Abstract
Background
Postoperative thrombocytopenia has been reported to be correlated with adverse events, but the prognostic value of baseline thrombocytopenia is unclear. This study was undertaken to evaluate the relationship between preoperative thrombocytopenia and adverse outcomes in patients with rheumatic heart disease who underwent valve replacement surgery.
Methods and Results
A total of 1789 patients with rheumatic heart disease undergoing valve replacement surgery were consecutively enrolled and postoperatively followed up for 1 year. Patients were stratified on the basis of presence (n=495) or absence (n=1294) of thrombocytopenia (platelet count, <150×109/L), according to hospital admission platelet counts. During the hospitalization period, 69 patients (3.9%) died. The in‐hospital all‐cause mortality rate was significantly higher in the thrombocytopenic group (6.9% versus 2.7%; P<0.001). Multivariate analyses revealed that thrombocytopenia was independently associated with in‐hospital all‐cause mortality (odds ratio, 2.21; 95% confidence interval, 1.29–3.80; P=0.004). Platelet counts could predict in‐hospital all‐cause mortality for patients both with and without previous atrial fibrillation (areas under the curve, 0.708 [P<0.001] and 0.610 [P=0.025], respectively). One‐year survival was significantly lower in patients with thrombocytopenia compared with controls (91.3% versus 96.1%; log‐rank=14.65; P<0.001). In addition, thrombocytopenia was an independent predictor for postoperative 1‐year all‐cause mortality in multivariate Cox regression analysis.
Conclusions
Platelet counts, as simple and inexpensive indexes, were reliable to be used as a preoperative risk assessment tool for patients with rheumatic heart disease undergoing valve replacement surgery.
Keywords: rheumatic heart disease, thrombocytopenia, valve replacement
Subject Categories: Cardiovascular Surgery, Prognosis, Rheumatic Heart Disease
Clinical Perspective
What Is New?
This study was the first to explore the prognostic value of baseline thrombocytopenia in patients with rheumatic heart disease.
Thrombocytopenia at hospital admission was an independent predictor of in‐hospital and 1‐year postoperative death.
What Are the Clinical Implications?
Platelet counts could be used as a preoperative risk assessment tool for patients with rheumatic heart disease undergoing valve replacement surgery.
Platelet count should be seriously considered by caregivers when evaluating initial patient workups.
Introduction
Rheumatic heart disease (RHD) remains a serious condition in developing countries, where causes are linked to permanent heart valve damage after group A streptococcal infections.1, 2 Despite diagnoses at earlier stages of disease and aggressive use of valve replacement surgery (VRS) techniques, a portion of patients with RHD experience postoperative mortality. In fact, data gathered by the European System for Cardiac Operative Risk Evaluation study group revealed that 4.7% (698/14 799) of patients died during, or shortly after, cardiac surgery.3 More attention should be paid to these high‐risk patients.
Platelets are a component of blood that have a critical effect on maintenance of hemostasis.4 Declines in platelet count, diagnosed as thrombocytopenic events, are commonly found in critically ill patients.5, 6 Postoperative thrombocytopenia was associated with worsened outcomes after VRS.7, 8 However, few studies have explored the prognostic value of baseline platelet counts in patients who underwent VRS, especially in the context of RHD. Patients with RHD frequently experience cardiac insufficiency, in which the platelets are overactivated and then aggregate.1, 9 Excessive consumption would cause a decrease in platelet counts. In addition, increased platelet activation and destruction were observed in patients with RHD.10 However, whether low platelet counts at hospital admission could be used to identify patients with RHD at high risk of adverse events remains uncertain. This study was undertaken to investigate whether a relationship existed between thrombocytopenic events presenting during hospital admission and in‐hospital and 1‐year mortality rates after VRS in patients with RHD.
Methods
For data privacy, the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure unless our agreement is obtained.
Patients and Setting
Patients with RHD consecutively admitted to Guangdong General Hospital (Guangzhou, Guangdong, China) between March 2009 and July 2013 were retrospectively identified. All patients underwent at least 1 VRS and underwent coronary artery bypass grafting when they had significant concomitant coronary artery disease, according to recommendations.11 RHD was diagnosed on the basis of having a history of acute rheumatic fever and/or symptoms of precordial abnormalities, the presence of a heart murmur, or valve abnormalities on echocardiographic scans.12 All of the patients underwent preoperative coronary angiography. Patients with any condition related to thrombocytopenia were excluded from these analyses, including the following: (1) previous VRS, (2) end‐stage renal disease, (3) infections or hematological or autoimmune diseases, and (4) general malignancy. The Institutional Ethics Committee of Guangdong General Hospital approved this protocol, with a waiver of informed consent because of retrospective analysis. The oral informed consent was obtained from patients themselves or their relatives through telephone and recorded by trained nurses in the follow‐up period.
