution of plant protein for animal protein decreases the established lipid targets low‐density lipoprotein cholesterol, non–high‐density lipoprotein cholesterol, and apolipoprotein B. More high‐quality randomized trials are needed to improve our estimates.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02037321.
Keywords: animal protein, cholesterol, dyslipidemia, lipids, meta‐analysis, protein, soy, systematic review, vegetable protein
Subject Categories: Lipids and Cholesterol, Meta Analysis
Clinical Perspective
What Is New?
Although the cholesterol‐lowering benefit of plant protein sources, such as soy, pulses, and nuts, is well documented, the overall cholesterol‐lowering benefit of plant protein in substitution for animal protein (as meat, dairy, and/or egg alternatives) has not been synthesized.
The available evidence from randomized controlled trials suggests that 1 to 2 servings of plant protein in substitution for animal protein decreases low‐density lipoprotein cholesterol, non–high‐density lipoprotein cholesterol, and apolipoprotein B by ≈4% in adults with and without hyperlipidemia.
Because of inconsistency or imprecision in the estimates, the overall quality (certainty) of the evidence is moderate by the Grading of Recommendations Assessment, Development, and Evaluation system, suggesting that more research will refine our estimates.
What Are the Clinical Implications?
Because the intake of plant protein from soy, nuts, and pulses remains low, there is an opportunity for people to realize the lipid‐lowering benefits of sustainable plant‐based dietary strategies that substitute plant protein for animal protein.
Plant protein, especially in combination with other cholesterol‐lowering foods (eg, viscous fiber and plant sterols) and/or as an adjunct to lipid‐lowering pharmacotherapy, may have a clinically meaningful benefit in helping people to achieve lipid targets and reduce cardiovascular risk.
Introduction
Cardiovascular disease (CVD) accounts for ≈48% of deaths attributable to noncommunicable disease worldwide and remains the number one cause of mortality.1, 2 Modification by diet and lifestyle of risk factors, particularly dyslipidemia, remains the cornerstone of therapy, according to major cardiovascular guidelines.3, 4
There has been increasing recent interest in plant‐based diets. Vegetarian and vegan dietary patterns and other plant‐based dietary patterns, such as the Mediterranean diet, have been established as dietary patterns that improve lipid profiles and reduce risks of CVD.5, 6, 7 Both the Scientific Report of the 2015 Dietary Guidelines Advisory Committee and 2016 Canadian Cardiovascular Society guidelines recently recommended a vegetarian dietary pattern and a Mediterranean dietary pattern for cardiovascular protection.3, 8 The mechanisms by which these dietary patterns improve cardiovascular risk likely include intrinsic and extrinsic pathways. Plant protein sources, such as soy, dietary pulses, and nuts, have all individually shown lipid‐lowering advantages through their specific components (specific protein fractions [7s‐globulin], viscous fibers, polyunsaturated fatty acids, and plant sterols). Replacement of animal protein with plant protein has also shown advantages through the displacement of saturated fatty acids.9 The combination allows for meaningful reductions in lipids in systematic reviews and meta‐analyses of randomized controlled trials (RCTs).9, 10, 11, 12
Despite the strong biological plausibility supporting their benefit and endorsement of plant‐based diets from recent guidelines, there is still uncertainty as to whether the benefit is attributable to the exchange of plant protein for animal protein or to other aspects of a plant‐based dietary pattern. It remains difficult to isolate specific mechanisms,13, 14, 15 and the strength of the evidence supporting the lipid‐lowering effects of plant protein remains disputed.16, 17, 18, 19 As a result, many authoritative guidelines do not specifically recommend substituting plant protein for animal protein for lipid‐lowering and cardiovascular protection.20, 21, 22, 23 To summarize and evaluate the available evidence, we conducted a systematic review and meta‐analysis using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system of the effect of substituting plant protein for animal protein on the established lipid targets for CVD prevention, low‐density lipoprotein cholesterol (LDL‐C), non– high‐density lipoprotein cholesterol (non–HDL‐C), and apolipoprotein B (Apo‐B), in RCTs.4, 24
Methods
This study was planned and conducted following the Cochrane Handbook for Systematic Review of Interventions.25 Data were reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines.26 The authors declare that all supporting data are available within the article (and its supplementary files).
Literature Search
We searched MEDLINE, EMBASE, and the Cochrane Register through September 9, 2017, for eligible trials. Table S1 shows our detailed search strategy.
Study Selection
We included randomized, long‐term, dietary intervention trials in human subjects comparing LDL‐C, non–HDL‐C, and/or Apo‐B parameters between plant and animal protein intervention arms. To be included, studies had to be at least 3 weeks in duration and performed in accordance with the minimum trial follow‐up requirement of the US Food and Drug Administration for lipid‐lowering health claims.27 Studies deliberately introducing confounding factors (eg, plant sterols or combined therapeutic interventions) to the plant protein arm were also excluded, including studies applying a broad vegetarian or vegan dietary pattern as opposed to a direct substitution of protein sources. No restrictions were placed on language.
Data Extraction
Study characteristics and results of eligible trials were each extracted by S.S.L. and a coextractor (L.L., S.B.M., S.E.S., E.V., or V.H.). Extracted characteristics include study setting, design, duration, blinding, sample size, participant characteristics, and plant and animal protein diet descriptions. Risk of bias of eligible trials was also assessed by S.S.L. and the same coextractor using the Cochrane risk of bias tool, which categorizes studies as high, low, or unclear risk of bias on the basis of criteria pertaining to selection bias, blinding, incomplete outcome data, and reporting bias.25 PlotDigitizer version 2.5.1 (Free Software Foundation, Boston, MA) was used to extract data from graphs, where applicable. Any discrepancies in data extraction or risk of bias assessment were reconciled by consensus.
Grading of the Evidence
The overall quality (certainty) of evidence was assessed using the GRADE system,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 which grades evidence as high, moderate, low, or very low quality. RCTs are graded as high‐quality evidence by default. Scores can then be downgraded on the basis of the following prespecified criteria: risk of bias (weight of studies shows important risk of bias), inconsistency (substantial unexplained interstudy heterogeneity of I2 >50%, P<0.10), indirectness (presence of factors that limit the generalizability of the results), imprecision (95% confidence interval [CI] for risk estimates are wide or overlap a minimally important difference of 0.1 mmol/L for LDL‐C and non‐HDL‐C and 0.04 g/L for Apo‐B), and publication bias (evidence of small‐study effects).
Statistical Analysis
We used Review Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) for primary analyses and Stata version 13 (StataCorp, College Station, TX) for meta‐regression and publication bias tests. Data were pooled using the generic inverse variance method with random‐effects models and are expressed as mean differences (MDs) with 95% CIs. All analyses were repeated using fixed‐effects models and parametric bootstrapping as sensitivity analyses. Where there were multiple plant or animal protein arms in a single trial, we pooled intervention arms to obtain a single pairwise comparison, to mitigate unit‐of‐analysis error25; where relevant, these arms were assessed separately for subgroup analyses.
Change‐from‐baseline values were favored, and differences in change‐from‐baseline values were used, where given; otherwise, we used end‐difference values, if reported, or calculated the differences from available data. Non–HDL‐C values were calculated by subtracting HDL‐C from total cholesterol values, where non–HDL‐C values were not directly reported, and the variance sum law was used to derive SDs for non–HDL‐C from total cholesterol and HDL‐C variance data.41 In crossover trials, missing variance data were calculated from t test P values using standard formulas; where P values were unavailable, a correlation coefficient of 0.5 was assumed as a conservative estimate and used to impute SE data.25, 42 Where no variance data were available, the average SD of the MDs across all other included trials was used to derive the SEM difference on the basis of the respective trial's sample size.
Interstudy heterogeneity was evaluated by the Cochran Q statistic and quantified using the I2 statistic. P<0.10 was considered significant; an I2 value of 50% or higher was considered substantial.25 Potential sources of heterogeneity were investigated by additional sensitivity analyses, in which we recalculated the pooled effect estimate after removing each individual trial, after removing all imputed data, and after imputing alternative correlation coefficients of 0.25 and 0.75. We additionally investigated potential sources of heterogeneity by subgroup analyses. Our a priori subgroups included study design, protein dose, plant and animal protein type, duration of follow‐up, and baseline lipid values. A post hoc analysis was also conducted for protein form (ie, whole food or protein isolate product). Between‐subgroup differences were assessed using meta‐regression with dummy variables.
A post hoc dose‐response analysis was conducted using a piecewise linear meta‐regression via the mkspline function, to assess potential dose thresholds for the continuous subgroup addressing grams of protein substitution.
Publication bias was assessed by inspection of funnel plots and by the use of Egger and Begg tests. Where publication bias was suspected, Duval and Tweedie nonparametric “trim‐and‐fill” analyses were also applied to assess the effect of the imputed “missing” studies.43
Results
Search Results
Figure 1 shows the trial selection process. Our search identified 3917 reports, of which 3689 were excluded on the basis of review of titles and abstracts. The remaining 228 articles were reviewed in full, of which 104 provided data for 112 trial comparisons for inclusion in our analyses.44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147
Figure 1.
Search summary.
Trial Characteristics
The Table summarizes characteristics of the included trials. Detailed characteristics are shown in Table S2. In total, 5774 participants (median age, 54 years) were included in this analysis. There were more women versus men overall (≈5:3 ratio), but this difference is largely attributable to a few large female‐only trials, and the median sex ratio in trials was relatively balanced (44% men). Sixty‐one trials were crossover, and all but 4 were in outpatient settings. Half of the trials were conducted in the United States and Canada (60 of 112), but trials were also distributed across European (24 trials), Asian (10 trials), Middle‐Eastern (9 trials), and South American (3 trials) countries, as well as Australia (6 trials). Of 112 trials, 34 recruited healthy subjects (including healthy postmenopausal women); 51 trials recruited subjects with hyperlipidemia, 4 of which also selected for additional conditions. The remaining 28 trials included participants with various conditions, including renal disease, overweight, obesity, type 2 diabetes mellitus, and hypertension. Average baseline LDL‐C, non–HDL‐C, and Apo‐B measures were 3.81 mmol/L, 4.42 mmol/L, and 1.16 g/L, respectively.
Table 1.
