Abstract
Background
The association of short‐term variability of fasting plasma glucose (FPG) and mortality has been well investigated. However, the relationships between visit‐to‐visit variability of FPG over longer periods of follow‐up and cardiovascular disease (CVD) and all‐cause mortality are unclear. This study aimed to investigate these relationships.
Methods and Results
The current analysis included 53 607 Chinese participants (mean age, 49.10 years) who were free of CVD in the Kailuan study. Participants were divided into 4 categories by quartiles of visit‐to‐visit variability of FPG. Visit‐to‐visit variability of FPG was defined as the coefficient of variation of 3 values of FPG that were measured from the examination periods of 2006 to 2007, 2008 to 2009, and 2010 to 2011. Cox proportional hazards models were used to calculate hazard ratios and 95% confidence intervals for CVD and all‐cause mortality. After a mean follow‐up of 4.93 years, 4261 individuals developed CVD and 1545 individuals died. The incidence of CVD and all‐cause mortality was 5.04 and 5.85 per 1000 person‐years, respectively. After adjusting for mean FPG and other potential confounders, individuals in the highest quartile of variability of FPG compared with participants in the lowest quartile showed a 26% greater risk of developing CVD (hazard ratio, 1.26; 95% confidence interval, 1.08–1.47) and a 46% greater risk for all‐cause mortality (hazard ratio, 1.46; 95% confidence interval, 1.25–1.70).
Conclusions
Independent of mean FPG and other baseline parameters, elevated visit‐to‐visit variability of FPG significantly increases the risk of CVD and all‐cause mortality in the general population. Measuring long‐term visit‐to‐visit variability of FPG is helpful for predicting the risk for CVD and all‐cause mortality.
Keywords: all‐cause mortality, cardiovascular events, death, glucose, variability, visit‐to‐visit
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Primary Prevention, Mortality/Survival
Clinical Perspective
What Is New?
Elevated visit‐to‐visit variability of fasting plasma glucose independently increases the risk of cardiovascular disease and all‐cause mortality in the general population.
The associations between variability of fasting plasma glucose and cardiovascular disease and all‐cause mortality are undifferentiated in individuals with and without diabetes mellitus.
What Are the Clinical Implications?
In predicting the risk for cardiovascular disease and all‐cause mortality in the general population, measuring long‐term visit‐to‐visit variability of fasting plasma glucose is helpful.
Introduction
Diabetes mellitus is highly prevalent worldwide. The presence of diabetes mellitus increases the incidence of cardiovascular diseases (CVDs) and risk of death. This represents a huge burden on health care and social economy.1 Management of fasting plasma glucose (FPG) levels associated with incident CVD and all‐cause mortality in the general population is an ongoing challenge for primary health care decision makers. Indeed, people with excessive high or low glucose levels have a high risk of CVD and all‐cause mortality.2, 3, 4, 5, 6, 7 Therefore, the main reason for CVD is thought to be not only chronic hyperglycemia or other traditional risk factors, but also frequent hypoglycemic and hyperglycemic episodes that accompany the disease's daily course. A new cardiovascular risk factor (ie, excessive glycemic variability) has been postulated.
Recent studies and a systematic review have shown that glycemic variability might play an important role in the pathogenesis of atherosclerosis, and may be an independent risk factor for ischemic stroke8 and all‐cause mortality in those with type 2 diabetes mellitus.9, 10 However, these previous studies on glycemic variability were all short‐term within 1 year or less and investigated in patients with type 2 diabetes mellitus. No literature had reported the prognostic significance of long‐term variability of FPG in the general population. Studies have demonstrated that elevated glycemic variability appears more important than admission glucose level and prior long‐term abnormal glycometabolic status in predicting 1‐year major adverse cardiac events in patients with acute myocardial infarction.11 Furthermore, availability of a pooled risk estimate for variability of FPG may help to determine the real high‐risk population and to design future studies for therapy. Therefore, to address these gaps in knowledge, we examined the prospective association between visit‐to‐visit variability (over 4 years) of FPG and incident CVD and all‐cause mortality in the general population during a median 4.93 years of follow‐up.
Methods
Study Design and Participants
The Kailuan study is a prospective cohort study that was conducted in the Kailuan community in Tangshan City, China. The detailed study design and characteristics of the study population have been described previously.12 From June 2006 to October 2007, 101 510 (81 110 men and 20 400 women; aged 18–98 years) employees (including the retired) in the community agreed to enroll in the Kailuan study. Participants underwent health examinations biennially until December 31, 2015. We excluded 3669 participants who were diagnosed as having preexisting myocardial infarction or stroke. A total of 54 398 individuals who participated in the 2 biennial follow‐up examinations of 2008 to 2009 and 2010 to 2011 and had complete FPG data were included. In addition, we also excluded 791 individuals who developed myocardial infarction or stroke before the 2010 to 2011 follow‐up. Finally, the remaining 53 607 participants who were free of CVD were included in the analysis (Figure 1). The protocol for the study was approved by the Ethics Committee of Kailuan General Hospital in compliance with the Declaration of Helsinki. All participants provided informed written consent with their signatures.
Figure 1.

Flow chart of the present study.
