Abstract
Background
We investigated whether cardiac parameters in young adulthood are associated with indicators of brain health in midlife.
Methods and Results
This study includes 648 participants from the CARDIA (Coronary Artery Risk Development in Young Adults) study (52% women, 38% black). We studied associations of cardiac parameters assessed by echocardiography (left ventricular ejection fraction, left atrial volume, and left ventricular mass) in young adulthood (mean age: 30 years) with brain measures obtained by magnetic resonance imaging (total brain, gray and white matter volume, white matter integrity, abnormal white matter) in midlife (mean age: 50 years). In 406 individuals with complete measurements, higher left atrial volume was associated with lower white matter fractional anisotropy, independent of traditional cardiovascular risk factors (β=−0.002; P <0.02). The association was strongest in black participants and in men.
Conclusions
Higher left atrial volume in early adulthood is associated with impairment of white matter integrity in midlife. Interventions to improve cardiac function in young adults may benefit brain health and should be targeted in particular at black men.
Keywords: brain, echocardiography, epidemiology, magnetic resonance imaging, white matter, young adulthood
Subject Categories: Aging, Epidemiology, Primary Prevention, Race and Ethnicity, Risk Factors
Clinical Perspective
What Is New?
In a biracial population‐based study, we found that higher left atrial volume in young adulthood was associated with a marker of structural damage of the brain in midlife.
This association was strongest for black participants and for men.
What Are the Clinical Implications?
Prevention of cardiac dysfunction early in life could benefit brain health.
The study suggests preventative strategies should be targeted to subgroups that may be at higher risk for cardiac dysfunction.
Intact cardiac function is needed to maintain cerebral perfusion in order to preserve brain health.1 Mounting evidence from general and patient populations indicates that individuals with heart failure have a greater risk of functional and structural pathologies of the brain, such as ischemic lesions, atrophy, cognitive impairment, and dementia.1, 2, 3, 4 In heart failure with reduced ejection fraction, decreased cardiac output could result in insufficient brain perfusion, leading to accelerated brain aging.1 Moreover, in heart failure with preserved ejection fraction, systemic inflammation and microvascular abnormalities, which induce diastolic dysfunction, likely affect the integrity of the brain.1
The importance of the pathophysiology of the heart–brain axis is not limited to patients with advanced heart failure. In cohorts of older adults without symptoms of heart failure, even subtle cardiac dysfunction is associated with lower brain volume, ischemic lesions, and impaired integrity of the white matter.5, 6, 7, 8, 9 Subclinical cardiac dysfunction frequently occurs in the community and is largely underdiagnosed.10 Its indicators, such as decreased left ventricular ejection fraction (LVEF), increased left atrial volume (LAV) and higher left ventricular (LV) mass may serve as reversible risk markers for compromised brain health.11
Recent studies suggest that cardiac dysfunction can already be detected in young adults, especially among black persons.12 There is a lack of data on whether cardiac function as early as in young adulthood affects brain health. We aimed to study whether markers of subclinical cardiac dysfunction obtained by echocardiography in young adulthood are associated with indicators of brain health measured by magnetic resonance imaging (MRI) in midlife. Because black patients, especially men, present with cardiac dysfunction at younger ages than white patients,12, 13 in secondary analyses, we investigated associations of echocardiographic parameters with brain MRI measures separately by race/ethnicity and sex.
Methods
Data
The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the procedure from the CARDIA (Coronary Artery Risk Development in Young Adults) Coordinating Center.14 CARDIA complies with data sharing requirements of the National Institutes of Health by providing limited‐access data sets from various CARDIA examinations to the National Heart, Lung and Blood Institute (NHLBI) bioLINCC.15 A description of the NHLBI policies governing the data and describing access to the data can be found online.16
Study Population
The participants are from the CARDIA study, which has been described previously.17 Briefly, CARDIA was established in 1985 at 4 centers in the United States (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) with the aim of examining the development and trajectories of cardiovascular disease in young adults. At baseline, participants were randomly selected within race/ethnicity, sex, and education strata and were contacted by telephone or door‐to‐door recruiters. The original cohort included 5115 persons (52% black, 55% women). Follow‐up examinations were performed after 2, 5, 7, 10, 15, 20, and 25 years; retention at these time points was 91%, 86%, 81%, 79%, 74%, 72%, and 72%, respectively, of the surviving cohort.
