Abstract
Background
We sought to examine the efficacy and safety of 2 PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors: alirocumab and evolocumab.
Methods and Results
We performed a systematic review and meta‐analysis of randomized controlled trials comparing treatment with and without PCSK9 inhibitors; 35 randomized controlled trials comprising 45 539 patients (mean follow‐up: 85.5 weeks) were included. Mean age was 61.0±2.8 years, and mean baseline low‐density lipoprotein cholesterol was 106±22 mg/dL. Compared with no PCSK9 inhibitor therapy, treatment with a PCSK9 inhibitor was associated with a lower rate of myocardial infarction (2.3% versus 3.6%; odds ratio [OR]: 0.72 [95% confidence interval (CI), 0.64–0.81]; P<0.001), stroke (1.0% versus 1.4%; OR: 0.80 [95% CI, 0.67–0.96]; P=0.02), and coronary revascularization (4.2% versus 5.8%; OR: 0.78 [95% CI, 0.71–0.86]; P<0.001). Overall, no significant change was observed in all‐cause mortality (OR: 0.71 [95% CI, 0.47–1.09]; P=0.12) or cardiovascular mortality (OR: 1.01 [95% CI, 0.85–1.19]; P=0.95). A significant association was observed between higher baseline low‐density lipoprotein cholesterol and benefit in all‐cause mortality (P=0.038). No significant change was observed in neurocognitive adverse events (OR: 1.12 [95% CI, 0.88–1.42]; P=0.37), myalgia (OR: 0.95 [95% CI, 0.75–1.20]; P=0.65), new onset or worsening of preexisting diabetes mellitus (OR: 1.05 [95% CI, 0.95–1.17]; P=0.32), and increase in levels of creatine kinase (OR: 0.84 [95% CI, 0.70–1.01]; P=0.06) or alanine or aspartate aminotransferase (OR: 0.96 [95% CI, 0.82–1.12]; P=0.61).
Conclusions
Treatment with a PCSK9 inhibitor is well tolerated and improves cardiovascular outcomes. Although no overall benefit was noted in all‐cause or cardiovascular mortality, such benefit may be achievable in patients with higher baseline low‐density lipoprotein cholesterol.
Keywords: alirocumab, evolocumab, hyperlipidemia, outcome, PCSK9
Subject Categories: Lipids and Cholesterol, Risk Factors, Primary Prevention, Secondary Prevention, Meta Analysis
Clinical Perspective
What Is New?
PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors appear safe and are associated with dramatically reduced atherogenic lipid fraction levels and lower incidence of myocardial infarction, stroke, and coronary revascularization.
Despite favorable early indications from lipid‐lowering trials, the available clinical data do not demonstrate a mortality benefit with PCSK9 inhibitors.
What Are the Clinical Implications?
Whether patient subgroups exist that can derive a significant mortality benefit from PCSK9 inhibitor treatment (eg, patients intolerant to statins or with familial hypercholesterolemia) needs to be further evaluated in randomized controlled trials.
Lipid‐lowering therapy with statins is highly beneficial for prevention of secondary and high‐risk primary atherosclerotic cardiovascular disease (ASCVD). Nevertheless, some patients cannot tolerate recommended statin doses1; a high proportion of patients do not achieve adequate reduction of low‐density lipoprotein cholesterol (LDL‐C), despite high‐intensity statin therapy2; and even patients who achieve guideline recommended reductions may have high residual ASCVD risk.3 Consequently, alternative therapies designed to lower LDL‐C and improve outcomes are needed. Improvements in cardiovascular outcomes were observed recently with combination treatment with ezetimibe; however, these improvements were modest, and outcome data on monotherapy with ezetimibe are limited.4 The PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors evolocumab and alirocumab have been associated with reduction of LDL‐C levels and recently with improved cardiovascular outcomes in the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial.5 However, questions remain about the patients who are most likely to derive the greatest clinical benefits and the safety profile of this class of drugs. We performed a systematic review and meta‐analysis of randomized controlled trials (RCTs) to examine the cumulative evidence on the clinical efficacy and safety of currently available PCSK9 inhibitors, with an emphasis on cardiovascular outcomes.
Methods
We conducted a systematic review of the literature and meta‐analysis of RCTs according to established methods and standards recommended by the Cochrane Collaboration6 and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement.7
Data Sources and Searches
We searched PubMed/Medline, Embase, CENTRAL (Cochrane Central Register of Controlled Trials), and ClinicalTrials.gov up to March 18, 2017. The following keywords were used, with the use of wildcard characters to account for variations in spelling and plurals: PCSK9 antibody/inhibitor, evolocumab, alirocumab, bococizumab, AMG145, REGN727, SAR236553, RN 316, and PF‐04950615. Citations were screened at the title and abstract levels and retrieved for full‐text evaluation if they were considered potentially relevant.
Study Selection
We included phase 2 or 3 RCTs comparing treatment with and without PCSK9 inhibitors in adults with hypercholesterolemia and reporting clinical outcomes. No restriction on language, follow‐up, or study size was applied. For phase 2 studies, only dosing regimens that were also tested in phase 3 studies were included. During the study‐selection phase of the trial, the phase 3 clinical development program for the PCSK9 inhibitor bococizumab (SPIRE [Studies of PCSK9 Inhibition and the Reduction of Vascular Events]) was discontinued without plans for future marketing of this drug; therefore, 3 published trials of bococizumab8, 9 were not included in our quantitative synthesis, so as to maintain the clinical relevance of our findings.
Clinical end points abstracted include all‐cause and cardiovascular mortality, myocardial infarction (MI), unstable angina requiring hospitalization, congestive heart failure exacerbation requiring hospitalization, stroke, coronary revascularization, neurocognitive adverse events, new onset or worsening of preexisting diabetes mellitus, increase in serum creatine kinase level (an increase of >3 times the upper limit of normal was preferentially abstracted), increase in serum alanine or aspartate aminotransferase levels (an increase in alanine aminotransferase >3 times the upper limit of normal was preferentially abstracted), myalgia, and treatment‐emergent serious adverse events. Lipid end points abstracted were percentage changes from baseline in LDL‐C, high‐density lipoprotein cholesterol, total cholesterol, apolipoprotein B, and lipoprotein(a). LDL‐C levels calculated using the Friedewald formula10 were preferentially abstracted.