Calculation of Long‐Term Variability of FPG
Visit‐to‐visit variability of FPG was defined as the coefficient of variation of 3 values of FPG that were measured from the examination periods of 2006 to 2007, 2008 to 2009, and 2010 to 2011. The coefficient of variation was the ratio of the standard deviation/the mean FPG.
Assessment of Potential Covariates
Demographic and clinical characteristics, including age, sex, alcohol use, education, and a history of disease, were collected via questionnaires. Educational attainment was categorized as illiteracy or primary school, middle school, and high school or above. Physical activity was classified as ≥4 times per week and ≥20 minutes at a time, <80 minutes per week, and none. Smoking status and drinking status were classified as never, former, or current, according to self‐reported information. Body mass index was calculated as kilograms per meter squared. Systolic blood pressure and diastolic blood pressure were measured 3 times in the seated position using a mercury sphygmomanometer. All blood samples were tested using a Hitachi 747 autoanalyzer (Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan General Hospital. FPG, triglyceride, total cholesterol, high‐density lipoprotein, low‐density lipoprotein, and serum creatinine levels were measured. The baseline estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.13
Incidence of CVD and All‐Cause Mortality
CVD included myocardial infarction, cerebral infarction, and cerebral hemorrhage. Each participant could contribute only 1 end point if he or she had >1 end points, as the first diagnosis of myocardial infarction, cerebral infarction, or cerebral hemorrhage. CVD in the Kailuan study was ascertained by surveying each year's discharge lists from local hospitals and death certificates from state vital statistics offices. CVD was also identified by contacting participants annually for a history of CVD. The criteria for myocardial infarction were based on combinations of chest pain symptoms, electrocardiographic changes, and cardiac enzyme levels.14 Stroke was diagnosed according to the World Health Organization criteria15 combined with brain computed tomography or magnetic resonance imaging for confirmation. Stroke was classified into 3 types: cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage. Neuroimages were reviewed by a single physician, with disagreements resolved by a second physician. Information on death was collected from death certificates from state vital statistics offices. We used the underlying cause of death International Classification of Diseases, Tenth Revision (ICD‐10), code on the Vital Statistics system to group causes of death.
Statistical Analyses
Continuous variables are described as mean±SD and were compared by ANOVA or Kruskal‐Wallis tests. Categorical variables are described as percentage and were compared using the χ2 tests. χ2 Tests for trend and Spearman rank correlation tests were used to test for a relationship between categorical or continuous baseline characteristics and quartiles of variability of FPG. Univariate survival analysis was performed by the Kaplan‐Meier method and the log‐rank test. Person‐years were calculated from the date that the 2010 interview was conducted to the date when the first occurrence of CVD was detected, date of death, or date of participating in the last interview in this analysis, whichever came first.
Cox proportional hazards regression was used to estimate the risk of CVD and all‐cause mortality by calculating the hazard ratios (HRs) and 95% confidence intervals. All participants were classified into 4 groups by quartiles of variability of FPG: quartile 1 (<5.27 mmol/L), quartile 2 (5.27–8.48 mmol/L), quartile 3 (8.49–13.11 mmol/L), and quartile 4 (>13.11 mmol/L). Model 1 was adjusted for age and sex at baseline and mean FPG of 3 time measurements. Model 2 was further adjusted for level of education, income, smoking, alcohol abuse, physical activity, and body mass index at baseline. Model 3 was further adjusted for a history of diabetes mellitus, hypertension, and dyslipidemia, glucose‐lowering treatment, and estimated glomerular filtration rate at baseline. For each model, a trend test was performed after the median value of each quintile was entered into the model and treated as a continuous variable. All these models were tested for proportionality assumption using scaled Schoenfeld residuals, and no appreciable violations were noted.
Stratified analyses were conducted among the following 3 groups: group of no diabetes mellitus, with FPG <5.6 mmol/L; group of no diabetes mellitus, with 5.6≤FPG<7.0 mmol/L; and group of diabetes mellitus. Diabetes mellitus was defined as any self‐reported diabetes mellitus or use of glucose‐lowering drugs or FPG ≥7 mmol/L at any examination of 2006 to 2007, 2008 to 2009, or 2010 to 2011. Interaction of variability of FPG and diabetes mellitus status was examined by adding their product terms into the full model using the likelihood ratio test for significance. Statistical analyses were performed using SAS 9.4. All statistical tests were 2 sided, and the significance level was set at 0.05.
Results
A total of 53 607 participants were eligible for inclusion in this study. The mean age was 49.10±11.85 years. The mean FPG level was 5.55±1.39 mmol/L. Individuals with a higher variability of FPG than those with a lower variability of FPG tended to be older, men, and current smokers; had a lower proportion of current drinkers; had a lower education level and monthly income; were more prevalent to perform active physical activity; had a higher prevalence of hypertension, diabetes mellitus, and dyslipidemia; had a higher body mass index, systolic and diastolic blood pressures, and mean FPG, triglyceride, and total cholesterol levels; and had a higher proportion of using glucose‐lowering treatment (Table 1).
Table 1.