At year 25, 719 individuals from the cohort underwent brain MRI examination at 3 of the CARDIA field centers: Birmingham, Minneapolis, and Oakland (CARDIA Brain MRI Substudy). The subsample for the CARDIA Brain MRI Substudy was selected at the time that the appointments for the examination at year 25 were made, with the aim of achieving a balance within 4 strata of ethnicity/race (black/white) and sex (men/women). Each of the 3 centers had a target sample size for the study. When this number was reached, enrollment was ended. Exclusion criteria were contraindications to MRI or a body size too large to enable the MRI examination.
All participants provided written informed consent at each exam, and the institutional review board from each participating institution annually approved the CARDIA study. For the CARDIA Brain MRI Substudy, separate approval was given by the institutional review boards of the participating sites and by the institutional review boards covering intramural research at the National Institute of Aging. Separate participant written consent for the CARDIA Brain MRI Substudy was obtained.
Cardiac Parameters
At follow‐up year 5 (1990), 2‐dimensional and guided M‐mode echocardiography was acquired using an Acuson ultrasound machine (Siemens Medical Solutions USA), as previously described.18 We studied 3 echocardiographic parameters that represent markers of cardiac dysfunction and predictors of incident heart failure10, 19, 20: LVEF, LAV, and LV mass. LAV was derived from M‐mode left atrial diameter and 2‐dimensional area of the left atrium in the apical 4‐chamber view, using a formula described by Desai.18 LV mass was calculated from an M‐mode measurement of LV diastolic dimension, interventricular septum thickness in diastole, and LV posterior wall thickness in diastole, according to a formula that was reported previously.18 LAV and LV mass were indexed to body surface area.
Brain Measures
At follow‐up year 25 (2010), brain MRI was performed using 3‐T MRI scanners located at 3 CARDIA sites (Siemens 3T Tim Trio/VB 15 platform, Siemens 3T Tim Trio/VB 15 platform, and Philips 3T Achieva/2.6.3.6 platform), as reported elsewhere.21 In the present analyses, parameters of interest were white matter fractional anisotropy (WMFA) and volumes of total brain, white matter, gray matter, and abnormal white matter (AWM), corrected for total intracranial volume.
WMFA was estimated from diffusion tensor imaging and is a measure of microstructural integrity and connectivity of the white matter. It allows estimation of diffusion of water along brain fibers and ranges from 0 and 1, where 0 is isotropy (random motion of water) and 1 anisotropy (diffusion of water along 1 axis; ie, white matter tract).22 Total brain volume was estimated as the sum of gray matter and white matter volumes from the sagittal 3‐dimensional T1 sequence. Total intracranial volume (a measure of head size) was estimated as the sum of gray matter, white matter, and cerebral spinal fluid volumes from the sagittal 3‐dimensional T1 sequence. AWM volume was estimated from the sagittal 3‐dimensional fluid‐attenuated inversion recovery T1 and T2 sequences and reflects tissue damaged by ischemia, demyelination, or inflammation and penumbra surrounding brain infarcts. The values of brain measures were assessed for outliers that could potentially influence results. WMFA and volumes of total brain, white matter, and gray matter followed a normal distribution. The volume of AWM, however, was skewed, thus we categorized this variable into 3 groups: no AWM (volume: 0 cm3), little AWM (volume: ≤0.3 cm3), and high AWM (volume: >0.3 cm3). The cutoff of 0.3 cm3 is the median value of AWM. The distributions of brain measures are presented in Table 1.
Table 1.