Summary Table of Characteristics
| Trial Characteristics | LDL‐C | Non–HDL‐C | Apo‐B |
|---|---|---|---|
| Trial number, N | 108 | 102 | 37 |
| Total participants | 5582 | 5401 | 1506 |
| Trial size (participants)a | 32 (4–352) | 32 (4–352) | 32 (4–130) |
| Male:female ratiob, c | 37:63 | 39:61 | 51:49 |
| Age, yc, d | 54 (44–59) | 54 (44–59) | 54 (43–60) |
| Inpatient:outpatient settingb | 4:96 | 3:97 | 3:97 |
| Baseline serum leveld, e | 3.7 (3.0–4.2) mmol/L | 4.4 (3.8–5.0) mmol/L | 1.2 (1–1.4) g/L |
| Crossover:parallel study designb | 54:46 | 54:46 | 57:43 |
| Amount of substitution, gd | 29 (23–49) | 30 (22–50) | 30 (25–50) |
| Follow‐up duration, wksa | 6 (3–208) | 6 (3–208) | 6 (3–52) |
| Funding sources (agency:industry:agency‐industry:NR)b | 23:19:48:9 | 23:19:49:10 | 19:32:43:5 |
| Plant protein source, N | Soy, 91; lupin, 3; legumes, 3; pinto beans, 2; pulses, 2; barley, 1; pea, 1; walnut, 1; various, 4 | Soy, 84; legumes, 3; lupin, 3; pinto beans, 2; pulses, 2; barley, 1; pea, 1; walnut, 1; various, 5 | Soy, 34; legumes, 1; walnut, 1; various, 1 |
| Animal protein source, N | Dairy, 70; meat, 10; chicken noodle soup, 2; egg, 1; various, 25 | Dairy, 64; meat, 10; chicken noodle soup, 2; egg, 1; various, 25 | Dairy, 25; meat, 3; egg, 1; various, 8 |
| Protein form, N | Whole food, 38; protein isolate, 72 | Whole food, 40; protein isolate, 63 | Whole food, 10; protein isolate, 28 |
Apo‐B indicates apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and NR, not reported.
Values are reported as medians (ranges).
Values are reported as percentage ratios.
Includes baseline data before dropouts, where final data were not available.
Values are reported as medians (interquartile ranges).
Baseline serum‐level data correspond to the respective lipid marker for each end point.
Of 112 trials, 94 used soy as the sole plant protein intervention, and 74 used dairy as the sole animal protein intervention. Other plant protein sources included various pulses, nuts, barley, and seeds; other animal protein sources included meat, fatty fish, and eggs. Seventy‐one trials used protein isolate products, 37 used whole foods, and 4 used a combination of the two. The median protein substitution was ≈30 g/d. Trial follow‐up ranged from 3 weeks to 4 years, with a median follow‐up of 6 weeks. Twenty‐five trials obtained funding from publicly funded agencies alone, 22 were supported by industry funding alone, and 55 used a combination of the two.
Most of our included trials were deemed to be “low risk of bias” or “unclear risk of bias” across most domains by the Cochrane Risk of Bias tool. Of the trials rated has high risk of bias, 3 were for allocation concealment, 3 were for blinding, 14 were for incomplete outcome data, and 5 were for selective outcome reporting; 1 trial was considered to have an alternative high‐risk source of bias (substantial macronutrient imbalance in protein interventions for tofu compared with cheese, in the trial by Meredith et al107). Detailed risk of bias assessment data can be found in Figure S1.
Effect on LDL‐C
Figure 2 and Figures S2 and S3 show the effect of plant protein in substitution for animal protein intake on LDL‐C across 108 trials. We found a significant reduction in LDL‐C (MD, −0.16 mmol/L [95% CI, −0.20 to −0.12 mmol/L]; P<0.00001), with evidence of substantial interstudy heterogeneity (I2 =55%; P<0.00001). Fixed‐effects model analysis, bootstrap analysis (Table S3), and sensitivity analyses did not alter the direction or significance of the effect estimates. Subgroup analyses were nonsignificant and failed to explain heterogeneity (Figure S4). Post hoc subgroup analyses (Figure S5) failed to identify significant effect modification by protein form on LDL‐C, and post hoc dose‐response analyses (Table S4) did not find a dose threshold for LDL‐C in continuous subgroup analyses.
Figure 2.
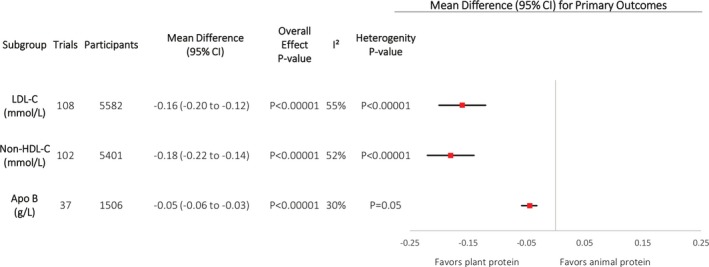
Primary analyses. Pooled effect estimates for each end point (squares) shown. Paired analyses were applied to all crossover trials. Data are expressed as mean differences (95% confidence intervals [CIs]), using generic inverse‐variance random‐effects models. Interstudy heterogeneity was tested using the Cochran Q statistic (χ2) at a significance level of P<0.10 and quantified by I2; levels of ≥50% represented substantial heterogeneity. All outcomes had significant pooled effect estimates. Heterogeneity was significant and substantial for low‐density lipoprotein cholesterol (LDL‐C) and non–high‐density lipoprotein cholesterol (HDL‐C), and significant but not substantial for apolipoprotein B (Apo‐B).
Effect on Non–HDL‐C
Figure 2 and Figures S6 and S7 show the effect of plant protein in substitution for animal protein intake on non–HDL‐C across 102 trials. We found a significant reduction in non–HDL‐C (MD, −0.18 mmol/L [95% CI, −0.22 to −0.14 mmol/L]; P<0.00001), with evidence of substantial interstudy heterogeneity (I2=52%; P<0.00001). Fixed‐effects model analysis, bootstrap analysis (Table S3), and sensitivity analyses did not alter the direction or significance of the effect estimates. Subgroup analyses, however, did reveal a greater reduction in non‐HDL‐C in trials with higher baseline non‐HDL‐C levels (between‐subgroup difference, −0.09 mmol/L [95% CI, −0.17 to −0.01 mmol/L]; P=0.03), with a residual I2=43% (Figure S8). Post hoc subgroup analyses (Figure S5) failed to identify significant effect modification by protein form on non–HDL‐C, and post hoc dose‐response analyses (Table S4) did not find a dose threshold in continuous subgroup analyses.
Effect on Apo‐B
Figure 2 and Figures S9 and S10 show the effect of plant protein in substitution for animal protein intake on Apo‐B across 37 trials. We found a significant reduction in Apo‐B by plant protein (MD, −0.05 g/L [95% CI, −0.06 to −0.03 g/L]; P<0.00001), with evidence of moderate interstudy heterogeneity (I2 =30%; P=0.05). Fixed‐effects model analysis, bootstrap analysis (Table S3), and sensitivity analyses did not alter the direction or significance of the effect estimates. Subgroup analyses also did not explain the heterogeneity (Figure S11). However, removal of the 2007 study by Azadbakht et al51 modified heterogeneity from significant to nonsignificant (I2 =21%; P=0.14). Post hoc subgroup analyses (Figure S5) failed to identify significant effect modification by protein form on non–HDL‐C, and post hoc dose‐response analyses (Table S4) did not find a dose threshold in continuous subgroup analyses.
Publication Bias
Figure S12 shows the funnel plots used to evaluate publication bias; on visual inspection, there was no evidence of asymmetry or small‐study effects for any outcome. The Egger test identified significant publication bias for LDL‐C (P=0.03), but the Begg test was nonsignificant. The Egger and Begg tests were nonsignificant across all other end points. Trim‐and‐fill analyses were conducted for LDL‐C, with data for 8 additional studies imputed to adjust for funnel plot asymmetry (Figure S13). There was no evidence of meaningful small‐study effects. The direction, significance, and size of the pooled effect estimate after inclusion of the imputed studies were not significantly altered (MD, −0.18 mmol/L [95% CI, −0.21 to −0.14 mmol/L]; P<0.001).
GRADE Assessment
Table S5 shows a summary of the GRADE assessments for each end point. The evidence for both LDL‐C and non–HDL‐C was rated moderate quality, on the basis of a downgrade for inconsistency in both analyses. The evidence for Apo‐B was rated moderate quality, on the basis of a downgrade for imprecision.
Discussion
We conducted a systematic review and meta‐analysis of 112 RCTs assessing the effect of plant protein versus animal protein on established lipid targets for CVD prevention in 5774 adult participants with and without hyperlipidemia. Plant protein substitution for animal protein led to modest reductions in LDL‐C (−0.16 mmol/L or ≈4%; 95% CI, ≈3%–5%), non–HDL‐C (−0.18 mmol/L or ≈4%; 95% CI, ≈3%–5%), and Apo‐B (−0.05 g/L or ≈3%; 95% CI, 2%–5%). On the basis of studies finding a one‐to‐one relationship between LDL‐C and cardiovascular risk reductions, these findings would translate to a 4% risk in major cardiovascular events.148, 149
Findings in Relation to the Literature
Our findings are supported by other systematic reviews and meta‐analyses of the effect of individual sources of plant protein in substitution for different macronutrients (not just animal protein) on blood lipids. We showed, in an updated analysis of an American Heart Association analysis, that soy protein produced similar decreases in LDL‐C (≈4%) in RCTs involving participants with and without hyperlipidemia.9 An individual patient‐level pooled analysis of RCTs showed that tree nuts decrease LDL‐C by ≈7%, along with other lipid end points.10 A systematic review and meta‐analysis of the effect of dietary pulses on established lipid targets showed an LDL‐C–lowering effect of ≈5% and a tendency for a non–HDL‐C–lowering effect.12
Our findings are also aligned with previous evidence related to plant protein as part of plant‐based dietary patterns. A systematic review of 13 observational studies and 14 RCTs trials demonstrated the lipid‐lowering benefits of plant‐based diets,6 and a recent systematic review and meta‐analysis of 11 RCTs found significant reductions in LDL‐C and non–HDL‐C following a vegetarian diet.150 We have shown that the Portfolio diet, which combines cholesterol‐lowering foods (including plant protein from soy, pulses, and nuts) along with viscous fibers and plant sterols, produces LDL‐C reductions comparable to lovastatin (−28.6% versus −30.9%) over 4 weeks when all foods were provided. 151 There were more modest reductions of 10% to 15% (with greater reductions seen with greater adherence) when the diet was administered as dietary advice under free living conditions over 6 months.152 Our Eco‐Atkins trial also found greater reductions in LDL‐C with a vegan low‐carbohydrate (“Eco‐Atkins”) diet that emphasizes plant proteins, compared with a high‐carbohydrate, low‐fat, lacto‐ovo vegetarian diet (treatment difference, −0.49 mmol/L).153
Furthermore, studies have found an association between plant‐based diets and cardiovascular disease. The PREDIMED (Prevención con Dieta Mediterránea) trial showed that a predominantly plant‐based Mediterranean diet supplemented with nuts as a source of plant protein decreases major cardiovascular events.154 Prospective cohort studies offer further support showing that dietary patterns high in plant proteins, such as Mediterranean and vegetarian dietary patterns, are associated with reduced cardiovascular events.155, 156, 157, 158 An analysis of the Harvard cohorts found that low‐carbohydrate and high‐protein diets were associated with increased mortality, but inversely correlated with mortality and particularly CVD mortality when based on plant protein.159 Other prospective cohort studies have also shown that plant‐based diets are associated with a mortality benefit.160 On the other hand, increased intake of animal protein sources has been associated with negative health outcomes. A pooled analysis of the Harvard cohorts found that red meat consumption was associated with increased risks of total, cardiovascular, and cancer mortality.161 Other large, prospective, cohort studies have found an association between animal protein sources and disease or mortality.162, 163, 164
There are several mechanisms by which plant protein may exert a lipid‐lowering effect. One explanation is that the plant protein source acts as a vehicle for other established antiatherogenic agents, such as plant sterols or soluble fiber; similarly, the displaced animal protein source could also act as a vehicle for hypercholesterolemic agents, such as saturated fat and cholesterol.13, 14, 15, 24 Interestingly, our post hoc subgroup analyses did not find a significant difference between protein isolate products and whole food sources for any given end point, suggesting that the cholesterol‐lowering effects are at least, in part, attributable to the plant protein itself rather than just the associated nutrients.