Baseline Characteristics Grouped by Quartiles of Variability of Fasting Plasma Glucose Levels
| Variable | Overall | Quartiles of Variability of Fasting Plasma Glucose | P Value for Trend | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Quartile range, mmol/L | ≤5.27 | 5.27–8.49 | 8.49–13.11 | >13.11 | ||
| No. of participants | 53 607 | 13 401 | 13 402 | 13 402 | 13 402 | |
| Age, y | 49.10±11.85 | 47.65±11.68 | 48.22±11.92 | 49.59±12.01 | 50.95±11.50 | <0.01 |
| Male sex, n (%) | 40 937 (76.37) | 9878 (73.71) | 10 081 (75.22) | 10 388 (77.51) | 10 590 (79.02) | <0.01 |
| High school or above, n (%) | 12 211 (22.78) | 3386 (25.27) | 3217 (24.00) | 3038 (22.67) | 2570 (19.18) | <0.01 |
| Income >800 Renminbi/month, n (%) | 8011 (14.94) | 2027 (15.13) | 2063 (15.39) | 2050 (15.30) | 1871 (13.96) | <0.01 |
| Current smoker, n (%) | 18 034 (33.64) | 4373 (32.63) | 4471 (33.36) | 4615 (34.44) | 4575 (34.14) | <0.01 |
| Current alcohol use, n (%) | 20 816 (38.83) | 5204 (38.83) | 5321 (39.70) | 5284 (39.43) | 5007 (37.36) | 0.01 |
| Active physical activity, n (%) | 7310 (13.64) | 1582 (11.81) | 1780 (13.28) | 1932 (14.42) | 2016 (15.04) | <0.01 |
| Hypertension, n (%) | 5520 (10.30) | 1062 (7.92) | 1237 (9.23) | 1446 (10.79) | 1775 (13.24) | <0.01 |
| Diabetes mellitus, n (%) | 1343 (2.51) | 115 (0.86) | 153 (1.14) | 247 (1.84) | 828 (6.18) | <0.01 |
| Dyslipidemia, n (%) | 2986 (5.57) | 604 (4.51) | 702 (5.24) | 781 (5.83) | 899 (6.71) | <0.01 |
| Body mass index, kg/m2 | 25.07±3.48 | 24.84±3.44 | 24.90±3.44 | 25.03±3.42 | 25.49±3.57 | <0.01 |
| Systolic blood pressure, mm Hg | 128.30±19.80 | 125.97±19.18 | 127.08±19.48 | 128.63±19.68 | 131.52±20.42 | <0.01 |
| Diastolic blood pressure, mm Hg | 82.52±11.34 | 81.50±11.15 | 81.93±11.25 | 82.62±11.24 | 84.04±11.58 | <0.01 |
| Fasting plasma glucose, mmol/L | 5.39±1.54 | 5.23±0.78 | 5.22±0.92 | 5.22±1.19 | 5.89±2.50 | 0.03 |
| Mean fasting plasma glucose, mmol/L | 5.55±1.39 | 5.25±0.77 | 5.30±0.88 | 5.39±1.09 | 6.24±2.14 | <0.01 |
| Total cholesterol, mmol/L | 4.92±1.13 | 4.91±1.04 | 4.91±1.09 | 4.93±1.11 | 4.94±1.26 | <0.01 |
| Triglycerides, mmol/L, | 1.68±1.36 | 1.55±1.23 | 1.60±1.28 | 1.68±1.33 | 1.88±1.56 | <0.01 |
| Low‐density lipoprotein, mmol/L | 2.29±0.90 | 2.29±0.81 | 2.30±0.88 | 2.29±0.90 | 2.27±1.02 | 0.63 |
| High‐density lipoprotein, mmol/L | 1.55±0.39 | 1.55±0.38 | 1.54±0.38 | 1.56±0.40 | 1.55±0.42 | 0.61 |
| Estimated glomerular filtration rate, mL/min | 84.13±25.10 | 84.26±24.68 | 84.32±24.44 | 83.77±22.67 | 84.17±28.29 | 0.14 |
| Glucose‐lowering treatment, n (%) | 2737 (5.11) | 214 (1.60) | 274 (2.04) | 467 (3.48) | 1782 (13.30) | <0.01 |
Data are given as mean±SD unless otherwise indicated.
A total of 47 920 participants were excluded. Compared with participants, the nonparticipants were older, predominantly men, less educated, and had lower monthly incomes; had a lower proportion of current smokers and current alcohol use, but more frequently performed physical activity; had a higher prevalence of hypertension, diabetes mellitus, and dyslipidemia; had higher baseline systolic and diastolic blood pressures and FPG, total cholesterol, and low‐density lipoprotein levels; and had a lower baseline high‐density lipoprotein level and estimated glomerular filtration rate (Table 2).
Table 2.