Description of the Analytical Sample (n=648): CARDIA Brain MRI Substudy
| Characteristic | Value |
|---|---|
| Demographic characteristics | |
| Age at year 5, y | 30.4±3.5 |
| Age at year 25, y | 50.4±3.5 |
| Women, n (%) | 339 (52) |
| Black, n (%) | 244 (38) |
| Education, y | 15.0±2.3 |
| Cardiac parameters | |
| LVEF, % (n=297) | 62.8±7.4 |
| LAV, mL/m2 (n=406) | 16.1±4.2 |
| LV mass, g/m2 (n=627) | 79.9±18.4 |
| Brain measures | |
| WMFA | 0.3±0.02 |
| Gray matter volume, cm3 | 518.5±53.7 |
| White matter volume, cm3 | 467.0±59.5 |
| Total brain volume, cm3 | 985.5±107.2 |
| AWM volume, n (%) | |
| None | 127 (20) |
| Little (≤0.3 cm3) | 246 (38) |
| High (>0.3 cm3) | 275 (42) |
| Covariates | |
| Systolic blood pressure, mm Hg | 117.2±14.3 |
| Diastolic blood pressure, mm Hg | 72.7±10.6 |
| Total cholesterol, mg/L | 193.2±35.3 |
| Fasting plasma glucose, mg/L | 96.4±28.8 |
| BMI | 28.7±5.7 |
| Sedentary behavior, h/d | 6.8±4.0 |
| Alcohol intake, mL/d, median (IQR) | 4.8 (0.0–17.0) |
| APOE ɛ4 allele, n (%) | 178 (29) |
| Smoker, n (%) | |
| Never | 393 (61) |
| Current | 95 (15) |
| Former | 153 (24) |
Data are presented as mean±SD unless otherwise noted. The information about cardiac parameters comes from year 5, and all other data come from year 25. AWM indicates abnormal white matter; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; IQR, interquartile range; LAV, left atrial volume; LV mass, left ventricular mass; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; WMFA, white matter fractional anisotropy.
Covariates
We selected 7 vascular risk factors, assessed at year 25, to include in our statistical models as confounders of the associations between the echocardiographic parameters and the brain MRI measures: systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), total cholesterol, fasting plasma glucose, smoking, and sedentary behavior.23, 24 Blood pressure was measured with a digital blood pressure monitor (Omron HEM‐907XL) 3 times, and the average of the second and third measurements of the SBP and DBP was used. BMI was calculated from the assessments of height and weight. Total plasma cholesterol and fasting plasma glucose were measured, as described previously.25, 26 Smoking status was self‐reported and categorized as nonsmoker, current smoker, or former smoker. Sedentary behavior was assessed by a questionnaire about daily activities and expressed as hours per week. Moreover, we controlled for self‐reported alcohol intake, which was expressed as total ethanol consumption in mL/day, and the presence of the APOE ɛ4 allele that was determined from plasma at year 10 by a modification of the method of Kamboh and colleagues.27 Other covariates were age, sex (men/women), race/ethnicity (black/white), field center (Birmingham, Chicago, Minneapolis, Oakland), and years of education.
Analytical Sample
Of the 719 people who participated in the CARDIA Brain MRI Substudy, 699 had complete data on brain MRI. Of those, 51 did not undergo echocardiography at year 5 and thus were excluded, giving a sample of 648 participants. Information was available on LVEF for 297 participants, on LAV for 406 participants, and on LV mass for 627 participants (Figure). There were more black participants among those with available values for LVEF (P=0.02) and LAV (P=0.03; Table S1). Given that data on LV mass were not available in only 21 individuals, the differences between those who had data and those who did not were not assessed. Compared with the rest of the population that underwent the follow‐up exam at year 25 but were not included in the analysis, the final analytical sample comprised participants with a lower burden of vascular risk factors and fewer black participants (Table S2).
Figure 1.