Data Extraction and Quality Assessment
Two investigators (A.K. and B.A.D.) independently abstracted data by using prespecified data collection forms. In case of discrepancies, consensus was achieved with the help of a third investigator (E.S.B.). Intensive background statin therapy was defined as daily use of atorvastatin ≥40 mg, rosuvastatin ≥20 mg, simvastatin ≥80 mg, or any statin plus ezetimibe. For studies in Japanese populations,11, 12, 13, 14 a modified definition of intensive background statin therapy was used (atorvastatin ≥10 mg, pitavastatin ≥2 mg, rosuvastatin ≥5 mg, simvastatin ≥20 mg, lovastatin ≥40 mg, fluvastatin ≥80 mg, pravastatin ≥40 mg, or any statin plus ezetimibe). Studies were classified as familial hypercholesterolemia (FH) studies if inclusion criteria required diagnosis of FH by genotyping or clinical criteria. The potential risk of bias of the RCTs was assessed using the Cochrane Collaboration guidelines.
Statistical Analyses
Efficacy outcomes were analyzed according to the intention‐to‐treat principle. For dichotomous data (cardiovascular and safety outcomes), odds ratios (ORs) pooled according to the Mantel‐Haenszel method were used as a summary statistic; for continuous data (lipid outcomes), mean difference (MD) of percentage change from baseline was used. Standard deviations were calculated from the standard error or confidence interval (CI) if not reported. Mean baseline LDL‐C was estimated from median and interquartile range if not reported.15 Heterogeneity and inconsistency were assessed by using the Cochran Q test and I2 statistic. Because included studies drew samples from clinically different populations, a random‐effects model was selected for the primary analysis. Both random‐ and fixed‐effects models were computed and shown as part of the sensitivity analysis. Publication bias was examined by means of funnel plots and the Egger test.
Primary stratification of the analyses was by type of PCSK9 inhibitor for cardiovascular and safety outcomes (alirocumab versus evolocumab) and by control for efficacy outcomes (placebo versus ezetimibe). Additional study‐level subgroup analyses by trial population (FH versus non‐FH/mixed) and background statin (on stable statin treatment versus statin intolerant/PCSK9 inhibitor monotherapy) were performed. Random‐effects metaregression was used to estimate the effect of baseline LDL‐C and treatment difference in percentage of LDL‐C change from baseline on clinical outcomes.
A 2‐tailed P value of <0.05 was considered statistically significant. All analyses were performed using Review Manager version 5.3 (RevMan; Cochrane Collaboration) and Comprehensive Meta‐Analysis Software version 3.3 (Biostat, Inc).
Results
Study Selection and Patient Population
The PRISMA study identification flowchart for the present analysis is shown in Figure S1. A total of 138 study arms from 35 studies were analyzed, comprising 45 539 patients (Table S1).5, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Alirocumab was used in 18 studies (28 treatment arms), and evolocumab was used in 17 studies (39 treatment arms; Figure 1); placebo was the most common control used (52 control arms), with ezetimibe used in 17 arms, and standard therapy in 2 arms. Eight studies were of an exclusively FH population, and 5 studies included only patients intolerant to statins. Mean treatment duration in the randomized population up to the time of reporting was 85.5 weeks (range: 8–113 weeks).
Figure 1.
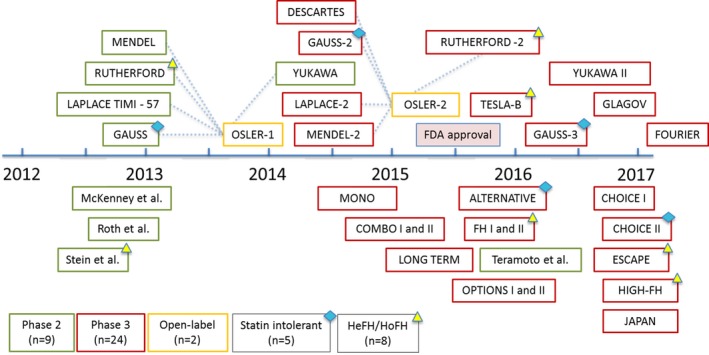
Timeline of randomized controlled trials of alirocumab and evolocumab. FDA indicates US Food and Drug Administration; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia.
Baseline patient characteristics for the study arms included are shown in Table S2. Mean age was 61.0±2.8 years, and 67.6% of participants were men; the mean baseline LDL‐C was 106.0±22.3 mg/dL (2.7±0.6 mmol/L). The majority of study participants (91.8%) were on stable statin therapy at baseline, and 58.4% were on an intensive statin regimen. From 45 539 total patients in the randomized population, safety data were available and abstracted for 45 503 (99.9%). Risk of methodological bias was assessed as low in most studies (Figure S2).
All‐Cause Mortality
Thirty‐five RCTs (45 503 participants) were included in the analysis of all‐cause mortality (Figure 2). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was not associated with a statistically significant change in mortality (crude rate, 1.9% versus 2.2%; OR: 0.71 [95% CI, 0.47–1.09]; P=0.12, I2=18%, heterogeneity P=0.26). Random effects metaregression showed a significant association between baseline LDL‐C and all‐cause mortality benefit (P=0.038; Figure 3).
Figure 2.
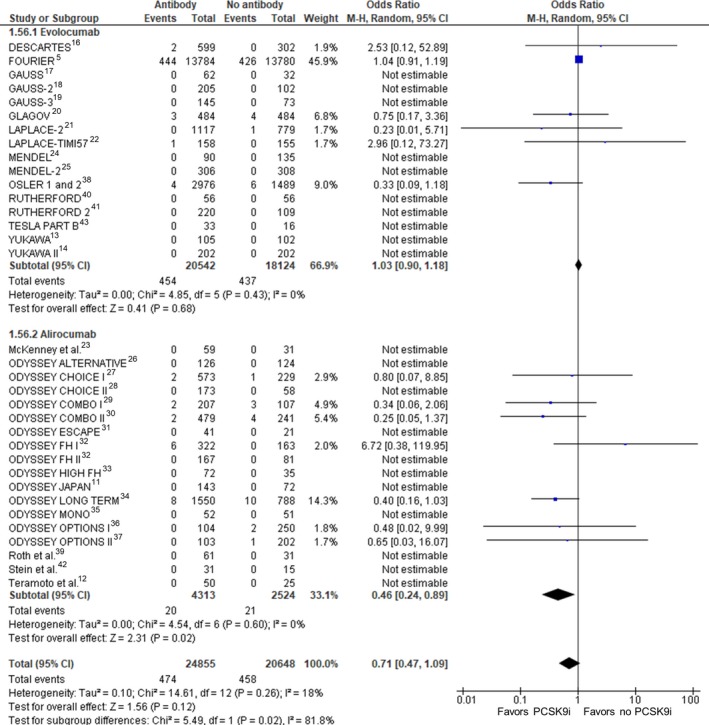
All‐cause mortality. Forrest plot showing the odds ratio for all‐cause mortality with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (PCSK9i) compared with no PCSK9i. The pooled odds ratio was calculated with random effects according to the Mantel‐Haenszel (M‐H) method. Marker size is proportional to the study weight. CI indicates confidence interval.