Data Collection
Platelet counts were estimated during hospital admission using an automated blood cell counter (model LH780; Beckman Coulter, Brea, CA). A thrombocytopenic event was defined as a platelet count of <150×109/L. Echocardiography was used to presurgically examine left ventricular ejection fraction levels; data were measured using the Simpson biplane method. Participant clinical features were collected using an electronic case report form. Data were gathered and randomly confirmed by 2 independent researchers. Anemic events were defined as hemoglobin levels of <130 g/L in men and <120 g/L in women. The Modification of Diet in Renal Disease equation was used to calculate the estimated glomerular filtration rate, with calibration set to a Chinese patient population.13 Patients with atrial fibrillation (AF) in their medical history and hospital admission physical examination findings were considered as having previous AF.
Outcome and Follow‐Up
The primary study end point was in‐hospital all‐cause death, with the exception of suicide. Patients were postoperatively followed up using telephone tracking methods and gathering hospital readmission clinical records and outpatient clinic interviews from April 2015 to July 2015. Postoperative events and all‐cause mortality within 1 year after surgery were considered secondary end points.
Statistical Analyses
Continuous variables with normal distributions were displayed as means±SDs. Data were compared using independent‐sample t tests. Nonnormally distributed data were expressed as medians (first and third quartiles), and data comparisons were performed using nonparametric tests. Categorical variables were presented as proportions and were compared using χ2 tests. Variables contributing to in‐hospital outcomes were evaluated using logistic regression analyses. A variable was selected for inclusion in the multivariate regression analyses if it showed a statistically significant change by univariate logistic regression analysis. Area under the curve (AUC) values were measured as indicators of predictive accuracy. Kaplan‐Meier curves were calculated to assess cumulative mortality rates during follow‐up. One‐year all‐cause mortality risk factors were corroborated by calculating multivariate Cox proportional hazard models. P<0.05 was considered significant. Statistical analyses were conducted by using SPSS software, version 13.0 (SPSS, Inc, Chicago, IL). The competing risk analysis method, proposed by Gray and Fine and Gray,14, 15 was used to compare the in‐hospital all‐cause mortality (discharge was considered as a competing event) and 1‐year cardiac‐related mortality (other reason–caused mortality was considered as a competing event).
Results
Baseline Clinical Characteristics of the Patients
A total of 1858 consecutively admitted patients with RHD undergoing VRS and preoperative coronary angiography were screened (Figure 1). Two patients committed suicide while in the hospital, and 18 patients missed routine presurgical blood testing. In addition, patients with previous VRS (n=35) and estimated glomerular filtration rate <30 mL/min per 1.73 m2 (n=14) were excluded to control for potential secondary effects on platelet counts. Thus, 1789 patients were analyzed in this study, of whom 67.3% were women; the mean±SD age was 58±6 years. At hospital admission, 495 patients (27.7%) were defined as thrombocytopenia (platelet counts, <150×109/L). Demographics of the eligible population are given in Table 1. Thrombocytopenic patients were significantly older (58.4±5.8 versus 57.3±5.6 years; P<0.001) and more likely to be men (42.8% versus 28.8%; P<0.001). The thrombocytopenic patient group more frequently had the following: New York Heart Association functional classification >II (50.1% versus 42.3%; P=0.003), tricuspid valve intervention (84.0% versus 75.7%; P<0.001), and previous AF (67.5% versus 61.4%; P=0.018). Higher total bilirubin (median, 22.1 [first‐third quartile, 17.0–30.2] μmol/L versus median, 17.8 [first‐third quartile, 13.7–23.7] μmol/L; P<0.001), serum creatinine levels (mean±SD, 84.0±23.9 versus 79.1±20.6 μmol/L; P<0.001), and right ventricular (RV) diameter (mean±SD, 53.4±7.9 versus 51.1±6.7 mm; P<0.001) were also observed in patients with thrombocytopenia, in whom the baseline platelet counts were lower (mean±SD, 121.5±21.1×109/L versus 207.8±46.7×109/L; P<0.001). Higher rates of multivalvular heart disease and multivalvular surgery (aortic and mitral valve replacement plus tricuspid valve intervention) were found in patients with thrombocytopenia. In addition, the thrombocytopenic group had a higher proportion with transfusion of ≥1 U platelets (30.7% versus 11.6%; P<0.001) or ≥5 U red blood cells (40.8% versus 34.1%; P=0.008) in the hospital. Longer hospital stay was found in patients with thrombocytopenia (25.3±13.5 versus 23.4±11.1 days; P=0.004).
Figure 1.