An alternative explanation relates to the amino acid breakdown encountered in plant proteins versus animal proteins; in particular, lysine, which is more prevalent in animal proteins, has been shown to increase cholesterol levels in animal models, whereas arginine, which is found more in plant proteins, has been found to have the opposite effect.165, 166, 167 The cholesterol‐lowering effect of arginine has also been demonstrated in a 5‐week arginine feeding trial in humans,168 but otherwise there are limited human studies investigating this subject. Proposed mechanisms for these effects involve bile acid production and binding of hepatic LDL receptors.166, 169
A Priori Subgroup Analyses
Our results appear to be robust to different trial conditions. Similar to a previous meta‐analysis by Anderson et al,170 we did find that increased baseline values amplified the effects seen in non–HDL‐C reduction. However, our overall analyses indicate that the lipid‐lowering effects of plant protein apply to both hypercholesterolemic and normal subjects, because the normocholesterolemic subgroup also showed a significant improvement in non–HDL‐C, and similar subgroup analyses in LDL‐C and Apo‐B were nonsignificant. The beneficial effects otherwise held across a range of ages and health statuses, and all other subgroup analyses were nonsignificant.
Strengths and Limitations
Our systematic review and meta‐analysis has several strengths and limitations. The strengths include the identification of all available evidence through a systematic search strategy, the inclusion of RCTs that provide the greatest protection against bias, quantitative syntheses of the data, and assessment of the overall quality of the evidence using the GRADE system.
The limitations of our systematic review and meta‐analysis relate to inconsistency in the treatment effects and imprecision. Evidence of unexplained inconsistency in treatment effects was seen for 2 of the established therapeutic lipid end points. There was substantial interstudy heterogeneity in our LDL‐C and non–HDL‐C analyses, which was not fully explained by sensitivity or subgroup analyses. Evidence of imprecision was seen in Apo‐B, because the 95% CI for effect estimates for Apo‐B overlapped the prespecified minimally important difference of 0.04 g/L. Apo‐B also showed evidence of moderate interstudy heterogeneity; however, the statistical significance of heterogeneity was eliminated by the removal of the 2007 study by Azadbakht et al.51 We also considered downgrading for indirectness of the evidence. A relatively large proportion of the available trials evaluated soy as the sole plant protein source (94 of 112 trials) and/or dairy as the sole animal protein source (74 of 112 trials). Subgroup analyses, however, did not reveal evidence of significant effect modification by protein sources across any of the 3 end points, which suggests that the effects seen apply across varying plant and animal protein sources. Several plant protein sources, however, were not evaluated, including wheat (gluten), rice, and other grains. In addition, there were limited studies with extended follow‐up duration, which would help assess issues of long‐term adherence.
Taking into account these strengths and limitations, the evidence was assessed by the GRADE system as moderate quality for a cholesterol‐lowering effect of plant protein in substitution for animal protein across LDL‐C, non–HDL‐C, and Apo‐B markers.
Implications
Current adult protein intakes average ≈80 to 100 g/d in the United States and Europe. Of this intake, ≈30% is from plant protein sources.171, 172 The median intervention of 30 g protein substitution per day across trials included in our analyses reflects the substitution of 1 to 2 servings of meat for plant protein substitutes or 3 250‐mL cups of dairy milk for soy milk. This additional substitution would mean a shift to diets with >50% plant protein, which can be attained by following healthy dietary patterns, such as vegetarian, Mediterranean, and Portfolio dietary patterns.173, 174, 175 Given the low current consumption of plant protein‐rich foods, such as soy and pulses, in Canada and the United States, there remains a significant opportunity to realize the benefits of making such dietary changes.176, 177, 178
Although the reductions in LDL‐C, non–HDL‐C, and Apo‐B on their own were modest (<5%), plant protein can still contribute to meaningful reductions in lipids. On the basis of the evidence from the Portfolio diet, the lipid‐lowering effects of individual food components, which include plant protein from soy, pulses, and nuts, are additive, such that the LDL‐C–lowering effect (≈5%–10%) of each of the 4 components of the Portfolio diet food can be summed to achieve meaningful reductions.3, 147, 148 Several large trials and cohort studies have shown that such reductions are associated with improved cardiovascular outcomes.179, 180, 181, 182, 183, 184, 185 The 2016 Canadian Cardiovascular Guidelines further highlighted the superior predictive value for CVD of non–HDL‐C and Apo‐B, both of which were reduced by plant protein.3 The implication is that plant protein as part of a comprehensive lipid‐lowering dietary pattern alone or as an add‐on to other lipid‐lowering therapy can help people achieve their lipid targets and reduce CVD risk.
Despite the existing evidence for benefit, current dietary guidelines do not wholly reflect the demonstrated benefits of plant protein versus animal protein and tend to place animal sources of protein on the same level as plant sources.20, 21, 22 In particular, the 2015 to 2020 Dietary Guidelines for Americans recommend seafood, meats, poultry, eggs, nuts, seeds, and soy products indiscriminately as options for protein sources and suggest that the vegetarian dietary patterns described are only for those already following a vegetarian diet (which is incongruent with the Scientific Report of the 2015 Dietary Guidelines Advisory Committee on which the the 2015 to 2020 Dietary Guidelines for Americans is based).8, 22, 23
Conclusions
In conclusion, our aggregate analyses demonstrate a benefit of plant protein in substitution for animal protein on established lipid targets for CVD prevention in adults with and without hyperlipidemia. To our knowledge, this is the first systematic review and meta‐analysis to directly evaluate the effects of plant protein as well as plant for animal protein replacement. These findings presents an opportunity for patients, clinicians, and guidelines to exploit the lipid‐lowering benefits of a sustainable plant‐based dietary strategy that is associated with improved overall health outcomes. Our confidence in the evidence for the LDL‐C–, non–HDL‐C–, and Apo‐B–lowering effects of plant protein, however, is limited by inconsistency for LDL‐C and non–HDL‐C and imprecision for Apo‐B. Further large, high‐quality, randomized controlled trials investigating plant protein sources beyond soy, particularly in young and healthy participants, would be useful to help better understand the role of plant protein in cardiovascular risk reduction.
Author Contributions
All authors had full access to all of the data (including statistical reports and tables) in this study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: Li and Sievenpiper. Analysis and interpretation of the data: Li, Blanco Mejia, de Souza, Leiter, Kendall, Jenkins, and Sievenpiper. Drafting of the article: Li. Critical revision of the article for important intellectual content: Li, Lytvyn, Blanco Mejia, Stewart, Viguiliouk, Ha, de Souza, Leiter, Kendall, Jenkins, and Sievenpiper. Final approval of the article: Li, Lytvyn, Blanco Mejia, Stewart, Viguiliouk, Ha, de Souza, Leiter, Kendall, Jenkins, and Sievenpiper. Statistical expertise: de Souza. Attainment of funding: Kendall, Jenkins, and Sievenpiper. Administrative, technical, or logistic support: Blanco Mejia. Collection and assembly of data: Li, Lytvyn, Blanco Mejia, Stewart, Viguiliouk, and Ha. Guarantor: Sievenpiper.
Sources of Funding
This work was funded by the Canadian Institutes of Health Research (CIHR; funding reference number 129920) through the Canada‐Wide Human Nutrition Trialists' Network. The Diet, Digestive Tract, and Disease Centre, funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation's Ontario Research Fund, provided the infrastructure for the conduct of this project. Jenkins was funded by the Government of Canada through the Canada Research Chair Endowment. Sievenpiper was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Diabetes Canada Clinician Scientist award, CIHR INMD/Canadian Nutrition Society New Investigator Partnership Prize, and Banting & Best Diabetes Centre Sun Life Financial New Investigator Award. Viguiliouk was supported by a Toronto 3D Knowledge Synthesis and Clinical Trials Foundation Internship Award. None of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the article or decision to publish.