Figure 3.
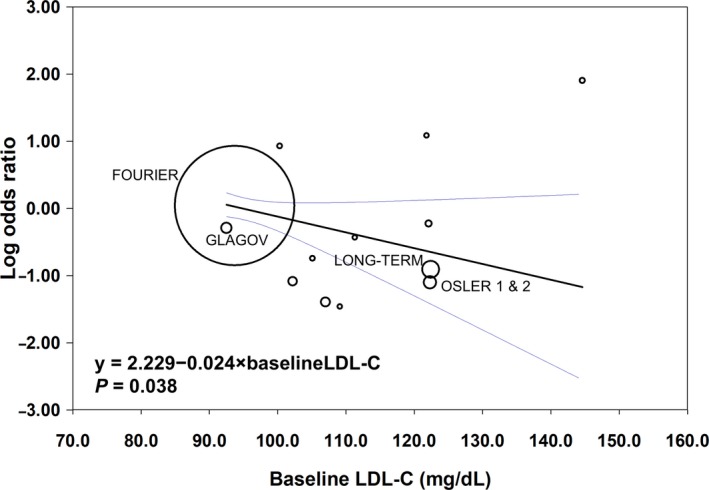
Study‐level metaregression analysis with random effects showing the relationship between baseline low‐density lipoprotein cholesterol (LDL‐C) and all‐cause mortality. Circle size is proportional to the study weight; 95% confidence intervals shown in blue.
Cardiovascular Mortality
Thirty‐four RCTs (44 701 participants) were included in the analysis of cardiovascular mortality (Figure 4). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was not associated with a statistically significant change in cardiovascular mortality (crude rate, 1.1% versus 1.3%; OR: 1.01 [95% CI, 0.85–1.19]; P=0.95, I2=0%, heterogeneity P=0.74).
Figure 4.
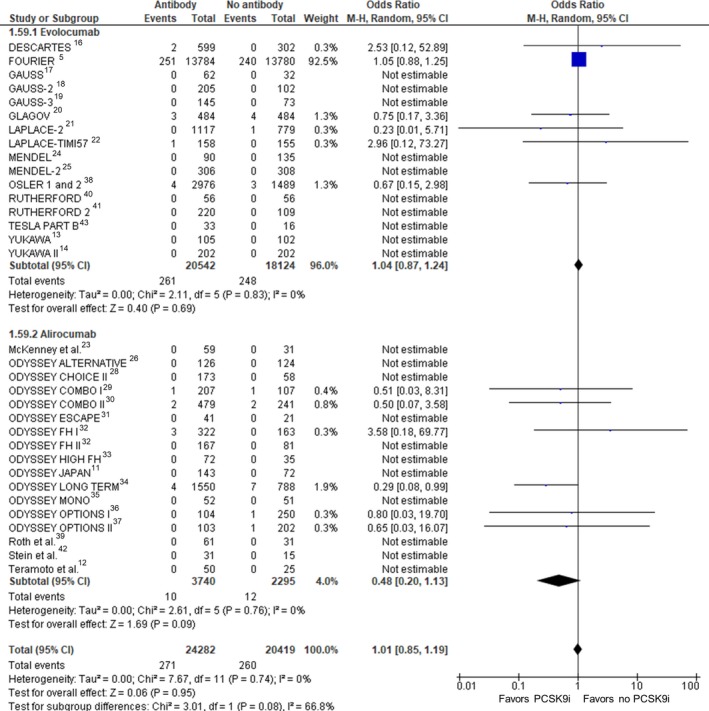
Cardiovascular mortality. Forrest plot showing the odds ratio for cardiovascular mortality with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (PCSK9i) compared with no PCSK9i. The pooled odds ratio was calculated with random effects according to the Mantel‐Haenszel (M‐H) method. Marker size is proportional to the study weight. CI indicates confidence interval.
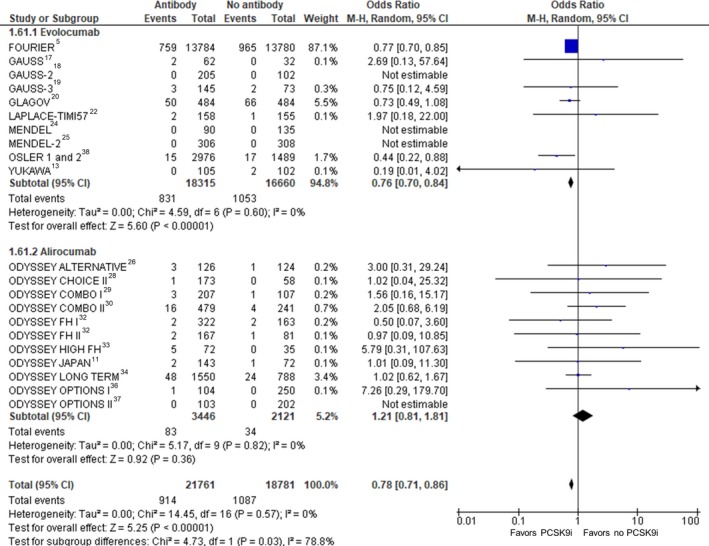
Myocardial Infarction
Twenty‐three RCTs (41 932 participants) were included in the analysis of MI (Figure 5). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was associated with a statistically significant reduction in MI (crude rate, 2.3% versus 3.6%; OR: 0.72 [95% CI, 0.64–0.81]; P<0.001, I2=0%, heterogeneity P=0.77).
Figure 5.