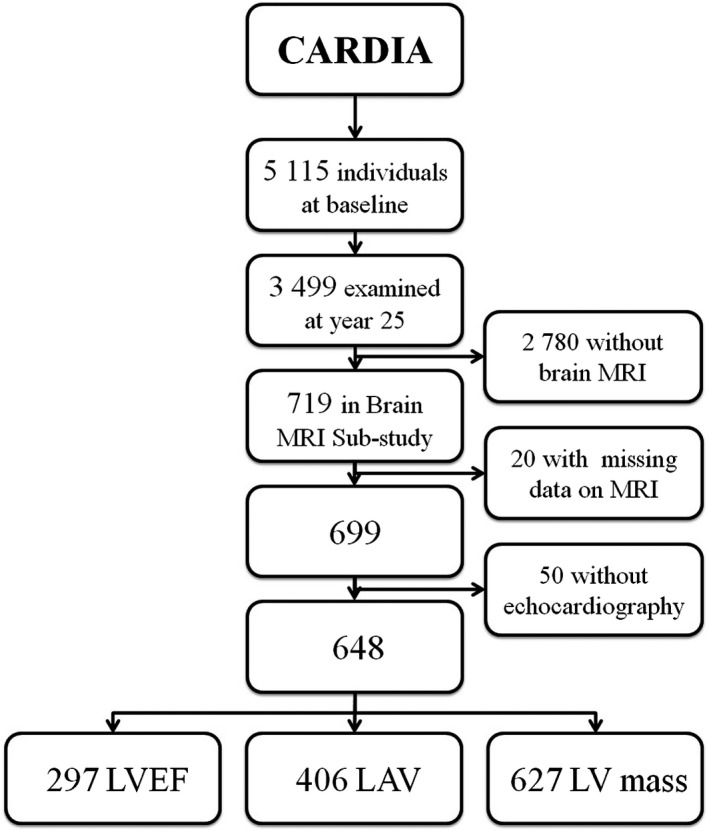
Selection of the study population: CARDIA (Coronary Artery Risk Development in Young Adults) Brain MRI Substudy. LAV indicates left atrial volume; LV mass, left ventricular mass; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging.
Statistical Analyses
Descriptive data of the study population as presented as mean±SD, median (25th–75th percentiles), or frequency (percentage). Linear regression analysis was applied to investigate associations of the echocardiographic parameters with WMFA, total brain volume, gray matter volume, and white matter volume. The fit of the models was assessed for homoscedasticity and normality of the error distribution. Multinomial logistic regression analysis was used to estimate odds ratios with 95% confidence intervals (CIs) for associations of the echocardiographic parameters with high and low AWM volume compared with no AWM. Echocardiographic parameters were used in all models after transformation into z scores.
The analyses were controlled for confounders in 2 steps. Model 1 was adjusted for age, sex, race/ethnicity, years of education, field center, and total intracranial volume. Model 2 was also adjusted for SBP, DBP, BMI, total cholesterol, fasting blood glucose, smoking status, sedentary behavior, alcohol intake, and the APOE ɛ4 allele.
In sensitivity analyses, we separately repeated the analyses of interest stratified by race/ethnicity, sex, and target blood pressure level of SBP/DBP ≥140/90 versus lower and SBP/DBP ≥120/80 versus lower; these factors in particular may influence the relationship between cardiac parameters and brain measures.12, 13, 24 Furthermore, we reran the analyses of interest for those individuals who had complete measures of LVEF (n=297) and LAV (n=406). Because data on LV mass were missing in only 21 people, we did not perform the sensitivity analysis in the sample of individuals specifically with measurements of LV mass. All analyses were performed using SPSS software version 22 (IBM Corp).
Results
Participants were, on average, 30 years old when they underwent echocardiography and 50 years old when they participated in the CARDIA Brain MRI Substudy (Table 1). There were 339 women (52%) and 244 black participants (38%). When compared with white participants, black participants were younger, were less educated, had higher blood pressure and BMI, spent more sedentary time, and were more frequently current smokers. Men were less educated and had higher blood pressure relative to women (Table S3).
Among the 406 participants with LAV measures, higher LAV was significantly associated with lower WMFA controlled for age, sex, race/ethnicity, years of education, field center, and intracranial volume (ß=−0.003; 95% CI, −0.004 to −0.001; Table 2, model 1). This association remained statistically significant after adjusting for SBP, DBP, BMI, total cholesterol, fasting blood glucose, sedentary time, smoking status, alcohol intake, and the APOE ɛ4 allele (ß=−0.002; 95% CI, −0.004 to −0.0004; Table 2, model 2).
Table 2.