Disclosures
Lytvyn is part of the Grading of Recommendations Assessment, Development, and Evaluation Working Group. Ha received support from a Canadian Institutes of Health Research (CIHR) doctoral award, David Sackett scholarship, and Ashbaugh Graduate scholarship. She has received payment from the World Health Organization (WHO) for work on a systematic review and meta‐analysis commissioned by the WHO for work on the relation of saturated fatty acids and polyunsaturated fatty acids with health outcomes. She and her peers received a cash prize for placing second in the regional “Mission Impulsible” Competition hosted by Pulse Canada, where they conceived and developed a marketable food product that contained dietary pulses. She received a travel award to attend the “Journey Through Science Day,” hosted by PepsiCo and the New York Academy of Sciences, and the Nutrica Travel Award from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD). de Souza has served as an external resource person to the World Health Organization's Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012‐2017 to present and discuss this work. He has also done contract research for the Canadian Institutes of Health Research's Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the World Health Organization for which he received remuneration. He has held a grant from the Canadian Foundation for Dietetic Research as a principal investigator, and is a co‐investigator on several funded team grants from Canadian Institutes of Health Research. He received compensation for a lecture on dietary fat given at McMaster Pediatric Nutrition Days in 2016. Kendall has received research support from the Advanced Foods and Materials Network, Agricultural Bioproducts Innovation Program through the Pulse Research Network, Agriculture and Agri‐Food Canada, Almond Board of California, Barilla, Calorie Control Council, CIHR, Canola Council of Canada, INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, Kellogg, Loblaw Companies Ltd., Pulse Canada, Saskatchewan Pulse Growers and Unilever. He has received consultant fees from American Pistachio Growers; speaker fees from American Peanut Council, Tate & Lyle and The WhiteWave Foods Company; and travel funding from Sabra Dipping Company, Tate & Lyle, International Tree Nut Council Research & Education Foundation, California Walnut Commission, Sun‐Maid, The Peanut Institute, General Mills, Oldways Foundation and International Nut and Dried Fruit Council Foundation. He is a member of the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the European Association for the Study of Diabetes (EASD), the Diabetes and Nutrition Study Group of the EASD and the International Carbohydrate Quality Consortium, and is the Director for the Toronto 3D Knowledge Synthesis and Clinical Trials Foundation. Jenkins has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever, Barilla, the Almond Board of California, Agriculture and Agri‐food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca‐Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, the Canola and Flax Councils of Canada, the Calorie Control Council, the CIHR, the Canada Foundation for Innovation and the Ontario Research Fund. He has received in‐kind supplies for trial as a research support from the Almond board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker's panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system, the Coca‐Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), FareWell, Verywell, True Health Initiative, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamental for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca‐Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi‐Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri‐Culture and Agri‐Food Canada, the Canadian Agri‐Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra‐Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife is a director and partner of Glycemic Index Laboratories, Inc., and his sister received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies.Sievenpiper has received research support from the Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), Canadian Nutrition Society (CNS), American Society for Nutrition (ASN), Calorie Control Council, INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, and The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers). He has received speaker fees and/or honoraria from Diabetes Canada, Canadian Nutrition Society (CNS), Dr. Pepper Snapple Group, Dairy Farmers of Canada, Nutrition Foundation of Italy (NFI), C3 Collaborating for Health, Sprim Brasil, WhiteWave Foods, Rippe Lifestyle, mdBriefcase, Alberta Milk, FoodMinds LLC, Memac Ogilvy & Mather LLC, PepsiCo, The Ginger Network LLC, International Sweeteners Association, and Pulse Canada. He has ad hoc consulting arrangements with Winston & Strawn LLP, Perkins Coie LLP, and Tate & Lyle. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), and Canadian Cardiovascular Society (CCS), as well as an expert writing panel of the American Society for Nutrition (ASN). He serves as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Unilever Canada. No competing interests were declared by Li, Blanco Mejia, Stewart, Viguiliouk, and Leiter. There are no patents, products in development, or marketed products to declare.
Supporting information
Table S1. Search Strategy
Table S2. Full Table of Characteristics
Table S3. Bootstrap Analyses
Table S4. Post‐Hoc Dose Response
Table S5. GRADE Assessment
Figure S1. Cochrane risk of bias. Risk of bias assessment using Cochrane Risk of Bias Tool.
Figure S2. LDL‐C forest plot, random‐effects model.
Figure S3. LDL‐C forest plot, fixed‐effects model.
Figure S4. LDL‐C visual subgroup. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S5. Post‐hoc subgroups. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S6. Non‐HDL‐C forest plot, random‐effects model.
Figure S7. Non‐HDL‐C forest plot, fixed‐effects model.
Figure S8. Non‐HDL‐C visual subgroup. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S9. Apo‐B forest plot, random‐effects model. HC indicates hypercholesterolemic; IF, isoflavones; LF, low‐fat; N, normal; NIF, no isoflavones. The pooled effect estimate (diamond) is shown. Paired analyses were applied to all crossover trials. Data are expressed as MDs with 95% CIs, using generic inverse‐variance random‐effects models. Inter‐study heterogeneity was tested using the Cochran Q statistic (chi‐square) at a significance level of P<0.10 and quantified by I2, levels of ≥50% represented substantial heterogeneity.
Figure S10. Apo‐B forest plot, fixed‐effects model. HC indicates hypercholesterolemic; IF, isoflavones; LF, low‐fat; N, normal; NIF, no isoflavones. The pooled effect estimate (diamond) is shown. Paired analyses were applied to all crossover trials. Data are expressed as MDs with 95% CIs, using generic inverse‐variance fixed‐effects models. Inter‐study heterogeneity was tested using the Cochran Q statistic (chi‐square) at a significance level of P<0.10 and quantified by I2, levels of ≥50% represented substantial heterogeneity.
Figure S11. Apo‐B visual subgroup. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S12. Funnel plots. Publication bias funnel plots for LDL (A), non‐HDL (B), and apolipoprotein B (C). The solid line represents the pooled effect estimate expressed as the weighted mean difference (MD) of each analysis, and dashed lines represent pseudo‐95% confidence limits. Circles represent effect estimates of included trials. P‐values of Egger and Begg tests for publication bias are shown at top right for each analysis. *Statistically significant (P<0.05).
Figure S13. LDL‐C trim‐and‐fill funnel plot. The horizontal line represents the pooled effect estimate expressed as a mean difference. The diagonal lines represent the pseudo 95% CIs of the mean difference. The clear circles represent effect estimates for each included study.
Acknowledgments
We thank Teruko Kishibe, an information specialist at the Scotiabank Health Sciences Library, St. Michael's Hospital, for help in the development of search terms used.
(J Am Heart Assoc. 2017;6:e006659 DOI: 10.1161/JAHA.117.006659.)29263032
References
- 1. World Health Statistics 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2. Global Status Report on Noncommunicable Diseases 2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3. Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GBJ, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 5. King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1–16. [PubMed] [Google Scholar]
- 6. Ferdowsian HR, Barnard ND. Effects of plant‐based diets on plasma lipids. Am J Cardiol. 2009;104:947–956. [DOI] [PubMed] [Google Scholar]
- 7. Mahon AK, Flynn MG, Stewart LK, McFarlin BK, Iglay HB, Mattes RD, Lyle RM, Considine RV, Campbell WW. Protein intake during energy restriction: effects on body composition and markers of metabolic and cardiovascular health in postmenopausal women. J Am Coll Nutr. 2008;26:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. USDA. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. In: DGAC‐USDA , editor. 2015. https://health.gov/dietaryguidelines/2015-scientific-report/pdfs/scientific-report-of-the-2015-dietary-guidelines-advisory-committee.pdf. Access date: June 3, 2017
- 9. Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, Abdulnour S, Sievenpiper JL, Kendall CW, Kris‐Etherton PM. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140:2302s–2311s. [DOI] [PubMed] [Google Scholar]
- 10. Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. [DOI] [PubMed] [Google Scholar]
- 11. Blanco Mejia S, Kendall CWC, Viguiliouk E, Augustin LS, Ha V, Cozma AI, Mirrahimi A, Maroleanu A, Chiavaroli L, Leiter LA, de Souza RJ, Jenkins DJA, Sievenpiper JL. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open. 2014;4:e004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha V, Sievenpiper JL, de Souza RJ, Jayalath VH, Mirrahimi A, Agarwal A, Chiavaroli L, Mejia SB, Sacks FM, Di Buono M, Bernstein AM, Leiter LA, Kris‐Etherton PM, Vuksan V, Bazinet RP, Josse RG, Beyene J, Kendall CW, Jenkins DJ. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta‐analysis of randomized controlled trials. CMAJ. 2014;186:E252–E262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. [DOI] [PubMed] [Google Scholar]