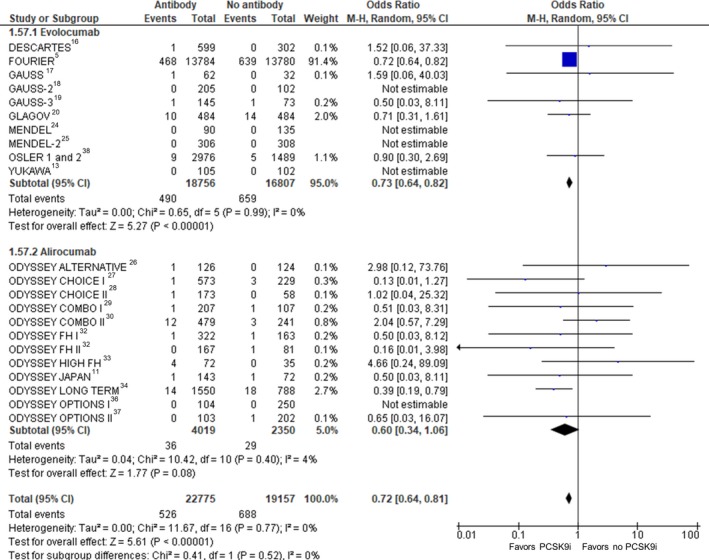
Myocardial infarction. Forrest plot showing the odds ratio for myocardial infarction with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (PCSK9i) compared with no PCSK9i. The pooled odds ratio was calculated with random effects according to the Mantel‐Haenszel (M‐H) method. Marker size is proportional to the study weight. CI indicates confidence interval.
Stroke
Twenty‐three RCTs (42 748 participants) were included in the analysis of stroke (Figure 6). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was associated with a statistically significant reduction in stroke (crude rate, 1.0% versus 1.4; OR: 0.80 [95% CI, 0.67–0.96]; P=0.02, I2=0%, heterogeneity P=0.92).
Figure 6.
Stroke. Forrest plot showing the odds ratio for stroke with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (PCSK9i) compared with no PCSK9i. The pooled odds ratio was calculated with random effects according to the Mantel‐Haenszel (M‐H) method. Marker size is proportional to the study weight. CI indicates confidence interval.
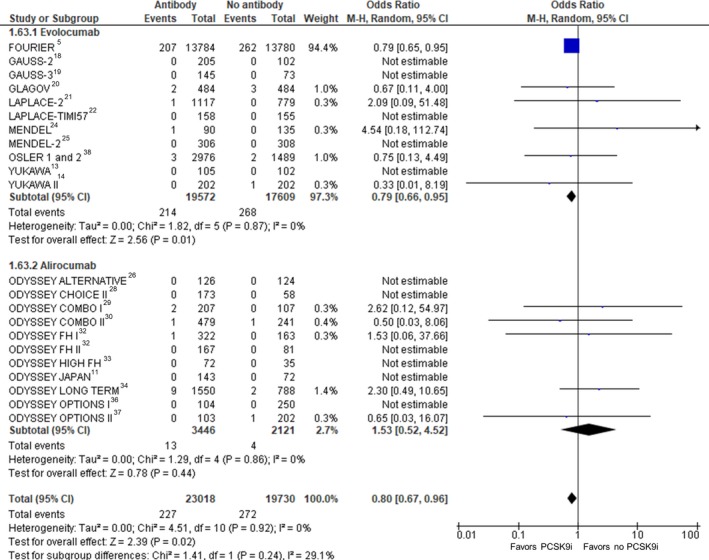
Coronary Revascularization
Twenty‐two RCTs (40 542 participants) were included in the analysis of coronary revascularization (Figure 7). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was associated with a statistically significant reduction in coronary revascularization (crude rate, 4.2% versus 5.8%; OR: 0.78 [95% CI, 0.71–0.86]; P<0.001, I2=0%, heterogeneity P=0.57). Random‐effects metaregression showed a significant association between higher treatment difference versus control in percentage of LDL‐C reduction from baseline and benefit in coronary revascularization (P=0.012; Table S3).
Figure 7.
Coronary revascularization. Forrest plot showing the odds ratio for coronary revascularization with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (PCSK9i) compared with no PCSK9i. The pooled odds ratio was calculated with random effects according to the Mantel‐Haenszel (M‐H) method. Marker size is proportional to the study weight. CI indicates confidence interval.
Unstable Angina
Twenty‐one RCTs (41 036 participants) were included in the analysis of unstable angina requiring hospitalization (Figure S3). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was not associated with a statistically significant change in unstable angina episodes requiring hospitalization (crude rate, 1.1% versus 1.3%; OR: 0.97 [95% CI, 0.81–1.16]; P=0.77, I2=0%, heterogeneity P=0.90).
Congestive Heart Failure Exacerbation
Twenty‐three RCTs (42 151 participants) were included in the analysis of congestive heart failure exacerbation requiring hospitalization (Figure S4). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was not associated with a statistically significant change in congestive heart failure exacerbations requiring hospitalization (crude rate, 1.8% versus 2.2%; OR: 0.98 [95% CI, 0.86–1.13]; P=0.79, I2=0%, heterogeneity P=0.95).
Neurocognitive Adverse Events
Twenty‐one RCTs (42 668 participants) were included in the analysis of neurocognitive adverse events (Figure 8). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was not associated with a statistically significant change in neurocognitive adverse events (crude rate, 1.2% versus 1.2%; OR: 1.12 [95% CI, 0.88–1.42]; P=0.37, I2=3%, heterogeneity P=0.42).
Figure 8.
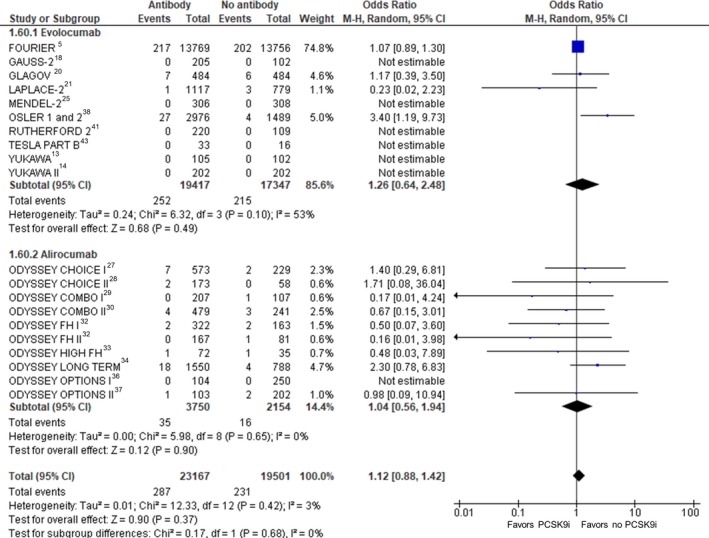
Neurocognitive adverse events. Forrest plot showing the odds ratio for neurocognitive adverse events with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (PCSK9i) compared with no PCSK9i. The pooled odds ratio was calculated with random effects according to the Mantel‐Haenszel (M‐H) method. Marker size is proportional to the study weight. CI indicates confidence interval.