Comparison of Baseline Characteristics Between Participants and Nonparticipants
| Variables | Participants | Nonparticipants | P Value |
|---|---|---|---|
| No. of participants | 53 607 | 47 902 | |
| Age, y | 49.10±11.85 | 55.10±12.80 | <0.01 |
| Male sex, n (%) | 40 937 (76.37) | 40 164 (83.85) | <0.01 |
| High school or above, n (%) | 12 211 (22.78) | 7400 (15.45) | <0.01 |
| Income ≥800 Renminbi/month, n (%) | 8011 (14.94) | 6004 (12.53) | <0.01 |
| Current smoker, n (%) | 18 034 (33.64) | 15 761 (32.90) | 0.01 |
| Current alcohol use, n (%) | 20 816 (38.83) | 15 836 (33.06) | <0.01 |
| Active physical activity, n (%) | 7310 (13.64) | 7971 (16.64) | <0.01 |
| Hypertension, n (%) | 5520 (10.30) | 7484 (15.62) | <0.01 |
| Diabetes mellitus, n (%) | 1343 (2.51) | 1907 (3.98) | <0.01 |
| Dyslipidemia, n (%) | 2986 (5.57) | 3274 (6.83) | <0.01 |
| Body mass index, kg/m2 | 25.07±3.48 | 25.02±3.51 | 0.08 |
| Systolic blood pressure, mm Hg | 128.30±19.80 | 134.22±22.02 | <0.01 |
| Diastolic blood pressure, mm Hg | 82.52±11.34 | 84.59±12.17 | <0.01 |
| Fasting plasma glucose, mmol/L | 5.55±1.39 | 5.67±1.76 | <0.01 |
| Total cholesterol, mmol/L | 4.92±1.13 | 4.98±1.17 | <0.01 |
| Triglycerides, mmol/L, | 1.68±1.36 | 1.68±1.38 | 0.10 |
| Low‐density lipoprotein, mmol/L | 2.29±0.90 | 2.41±0.92 | <0.01 |
| High‐density lipoprotein, mmol/L | 1.55±0.39 | 1.54±0.41 | <0.01 |
| Estimated glomerular filtration rate, mL/min | 84.13±25.10 | 79.38±26.02 | <0.01 |
Data are mean±SD unless otherwise indicated.
After a mean follow‐up of 4.93 years, 4261 individuals developed CVD and 1545 individuals died. The incidence of CVD and all‐cause mortality was 5.04 and 5.85 per 1000 person‐years, respectively, and it significantly increased with a higher variability of FPG. From the lowest variability of FPG quartile to the second, third and highest quartiles, the incidence of CVD increased from 3.86 to 4.29, 4.82, and 7.18 per 1000 person‐years, respectively. The incidence of all‐cause mortality increased from 3.95 to 5.08, 5.78, and 8.59 per 1000 person‐years, respectively (Table 3). Table 2 shows HRs of CVD and all‐cause mortality grouped by quartiles of variability of FPG. Compared with participants with the lowest quartile, after adjustment for potential confounding factors, the HRs (95% confidence intervals) for CVD in the second, third, and highest quartiles of variability of FPG were 1.06 (0.89–1.25), 1.07 (0.91–1.27), and 1.26 (1.07–1.47), respectively. The adjusted HRs (95% confidence intervals) for all‐cause mortality in the second, third, and highest quartiles of variability of FPG were 1.18 (1.00–1.39), 1.20 (1.02–1.40), and 1.46 (1.25–1.70), respectively.
Table 3.
HRs of Cardiovascular Disease and All‐Cause Mortality Grouped by Quartiles of Variability of Fasting Plasma Glucose Levels
| Variable | HR (95% CI) for Quartiles of Variability of Fasting Plasma Glucose | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value for Trend | |
| Cardiovascular disease | |||||
| Case, n(%) | 255 (1.90) | 284 (2.12) | 319 (2.38) | 474 (3.54) | <0.01 |
| Incidence, per 1000 person‐y | 3.86 | 4.29 | 4.82 | 7.18 | |
| Cox regression models | |||||
| Model 1 | Reference | 1.06 (0.83–1.22) | 1.09 (0.92–1.28) | 1.33 (1.14–1.56) | <0.01 |
| Model 2 | Reference | 1.06 (0.90–1.26) | 1.09 (0.92–1.28) | 1.29 (1.10–1.51) | <0.01 |
| Model 3 | Reference | 1.06 (0.89–1.25) | 1.07 (0.91–1.27) | 1.26 (1.07–1.47) | <0.01 |
| All‐cause mortality | |||||
| Case, n(%) | 261 (1.95) | 336 (2.51) | 382 (2.85) | 566 (4.22) | <0.01 |
| Incidence, per 1000 person‐y | 3.95 | 5.08 | 5.78 | 8.59 | |
| Cox regression models | |||||
| Model 1 | Reference | 1.19 (1.01–1.39) | 1.19 (1.02–1.39) | 1.48 (1.27–1.72) | <0.01 |
| Model 2 | Reference | 1.19 (1.01–1.40) | 1.20 (1.03–1.41) | 1.49 (1.28–1.74) | <0.01 |
| Model 3 | Reference | 1.18 (1.00–1.39) | 1.20 (1.02–1.40) | 1.46 (1.25–1.70) | <0.01 |
Model 1, adjusted for age and sex at baseline and mean fasting plasma glucose of 3 time measurements. Model 2, adjusted for variables in model 1 plus level of education, income, smoking, alcohol abuse, physical activity, and body mass index at baseline. Model 3, adjusted for variables in model 2 plus a history of hypertension, diabetes mellitus, and dyslipidemia, glucose‐lowering treatment, and estimated glomerular filtration rate at baseline. CI indicates confidence interval; and HR, hazard ratio.
Figure 2A and 2B show the Kaplan‐Meier cumulative risk for CVD and all‐cause mortality within subgroups defined by variability of FPG. Individuals in the top quartile of variability of FPG experienced a higher risk than did participants of the other quartiles during the 4.93‐year follow‐up for CVD (log‐rank test, P<0.001; Figure 2A) and all‐cause mortality (log‐rank test, P<0.001; Figure 2B).
Figure 2.