- 14. Solà R, Bruckert E, Valls R‐M, Narejos S, Luque X, Castro‐Cabezas M, Doménech G, Torres F, Heras M, Farrés X, Vaquer J‐V, Martínez J‐M, Almaraz M‐C, Anguera A. Soluble fibre (Plantago ovata husk) reduces plasma low‐density lipoprotein (LDL) cholesterol, triglycerides, insulin, oxidised LDL and systolic blood pressure in hypercholesterolaemic patients: a randomised trial. Atherosclerosis. 2010;211:630–637. [DOI] [PubMed] [Google Scholar]
- 15. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta‐analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. [DOI] [PubMed] [Google Scholar]
- 16. Vega‐López S, Lichtenstein AH. Dietary protein type and cardiovascular disease risk factors. Prev Cardiol. 2005;8:31–40. [DOI] [PubMed] [Google Scholar]
- 17. Forsythe WA, Green MS, Anderson JJ. Dietary protein effects on cholesterol and lipoprotein concentrations: a review. J Am Coll Nutr. 1986;5:533–549. [DOI] [PubMed] [Google Scholar]
- 18. Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris‐Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. [DOI] [PubMed] [Google Scholar]
- 19. EFSA Panel on Dietetic Products N, Allergies . Scientific Opinion on the substantiation of a health claim related to isolated soy protein and reduction of blood LDL‐cholesterol concentrations pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2012;10:2555 [17 pp.]. [Google Scholar]
- 20. The American Heart Association's Diet and Lifestyle Recommendations. Chicago, IL: American Heart Association; 2014. [Google Scholar]
- 21. Canada's Food Guide. Health Canada; Ottawa, Ontario: 2007. [Google Scholar]
- 22. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans 8 ed December 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/. Access date: 3 June 2017. [Google Scholar]
- 23. Katz D. 2015 dietary guidelines: a plate full of politics. TheHuffingtonPost.com; 2016. http://www.huffingtonpost.com/david-katz-md/2015-dietary-guidelines-a_b_8930098.html. Access date: March 16, 2016
- 24. Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GBJ, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 Update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 510. United Kingdom: The Cochrane Collaboration; 2011. [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman D; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guidance for Industry: Evidence‐Based Review System for the Scientific Evaluation of Health Claims. In: Food and Drug Administration: Silver Springs, MD; 2009. https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm073332.htm. Access date: June 3, 2017 [Google Scholar]
- 28. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck‐Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schunemann HJ. GRADE guidelines, 1: introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck‐Ytter Y, Schunemann HJ. GRADE guidelines, 2: framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 30. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck‐Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines, 3: rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, Montori V, Akl EA, Djulbegovic B, Falck‐Ytter Y, Norris SL, Williams JW Jr, Atkins D, Meerpohl J, Schunemann HJ. GRADE guidelines, 4: rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso‐Coello P, Djulbegovic B, Atkins D, Falck‐Ytter Y, Williams JW Jr, Meerpohl J, Norris SL, Akl EA, Schunemann HJ. GRADE guidelines, 5: rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 33. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW Jr, Murad MH, Sinclair D, Falck‐Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann HJ. GRADE guidelines 6: rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. [DOI] [PubMed] [Google Scholar]
- 34. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso‐Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck‐Ytter Y, Schunemann HJ; GRADE Working Group . GRADE guidelines, 7: rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 35. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso‐Coello P, Falck‐Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schunemann HJ; GRADE Working Group . GRADE guidelines, 8: rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303–1310. [DOI] [PubMed] [Google Scholar]
- 36. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, Atkins D, Kunz R, Brozek J, Montori V, Jaeschke R, Rind D, Dahm P, Meerpohl J, Vist G, Berliner E, Norris S, Falck‐Ytter Y, Murad MH, Schunemann HJ; GRADE Working Group . GRADE guidelines, 9: rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. [DOI] [PubMed] [Google Scholar]
- 37. Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, Sisk J, Ruiz F, Hill S, Guyatt GH, Jaeschke R, Helfand M, Harbour R, Davoli M, Amato L, Liberati A, Schunemann HJ. GRADE guidelines, 10: considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66:140–150. [DOI] [PubMed] [Google Scholar]
- 38. Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, Atkins D, Kunz R, Montori V, Jaeschke R, Rind D, Dahm P, Akl EA, Meerpohl J, Vist G, Berliner E, Norris S, Falck‐Ytter Y, Schunemann HJ. GRADE guidelines, 11: making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–157. [DOI] [PubMed] [Google Scholar]
- 39. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B, Alonso‐Coello P, Post PN, Busse JW, Glasziou P, Christensen R, Schunemann HJ. GRADE guidelines, 12: preparing summary of findings tables‐binary outcomes. J Clin Epidemiol. 2013;66:158–172. [DOI] [PubMed] [Google Scholar]
- 40. Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, Johnston BC, Karanicolas P, Akl EA, Vist G, Kunz R, Brozek J, Kupper LL, Martin SL, Meerpohl JJ, Alonso‐Coello P, Christensen R, Schunemann HJ. GRADE guidelines, 13: preparing summary of findings tables and evidence profiles‐continuous outcomes. J Clin Epidemiol. 2013;66:173–183. [DOI] [PubMed] [Google Scholar]
- 41. Lane DM. Online statistics education: a multimedia course of study. 2007. http://onlinestatbook.com/. Access date: June 3, 2017
- 42. Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. [DOI] [PubMed] [Google Scholar]
- 43. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 44. Abd‐Mishani M, Hosseinpour‐Niazi S, Delshad H, Bahadori‐Monfared A, Mirmiran P, Azizi F. Effect of modified diet on lipid profiles in type 2 diabetic patients. Iran J Endocrinol Metab. 2014;16:103–110. [Google Scholar]
- 45. Abete I, Parra D, Martinez JA. Legume‐, fish‐, or high‐protein‐based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J Med Food. 2009;12:100–108. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed MS, Calabria AC, Kirsztajn GM. Short‐term effects of soy protein diet in patients with proteinuric glomerulopathies. J Bras Nefrol. 2011;33:150–159. [DOI] [PubMed] [Google Scholar]
- 47. Allen JK, Becker DM, Kwiterovich PO, Lindenstruth KA, Curtis C. Effect of soy protein‐containing isoflavones on lipoproteins in postmenopausal women. Menopause. 2007;14:106–114. [DOI] [PubMed] [Google Scholar]
- 48. Appt SE, Tormala R, Franke AA, Mikkola TS, Tikkanen MJ, Ylikorkala O, Clarkson TB. Soy‐tibolone combination: effect on lipids in postmenopausal monkeys and women. Maturitas. 2008;60:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashton E, Ball M. Effects of soy as tofu vs meat on lipoprotein concentrations. Eur J Clin Nutr. 2000;54:14–19. [DOI] [PubMed] [Google Scholar]
- 50. Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill‐Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003;57:1292–1294. [DOI] [PubMed] [Google Scholar]
- 51. Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85:735–741. [DOI] [PubMed] [Google Scholar]
- 52. Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C‐reactive protein in type 2 diabetes with nephropathy. Diabetes Care. 2008;31:648–654. [DOI] [PubMed] [Google Scholar]
- 53. Bähr M, Fechner A, Krämer J, Kiehntopf M, Jahreis G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: a randomized, controlled crossover study. Nutr J. 2013;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bähr M, Fechner A, Kiehntopf M, Jahreis G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: a randomized controlled trial. Clin Nutr. 2014;34:7–14. [DOI] [PubMed] [Google Scholar]
- 55. Bakhit RM, Klein BP, Essex‐Sorlie D, Ham JO, Erdman JW Jr, Potter SM. Intake of 25 g of soybean protein with or without soybean fiber alters plasma lipids in men with elevated cholesterol concentrations. J Nutr. 1994;124:213–222. [DOI] [PubMed] [Google Scholar]
- 56. Basaria S, Wisniewski A, Dupree K, Bruno T, Song MY, Yao F, Ojumu A, John M, Dobs AS. Effect of high‐dose isoflavones on cognition, quality of life, androgens, and lipoprotein in post‐menopausal women. J Endocrinol Invest. 2009;32:150–155. [DOI] [PubMed] [Google Scholar]
- 57. Baum JA, Teng H, Erdman JW, Weigel RM, Klein BP, Persky VW, Freels S, Surya P, Bakhit RM, Ramos E, Shay NF, Potter SM. Long‐term intake of soy protein improves blood lipid profiles and increases mononuclear cell low‐density‐lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am J Clin Nutr. 1998;68:545–551. [DOI] [PubMed] [Google Scholar]
- 58. Beavers KM, Serra MC, Beavers DP, Hudson GM, Willoughby DS. The lipid‐lowering effects of 4 weeks of daily soymilk or dairy milk ingestion in a postmenopausal female population. J Med Food. 2010;13:650–656. [DOI] [PubMed] [Google Scholar]
- 59. Blum A, Lang N, Peleg A, Vigder F, Israeli P, Gumanovsky M, Lupovitz S, Elgazi A, Ben‐Ami M. Effects of oral soy protein on markers of inflammation in postmenopausal women with mild hypercholesterolemia. Am Heart J. 2003;145:e7. [DOI] [PubMed] [Google Scholar]
- 60. Borodin EA, Menshikova IG, Dorovskikh VA, Feoktistova NA, Shtarberg MA, Yamamoto T, Takamatsu K, Mori H, Yamamoto S. Effects of two‐month consumption of 30 g a day of soy protein isolate or skimmed curd protein on blood lipid concentration in Russian adults with hyperlipidemia. J Nutr Sci Vitaminol (Tokyo). 2009;55:492–497. [DOI] [PubMed] [Google Scholar]
- 61. Bricarello LP, Kasinski N, Bertolami MC, Faludi A, Pinto LA, Relvas WG, Izar MC, Ihara SS, Tufik S, Fonseca FA. Comparison between the effects of soy milk and non‐fat cow milk on lipid profile and lipid peroxidation in patients with primary hypercholesterolemia. Nutrition. 2004;20:200–204. [DOI] [PubMed] [Google Scholar]