Diabetes Mellitus
Fifteen RCTs (27 905 participants) were included in the analysis of new onset or worsening of preexisting diabetes mellitus (Figure S5). Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was not associated with a statistically significant change in new onset or worsening of preexisting diabetes mellitus (crude rate, 5.6% versus 5.9%; OR: 1.05 [95% CI, 0.95–1.17]; P=0.32, I2=0%, heterogeneity P=0.86).
Other Safety End Points
Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was associated with a trend of fewer increases in creatine kinase (OR: 0.84 [95% CI, 0.70–1.01]; P=0.06) and was not associated with a statistically significant change in the rates of myalgia (OR: 0.95 [95% CI, 0.75–1.20]; P=0.65), increase in alanine or aspartate aminotransferase (OR: 0.96 [95% CI, 0.82–1.12]; P=0.61), or treatment‐emergent serious adverse events (OR: 0.99 [95% CI, 0.95–1.05]; P=0.84; Figures S6 through S9).
Lipid End Points
Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was associated with a significant percentage of reduction in LDL‐C from baseline (MD: −54.77% [95% CI, −58.27% to −51.27%]; P<0.001; Figure S10). LDL‐C reduction was significantly greater in study arms controlled by placebo compared with those controlled by ezetimibe (MD: −60.91 [95% CI, −63.24 to −58.58] versus MD: −31.32% [95% CI, −34.83 to −27.81]; P<0.001).
Compared with no treatment with a PCSK9 inhibitor, treatment with a PCSK9 inhibitor was also associated with favorable changes in high‐density lipoprotein cholesterol (MD: 6.85 [95% CI, 6.10–7.60]; P<0.001), total cholesterol (MD: −34.95 [95% CI, −37.53 to −32.37]; P<0.001), lipoprotein(a) (MD: −26.45 [95% CI, −28.88 to −24.03]; P<0.001), and apolipoprotein B (MD: −45.50 [95% CI, −48.35 to −42.64]; P<0.001; Figures S11 through S14).
Unless specified earlier, other subgroup and sensitivity analyses were consistent with the primary results (Tables S3 through S5). Visual inspection of funnel plots (Figures S15 through S32) and the Egger test did not indicate publication bias (Table S6).
Discussion
The main findings of this meta‐analysis are that, compared with no PCSK9 inhibitor use, treatment with PCSK9 inhibitors (1) is associated with a statistically significant reduction in MI, stroke, and coronary revascularization; (2) is not significantly associated with all‐cause or cardiovascular mortality, neurocognitive adverse events, incident or worsening of preexisting diabetes mellitus, creatine kinase increase, myalgia, increase in alanine or aspartate aminotransferase, or treatment‐emergent serious adverse events; and (3) is associated with consistent and favorable changes in lipid fractions.
In 2006, Cohen et al published initial reports linking loss‐of‐function genetic variants impairing the PCSK9 protein activity to lifelong reductions in LDL‐C and resultant protection against ASCVD.44 In contrast, gain‐of‐function mutations of PCSK9 resulted in a phenotype similar to FH.45 These findings sparked the development of antibodies against PCSK9, 2 of which (alirocumab and evolocumab) are currently commercially available.
Until recently, the highest quality of evidence surrounding alirocumab and evolocumab stemmed from phase 2 and 3 lipid‐lowering trials and their meta‐analyses.46, 47 The encouraging results led to the 2015 US Food and Drug Administration fast‐track approval for use of PCSK9 inhibitors as adjuncts to diet and maximally tolerated statin for patients with FH and clinical ASCVD. In 2016, the American College of Cardiology released an expert consensus decision pathway regarding the role of nonstatin therapies for the treatment of hypercholesterolemia,48 according to which treatment with PCSK9 inhibitors should be considered (as first or second line) for patients with clinical ASCVD and patients with baseline LDL‐C ≥190 mg/dL not due to secondary modifiable causes who have not achieved an optimal LDL‐C reduction on a maximally tolerated statin therapy (<50% or <70–100 mg/dL).
The recently released results of the FOURIER trial, a cardiovascular outcomes study that randomized 27 564 adults aged 40 to 85 years with clinical ASCVD and baseline LDL‐C >70 mg/dL on background statin therapy to evolocumab or placebo, demonstrated a 15% reduction in the primary end point of cardiovascular death, MI, stroke, unstable angina, or coronary revascularization and a 20% reduction in the key secondary end point of cardiovascular death, MI, or stroke. However, no benefit was observed in all‐cause mortality (P=0.54) or cardiovascular mortality (P=0.62).5
These findings are corroborated by our meta‐analysis of 45 539 participants of all available phase 2 and 3 trials of evolocumab and alirocumab. Importantly, although we found significant relative improvement in the risk of MI, stroke, and coronary revascularization, the absolute risk reduction was relatively small, especially for stroke (absolute risk reduction: 0.4%; number needed to treat: 255). No statistically significant differences were identified in study‐level subgroup analyses including patients on or off treatment with a background statin and patients with FH. In contrast with a previous meta‐analysis from lipid‐lowering trials,47 we found no benefit in all‐cause mortality with PCSK9 inhibitor therapy. This finding may be attributable to the fact that our analysis included 2 large trials of populations with a baseline LDL‐C of <100 mg/dL,5, 20 contributing to an overall baseline LDL‐C of 106 mg/dL in our pooled sample. Taken in the context of this relatively low baseline LDL‐C, this lack of benefit in mortality is concordant with prior studies examining further LDL‐C reduction with high‐intensity statins49, 50 or ezetimibe.4 Notably, using random‐effects metaregression at the study level, we found that there was a significant association between higher baseline LDL‐C levels and all‐cause mortality benefit derived. This generates the hypothesis that reduction in all‐cause mortality may be possible with PCSK9 inhibitors in patients with higher baseline LDL‐C, such as patients with FH or patients with high LDL‐C levels who are intolerant to statins. Further research is needed to determine whether there is an LDL‐C cutoff at which PCSK9 inhibitors are associated with a mortality benefit.