Associations of Cardiac Parameters With WMFA: CARDIA Brain MRI Substudy
| Cardiac Parameter | Model 1 | Model 2 | ||
|---|---|---|---|---|
| ß (95% CI) | P Value | ß (95% CI) | P Value | |
| LVEF, per SD (n=297) | −0.0003 (−0.002 to 0.002) | 0.80 | 0.0001 (−0.002 to 0.002) | 0.94 |
| LAV, per SD (n=406) | −0.003 (−0.004 to −0.001) | 0.004a | −0.002 (−0.004 to −0.0004) | 0.016a |
| LV mass, per SD (n=627) | −0.002 (−0.003 to 0.0001) | 0.06 | −0.001 (−0.003 to 0.0003) | 0.11 |
Each cardiac parameter was transformed into a z score and entered into each model separately. ß is the coefficient for an association of the cardiac parameter (per SD) with the measure of aging brain. Model 1 is adjusted for age, sex, race/ethnicity, field center, years of education, and intracranial volume. Model 2 is adjusted for age, sex, race, field center, years of education, intracranial volume, systolic blood pressure, diastolic blood pressure, body mass index, total cholesterol, fasting plasma glucose, smoking status, sedentary time, alcohol intake, and APOE ɛ4 allele. CI indicates confidence interval; CARDIA, Coronary Artery Risk Development in Young Adults; LAV, left atrial volume; LV mass, left ventricular mass; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; WMFA, white matter fractional anisotropy.
Statistically significant at P<0.05.
When stratified by race/ethnicity, LAV was associated with lower WMFA only in black participants, independent of all potential confounders (ß=−0.004; 95% CI, −0.007 to −0.001; Table 3, model 2). When stratified by sex, LAV was associated with WMFA only in men (ß=−0.003; 95% CI, −0.005 to −0.0003; Table 3, model 2). There were no differences in association by level of blood pressure between LAV and WMFA (not shown). LAV was associated with lower WMFA in participants with available measurements of LVEF (n=289; model 1: β=−0.0022 [95% CI, −0.004 to −0.0001]; P=0.04; not shown). LVEF and LV mass were not associated with WMFA. In addition, none of the cardiac parameters were associated with gray matter, white matter, total brain volume, or AWM volume (Table S4).
Table 3.
Associations of LAV With WMFA Stratified by Race/Ethnicity and Sex: CARDIA Brain MRI Substudy
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| ß (95% CI) | P Value | ß (95% CI) | P Value | |
| Race/ethnicity | ||||
| White (n=240) | −0.001 (−0.004 to 0.001) | 0.25 | −0.001 (−0.003 to 0.001) | 0.42 |
| Black (n=166) | −0.004 (−0.007 to −0.001) | 0.004a | −0.004 (−0.007 to −0.001) | 0.007a |
| Sex | ||||
| Male (n=197) | −0.003 (−0.006 to −0.001) | 0.003a | −0.003 (−0.005 to −0.0003) | 0.026a |
| Female (n=209) | −0.002 (−0.005 to 0.001) | 0.21 | −0.002 (−0.005 to 0.001) | 0.23 |
ß is the coefficient for an association of left atrial volume (per SD) with WMFA. When stratified by race/ethnicity, model 1 is adjusted for age, sex, field center, years of education, and intracranial volume; model 2 is also adjusted for systolic blood pressure, diastolic blood pressure, BMI, total cholesterol, fasting plasma glucose, smoking status, sedentary time, alcohol intake, and APOE ɛ4 allele. When stratified by sex, model 1 is adjusted for age, race/ethnicity, field center, years of education, and intracranial volume; model 2 is also adjusted for systolic blood pressure, diastolic blood pressure, BMI, total cholesterol, fasting plasma glucose, smoking status, sedentary time, alcohol intake, and APOE ɛ4 allele. BMI indicates body mass index; CI, confidence interval; CARDIA, Coronary Artery Risk Development in Young Adults; LAV, left atrial volume; MRI, magnetic resonance imaging; WMFA, white matter fractional anisotropy.
Statistically significant at P<0.05.
Discussion
We investigated whether markers of cardiac function assessed at age 30 years by echocardiography were linked to indicators of brain health measured by MRI on average 20 years later. We found that higher LAV was associated with lower WMFA, particularly in black men. The association extends the effect of traditional vascular risk factors and indicates an independent relationship between the size of the left atrium and the integrity of the white matter. This study suggests that cardiac function in young adulthood may influence brain health later in life. This finding is of interest in the context of identifying early markers of a potentially increased risk for later‐life cognitive decline and dementia.