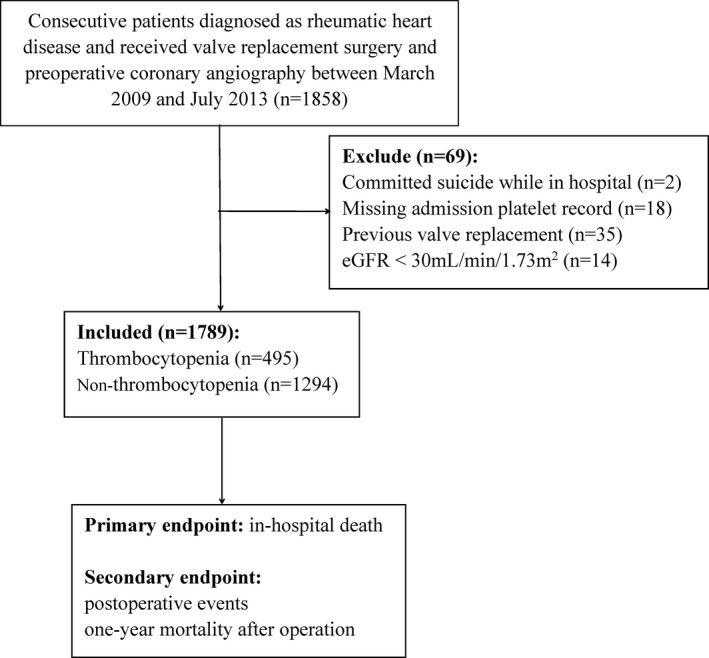
Flow diagram of included population. eGFR indicates estimated glomerular filtration rate.
Table 1.
Clinical Characteristics of the Included Population
| Clinical Variables | Overall (N=1789) | Patients With Thrombocytopenia (n=495) | Patients Without Thrombocytopenia (n=1294) | P Value |
|---|---|---|---|---|
| Age, y | 57.6±5.7 | 58.4±5.8 | 57.3±5.6 | <0.001 |
| Male sex | 585 (32.7) | 212 (42.8) | 373 (28.8) | <0.001 |
| Previous diabetes mellitus | 106 (5.9) | 28 (5.7) | 78 (6.0) | 0.766 |
| Previous AFa | 1129 (63.1) | 334 (67.5) | 795 (61.4) | 0.018 |
| NYHA class >II | 796 (44.5) | 248 (50.1) | 548 (42.3) | 0.003 |
| ALT, U/L | 21.0 (16.0–28.0) | 20.0 (16.0–27.0) | 21.0 (16.8–28.0) | 0.180 |
| Total bilirubin, μmol/L | 19.1 (14.3–25.4) | 22.1 (17.0–30.2) | 17.8 (13.7–23.7) | <0.001 |
| Hemoglobin, g/L | 134.4±15.7 | 133.5±15.7 | 134.8±15.8 | 0.125 |
| Serum creatinine, μmol/L | 80.4±21.6 | 84.0±23.9 | 79.1±20.6 | <0.001 |
| Platelet counts, ×109/L | 183.9±56.5 | 121.5±21.1 | 207.8±46.7 | <0.001 |
| LA thrombus | 294 (16.4) | 81 (16.4) | 213 (16.5) | 0.961 |
| LVEF, % | 61.6±9.2 | 61.1±9.0 | 61.7±9.3 | 0.208 |
| Multivalvular heart disease | 1518 (84.9) | 444 (89.1) | 1077 (83.2) | 0.002 |
| RV diameter, mm | 51.7±7.1 | 53.4±7.9 | 51.1±6.7 | <0.001 |
| Valve surgery | ||||
| AVR | 750 (41.9) | 218 (44.0) | 532 (41.1) | 0.262 |
| MVR | 1704 (95.2) | 470 (94.9) | 1234 (95.4) | 0.713 |
| TVI | 1395 (78.0) | 416 (84.0) | 979 (75.7) | <0.001 |
| AVR+MVR | 670 (37.5) | 196 (39.6) | 474 (36.6) | 0.246 |
| AVR+MVR+TVI | 542 (30.3) | 168 (33.9) | 374 (28.9) | 0.038 |
| CABG surgery | 82 (4.6) | 15 (3.0) | 67 (5.2) | 0.052 |
| Transfusion | ||||
| ≥1 U platelets in hospital | 302 (16.9) | 152 (30.7) | 150 (11.6) | <0.001 |
| ≥1 U RBCs in hospital | 1377 (77.0) | 369 (74.5) | 1008 (77.9) | 0.132 |
| ≥5 U RBCs in hospital | 643 (35.9) | 202 (40.8) | 441 (34.1) | 0.008 |
| Hospital stay, d | 23.9±11.9 | 25.3±13.5 | 23.4±11.1 | 0.004 |
| Postoperative events | ||||
| AKI | 1014 (56.7) | 298 (61.1) | 716 (56.6) | 0.090 |
| Dialysis | 43 (2.4) | 23 (4.6) | 20 (1.5) | <0.001 |
| Rethoracotomy for hemostasis | 81 (4.5) | 38 (7.7) | 43 (3.3) | <0.001 |
| Stroke | 25 (1.4) | 8 (1.6) | 17 (1.3) | 0.626 |
| In‐hospital all‐cause death | 69 (3.9) | 34 (6.9) | 35 (2.7) | <0.001 |
Data are given as mean±SD, number (percentage), or median (first‐third quartile). AF indicates atrial fibrillation; AKI, acute kidney injury; ALT, alanine transaminase; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; LA, left atrial; LVEF, left ventricular ejection fraction; MVR, mitral valve replacement; NYHA, New York Heart Association; RBC, red blood cell; RV, right ventricular; and TVI, tricuspid valve intervention.