Our analysis revealed a statistically significant reduction in the odds for coronary revascularization in the evolocumab pool compared with alirocumab. However, this finding should be interpreted in the context of lack of a powered cardiovascular outcome trial for alirocumab, resulting in a much smaller number of coronary revascularization events compared with evolocumab. ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) is an ongoing cardiovascular outcome trial of 18 000 patients investigating alirocumab every 2 weeks versus placebo in patients with hospitalization caused by a recent acute coronary syndrome and is expected to clarify the long‐term efficacy and safety of alirocumab and any potential differences between the 2 PCSK9 inhibitors.51
In addition to the high cost and uncertainty about the exact benefits in terms of cardiovascular outcomes, other obstacles to expanded use of PCSK9 inhibitors include safety concerns about neurocognitive adverse events and incident diabetes mellitus. De novo glial synthesis of cholesterol in the brain is postulated to be important for synapse formation and function; observational studies and RCTs of statins have been inconsistent in their demonstration of an association between statin utilization and impaired neurocognitive function.52, 53, 54, 55 PCSK9 inhibitors are not known to inhibit de novo cholesterol synthesis or to cross the blood–brain barrier; nevertheless, imbalances in neurocognitive side effects between PCSK9 inhibitors and control groups were detected in OSLER (Open‐Label Study of Long‐Term Evaluation Against LDL‐C) 1 and 2 (pooled rate: 0.9% versus 0.3%) and in ODYSSEY LONG‐TERM (1.2% versus 0.5%), as well as 2 large meta‐analyses of lipid‐lowering trials.46, 56 However, these findings were limited by heterogeneity of the examined populations, small numbers of events, and differences in the definition and assessment of neurocognitive events. Neurocognitive adverse events were recorded in FOURIER, with no imbalance shown between treatment and control arms. In addition, EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects), a dedicated noninferiority neurocognitive substudy of FOURIER, assessed neurocognitive function with formal testing and showed no statistically significant difference between evolocumab and placebo, even in patients with a nadir achieved LDL‐C <25 mg/dL.57 These findings are corroborated by our meta‐analysis, which showed no significant association between PCSK9 inhibitors and neurocognitive adverse events, including in metaregression analysis using baseline LDL‐C and treatment difference versus control in percentage of LDL‐C reduction from baseline as moderator variables.
Given the dramatic decrease in LDL‐C achieved with PCSK9 inhibitors, it has been hypothesized that they may adversely affect glycemic control similarly to statins.58 Although genetic polymorphism data suggest that polymorphisms of PCSK9 are associated with a similar risk for diabetes mellitus as polymorphisms of HMGCR (3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase) for a given decrease in LDL‐C,59 analysis of PCSK9 inhibitor trials does not support this association. A pooled analysis of 10 phase 3 studies from the ODYSSEY program found no significant association between incident diabetes mellitus or diabetic complications and treatment with alirocumab compared with placebo or ezetimibe.60 Similarly, no statistically significant effect was identified in the present meta‐analysis.
SPIRE was the phase 3 clinical trial program incorporating 8 RCTs investigating bococizumab, a humanized monoclonal antibody against PCSK9. The 2 cardiovascular outcome trials, SPIRE‐1 and SPIRE‐2, collectively enrolled 16 187 patients with variable baseline lipid‐lowering therapy (including patients with statin intolerance) and ASCVD status (including patients in the high‐risk, primary prevention setting) before the trial was terminated.9 Bococizumab showed a propensity for development of antidrug antibodies, which may explain the high individual variability in percentage of change in LDL‐C, attenuation in LDL‐C reduction over time, and comparatively high rate of injection‐site reactions. Because this PCSK9 inhibitor will not become available for clinical use, we elected to not include bococizumab trials in our study.
Our meta‐analysis has several important limitations. First, pooling of the data was performed at the study level and not at the patient level, limiting the potential for subgroup analyses. In addition, despite the low degree of statistical heterogeneity detected, inherent methodological heterogeneity is present because of the pooling of results from studies of different populations. Some definitions of outcomes were nonuniform among various trials.
In conclusion, our comprehensive meta‐analysis of 35 RCTs shows that, compared with no PCSK9 inhibitor administration, treatment with PCSK9 inhibitors results in improvement in cardiovascular outcomes, including MI, stroke, and coronary revascularization; no statistically significant change in the rate of adverse events, including neurocognitive adverse events, and incident or worsening of preexisting diabetes mellitus; and dramatic reductions in atherogenic lipid fractions. Although there was no statistically significant improvement in mortality, metaregression analysis revealed an association between higher baseline LDL‐C and an all‐cause mortality benefit, which needs further evaluation in RCTs.
Disclosures
Dr Ahmad has received research grants from NIH and Regeneron (modest); honoraria from Genzyme and Sanofi (modest); and serves as consultant/advisory board for Genzyme (modest). Dr Banerjee has received research grants from Boston Scientific and Merck (significant); honoraria from Medtronic, Cardiovascular Systems, Inc., and GORE (significant); and has ownership in HygeiaTel and MDCARE Global (spouse, significant). Dr Brilakis has received research grants from Boston Scientific and InfraRedx (significant); honoraria from Abbott Vascular and GE Healthcare (significant); honoraria from Asahi, Cardinal Health, and Elsevier (modest); serves as consultant/advisory board for Abbott Vascular (modest); and is employed by Medtronic (spouse, significant). The remaining authors have no disclosures.
Supporting information
Table S1. Study Characteristics
Table S2. Baseline Patient Characteristics
Table S3. Random Effects Metaregression Analysis Showing the Study‐Level Association Between Baseline Low‐Density Lipoprotein Cholesterol (LDL‐C; Left) and Treatment Difference Versus Control in Percent LDL‐C Reduction From Baseline (Right) and Cardiovascular/Safety End Points
Table S4. Subgroup Analyses for Cardiovascular/Safety End Points Stratified by Familial Hypercholesterolemia and Background Statin Therapy
Table S5. Random‐ and Fixed‐Effects Models for Cardiovascular/Safety End Points
Table S6. Egger Regression Test for Cardiovascular/Safety End Points
Figure S1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) meta‐analysis flowchart.