The association of LAV with WMFA is indicative of a modest effect of subclinical cardiac dysfunction in young adulthood on the brain 25 years later. Specifically, this study suggests that increase in 1 standardized unit of LAV may result in a decrease in WMFA by 0.002 on a scale between 0 and 1. This effect size is smaller than the magnitude of the associations of age and traditional vascular risk factors on WMFA that were previously studied in this middle‐aged population.17 For example, WMFA was reported to decline by 0.008 with every increasing mm Hg of SBP and DBP and by 0.03 with every additional year of age.17 The aggregation of several risk factors is likely a key factor leading to clinically meaningful brain damage.24 Lower WMFA may be an early sign of microvascular pathology in the white matter as it may precede the development of white matter lesions, which increase the risk of future cognitive impairment and dementia.28
Higher LAV relates to LV filling pressures and is associated with diastolic dysfunction, reflecting long‐term exposure to vascular risk factors, particularly high blood pressure and obesity.19 Enlargement of the left atrium was previously linked to ischemic lesions, loss of brain tissue, and cognitive impairment in older patients.11, 29, 30 In previous studies on older adults, LVEF and LV mass were associated with indicators of brain pathology11; we did not find this in this younger cohort, possibly because other markers of cardiac disease occur later.31
Recent studies report that patients with dementia are predominantly affected by heart failure with preserved ejection fraction and diastolic cardiac dysfunction, indicating its relevance for and possible influence on brain health.32, 33 A new paradigm of heart failure with preserved ejection fraction proposed that conditions such as high blood pressure, obesity, diabetes mellitus, and other comorbidities induce a systemic inflammatory state.34 This affects the coronary endothelium and triggers its dysfunction, which contributes to cardiac remodeling and eventually results in diastolic heart failure.34
We propose following mechanisms that can explain the association of LAV with WMFA. First, the state of chronic inflammation and immune response, as likely present in our study sample, may induce endothelial dysfunction and damage of the blood–brain barrier, possibly leading to increased permeability and failure in maintaining microcirculation in the white matter. Second, because increased LAV strongly correlates with atrial dysfunction and atrial fibrillation,19 lower WMFA may be a result of microembolism or reduced brain perfusion due to insufficient cardiac output in the setting of silent atrial fibrillation. We were unable to test this possibility because the numbers of clinical events of atrial fibrillation are too low for meaningful analysis in this relatively young cohort.35
This study uniquely includes a well‐characterized sample of participants who have been followed up for 25 years, with echocardiograms in young adulthood and state‐of‐the‐art brain imaging in midlife. Another strength is the biracial nature of the sample; black patients have been underrepresented in previous studies investigating the heart–brain axis. This study has several limitations. One is the varying number of participants with values on LVEF and LAV; however, we assessed whether the smaller samples showed associations similar to those based on larger samples.
Another limitation is that the study sample was a healthier group and included fewer black participants than the total CARDIA cohort. Furthermore, participants who had more severe cardiac dysfunction in young adulthood12, 18 were underrepresented in our final analytical sample. This could have created selection bias from demographic and clinical characteristics of the participants. If the associations of cardiac parameters with brain measures are similar in those included and excluded from the analyses, this could lead to underestimation of the association between LAV and WMFA that we found in the studied sample. Moreover, there is a possibility that we did not account for all confounders, and it is unknown how factors not included in the analyses, such as treatment for vascular risk factors, affected the outcome.
Previous studies suggest that black persons are disproportionately burdened by heart failure and dementia.12, 36 The present study indicates that young black men in particular may be at risk for compromised brain health due to cardiac dysfunction. Because an enlarged left atrium is associated with previous chronic exposure to vascular risk factors, especially elevated blood pressure and obesity,19 we propose that interventions aimed at their reduction to improve cardiac function may also benefit brain health. We suggest that such interventions should be targeted early in life and may have the largest benefit for black men.
Sources of Funding
The CARDIA (Coronary Artery Risk Development in Young Adults) study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra‐agency agreement between NIA and NHLBI (AG0005). Cermakova was supported by the Alzheimer Foundation–Czech Republic, the Swedish Research Council (grant 2012‐2291), and project “Sustainability for the National Institute of Mental Health” (grant LO1611), with a financial support from the Ministry of Education, Youth and Sports of the Czech Republic. Religa was supported by the Swedish Research Council (grant 2012‐2291). This article was reviewed by CARDIA for scientific content.