Patients with AF in medical history and hospital admission physical examination findings.
Postoperative acute kidney injury occurred in 56.7% of patients. Although a slight increase in postoperative acute kidney injury was observed in the thrombocytopenic group, differences between the 2 groups were not statistically significant. However, more patients with thrombocytopenia received dialysis than patients without thrombocytopenia (4.6% versus 1.5%; P<0.001). Rethoracotomy for hemostasis was performed in 81 patients (4.5%), which was significantly different between the 2 groups (7.7% versus 3.3%; P<0.001). During hospitalization periods, 69 patients (3.9%) died (47 experienced a cardiac‐related mortality), with 34 (6.9%) in the thrombocytopenic group and 35 (2.7%) in the nonthrombocytopenic group. The difference in the proportion of all‐cause death during hospitalization was statistically significant (P<0.001; Table 1).
Thrombocytopenia and In‐Hospital Death
Different platelet reactivity was found in patients with and without AF.16 Therefore, receiver operating characteristic curve analyses were performed after stratifying the patient population by occurrence of AF. In patients without AF, the AUC was 0.708 (95% confidence interval [CI], 0.672–0.743; P<0.001; Table 2 and Figure 2A) for platelet counts as predictors of in‐hospital all‐cause mortality. Platelet counts of <178×109/L had higher sensitivities and specificities (82.1% and 52.2%, respectively). For patients with AF, platelet counts of <148×109/L had sensitivities and specificities for predicting in‐hospital all‐cause death at levels of 51.2% and 72.8%, respectively (AUC, 0.610; P=0.025; Table 2 and Figure 2B). The difference of AUC in patients with and without AF was analyzed through a z‐test. The result showed that, although a slightly increased AUC was found in patients without AF, there was no statistical difference (AUC, 0.708 versus 0.610; P=0.156). In addition, the receiver operating characteristic curve showing the predictive value of platelet count in the overall population is in Figure 3. Platelet counts could predict in‐hospital all‐cause death (AUC, 0.649; 95% CI, 0.626–0.671; P<0.001; Figure 3A and Table 2).
Table 2.
AUC and 95% CI of Platelet Counts for In‐Hospital 1‐Year Death
| Group | AUC | 95% CI | P Value |
|---|---|---|---|
| In‐hospital death | |||
| Overall | 0.649 | 0.626–0.671 | <0.001 |
| Patients with AF | 0.610 | 0.581–0.638 | 0.025 |
| Patients without AF | 0.708 | 0.672–0.743 | <0.001 |
| 1‐y Death | |||
| Overall | 0.634 | 0.610–0.658 | <0.001 |
| Patients with AF | 0.613 | 0.582–0.642 | 0.015 |
| Patients without AF | 0.666 | 0.626–0.704 | <0.001 |
AF indicates atrial fibrillation; AUC, area under the curve; and CI, confidence interval.
Figure 2.
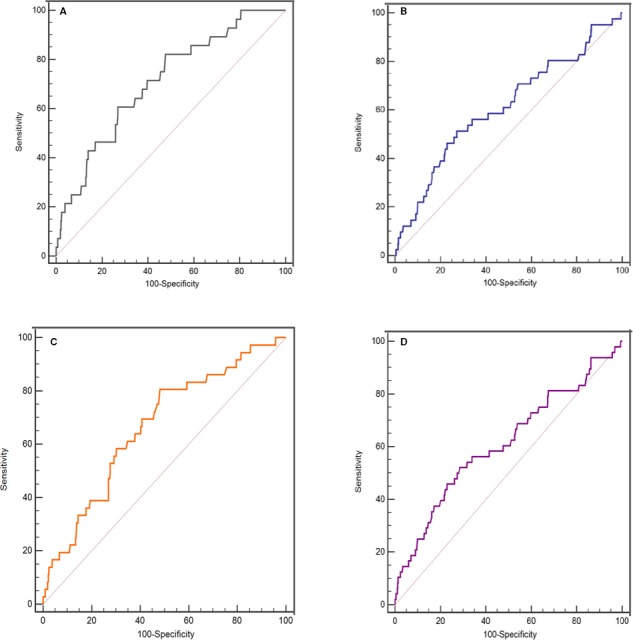
Receiver operating characteristic curve for platelet counts in predicting in‐hospital and 1‐year mortality. A, In‐hospital death for patients without atrial fibrillation (AF). B, 1‐year death for patients without AF. C, In‐hospital death for patients with AF. D, 1‐year death for patients with AF.