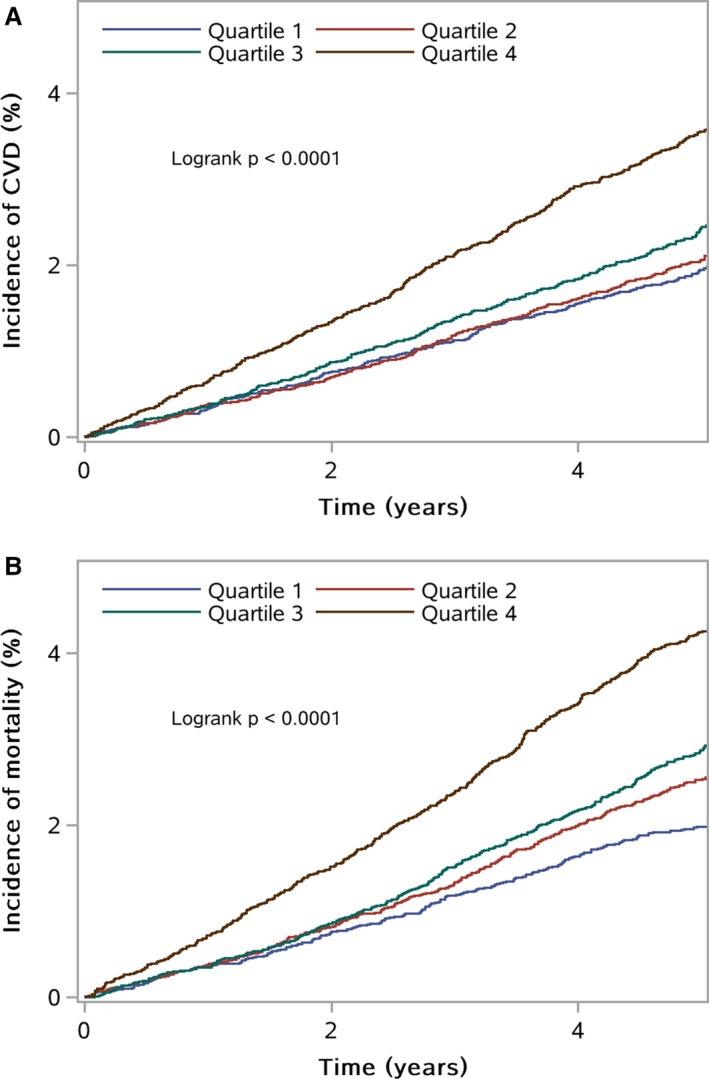
A, Kaplan‐Meier estimates of cardiovascular disease (CVD) grouped by quartiles of variability of fasting plasma glucose levels. B, Kaplan‐Meier estimates of survival probability grouped by quartiles of variability of fasting plasma glucose levels.
In our secondary analysis, Cox regression models were recalculated in participants of no diabetes mellitus with FPG <5.6 mmol/L, participants of no diabetes mellitus with 5.6≤FPG<7.0 mmol/L, and participants of diabetes mellitus (Table 4). In the group of no diabetes mellitus with FPG <5.6 mmol/L and another group of diabetes mellitus, variability of FPG was not associated with a risk of CVD events and all‐cause mortality. In the group of no diabetes mellitus with 5.6≤FPG<7.0 mmol/L, variability of FPG was associated with all‐cause mortality, but not associated with a risk of CVD events. In this group, the highest quartile group had an HR (95% confidence interval) of 1.55 (1.19–2.02) for all‐cause mortality in model 3 compared with the lowest quartile group. There was no interaction between variability of FPG and diabetes mellitus status for the risk for CVD and all‐cause mortality (P>0.05).
Table 4.
HRs of Cardiovascular Disease and All‐Cause Mortality, Grouped by Quartiles of Variability of FPG Levels in the Kailuan Study and Stratified by Diabetes Mellitus Status
| Variable | HR (95% CI) for Quartiles of Variability of FPG | |||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value for Trend | P Value for Interaction | |
| No diabetes mellitus, with FPG <5.6 mmol/L (n=24 970) | 6234 | 6251 | 6243 | 6242 | ||
| Cardiovascular diseases, case, n(%) | 100 (1.60) | 101 (1.62) | 111 (1.78) | 150 (2.40) | ||
| Incidence, per 1000 person‐y | 3.23 | 3.25 | 3.58 | 4.83 | <0.01 | |
| Cox regression models | ||||||
| Model 1 | Reference | 0.99 (0.75–1.31) | 1.03 (0.78–1.36) | 1.28 (0.98–1.69) | 0.07 | 0.83 |
| Model 2 | Reference | 1.00 (0.76–1.32) | 1.05 (0.80–1.38) | 1.27 (0.97–1.68) | 0.07 | 0.79 |
| Model 3 | Reference | 1.02 (0.77–1.34) | 1.05 (0.80–1.39) | 1.26 (0.96–1.66) | 0.09 | 0.81 |
| All‐cause mortality, case, n(%) | 114 (1.83) | 116 (1.86) | 153 (2.45) | 188 (3.01) | ||
| Incidence, per 1000 person‐y | 3.69 | 3.74 | 4.94 | 6.07 | <0.01 | |
| Cox regression models | ||||||
| Model 1 | Reference | 0.96 (0.74–1.24) | 1.10 (0.86–1.41) | 1.16 (0.90–1.48) | 0.14 | 0.86 |
| Model 2 | Reference | 0.96 (0.74–1.26) | 1.11 (0.86–1.42) | 1.18 (0.92–1.51) | 0.11 | 0.90 |
| Model 3 | Reference | 0.97 (0.75–1.25) | 1.10 (0.86–1.41) | 1.16 (0.90–1.49) | 0.15 | 0.92 |
| No diabetes mellitus, with 5.6≤FPG<7.0 mmol/L (n=20 650) | 5162 | 5165 | 5161 | 5162 | ||
| Cardiovascular diseases, case, n(%) | 124 (2.40) | 119 (2.30) | 105 (2.03) | 136 (2.63) | ||
| Incidence, per 1000 person‐y | 4.92 | 4.69 | 4.12 | 5.32 | 0.88 | |