Disclosures
None.
Supporting information
Table S1. Among Those in the CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy, Differences in the Analytical Sample Between Participants With and Without Available Measurements of Left Ventricular Ejection Fraction and Left Atrial Volume
Table S2. Differences Between the Study Sample and Other Participants at Year 25: CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy
Table S3. Differences Between White and Black Participants and Between Men and Women: CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy
Table S4. Associations of Cardiac Parameters With Brain Volumes: CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy
(J Am Heart Assoc. 2017;6:e006750 DOI: 10.1161/JAHA.117.006750.)29246962
References
- 1. Cermakova P, Eriksdotter M, Lund LH, Winblad B, Religa P, Religa D. Heart failure and Alzheimer's disease. J Intern Med. 2015;277:406–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adelborg K, Horvath‐Puho E, Ording A, Pedersen L, Toft Sorensen H, Henderson VW. Heart failure and risk of dementia: a Danish nationwide population‐based cohort study. Eur J Heart Fail. 2017;19:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huijts M, van Oostenbrugge RJ, Duits A, Burkard T, Muzzarelli S, Maeder MT, Schindler R, Pfisterer ME, Brunner‐La Rocca HP. Cognitive impairment in heart failure: results from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME‐CHF) randomized trial. Eur J Heart Fail. 2013;15:699–707. [DOI] [PubMed] [Google Scholar]
- 4. van den Hurk K, Reijmer YD, van den Berg E, Alssema M, Nijpels G, Kostense PJ, Stehouwer CD, Paulus WJ, Kamp O, Dekker JM, Biessels GJ. Heart failure and cognitive function in the general population: the Hoorn Study. Eur J Heart Fail. 2011;13:1362–1369. [DOI] [PubMed] [Google Scholar]
- 5. de Bruijn RF, Portegies ML, Leening MJ, Bos MJ, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW, Franco OH, Koudstaal PJ, Ikram MA. Subclinical cardiac dysfunction increases the risk of stroke and dementia: the Rotterdam Study. Neurology. 2015;84:833–840. [DOI] [PubMed] [Google Scholar]
- 6. Zonneveld HI, Ikram MA, Hofman A, Niessen WJ, van der Lugt A, Krestin GP, Franco OH, Vernooij MW. N‐terminal pro‐B‐type natriuretic peptide and subclinical brain damage in the general population. Radiology. 2017;283:205–214. [DOI] [PubMed] [Google Scholar]
- 7. Sabayan B, van Buchem MA, Sigurdsson S, Zhang Q, Harris TB, Gudnason V, Arai AE, Launer LJ. Cardiac hemodynamics are linked with structural and functional features of brain aging: the age, gene/environment susceptibility (AGES)‐Reykjavik Study. J Am Heart Assoc. 2015;4:e001294 DOI: 10.1161/JAHA.114.001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O'Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O'Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am J Cardiol. 2011;108:1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 11. Arangalage D, Ederhy S, Dufour L, Joffre J, Van der Vynckt C, Lang S, Tzourio C, Cohen A. Relationship between cognitive impairment and echocardiographic parameters: a review. J Am Soc Echocardiogr. 2015;28:264–274. [DOI] [PubMed] [Google Scholar]
- 12. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR Jr, Sidney S, Wu CO, Cook NL, Lewis CE, Schreiner PJ, Isogawa A, Liu K, Lima JA. Race‐ethnic and sex differences in left ventricular structure and function: the coronary artery risk development in young adults (CARDIA) study. J Am Heart Assoc. 2015;4:e001264 DOI: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. CARDIA . CARDIA Coordinating Center. 2015. Available at: http://www.cardia.dopm.uab.edu/contact-cardia. Accessed March 01, 2015.
- 15. National Heart, Lung and Blood Institute . Biospecimen and Data Resources. 2015. Available at: https://biolincc.nhlbi.nih.gov/home/. Accessed March 01, 2015.
- 16. CARDIA . NHLBI Data Repository. 2015. Available at: http://www.cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data. Accessed March 01, 2015.