Figure 3.
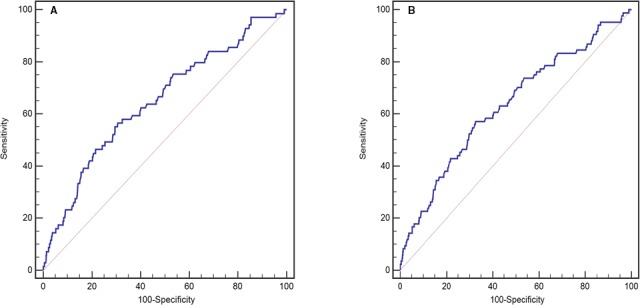
Receiver operating characteristic curves of platelet counts in predicting in‐hospital (A) and 1‐year (B) mortality in the overall population.
The results of univariate and multiple logistic regression analyses are given in Table 3. Thrombocytopenia, older age, previous diabetes mellitus, New York Heart Association class III to IV, alanine transaminase level, total bilirubin level, anemia, estimated glomerular filtration rate <60 mL/min per 1.73 m2, RV diameter, and treatment with coronary artery bypass grafting surgery were significantly associated with in‐hospital all‐cause mortality. These statistically meaningful variables were included in the multiple regression models. Thrombocytopenia remained an independent predictor of in‐hospital all‐cause death (odds ratio [OR], 2.21; 95% CI, 1.29–3.80; P=0.004). Other independent risk factors included older age (OR, 1.08; 95% CI, 1.03–1.13; P=0.001), previous diabetes mellitus (OR, 2.36; 95% CI, 1.06–5.25; P=0.036), New York Heart Association class III to IV (OR, 1.80; 95% CI, 1.05–3.10; P=0.033), and treatment with coronary artery bypass grafting surgery (OR, 2.99; 95% CI, 1.28–7.00; P=0.012).
Table 3.
Univariate Analysis and Multiple Logistic Regression Analysis for In‐Hospital Mortality
| Clinical Variables | Univariate Analysis | Multiple Logistic Regression | |||
|---|---|---|---|---|---|
| OR | P Value | OR | 95% CI | P Value | |
| Thrombocytopenia | 2.65 | <0.001 | 2.21 | 1.29–3.80 | 0.004 |
| Age | 1.10 | <0.001 | 1.08 | 1.03–1.13 | 0.001 |
| Female sex | 0.80 | 0.369 | … | … | … |
| Previous diabetes mellitus | 2.51 | 0.013 | 2.36 | 1.06–5.25 | 0.036 |
| Previous AF | 0.85 | 0.518 | … | … | … |
| NYHA class III–IV | 1.99 | 0.006 | 1.80 | 1.05–3.10 | 0.033 |
| ALT | 1.01 | 0.021 | 1.01 | 1.00–1.01 | 0.149 |
| Total bilirubin | 1.02 | 0.001 | 1.02 | 1.00–1.03 | 0.095 |
| Anemia | 1.86 | 0.019 | 1.67 | 0.94–2.95 | 0.079 |
| eGFR <60 mL/min per 1.73 m2 | 2.17 | 0.015 | 1.29 | 0.62–2.67 | 0.501 |
| LA thrombus | 1.48 | 0.283 | … | … | … |
| Multivalvular heart disease | 1.38 | 0.403 | … | … | … |
| RV diameter | 1.04 | 0.004 | 1.03 | 0.99–1.06 | 0.111 |
| AVR | 1.39 | 0.178 | … | … | … |
| MVR | 0.51 | 0.123 | … | … | … |
| TVI | 1.92 | 0.071 | … | … | … |
| AVR+MVR | 1.22 | 0.424 | … | … | … |
| AVR+MVR+TVI | 1.50 | 0.106 | … | … | … |
| CABG surgery | 2.92 | 0.007 | 2.99 | 1.28–7.00 | 0.012 |
AF indicates atrial fibrillation; ALT, alanine transaminase; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CI, confidence interval; eGFR, estimated glomerular filtration rate; LA, left atrial; MVR, mitral valve replacement; NYHA, New York Heart Association; OR, odds ratio; RV, right ventricular; and TVI, tricuspid valve intervention.
Competing risk analysis for in‐hospital mortality indicated that patients with thrombocytopenia have a significantly higher risk of death than those without thrombocytopenia (subdistribution hazard ratio, 2.57; 95% CI, 1.61–4.12; P<0.001; Figure 4).
Figure 4.