| Cox regression models | ||||||
| Model 1 | Reference | 1.01 (0.78–1.30) | 0.91 (0.70–1.19) | 1.23 (0.94–1.60) | 0.24 | |
| Model 2 | Reference | 1.02 (0.79–1.32) | 0.92 (0.71–1.21) | 1.21 (0.93–1.58) | 0.28 | |
| Model 3 | Reference | 1.02 (0.79–1.31) | 0.91 (0.69–1.19) | 1.20 (0.91–1.56) | 0.34 | |
| All‐cause mortality, case, n(%) | 110 (2.13) | 132 (2.56) | 132 (2.56) | 173 (3.35) | ||
| Incidence, per 1000 person‐y | 4.36 | 5.21 | 5.20 | 6.79 | <0.01 | |
| Cox regression models | ||||||
| Model 1 | Reference | 1.21 (0.94–1.56) | 1.21 (0.94–1.58) | 1.58 (1.22–2.06) | <0.01 | |
| Model 2 | Reference | 1.19 (0.92–1.53) | 1.21 (0.93–1.57) | 1.57 (1.21–2.05) | <0.01 | |
| Model 3 | Reference | 1.18 (0.91–1.53) | 1.20 (0.92–1.56) | 1.55 (1.19–2.02) | <0.01 | |
| Diabetes mellitus (n=7987) | 1996 | 1997 | 1997 | 1997 | ||
| Cardiovascular diseases, case, n(%) | 102 (5.11) | 93 (4.66) | 84 (4.21) | 107 (5.36) | ||
| Incidence, per 1000 person‐y | 10.60 | 9.61 | 8.57 | 11.00 | 0.11 | |
| Cox regression models | ||||||
| Model 1 | Reference | 0.90 (0.68–1.19) | 0.84 (0.63–1.12) | 1.01 (0.76–1.32) | 0.91 | |
| Model 2 | Reference | 0.90 (0.68–1.19) | 0.82 (0.62–1.10) | 1.00 (0.76–1.31) | 0.84 | |
| Model 3 | Reference | 0.90 (0.68–1.20) | 0.84 (0.63–1.12) | 1.03 (0.78–1.35) | 0.99 | |
| All‐cause mortality, case, n(%) | 97 (4.86) | 107 (5.36) | 96 (4.81) | 127 (6.36) | ||
| Incidence, per 1000 person‐y | 10.04 | 11.04 | 9.81 | 13.10 | 0.13 | |
| Cox regression models | ||||||
| Model 1 | Reference | 1.06 (0.81–1.40) | 1.04 (0.79–1.38) | 1.29 (0.98–1.69) | 0.08 | |
| Model 2 | Reference | 1.05 (0.80–1.38) | 1.03 (0.78–1.37) | 1.27 (0.97–1.67) | 0.10 | |
| Model 3 | Reference | 1.05 (0.80–1.39) | 1.04 (0.78–1.38) | 1.28 (0.98–1.67) | 0.09 | |
Diabetes mellitus was defined as any self‐reported diabetes mellitus or use of glucose‐lowering drugs or FPG ≥7 mmol/L at any examination of 2006 to 2007, 2008 to 2009, or 2010 to 2011. Model 1, adjusted for age and sex at baseline and mean FPG of 3 time measurements. Model 2, adjusted for variables in model 1 plus level of education, income, smoking, alcohol abuse, physical activity, and body mass index at baseline. Model 3, adjusted for variables in model 2 plus a history of hypertension and dyslipidemia and estimated glomerular filtration rate at baseline. CI indicates confidence interval; FPG, fasting plasma glucose; and HR, hazard ratio.
Discussion
The primary findings from this large, prospective, population‐based cohort study showed that higher visit‐to‐visit variability of FPG was associated with an increased risk for CVD events and all‐cause mortality over a 4.93‐year follow‐up. These findings were obtained after adjustment for traditional CVD risk factors, including a history of diabetes mellitus, hypertension, dyslipidemia, and mean FPG levels.