Figure S2. Risk of bias assessment of included studies.
Figure S3. Unstable angina.
Figure S4. Congestive heart failure exacerbation.
Figure S5. Diabetes mellitus.
Figure S6. Increase in creatine kinase.
Figure S7. Myalgia.
Figure S8. Alanine or aspartate aminotransferase increase.
Figure S9. Treatment‐emergent serious adverse events.
Figure S10. Low‐density lipoprotein cholesterol percentage of change from baseline.
Figure S11. High‐density lipoprotein cholesterol percentage of change from baseline.
Figure S12. Total cholesterol percentage of change from baseline.
Figure S13. Lipoprotein(a) percentage of change from baseline.
Figure S14. Apolipoprotein B percentage of change from baseline.
Figure S15. Funnel plot: all‐cause mortality.
Figure S16. Funnel plot: cardiovascular mortality.
Figure S17. Funnel plot: myocardial infarction.
Figure S18. Funnel plot: stroke.
Figure S19. Funnel plot: coronary revascularization.
Figure S20. Funnel plot: unstable angina.
Figure S21. Funnel plot: congestive heart failure exacerbation.
Figure S22. Funnel plot: neurocognitive adverse events.
Figure S23. Funnel plot: diabetes mellitus.
Figure S24. Funnel plot: increase in creatine kinase.
Figure S25. Funnel plot: myalgia.
Figure S26. Funnel plot: increase in alanine or aspartate aminotransferase.
Figure S27. Funnel plot: treatment‐emergent serious adverse events.
Figure S28. Funnel plot: low‐density lipoprotein cholesterol.
Figure S29. Funnel plot: high‐density lipoprotein cholesterol.
Figure S30. Funnel plot: total cholesterol.
Figure S31. Funnel plot: lipoprotein(a).
Figure S32. Funnel plot: apolipoprotein B.
(J Am Heart Assoc. 2017;6:e006910 DOI: 10.1161/JAHA.117.006910.)29223954
References
- 1. Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high‐dosage statin therapy in hyperlipidemic patients–the primo study. Cardiovasc Drugs Ther. 2005;19:403–414. [DOI] [PubMed] [Google Scholar]
- 2. Jones PH, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin‐treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1:e001800 DOI: 10.1161/JAHA.112.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, Deedwania P, Kastelein JJ, Waters DD. Determinants of residual risk in secondary prevention patients treated with high‐ versus low‐dose statin therapy: the Treating to New Targets (TNT) study. Circulation. 2012;125:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 6. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: The prisma statement. Ann Intern Med. 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 8. Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, Sweeney K, Kaila N, Vincent J, Bays H. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo‐controlled, dose‐ranging study in statin‐treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–1221. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC; SPIRE Cardiovascular Outcome Investigators . Cardiovascular efficacy and safety of bococizumab in high‐risk patients. N Engl J Med. 2017;376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 10. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 11. Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, Uno K, Baccara‐Dinet MT, Nohara A. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins—ODYSSEY JAPAN randomized controlled trial. Circ J. 2016;80:1980–1987. [DOI] [PubMed] [Google Scholar]
- 12. Teramoto T, Kobayashi M, Uno K, Takagi Y, Matsuoka O, Sugimoto M, Inoue S, Minami F, Baccara‐Dinet MT. Efficacy and safety of alirocumab in Japanese subjects (Phase 1 and 2 Studies). Am J Cardiol. 2016;118:56–63. [DOI] [PubMed] [Google Scholar]
- 13. Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, Teramoto T. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin‐treated Japanese patients at high cardiovascular risk—primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–1082. [DOI] [PubMed] [Google Scholar]
- 14. Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A. A phase 3 study of evolocumab (AMG 145) in statin‐treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117:40–47. [DOI] [PubMed] [Google Scholar]
- 15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA; DESCARTES Investigators . A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 17. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. Effect of a monoclonal antibody to PCSK9 on low‐density lipoprotein cholesterol levels in statin‐intolerant patients: the gauss randomized trial. JAMA. 2012;308:2497–2506. [DOI] [PubMed] [Google Scholar]
- 18. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M; GAUSS‐2 Investigators . Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. [DOI] [PubMed] [Google Scholar]
- 19. Nissen SE, Dent‐Acosta RE, Rosenson RS, Stroes E, Sattar N, Preiss D, Mancini GB, Ballantyne CM, Catapano A, Gouni‐Berthold I, Stein EA, Xue A, Wasserman SM, Scott R, Thompson PD; GAUSS‐3 Investigators . Comparison of PCSK9 inhibitor evolocumab vs ezetimibe in statin‐intolerant patients: design of the goal achievement after utilizing an anti‐PCSK9 antibody in statin‐intolerant subjects 3 (GAUSS‐3) trial. Clin Cardiol. 2016;39:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin‐treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 21. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R; LAPLACE‐2 Investigators . Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 22. Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, Abrahamsen TE, Wasserman SM, Scott R, Sabatine MS; LAPLACE‐TIMI 57 Investigators . Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE‐TIMI 57): a randomised, placebo‐controlled, dose‐ranging, phase 2 study. Lancet. 2012;380:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 24. Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, Bolognese M, Wasserman SM. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet. 2012;380:1995–2006. [DOI] [PubMed] [Google Scholar]
- 25. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H; MENDEL‐2 Investigators . Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. [DOI] [PubMed] [Google Scholar]
- 26. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara‐Dinet MT, Du Y, Pordy R, Gipe DA; ODYSSEY ALTERNATIVE Investigators . Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the odyssey alternative randomized trial. J Clin Lipidol. 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 27. Roth EM, Moriarty PM, Bergeron J, Langslet G, Manvelian G, Zhao J, Baccara‐Dinet MT, Rader DJ. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add‐on to statin: ODYSSEY CHOICE I. Atherosclerosis. 2016;254:254–262. [DOI] [PubMed] [Google Scholar]
- 28. Stroes E, Guyton JR, Lepor N, Civeira F, Gaudet D, Watts GF, Baccara‐Dinet MT, Lecorps G, Manvelian G, Farnier M. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the odyssey choice II study. J Am Heart Assoc. 2016;5:e003421 DOI: 10.1161/JAHA.116.003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906–915.e913. [DOI] [PubMed] [Google Scholar]