- 62. Burns‐Whitmore B, Haddad E, Sabaté J, Rajaram S. Effects of supplementing n‐3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free‐living lacto‐ovo‐vegetarians: a randomized, crossover, free‐living intervention study. Nutr J. 2014;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Campbell SC, Khalil DA, Payton ME, Arjmandi BH. One‐year soy protein supplementation does not improve lipid profile in postmenopausal women. Menopause. 2010;17:587–593. [DOI] [PubMed] [Google Scholar]
- 64. Chen ST, Ferng SH, Yang CS, Peng SJ, Lee HR, Chen JR. Variable effects of soy protein on plasma lipids in hyperlipidemic and normolipidemic hemodialysis patients. Am J Kidney Dis. 2005;46:1099–1106. [DOI] [PubMed] [Google Scholar]
- 65. Chen ST, Chen JR, Yang CS, Peng SJ, Ferng SH. Effect of soya protein on serum lipid profile and lipoprotein concentrations in patients undergoing hypercholesterolaemic haemodialysis. Br J Nutr. 2006;95:366–371. [DOI] [PubMed] [Google Scholar]
- 66. Crouse JR III, Morgan T, Terry JG, Ellis J, Vitolins M, Burke GL. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med. 1999;159:2070–2076. [DOI] [PubMed] [Google Scholar]
- 67. Cuevas AM, Irribarra VL, Castillo OA, Yanez MD, Germain AM. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr. 2003;57:889–894. [DOI] [PubMed] [Google Scholar]
- 68. Dent SB, Peterson CT, Brace LD, Swain JH, Reddy MB, Hanson KB, Robinson JG, Alekel DL. Soy protein intake by perimenopausal women does not affect circulating lipids and lipoproteins or coagulation and fibrinolytic factors. J Nutr. 2001;131:2280–2287. [DOI] [PubMed] [Google Scholar]
- 69. Duane WC. Effects of soybean protein and very low dietary cholesterol on serum lipids, biliary lipids, and fecal sterols in humans. Metabolism. 1999;48:489–494. [DOI] [PubMed] [Google Scholar]
- 70. Dunn C, Liebman M. Plasma lipid alterations in vegetarian males resulting from the substitution of tofu for cheese. Nutr Res. 1986;6:1343–1352. [Google Scholar]
- 71. Finley JW, Burrell JB, Reeves PG. Pinto bean consumption changes SCFA profiles in fecal fermentations, bacterial populations of the lower bowel, and lipid profiles in blood of humans. J Nutr. 2007;137:2391–2398. [DOI] [PubMed] [Google Scholar]
- 72. Gardner CD, Newell KA, Cherin R, Haskell WL. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:728–735. [DOI] [PubMed] [Google Scholar]
- 73. Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: a randomized trial. J Am Coll Nutr. 2007;26:669–677. [DOI] [PubMed] [Google Scholar]
- 74. Giovannetti PM, Carroll KK, Wolfe BM. Constancy of fasting serum cholesterol of healthy young women upon substitution of soy protein isolate for meat and dairy protein in medium and low fat diets. Nutr Res. 1986;6:609–618. [Google Scholar]
- 75. Goldberg AP, Lim A, Kolar JB, Grundhauser JJ, Steinke FH, Schonfeld G. Soybean protein independently lowers plasma cholesterol levels in primary hypercholesterolemia. Atherosclerosis. 1982;43:355–368. [DOI] [PubMed] [Google Scholar]
- 76. Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Probiotic consumption does not enhance the cholesterol‐lowering effect of soy in postmenopausal women. J Nutr. 2004;134:3277–3283. [DOI] [PubMed] [Google Scholar]
- 77. Haub MD, Wells AM, Campbell WW. Beef and soy‐based food supplements differentially affect serum lipoprotein‐lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive‐train. Metabolism. 2005;54:769–774. [DOI] [PubMed] [Google Scholar]
- 78. Hermansen K, Søndergaard M, Høie L, Carstensen M, Brock B. Beneficial effects of a soy‐based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care. 2001;24:228–233. [DOI] [PubMed] [Google Scholar]
- 79. Hill AM, Harris Jackson KA, Roussell MA, West SG, Kris‐Etherton PM. Type and amount of dietary protein in the treatment of metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. 2015;102:757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hoie LH, Morgenstern EC, Gruenwald J, Graubaum HJ, Busch R, Luder W, Zunft HJ. A double‐blind placebo‐controlled clinical trial compares the cholesterol‐lowering effects of two different soy protein preparations in hypercholesterolemic subjects. Eur J Nutr. 2005;44:65–71. [DOI] [PubMed] [Google Scholar]
- 81. Høie LH, Graubaum H‐J, Harde A, Gruenwald J, Wernecke K‐D. Lipid‐lowering effect of 2 dosages of a soy protein supplement in hypercholesterolemia. Adv Ther. 2005;22:175–186. [DOI] [PubMed] [Google Scholar]
- 82. Hoie LH, Guldstrand M, Sjoholm A, Graubaum HJ, Gruenwald J, Zunft HJ, Lueder W. Cholesterol‐lowering effects of a new isolated soy protein with high levels of nondenaturated protein in hypercholesterolemic patients. Adv Ther. 2007;24:439–447. [DOI] [PubMed] [Google Scholar]
- 83. Hosseinpour‐Niazi S, Mirmiran P, Hedayati M, Azizi F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: a cross‐over randomized clinical trial. Eur J Clin Nutr. 2015;69:592–597. [DOI] [PubMed] [Google Scholar]
- 84. Huff MW, Giovannetti PM, Wolfe BM. Turnover of very low‐density lipoprotein‐apoprotein B is increased by substitution of soybean protein for meat and dairy protein in the diets of hypercholesterolemic men. Am J Clin Nutr. 1984;39:888–897. [DOI] [PubMed] [Google Scholar]
- 85. Jenkins DJA, Wolever TMS, Spiller G, Buckley G, Lam Y, Jenkins AL, Josse RG. Hypocholesterolemic effect of vegetable protein in a hypocaloric diet. Atherosclerosis. 1989;78:99–107. [DOI] [PubMed] [Google Scholar]
- 86. Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, Vidgen E, Cunnane SC, Leiter LA, Josse RG. Effects of high‐ and low‐isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76:365–372. [DOI] [PubMed] [Google Scholar]
- 87. Jenkins DJ, Srichaikul K, Wong JM, Kendall CW, Bashyam B, Vidgen E, Lamarche B, Rao AV, Jones PJ, Josse RG, Jackson CJ, Ng V, Leong T, Leiter LA. Supplemental barley protein and casein similarly affect serum lipids in hypercholesterolemic women and men. J Nutr. 2010;140:1633–1637. [DOI] [PubMed] [Google Scholar]
- 88. Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free‐living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr. 1989;50:280–287. [DOI] [PubMed] [Google Scholar]
- 89. Kjolbaek L, Sorensen LB, Sondertoft NB, Rasmussen CK, Lorenzen JK, Serena A, Astrup A, Larsen LH. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double‐blinded trial. Am J Clin Nutr. 2017;106:684–697. [DOI] [PubMed] [Google Scholar]
- 90. Kreijkamp‐Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. [DOI] [PubMed] [Google Scholar]
- 91. Kurowska EM, Jordan J, Spence JD, Wetmore S, Piche LA, Radzikowski M, Dandona P, Carroll KK. Effects of substituting dietary soybean protein and oil for milk protein and fat in subjects with hypercholesterolemia. Clin Invest Med. 1997;20:162–170. [PubMed] [Google Scholar]
- 92. Laidlaw M, Mercer NJH. Serum cholesterol, triglyceride and lipoprotein response in hypercholesterolemic males to replacement of cow's milk with a soy beverage. Fed Proc. 1985;44(5):No. 6360. [Google Scholar]
- 93. Laurin D, Jacques H, Moorjani S, Steinke FH, Gagne C, Brun D, Lupien PJ. Effects of a soy‐protein beverage on plasma lipoproteins in children with familial hypercholesterolemia. Am J Clin Nutr. 1991;54:98–103. [DOI] [PubMed] [Google Scholar]
- 94. Li J, Armstrong CL, Campbell WW. Effects of dietary protein source and quantity during weight loss on appetite, energy expenditure, and cardio‐metabolic responses. Nutrients. 2016;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liao FH, Shieh MJ, Yang SC, Lin SH, Chien YW. Effectiveness of a soy‐based compared with a traditional low‐calorie diet on weight loss and lipid levels in overweight adults. Nutrition. 2007;23:551–556. [DOI] [PubMed] [Google Scholar]
- 96. Lichtenstein AH, Jalbert SM, Adlercreutz H, Goldin BR, Rasmussen H, Schaefer EJ, Ausman LM. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol. 2002;22:1852–1858. [DOI] [PubMed] [Google Scholar]
- 97. Liu ZM, Ho SC, Chen YM, Ho YP. The effects of isoflavones combined with soy protein on lipid profiles, C‐reactive protein and cardiovascular risk among postmenopausal Chinese women. Nutr Metab Cardiovasc Dis. 2012;22:712–719. [DOI] [PubMed] [Google Scholar]
- 98. Liu ZM, Ho SC, Chen YM, Ho S, To K, Tomlinson B, Woo J. Whole soy, but not purified daidzein, had a favorable effect on improvement of cardiovascular risks: a 6‐month randomized, double‐blind, and placebo‐controlled trial in equol‐producing postmenopausal women. Mol Nutr Food Res. 2014;58:709–717. [DOI] [PubMed] [Google Scholar]
- 99. Lovati MR, Manzoni C, Canavesi A, Sirtori M, Vaccarino V, Marchi M, Gaddi G, Sirtori CR. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. J Clin Invest. 1987;80:1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ma Y, Chiriboga D, Olendzki BC, Nicolosi R, Merriam PA, Ockene IS. Effect of soy protein containing isoflavones on blood lipids in moderately hypercholesterolemic adults: a randomized controlled trial. J Am Coll Nutr. 2005;24:275–285. [DOI] [PubMed] [Google Scholar]
- 101. Ma L, Grann K, Li M, Jiang Z. A pilot study to evaluate the effect of soy isolate protein on the serum lipid profile and other potential cardiovascular risk markers in moderately hypercholesterolemic Chinese adults. Ecol Food Nutr. 2011;50:473–485. [DOI] [PubMed] [Google Scholar]
- 102. Maki KC, Butteiger DN, Rains TM, Lawless A, Reeves MS, Schasteen C, Krul ES. Effects of soy protein on lipoprotein lipids and fecal bile acid excretion in men and women with moderate hypercholesterolemia. J Clin Lipidol. 2010;4:531–542. [DOI] [PubMed] [Google Scholar]
- 103. Markova MHS, Sucher S, Pivovarova O, Pfeiffer A. Metabolic and molecular effects of a high‐protein diet in subjects with type 2 diabetes. Diabetologia. 2015;58:S335. [Google Scholar]
- 104. Matthan NR, Jalbert SM, Ausman LM, Kuvin JT, Karas RH, Lichtenstein AH. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:960–966. [DOI] [PubMed] [Google Scholar]
- 105. McVeigh BL, Dillingham BL, Lampe JW, Duncan AM. Effect of soy protein varying in isoflavone content on serum lipids in healthy young men. Am J Clin Nutr. 2006;83:244–251. [DOI] [PubMed] [Google Scholar]
- 106. Mercer NJH, Carroll KK, Giovannetti PM. Effects on human plasma lipids of substituting soybean protein isolate for milk protein in the diet. Nutr Rep Int. 1987;35:279–287. [Google Scholar]
- 107. Meredith L, Liebman M, Graves K. Alterations in plasma lipid levels resulting from tofu and cheese consumption in adult women. J Am Coll Nutr. 1989;8:573–579. [DOI] [PubMed] [Google Scholar]