In accordance with our findings, the Verona Diabetes study16 and a dynamic cohort study in China9 reported that fasting glycemic variability may be an independent predictor of mortality in patients with type 2 diabetes mellitus. In addition, multiple studies from the intensive care unit setting have shown a strong relationship between glycemic variability and mortality.17, 18, 19, 20, 21, 22, 23, 24 Critically ill patients with a wide glycemic variation appear to be at significantly higher risk for death, regardless of whether they are hospitalized in medical, surgical, pediatric, trauma, or burn units. Adjustment for demographic and clinical characteristics attenuated these relationships to a modest degree in previous studies.17, 18, 19, 20, 21, 22, 23, 24 However, there is still an extensive debate about glycemic variability as a risk factor for CVD and mortality.25, 26 Reanalysis from the HEART2D (Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus) study25 showed that targeting postprandial glucose‐decreased intraday glycemic variability would not be beneficial in reducing adverse CVD in patients with acute myocardial infarction. Lipska et al suggested that glycemic variability does not provide additional prognostic value above and beyond already recognized risk factors for mortality in patients with acute myocardial infarction.26 In our study, FPG variability over 4 years was an independent predictor of CVD and all‐cause mortality in the general population.
A systematic review reported that there were 13 different indicators to measure glucose variability.10 SD was one of the most common indicators. In this study, we defined glucose variability as the variation coefficient of 3 FPG values on 3 consecutive visit examinations, which was calculated by the ratio of SD/the mean FPG. Compared with other indicators, our visit‐to‐visit variability of FPG reflected the long‐term variability of FPG across 3 years. Few studies specifically accounted for the relationship between variability of FPG and outcomes in the nondiabetic population.18, 22, 24 In our secondary analysis, we examined the relationship between variability of FPG with CVD and all‐cause mortality in subgroups stratified by diabetes mellitus status. However, there was no evidence for interactions between FPG variability and diabetes mellitus status on the risk of CVD and all‐cause mortality. The relationship between FPG variability and CVD and all‐cause mortality was undifferentiated in individuals with and without diabetes mellitus. In contrast to our findings, another study showed that variability of FPG was not independently associated with all‐cause mortality in the whole population with diabetes mellitus. However, its stratified analysis found a significant association between variability of FPG and all‐cause mortality in patients whose glucose control was poor.9 Indeed, a previous study showed that participants with low FPG levels also had a high risk of CVD and all‐cause mortality.2 Therefore, we suggest that not only FPG levels, but also longitudinal variability in FPG levels, predict a high risk of CVD and all‐cause mortality that is compatible or beyond mean FPG levels. Therefore, monitoring longitudinal patterns of FPG levels in clinical practice is important.
The pathophysiological mechanisms that mediate the link between variability of FPG and CVD and all‐cause mortality are unknown and require further investigation. There are several potential explanations for why increased variability of FPG may be a risk factor for CVD and all‐cause mortality. First, the underlying mechanisms have been proposed to involve oxidative stress,27 release of inflammatory cytokines,28 and endothelial dysfunction.29 Second, individuals with a higher variability of FPG tend to have a higher prevalence of traditional risk factors for CVD and all‐cause mortality, including older age; a higher prevalence of hypertension, diabetes mellitus, and dyslipidemia; increased systolic and diastolic blood pressures; higher fasting blood glucose levels; and a higher body mass index. Third, severe glycemic disorders, as assessed by increasing variability of FPG, may adversely affect sympathetic dysfunction, which is associated with mortality and morbidity of CVD.30
Our study has several strengths. This was the first prospective study to examine the association between long‐term visit‐to‐visit variability of FPG and the risk of CVD and all‐cause mortality in the general population. However, there are some inherent limitations. First, we did not distinguish CVD as myocardial infarction, ischemic stroke, and hemorrhagic stroke for the current analysis because of a lack of statistical power. There were few cases of incident myocardial infarction or hemorrhagic stroke during the follow‐up. Second, we did not measure hemoglobin A1c levels at baseline because of the high cost of a screening test in the general population. Third, we did not collect information on specific causes of death. Fourth, although we adjusted for drug effects on plasma glucose variability and the risk for CVD and death, residual confounding from nonpharmacological factors cannot be completely excluded. People with diabetes mellitus or hypertension are often encouraged to reduce weight, quit smoking, and restrict intake of sugar. These behavioral factors may favorably modify blood glucose levels. Fifth, the nonparticipants had more traditional CVD risk factors than the participants. These differences may bias the present findings. In addition, the exclusion of a large proportion of the initial study population may result in an inadequate statistical power for this study. Sixth, our study calculates the visit‐to‐visit variability of FPG at only 3 points, and the follow‐up is shorter compared with other longitudinal studies. It would be valuable to include more visits and a more long‐term follow‐up. Finally, all participants were recruited from Tangshan City (an industrial city located in northern China), most of them were men, and a large proportion were manual workers, including coal miners. The cohort is not nationally representative. Although sex has been adjusted as a confounder, coal‐relevant industrial diseases have not been adjusted because of data deficiency. Therefore, the findings on increased variability of FPG and CVD or all‐cause mortality are not generalizable to other parts of China.
In conclusion, long‐term visit‐to‐visit variability of FPG is a strong independent predictor of CVD and all‐cause mortality in the general population. Evidence from the current study suggests that visit‐to‐visit variability of FPG over longer periods of follow‐up may have important prognostic value. This may help to determine the real high‐risk population and to design future studies for therapy.
Sources of Funding
This study was supported by grants from the Ministry of Science and Technology of the People's Republic of China (2011BAI08B02 and 2008ZX09312‐008), the National Natural Science Foundation of China (81322019), Beijing Municipal Science and Technology Commission (D151100002015001), and Beijing Biobank of Cerebral Vascular Disease (D131100005313003).