- 30. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM; ODYSSEY COMBO II Investigators . Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moriarty PM, Parhofer KG, Babirak SP, Cornier MA, Duell PB, Hohenstein B, Leebmann J, Ramlow W, Schettler V, Simha V, Steinhagen‐Thiessen E, Thompson PD, Vogt A, von Stritzky B, Du Y, Manvelian G. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J. 2016;37:3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara‐Dinet MT, Gipe DA, Geiger MJ, Farnier M. Odyssey FH I and fh II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara‐Dinet MT, Lorenzato C, Pordy R, Stroes E. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL‐C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 35. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara‐Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double‐blind, randomized phase 3 trial. Int J Cardiol. 2014;176:55–61. [DOI] [PubMed] [Google Scholar]
- 36. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni‐Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farnier M, Jones P, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM, Du Y, Hanotin C, Donahue S. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 38. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA; Open‐Label Study of Long‐Term Evaluation against LDLCI . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 39. Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–1900. [DOI] [PubMed] [Google Scholar]
- 40. Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low‐density lipoprotein cholesterol‐lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL‐C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. [DOI] [PubMed] [Google Scholar]
- 41. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni‐Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D; Investigators R . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 42. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low‐density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. [DOI] [PubMed] [Google Scholar]
- 43. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA; TESLA Investigators . Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:341–350. [DOI] [PubMed] [Google Scholar]
- 44. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 45. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. [DOI] [PubMed] [Google Scholar]
- 46. Lipinski MJ, Benedetto U, Escarcega RO, Biondi‐Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R. The impact of proprotein convertase subtilisin‐kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta‐analysis. Eur Heart J. 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 47. Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB Sr, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta‐analysis. Ann Intern Med. 2015;163:40–51. [DOI] [PubMed] [Google Scholar]
- 48. Writing C, Lloyd‐Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. 2016 ACC expert consensus decision pathway on the role of non‐statin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- 49. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets I . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 50. Study of the Effectiveness of Additional Reductions in C, Homocysteine Collaborative G , Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double‐blind randomised trial. Lancet. 2010;376:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long‐term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. [DOI] [PubMed] [Google Scholar]
- 52. Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Effects of lovastatin on cognitive function and psychological well‐being. Am J Med. 2000;108:538–546. [DOI] [PubMed] [Google Scholar]
- 53. Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117:823–829. [DOI] [PubMed] [Google Scholar]
- 54. Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688–697. [DOI] [PubMed] [Google Scholar]
- 55. Trompet S, van Vliet P, de Craen AJ, Jolles J, Buckley BM, Murphy MB, Ford I, Macfarlane PW, Sattar N, Packard CJ, Stott DJ, Shepherd J, Bollen EL, Blauw GJ, Jukema JW, Westendorp RG. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. [DOI] [PubMed] [Google Scholar]
- 56. Khan AR, Bavishi C, Riaz H, Farid TA, Khan S, Atlas M, Hirsch G, Ikram S, Bolli R. Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin‐kexin type 9 inhibitors. Circ Cardiovasc Qual Outcomes. 2017;10:e003153. [DOI] [PubMed] [Google Scholar]
- 57. Giugliano RP, Mach F, Zavitz K, Kurtz C, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Honarpour N, Wasserman SM, Ott BR; Investigators E . Design and rationale of the EBBINGHAUS trial: a phase 3, double‐blind, placebo‐controlled, multicenter study to assess the effect of evolocumab on cognitive function in patients with clinically evident cardiovascular disease and receiving statin background lipid‐lowering therapy‐A cognitive study of patients enrolled in the FOURIER trial. Clin Cardiol. 2017;40:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, Sabatine MS. Variation in PCSK9 and hmgcr and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–2153. [DOI] [PubMed] [Google Scholar]
- 60. Colhoun HM, Ginsberg HN, Robinson JG, Leiter LA, Muller‐Wieland D, Henry RR, Cariou B, Baccara‐Dinet MT, Pordy R, Merlet L, Eckel RH. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY phase 3 studies. Eur Heart J. 2016;37:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study Characteristics
Table S2. Baseline Patient Characteristics
Table S3. Random Effects Metaregression Analysis Showing the Study‐Level Association Between Baseline Low‐Density Lipoprotein Cholesterol (LDL‐C; Left) and Treatment Difference Versus Control in Percent LDL‐C Reduction From Baseline (Right) and Cardiovascular/Safety End Points
Table S4. Subgroup Analyses for Cardiovascular/Safety End Points Stratified by Familial Hypercholesterolemia and Background Statin Therapy
Table S5. Random‐ and Fixed‐Effects Models for Cardiovascular/Safety End Points
Table S6. Egger Regression Test for Cardiovascular/Safety End Points
Figure S1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) meta‐analysis flowchart.
Figure S2. Risk of bias assessment of included studies.
Figure S3. Unstable angina.
Figure S4. Congestive heart failure exacerbation.
Figure S5. Diabetes mellitus.
Figure S6. Increase in creatine kinase.
Figure S7. Myalgia.
Figure S8. Alanine or aspartate aminotransferase increase.
Figure S9. Treatment‐emergent serious adverse events.
Figure S10. Low‐density lipoprotein cholesterol percentage of change from baseline.
Figure S11. High‐density lipoprotein cholesterol percentage of change from baseline.
Figure S12. Total cholesterol percentage of change from baseline.
Figure S13. Lipoprotein(a) percentage of change from baseline.
Figure S14. Apolipoprotein B percentage of change from baseline.
Figure S15. Funnel plot: all‐cause mortality.
Figure S16. Funnel plot: cardiovascular mortality.
Figure S17. Funnel plot: myocardial infarction.
Figure S18. Funnel plot: stroke.
Figure S19. Funnel plot: coronary revascularization.
Figure S20. Funnel plot: unstable angina.
Figure S21. Funnel plot: congestive heart failure exacerbation.
Figure S22. Funnel plot: neurocognitive adverse events.
Figure S23. Funnel plot: diabetes mellitus.
Figure S24. Funnel plot: increase in creatine kinase.
Figure S25. Funnel plot: myalgia.
Figure S26. Funnel plot: increase in alanine or aspartate aminotransferase.
Figure S27. Funnel plot: treatment‐emergent serious adverse events.
Figure S28. Funnel plot: low‐density lipoprotein cholesterol.
Figure S29. Funnel plot: high‐density lipoprotein cholesterol.
Figure S30. Funnel plot: total cholesterol.
Figure S31. Funnel plot: lipoprotein(a).
Figure S32. Funnel plot: apolipoprotein B.