- 17. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 18. Desai CS, Colangelo LA, Liu K, Jacobs DR Jr, Cook NL, Lloyd‐Jones DM, Ogunyankin KO. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol. 2013;177:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 20. de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–747. [DOI] [PubMed] [Google Scholar]
- 21. Launer LJ, Lewis CE, Schreiner PJ, Sidney S, Battapady H, Jacobs DR, Lim KO, D'Esposito M, Zhang Q, Reis J, Davatzikos C, Bryan RN. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong Q, Welsh RC, Chenevert TL, Carlos RC, Maly‐Sundgren P, Gomez‐Hassan DM, Mukherji SK. Clinical applications of diffusion tensor imaging. J Magn Reson Imaging. 2004;19:6–18. [DOI] [PubMed] [Google Scholar]
- 23. Deckers K, van Boxtel MPJ, Schiepers OJG, de Vugt M, Sanchez JLM, Anstey KJ, Brayne C, Dartigues JF, Engedal K, Kivipelto M, Ritchie K, Starr JM, Yaffe K, Irving K, Verhey FRJ, Kohler S. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. [DOI] [PubMed] [Google Scholar]
- 24. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age‐related cognitive decline. Nat Rev Cardiol. 2015;12:267–277. [DOI] [PubMed] [Google Scholar]
- 25. Bild DE, Sholinsky P, Smith DE, Lewis CE, Hardin JM, Burke GL. Correlates and predictors of weight loss in young adults: the CARDIA study. Int J Obes Relat Metab Disord. 1996;20:47–55. [PubMed] [Google Scholar]
- 26. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamboh MI, Ferrell RE, Sepehrnia B. Genetic studies of human apolipoproteins. IV. Structural heterogeneity of apolipoprotein H (beta 2‐glycoprotein I). Am J Hum Genet. 1988;42:452–457. [PMC free article] [PubMed] [Google Scholar]
- 28. de Groot M, Verhaaren BF, de Boer R, Klein S, Hofman A, van der Lugt A, Ikram MA, Niessen WJ, Vernooij MW. Changes in normal‐appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–1042. [DOI] [PubMed] [Google Scholar]
- 29. Karadag B, Ozyigit T, Ozben B, Kayaoglu S, Altuntas Y. Relationship between left atrial volume index and cognitive decline in elderly patients with sinus rhythm. J Clin Neurosci. 2013;20:1074–1078. [DOI] [PubMed] [Google Scholar]
- 30. Oh JE, Shin JW, Sohn EH, Jung JO, Jeong SH, Song HJ, Kim JM, Lee AY. Effect of cardiac function on cognition and brain structural changes in dementia. J Clin Neurol. 2012;8:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong AC, Liu K, Lewis CE, Sidney S, Colangelo LA, Kishi S, Ambale‐Venkatesh B, Arynchyn A, Jacobs DR Jr, Correia LC, Gidding SS, Lima JA. Left atrial dimension and traditional cardiovascular risk factors predict 20‐year clinical cardiovascular events in young healthy adults: the CARDIA study. Eur Heart J Cardiovasc Imaging. 2014;15:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cermakova P, Lund LH, Fereshtehnejad SM, Johnell K, Winblad B, Dahlstrom U, Eriksdotter M, Religa D. Heart failure and dementia: survival in relation to types of heart failure and different dementia disorders. Eur J Heart Fail. 2015;17:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Troncone L, Luciani M, Coggins M, Wilker EH, Ho CY, Codispoti KE, Frosch MP, Kayed R, Del Monte F. Abeta amyloid pathology affects the hearts of patients with Alzheimer's disease: mind the heart. J Am Coll Cardiol. 2016;68:2395–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 35. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Among Those in the CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy, Differences in the Analytical Sample Between Participants With and Without Available Measurements of Left Ventricular Ejection Fraction and Left Atrial Volume
Table S2. Differences Between the Study Sample and Other Participants at Year 25: CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy
Table S3. Differences Between White and Black Participants and Between Men and Women: CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy
Table S4. Associations of Cardiac Parameters With Brain Volumes: CARDIA (Coronary Artery Risk Development in Young Adults) Brain Magnetic Resonance Imaging Substudy