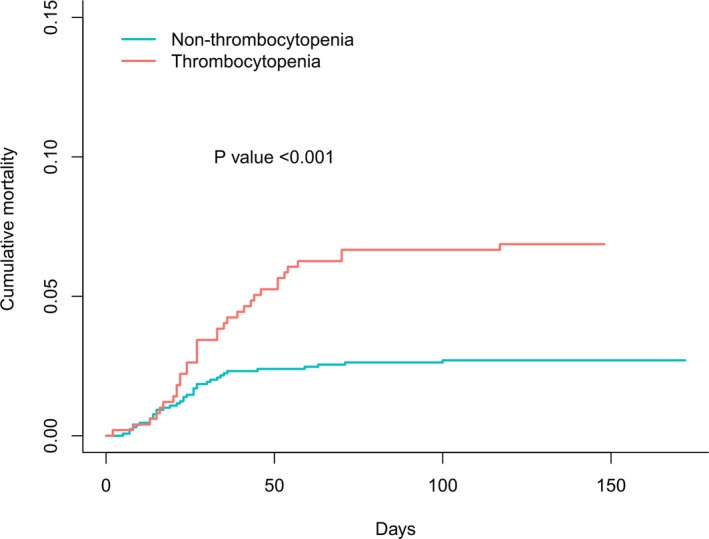
Kaplan‐Meier curve for cumulative rate of in‐hospital all‐cause mortality, according to with or without thrombocytopenia.
Thrombocytopenia and 1‐Year Death
After surgery, 1596 patients (89.2%) were followed up for 1 year. One‐year survival rate was significantly lower in thrombocytopenic patients compared with those without thrombocytopenia (91.3% versus 96.1%; log‐rank=14.65; P<0.001; Figure 5). Univariate and multivariate Cox proportional hazard analyses were used to test for potential confounding factors that could affect the prognostic value of thrombocytopenia (Tables 4 and 5). Thrombocytopenia remained independently associated with 1‐year all‐cause death rate (hazard ratio, 2.02; 95% CI, 1.30–3.14; P=0.002; model 1, Table 5) after adjusting for age, previous diabetes mellitus, New York Heart Association class III to IV, estimated glomerular filtration rate <60 mL/min per 1.73 m2, and coronary artery bypass grafting surgery. Similar results were found when additionally adjusting for aortic valve replacement and RV diameter (model 2), mitral valve replacement and anemia (model 3), and total bilirubin level (model 4, Table 5). Total platelet numbers was also a predictor of 1‐year postoperative all‐cause mortality (AUCs, 0.613 [P=0.015] and 0.666 [P<0.001] for patients with or without AF, respectively; Figure 2C and 2D). For overall population, the AUC was 0.634 (P<0.001; Table 2 and Figure 3B).
Figure 5.
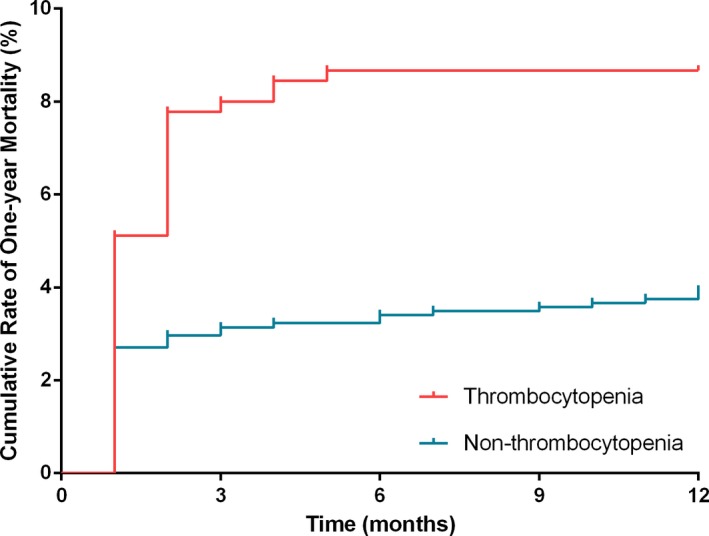
Kaplan‐Meier curve for cumulative rate of 1‐year all‐cause mortality, according to with or without thrombocytopenia.
Table 4.