Disclosures
None.
Acknowledgments
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group; and the project development and management teams at the Beijing Tiantan Hospital and the Kailuan Group.
(J Am Heart Assoc. 2017;6:e006757 DOI: 10.1161/JAHA.117.006757.)29187392
References
- 1. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating G roup (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet. 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 2. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Stern MP, Blair SN. Low fasting plasma glucose level as a predictor of cardiovascular disease and all‐cause mortality. Circulation. 2000;101:2047–2052. [DOI] [PubMed] [Google Scholar]
- 3. Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all‐cause and cardiovascular mortality: the San Antonio Heart Study. Diabetes Care. 1998;21:1167–1172. [DOI] [PubMed] [Google Scholar]
- 4. Lowe LP, Liu K, Greenland P, Metzger BE, Dyer AR, Stamler J. Diabetes, asymptomatic hyperglycemia, and 22‐year mortality in black and white men: the Chicago Heart Association Detection Project in Industry Study. Diabetes Care. 1997;20:163–169. [DOI] [PubMed] [Google Scholar]
- 5. Oguoma VM, Nwose EU, Ulasi II, Akintunde AA, Chukwukelu EE, Bwititi PT, Richards RS, Skinner TC. Cardiovascular disease risk factors in a Nigerian population with impaired fasting blood glucose level and diabetes mellitus. BMC Public Health. 2017;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bragg F, Li L, Bennett D, Guo Y, Lewington S, Bian Z, Yang L, Chen J, Chen Y, Collins R, Peto R, Zhu B, Yin J, Hu X, Zhou L, Pan Y, Chen Z; China Kadoorie Biobank (CKB) Collaborative Group . Association of random plasma glucose levels with the risk for cardiovascular disease among Chinese adults without known diabetes. JAMA Cardiol. 2016;1:813–823. [DOI] [PubMed] [Google Scholar]
- 7. Muggeo M, Verlato G, Bonora E, Zoppini G, Corbellini M, de Marco R. Long‐term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non‐insulin‐dependent diabetes mellitus: the Verona Diabetes Study. Circulation. 1997;96:1750–1754. [DOI] [PubMed] [Google Scholar]
- 8. Lin CC, Yang CP, Li CI, Liu CS, Chen CC, Lin WY, Hwang KL, Yang SY, Li TC. Visit‐to‐visit variability of fasting plasma glucose as predictor of ischemic stroke: competing risk analysis in a national cohort of Taiwan diabetes study. BMC Med. 2014;12:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu D, Fang H, Xu W, Yan Y, Liu Y, Yao B. Fasting plasma glucose variability and all‐cause mortality among type 2 diabetes patients: a dynamic cohort study in Shanghai, China. Sci Rep. 2016;6:39633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eslami S, Taherzadeh Z, Schultz MJ, Abu‐Hanna A. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med. 2011;37:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, Zhou Y, Tian L. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36:1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, Wu S, Zhao X. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; for the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI:10.7326/0003‐4819‐150‐9‐200905050‐00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4‐year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5:487–493. [DOI] [PubMed] [Google Scholar]
- 15. Stroke–1989: recommendations on stroke prevention, diagnosis, and therapy: report of the WHO Task Force on Stroke and Other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. DOI: 10.1161/01.STR.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 16. Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, Verlato G. Fasting plasma glucose variability predicts 10‐year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45–50. [DOI] [PubMed] [Google Scholar]
- 17. Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38:1021–1029. [DOI] [PubMed] [Google Scholar]
- 18. Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–179. [DOI] [PubMed] [Google Scholar]
- 19. Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM Jr, May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74:679–685; discussion 685. [DOI] [PubMed] [Google Scholar]
- 20. Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. [DOI] [PubMed] [Google Scholar]
- 21. Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–366. [DOI] [PubMed] [Google Scholar]
- 22. Ali NA, O'Brien JM Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF Jr, Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pidcoke HF, Wanek SM, Rohleder LS, Holcomb JB, Wolf SE, Wade CE. Glucose variability is associated with high mortality after severe burn. J Trauma. 2009;67:990–995. [DOI] [PubMed] [Google Scholar]
- 24. Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–842. [DOI] [PubMed] [Google Scholar]
- 25. Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care. 2011;34:855–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipska KJ, Venkitachalam L, Gosch K, Kovatchev B, Van den Berghe G, Meyfroidt G, Jones PG, Inzucchi SE, Spertus JA, DeVries JH, Kosiborod M. Glucose variability and mortality in patients hospitalized with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:550–557. [DOI] [PubMed] [Google Scholar]
- 27. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. [DOI] [PubMed] [Google Scholar]
- 28. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 29. Horvath EM, Benko R, Kiss L, Muranyi M, Pek T, Fekete K, Barany T, Somlai A, Csordas A, Szabo C. Rapid “glycaemic swings” induce nitrosative stress, activate poly(ADP‐ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52:952–961. [DOI] [PubMed] [Google Scholar]
- 30. Takei Y, Tomiyama H, Tanaka N, Yamashina A. Close relationship between sympathetic activation and coronary microvascular dysfunction during acute hyperglycemia in subjects with atherosclerotic risk factors. Circ J. 2007;71:202–206. DOI: 10.1253/circj.71.202. [DOI] [PubMed] [Google Scholar]