- 108. Meyer BJ, Larkin TA, Owen AJ, Astheimer LB, Tapsell LC, Howe PR. Limited lipid‐lowering effects of regular consumption of whole soybean foods. Ann Nutr Metab. 2004;48:67–78. [DOI] [PubMed] [Google Scholar]
- 109. Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35:1981–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Napora JK, Short RG, Muller DC, Carlson OD, Odetunde JO, Xu X, Carducci M, Travison TG, Maggio M, Egan JM, Basaria S. High dose isoflavones do not improve metabolic and inflammatory parameters in androgen deprived men with prostate cancer. J Androl. 2011;32:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Onning G, Akesson B, Oste R, Lundquist I. Effects of consumption of oat milk, soya milk, or cow's milk on plasma lipids and antioxidative capacity in healthy subjects. Ann Nutr Metab. 1998;42:211–220. [DOI] [PubMed] [Google Scholar]
- 112. Padhi EM, Blewett HJ, Duncan AM, Guzman RP, Hawke A, Seetharaman K, Tsao R, Wolever TM, Ramdath DD. Whole soy flour incorporated into a muffin and consumed at 2 doses of soy protein does not lower LDL cholesterol in a randomized, double‐blind controlled trial of hypercholesterolemic adults. J Nutr. 2015;145:2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pipe EA, Gobert CP, Capes SE, Darlington GA, Lampe JW, Duncan AM. Soy protein reduces serum LDL cholesterol and the LDL cholesterol:HDL cholesterol and apolipoprotein B:apolipoprotein A‐I ratios in adults with type 2 diabetes. J Nutr. 2009;139:1700–1706. [DOI] [PubMed] [Google Scholar]
- 114. Potter SM, Bakhit RM, Essex‐Sorlie DL, Weingartner KE, Chapman KM, Nelson RA, Prabhudesai M, Savage WD, Nelson AI, Winter LW. Depression of plasma cholesterol in men by consumption of baked products containing soy protein. Am J Clin Nutr. 1993;58:501–506. [DOI] [PubMed] [Google Scholar]
- 115. Puska P, Korpelainen V, Hoie LH, Skovlund E, Lahti T, Smerud KT. Soy in hypercholesterolaemia: a double‐blind, placebo‐controlled trial. Eur J Clin Nutr. 2002;56:352–357. [DOI] [PubMed] [Google Scholar]
- 116. Puska P, Korpelainen V, Hoie LH, Skovlund E, Smerud KT. Isolated soya protein with standardised levels of isoflavones, cotyledon soya fibres and soya phospholipids improves plasma lipids in hypercholesterolaemia: a double‐blind, placebo‐controlled trial of a yoghurt formulation. Br J Nutr. 2004;91:393–401. [DOI] [PubMed] [Google Scholar]
- 117. Roughead ZK, Hunt JR, Johnson LK, Badger TM, Lykken GI. Controlled substitution of soy protein for meat protein: effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J Clin Endocrinol Metab. 2005;90:181–189. [DOI] [PubMed] [Google Scholar]
- 118. Santo AS, Cunningham AM, Alhassan S, Browne RW, Burton H, Leddy JJ, Grandjean PW, Horvath SM, Horvath PJ. NMR analysis of lipoprotein particle size does not increase sensitivity to the effect of soy protein on CVD risk when compared with the traditional lipid profile. Appl Physiol Nutr Metab. 2008;33:489–500. [DOI] [PubMed] [Google Scholar]
- 119. Shidfar F, Ehramphosh E, Heydari I, Haghighi L, Hosseini S, Shidfar S. Effects of soy bean on serum paraoxonase 1 activity and lipoproteins in hyperlipidemic postmenopausal women. Int J Food Sci Nutr. 2009;60:195–205. [DOI] [PubMed] [Google Scholar]
- 120. Shige H, Ishikawa T, Higashi K, Yamashita T, Tomiyasu K, Yoshida H, Hosoai H, Ito T, Nakajima K, Ayaori M, Yonemura A, Suzukawa M, Nakamura H. Effects of soy protein isolate (SPI) and casein on the postprandial lipemia in normolipidemic men. J Nutr Sci Vitaminol. 1998;44:113–127. [DOI] [PubMed] [Google Scholar]
- 121. Sirtori CR, Agradi E, Conti F. Soybean protein diet in the treatment of type II hyperlipoproteinaemia. Lancet. 1977;1:275–277. [DOI] [PubMed] [Google Scholar]
- 122. Sirtori CR, Pazzucconi F, Colombo L, Battistin P, Bondioli A, Descheemaeker K. Double‐blind study of the addition of high‐protein soya milk v. cows' milk to the diet of patients with severe hypercholesterolaemia and resistance to or intolerance of statins. Br J Nutr. 1999;82:91–96. [PubMed] [Google Scholar]
- 123. Sirtori CR, Bosisio R, Pazzucconi F, Bondioli A, Gatti E, Lovati MR, Murphy P. Soy milk with a high glycitein content does not reduce low‐density lipoprotein cholesterolemia in type II hypercholesterolemic patients. Ann Nutr Metab. 2002;46:88–92. [DOI] [PubMed] [Google Scholar]
- 124. Steele MG. The effect on serum cholesterol levels of substituting milk with a soya beverage. Aust J Nutr Diet. 1992;49:24–28. [Google Scholar]
- 125. Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr. 2003;78:123–130. [DOI] [PubMed] [Google Scholar]
- 126. Sucher S, Markova M, Hornemann S, Pivovarova O, Rudovich N, Thomann R, Schneeweiss R, Rohn S, Pfeiffer AFH. Comparison of the effects of diets high in animal or plant protein on metabolic and cardiovascular markers in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2017;19:944–952. [DOI] [PubMed] [Google Scholar]
- 127. Tabibi H, Imani H, Hedayati M, Atabak S, Rahmani L. Effects of soy consumption on serum lipids and apoproteins in peritoneal dialysis patients: a randomized controlled trial. Perit Dial Int. 2010;30:611–618. [DOI] [PubMed] [Google Scholar]
- 128. Takahira M, Noda K, Fukushima M, Zhang B, Mitsutake R, Uehara Y, Ogawa M, Kakuma T, Saku K. Randomized, double‐blind, controlled, comparative trial of formula food containing soy protein vs. milk protein in visceral fat obesity: FLAVO study. Circ J. 2011;75:2235–2243. [DOI] [PubMed] [Google Scholar]
- 129. Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo‐controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060. [DOI] [PubMed] [Google Scholar]
- 130. Teixeira SR, Potter SM, Weigel R, Hannum S, Erdman JW, Hasler CM. Effects of feeding 4 levels of soy protein for 3 and 6 wk on blood lipids and apolipoproteins in moderately hypercholesterolemic men. Am J Clin Nutr. 2000;71:1077–1084. [DOI] [PubMed] [Google Scholar]
- 131. Teixeira SR, Tappenden KA, Carson L, Jones R, Prabhudesai M, Marshall WP, Erdman JW. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134:1874–1880. [DOI] [PubMed] [Google Scholar]
- 132. Thorp AA, Howe PR, Mori TA, Coates AM, Buckley JD, Hodgson J, Mansour J, Meyer BJ. Soy food consumption does not lower LDL cholesterol in either equol or nonequol producers. Am J Clin Nutr. 2008;88:298–304. [DOI] [PubMed] [Google Scholar]
- 133. Tonstad S, Smerud K, Hoie L. A comparison of the effects of 2 doses of soy protein or casein on serum lipids, serum lipoproteins, and plasma total homocysteine in hypercholesterolemic subjects. Am J Clin Nutr. 2002;76:78–84. [DOI] [PubMed] [Google Scholar]
- 134. Van Horn L, Liu K, Gerber J, Garside D, Schiffer L, Gernhofer N, Greenland P. Oats and soy in lipid‐lowering diets for women with hypercholesterolemia: is there synergy? J Am Diet Assoc. 2001;101:1319–1325. [DOI] [PubMed] [Google Scholar]
- 135. van Nielen M, Feskens EJM, Rietman A, Siebelink E, Mensink M. Partly replacing meat protein with soy protein alters insulin resistance and blood lipids in postmenopausal women with abdominal obesity. J Nutr. 2014;144:1423–1429. [DOI] [PubMed] [Google Scholar]
- 136. van Raaij JM, Katan MB, Hautvast JG, Hermus RJ. Effects of casein versus soy protein diets on serum cholesterol and lipoproteins in young healthy volunteers. Am J Clin Nutr. 1981;34:1261–1271. [DOI] [PubMed] [Google Scholar]
- 137. van Raaij JM, Katan MB, West CE, Hautvast JG. Influence of diets containing casein, soy isolate, and soy concentrate on serum cholesterol and lipoproteins in middle‐aged volunteers. Am J Clin Nutr. 1982;35:925–934. [DOI] [PubMed] [Google Scholar]
- 138. Vega‐Lopez S, Matthan NR, Ausman LM, Harding SV, Rideout TC, Ai M, Otokozawa S, Freed A, Kuvin JT, Jones PJ, Schaefer EJ, Lichtenstein AH. Altering dietary lysine:arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atherosclerosis. 2010;210:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vigna GB, Pansini F, Bonaccorsi G, Albertazzi P, Donega P, Zanotti L, De Aloysio D, Mollica G, Fellin R. Plasma lipoproteins in soy‐treated postmenopausal women: a double‐blind, placebo‐controlled trial. Nutr Metab Cardiovasc Dis. 2000;10:315–322. [PubMed] [Google Scholar]
- 140. Weisse K, Brandsch C, Zernsdorf B, Nkengfack Nembongwe GS, Hofmann K, Eder K, Stangl GI. Lupin protein compared to casein lowers the LDL cholesterol:HDL cholesterol‐ratio of hypercholesterolemic adults. Eur J Nutr. 2010;49:65–71. [DOI] [PubMed] [Google Scholar]
- 141. West SG, Hilpert KF, Juturu V, Bordi PL, Lampe JW, Mousa SA, Kris‐Etherton PM. Effects of including soy protein in a blood cholesterol‐lowering diet on markers of cardiac risk in men and in postmenopausal women with and without hormone replacement therapy. J Womens Health (Larchmt). 2005;14:253–262. [DOI] [PubMed] [Google Scholar]
- 142. Wheeler ML, Fineberg SE, Fineberg NS, Gibson RG, Hackward LL. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria: effects on renal, glycemic, and lipid parameters. Diabetes Care. 2002;25:1277–1282. [DOI] [PubMed] [Google Scholar]
- 143. Wiebe SL, Bruce VM, McDonald BE. A comparison of the effect of diets containing beef protein and plant proteins on blood lipids of healthy young men. Am J Clin Nutr. 1984;40:982–989. [DOI] [PubMed] [Google Scholar]
- 144. Wofford MR, Rebholz CM, Reynolds K, Chen J, Chen CS, Myers L, Xu J, Jones DW, Whelton PK, He J. Effect of soy and milk protein supplementation on serum lipid levels: a randomized controlled trial. Eur J Clin Nutr. 2012;66:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wolfe BM, Giovannetti PM, Cheng DCH. Hypolipidemic effect of substituting soybean protein isolate for all meat and dairy protein in the diets of hypercholesterolemic men. Nutr Rep Int. 1981;24:1187–1198. [Google Scholar]
- 146. Wolfe BM, Giovannetti PM. Elevation of VLDL‐cholesterol during substitution of soy protein for animal protein in diets of hypercholesterolemic Canadians. Nutr Rep Int. 1985;32:1057–1065. [Google Scholar]
- 147. Wong WW, Smith EO, Stuff JE, Hachey DL, Heird WC, Pownell HJ. Cholesterol‐lowering effect of soy protein in normocholesterolemic and hypercholesterolemic men. Am J Clin Nutr. 1998;68:1385S–1389S. [DOI] [PubMed] [Google Scholar]
- 148. Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta‐regression analysis. J Am Coll Cardiol. 2005;46:1855–1862. [DOI] [PubMed] [Google Scholar]