Univariate Cox Proportional Hazard of 1‐Year Mortality
| Clinical Variables | HR | 95% CI | P Value |
|---|---|---|---|
| Thrombocytopenia | 2.34 | 1.46–3.44 | <0.001 |
| Age | 1.10 | 1.06–1.14 | <0.001 |
| Female sex | 0.71 | 0.46–1.10 | 0.123 |
| Previous diabetes mellitus | 2.13 | 1.10–4.12 | 0.025 |
| Previous AF | 0.78 | 0.51–1.20 | 0.257 |
| NYHA class III–IV | 1.96 | 1.27–3.04 | 0.003 |
| ALT | 1.01 | 1.00–1.01 | 0.112 |
| Total bilirubin | 1.02 | 1.01–1.03 | <0.001 |
| Anemia | 2.09 | 1.33–3.29 | 0.001 |
| eGFR <60 mL/min per 1.73 m2 | 1.99 | 1.14–3.48 | 0.016 |
| LA thrombus | 1.16 | 0.67–2.03 | 0.599 |
| Multivalvular heart disease | 1.18 | 0.62–2.22 | 0.618 |
| RV diameter | 1.04 | 1.02–1.07 | 0.001 |
| AVR | 1.57 | 1.02–2.41 | 0.039 |
| MVR | 0.43 | 0.22–0.86 | 0.017 |
| TVI | 1.38 | 0.78–2.45 | 0.272 |
| AVR+MVR | 1.30 | 0.85–2.00 | 0.233 |
| AVR+MVR+TVI | 1.46 | 0.94–2.26 | 0.094 |
| CABG surgery | 2.89 | 1.49–5.59 | 0.002 |
AF indicates atrial fibrillation; ALT, alanine transaminase; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LA, left atrial; MVR, mitral valve replacement; NYHA, New York Heart Association; RV, right ventricular; and TVI, tricuspid valve intervention.
Table 5.
Adjusted HR and 95% CI of Thrombocytopenia for 1‐Year Death
| Thrombocytopenia | HR | 95% CI | P Value |
|---|---|---|---|
| Model 1 | 2.02 | 1.30–3.14 | 0.002 |
| Model 2 | 1.75 | 1.11–2.75 | 0.017 |
| Model 3 | 1.95 | 1.25–3.04 | 0.003 |
| Model 4 | 1.72 | 1.09–2.70 | 0.019 |
Model 1 adjusted for age, previous diabetes mellitus, New York Heart Association class III to IV, estimated glomerular filtration rate <60 mL/min per 1.73 m2, and coronary artery bypass grafting surgery. Model 2 adjusted for model 1 plus aortic valve replacement and right ventricular diameter. Model 3 adjusted for model 1 plus mitral valve replacement and anemia. Model 4 adjusted for model 1 plus total bilirubin. CI indicates confidence interval; and HR, hazard ratio.
The 1‐year postoperative all‐cause mortality was 4.7% (84/1512; 10.8% were unavailable for follow‐up), in which 54 deaths (64.2%) were cardiac related. Competing risk analysis showed that a slightly increased risk of cardiac‐related mortality was found in the thrombocytopenic group (subdistribution hazard ratio, 1.39; 95% CI, 0.80–2.41; P=0.245; Figure 6).
Figure 6.
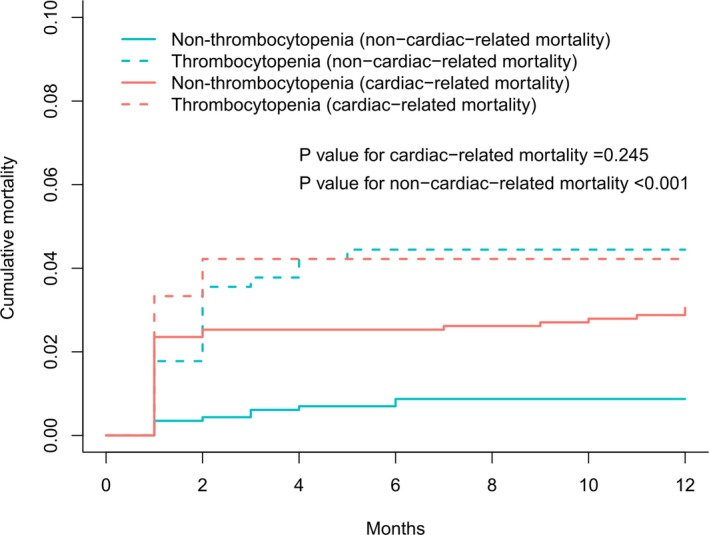
Kaplan‐Meier curve for cumulative rate of 1‐year cardiac‐related mortality.
Discussion
This study, to our best knowledge, was the first to explore the prognostic value of thrombocytopenia at hospital admission in patients with RHD. Our results indicated that thrombocytopenia was an independent predictor of in‐hospital and 1‐year postoperative death rates in patients with RHD undergoing VRS.