- 149. Manson JE, Tosteson H, Ridker PM, Satterfield S, Hebert P, O'Connor GT, Buring JE, Hennekens CH. The primary prevention of myocardial infarction. N Engl J Med. 1992;326:1406–1416. [DOI] [PubMed] [Google Scholar]
- 150. Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002408 DOI: 10.1161/JAHA.115.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, Emam A, Parker TL, Vidgen E, Lapsley KG, Trautwein EA, Josse RG, Leiter LA, Connelly PW. Effects of a dietary portfolio of cholesterol‐lowering foods vs lovastatin on serum lipids and C‐reactive protein. JAMA. 2003;290:502–510. [DOI] [PubMed] [Google Scholar]
- 152. Jenkins DJ, Jones PJ, Lamarche B, Kendall CW, Faulkner D, Cermakova L, Gigleux I, Ramprasath V, de Souza R, Ireland C, Patel D, Srichaikul K, Abdulnour S, Bashyam B, Collier C, Hoshizaki S, Josse RG, Leiter LA, Connelly PW, Frohlich J. Effect of a dietary portfolio of cholesterol‐lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: a randomized controlled trial. JAMA. 2011;306:831–839. [DOI] [PubMed] [Google Scholar]
- 153. Jenkins DJA, Wong JMW, Kendall CWC, Esfahani A, Ng VWY, Leong TCK, Faulkner DA, Vidgen E, Paul G, Mukherjea R, Krul ES, Singer W. Effect of a 6‐month vegan low‐carbohydrate (“Eco‐Atkins”) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial. BMJ Open. 2014;4:e003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Estruch R, Ros E, Salas‐Salvadó J, Covas M‐I, Corella D, Arós F, Gómez‐Gracia E, Ruiz‐Gutiérrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez‐González MA. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 155. Knoops KB, de Groot LM, Kromhout D, Perrin AE, Moreiras‐Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10‐year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. [DOI] [PubMed] [Google Scholar]
- 156. Kris‐Etherton P, Eckel RH, Howard BV, St. Jeor S, Bazzarre TL. Lyon Diet Heart Study: benefits of a Mediterranean‐style, National Cholesterol Education Program/American Heart Association Step I dietary pattern on cardiovascular disease. Circulation. 2001;103:1823–1825. [DOI] [PubMed] [Google Scholar]
- 157. Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, Burr ML, Chang‐Claude J, Frentzel‐Beyme R, Kuzma JW, Mann J, McPherson K. Mortality in vegetarians and non‐vegetarians: a collaborative analysis of 8300 deaths among 76,000 men and women in five prospective studies. Public Health Nutr. 1998;1:33–41. [DOI] [PubMed] [Google Scholar]
- 158. Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC‐Oxford cohort study. Am J Clin Nutr. 2013;97:597–603. [DOI] [PubMed] [Google Scholar]
- 159. Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low‐carbohydrate diets and all‐cause and cause‐specific mortality two cohort studies. Ann Intern Med. 2010;153:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kushi LH, Lew RA, Stare FJ, Ellison CR, el Lozy M, Bourke G, Daly L, Graham I, Hickey N, Mulcahy R, Kevaney J. Diet and 20‐year mortality from coronary heart disease. N Engl J Med. 1985;312:811–818. [DOI] [PubMed] [Google Scholar]
- 161. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Snowdon DA. Animal product consumption and mortality because of all causes combined, coronary heart disease, stroke, diabetes, and cancer in Seventh‐day Adventists. Am J Clin Nutr. 1988;48:739–748. [DOI] [PubMed] [Google Scholar]
- 163. Kelemen LE, Kushi LH, Jacobs DR, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161:239–249. [DOI] [PubMed] [Google Scholar]
- 164. Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–631. [DOI] [PubMed] [Google Scholar]
- 165. Sanchez A, Rubano DA, Shavlik GW, Hubbard R, Horning MC. Cholesterolemic effects of the lysine/arginine ratio in rabbits after initial early growth. Arch Latinoam Nutr. 1988;38:229–238. [PubMed] [Google Scholar]
- 166. Kurowska EM, Carroll KK. Effect of high levels of selected dietary essential amino acids on hypercholesterolemia and down‐regulation of hepatic LDL receptors in rabbits. Biochim Biophys Acta. 1992;1126:185–191. [DOI] [PubMed] [Google Scholar]
- 167. Park MS, Liepa GU. Effects of dietary protein and amino acids on the metabolism of cholesterol‐carrying lipoproteins in rats. J Nutr. 1982;112:1892–1898. [DOI] [PubMed] [Google Scholar]
- 168. Kohls KJ, Kies C, Fox HM. Blood serum lipid levels of humans given arginine, lysine and tryptophan supplements without food. Nutr Rep Int. 1987;35:5–11. [Google Scholar]
- 169. Berryman CE, Preston AG, Karmally W, Deckelbaum RJ, Kris‐Etherton PM. Effects of almond consumption on the reduction of LDL‐cholesterol: a discussion of potential mechanisms and future research directions. Nutr Rev. 2011;69:171–185. [DOI] [PubMed] [Google Scholar]
- 170. Anderson JW, Johnstone BM, Cook‐Newell ME. Meta‐analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–282. [DOI] [PubMed] [Google Scholar]
- 171. Pasiakos S, Agarwal S, Lieberman H, Fulgoni V. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007–2010. Nutrients. 2015;7:5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Halkjar J, Olsen A, Bjerregaard LJ, Deharveng G, Tjonneland A, Welch AA, Crowe FL, Wirfalt E, Hellstrom V, Niravong M, Touvier M, Linseisen J, Steffen A, Ocke MC, Peeters PHM, Chirlaque MD, Larranaga N, Ferrari P, Contiero P, Frasca G, Engeset D, Lund E, Misirli G, Kosti M, Riboli E, Slimani N, Bingham S. Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 2015;63:S16–S36. [DOI] [PubMed] [Google Scholar]
- 173. Rizzo NS, Jaceldo‐Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Estruch R, Martínez‐González MA, Corella D, Salas‐Salvadó J, Ruiz‐Gutiérrez V, Covas MI, Fiol M, Gómez‐Gracia E, López‐Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E. Effects of a Mediterranean‐style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. [DOI] [PubMed] [Google Scholar]
- 175. Jenkins DJA, Kendall CWC, Faulkner D, Vidgen E, Trautwein EA, Parker TL, Marchie A, Koumbridis G, Lapsley KG, Josse RG, Leiter LA, Connelly PW. A dietary portfolio approach to cholesterol reduction: combined effects of plant sterols, vegetable proteins, and viscous fibers in hypercholesterolemia. Metabolism. 2002;51:1596–1604. [DOI] [PubMed] [Google Scholar]
- 176. Mudryj AN, Yu N, Hartman TJ, Mitchell DC, Lawrence FR, Aukema HM. Pulse consumption in Canadian adults influences nutrient intakes. Br J Nutr. 2012;108(suppl 1):S27–S36. [DOI] [PubMed] [Google Scholar]
- 177. Mudryj AN, Aukema HM, Yu N. Intake patterns and dietary associations of soya protein consumption in adults and children in the Canadian Community Health Survey, Cycle 2.2. Br J Nutr. 2015;113:299–309. [DOI] [PubMed] [Google Scholar]
- 178. Asif M, Rooney LW, Ali R, Riaz MN. Application and opportunities of pulses in food system: a review. Crit Rev Food Sci Nutr. 2013;53:1168–1179. [DOI] [PubMed] [Google Scholar]
- 179. Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 180. Rossouw JE, Lewis B, Rifkind BM. The value of lowering cholesterol after myocardial infarction. N Engl J Med. 1990;323:1112–1119. [DOI] [PubMed] [Google Scholar]
- 181. Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356 222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986;256:2823–2828. [PubMed] [Google Scholar]
- 182. The Lipid Research Clinics Coronary Primary Prevention Trial results, I: reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. [DOI] [PubMed] [Google Scholar]
- 183. The Lipid Research Clinics Coronary Primary Prevention Trial results, II: the relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 184. Cholesterol Treatment Trialists' (CTT) Collaboration , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy
Table S2. Full Table of Characteristics
Table S3. Bootstrap Analyses
Table S4. Post‐Hoc Dose Response
Table S5. GRADE Assessment
Figure S1. Cochrane risk of bias. Risk of bias assessment using Cochrane Risk of Bias Tool.
Figure S2. LDL‐C forest plot, random‐effects model.
Figure S3. LDL‐C forest plot, fixed‐effects model.
Figure S4. LDL‐C visual subgroup. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S5. Post‐hoc subgroups. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S6. Non‐HDL‐C forest plot, random‐effects model.
Figure S7. Non‐HDL‐C forest plot, fixed‐effects model.
Figure S8. Non‐HDL‐C visual subgroup. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S9. Apo‐B forest plot, random‐effects model. HC indicates hypercholesterolemic; IF, isoflavones; LF, low‐fat; N, normal; NIF, no isoflavones. The pooled effect estimate (diamond) is shown. Paired analyses were applied to all crossover trials. Data are expressed as MDs with 95% CIs, using generic inverse‐variance random‐effects models. Inter‐study heterogeneity was tested using the Cochran Q statistic (chi‐square) at a significance level of P<0.10 and quantified by I2, levels of ≥50% represented substantial heterogeneity.
Figure S10. Apo‐B forest plot, fixed‐effects model. HC indicates hypercholesterolemic; IF, isoflavones; LF, low‐fat; N, normal; NIF, no isoflavones. The pooled effect estimate (diamond) is shown. Paired analyses were applied to all crossover trials. Data are expressed as MDs with 95% CIs, using generic inverse‐variance fixed‐effects models. Inter‐study heterogeneity was tested using the Cochran Q statistic (chi‐square) at a significance level of P<0.10 and quantified by I2, levels of ≥50% represented substantial heterogeneity.
Figure S11. Apo‐B visual subgroup. Point estimates for each subgroup level (squares) are the pooled effect estimates. The dashed line represents the pooled effect estimate for the overall (total) analysis. The residual I2 value indicates the interstudy heterogeneity unexplained by the subgroup. Statistically significant pairwise subgroup effect modification by meta‐regression analyses at P<0.05.
Figure S12. Funnel plots. Publication bias funnel plots for LDL (A), non‐HDL (B), and apolipoprotein B (C). The solid line represents the pooled effect estimate expressed as the weighted mean difference (MD) of each analysis, and dashed lines represent pseudo‐95% confidence limits. Circles represent effect estimates of included trials. P‐values of Egger and Begg tests for publication bias are shown at top right for each analysis. *Statistically significant (P<0.05).
Figure S13. LDL‐C trim‐and‐fill funnel plot. The horizontal line represents the pooled effect estimate expressed as a mean difference. The diagonal lines represent the pseudo 95% CIs of the mean difference. The clear circles represent effect estimates for each included study.