Platelets, which derive from megakaryocytes, function in the blood‐clotting process. Thrombocytopenia is defined as low platelet count and is induced by either decreased production or increased destruction of platelets. In this analysis, 27.7% of patients with RHD presented with thrombocytopenia (platelet count, <150×109/L) at hospital admission. The following reasons might account for this phenomenon. First, thrombocytopenia may be a consequence of platelet overdestruction secondary to aberrant platelet activation. In a previous study, increased platelet activation and destruction were observed in patients with RHD.10 It is well known that RHD is a complication of rheumatic fever, which can result in permanent damage to heart valves.17 Shear stress from impaired valves was postulated to activate the platelet and shorten its life span.18, 19 In addition, the present study demonstrated that the thrombocytopenic patients more frequently had a history of AF. Concomitant AF may activate the platelet and predispose it to thromboembolism by facilitating stasis of intracardiac blood flow.20 Furthermore, heart failure caused by impaired valves brought about hemodynamic change. Subsequently, altered blood flow would increase circulating catecholamines and activate the rennin‐angiotensin system, both of which contribute to platelet activation.9, 21 Second, platelets play other roles in several fields in addition to prevention of bleeding, such as inflammation and immune responses.22 RHD is an autoimmune disease, and inflammatory response would be ongoing, even in its chronic phase.23, 24 Persistent inflammation might cause excessive consumption of platelets. Third, RHD is known to be induced by complex immune events.25 The increment of immune‐mediated platelet destruction might be another reason for thrombocytopenia. There are some conditions, like tumors, viral infections, or immunological diseases, that could induce immune‐mediated thrombocytopenia. In rheumatic disease, the immune system might produce abundant platelet antibodies; they specifically bind receptors of the blood platelet surface membrane, resulting in the increment of platelet destruction and thrombocytopenia.26, 27, 28
Our results also revealed that thrombocytopenia at hospital admission could serve as a prognostic marker in patients with RHD undergoing VRS. This observation could be explained by the following data. First, the main function of platelets is to perform thrombosis. Previous studies have demonstrated that declines in preoperative platelet counts predicted excessive bleeding events after cardiac surgeries.29, 30 In the present study, 7.7% of patients with thrombocytopenia underwent rethoracotomy for hemostasis, which was higher than nonthrombocytopenic patients. In addition, a higher proportion of blood transfusions in the hospital were found in the thrombocytopenic group. Operative patients with high blood loss experienced adverse outcomes.31 Second, the large epidemiological data revealed that thrombocytopenia itself may also directly affect prognosis.32 Third, all the included patients required an operation, which pointed out the fact that patients experienced symptoms of cardiac insufficiency. Thrombocytopenia might reflect more severe right‐sided heart failure and abnormal hemodynamics (related to hepatic congestion and resultant hypersplenism) and, therefore, poor prognosis.33 In this analysis, RV diameter, which was an indicator for RV dysfunction,34 was significantly higher in thrombocytopenic patients. Chronic heart failure, leading to chronic passive congestion of the liver, leads to a liver function panel consistent with cholestasis.35 Total bilirubin level was an independent indicator for development of RV failure after left ventricular assist device implantation.36 In our study, a higher total bilirubin level was found in patients with thrombocytopenia, which encouraged us to speculate that low platelet counts might reflect RV dysfunction. Previous study has proved that RV failure was a strong marker of cardiovascular morbidity and mortality in patients with heart failure.37 Finally, a decline in platelet counts was considered a sign of hematologic dysfunction. Thrombocytopenia has been an early prognostic marker used in critically ill patients.6 For example, thrombocytopenic events were important indicators of worsened outcomes when determining Sequential Organ Failure Assessment score38 and Multiple Organ Dysfunction Score.39 Taken together, these results supported thrombocytopenia at hospital admission as being a prognostic marker of VRS outcomes in these patients with RHD.
Limitations
Several important limitations of these data should be considered. First, although the models were adjusted for potential risk factors using multiple regression analysis techniques, some residual confounding remained. Second, the cause of thrombocytopenia was not clearly elucidated, which is an underlying weakness of retrospective study designs. Finally, platelet count was not a strong predictor of events, with an AUC of ≈0.7. However, the aim of the present study was to demonstrate the value of thrombocytopenia for clinical outcomes in patients with RHD and then report a new biomarker or risk factor independent of previous factors for patients with RHD. It might be helpful for future research to establish the risk scores of patients with RHD, which included this simple biomarker. A future prospective trial is warranted to determine whether increased platelet counts that exceed the cutoff points determined in these models improve prognosis for patients with RHD undergoing VRS.
Conclusion
This study demonstrated that thrombocytopenia at hospital admission was independently associated with increased in‐hospital and 1‐year death rates in patients with RHD undergoing VRS. Platelet counts have the potential to become a simple, quick, and cheap preoperative risk assessment tool for these patients. Platelet counts should be seriously considered by caregivers when evaluating initial patient workups.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006988 DOI: 10.1161/JAHA.117.006988.)29203580
Contributor Information
Ning Tan, Email: gdydq100@126.com.
Dan‐qing Yu, Email: gdydq100@126.com.
References
- 1. Remenyi B, ElGuindy A, Smith SJ, Yacoub M, Holmes DJ. Valvular aspects of rheumatic heart disease. Lancet. 2016;387:1335–1346. [DOI] [PubMed] [Google Scholar]
- 2. He VY, Condon JR, Ralph AP, Zhao Y, Roberts K, de Dassel JL, Currie BJ, Fittock M, Edwards KN, Carapetis JR. Long‐term outcomes from acute rheumatic fever and rheumatic heart disease: a data‐linkage and survival analysis approach. Circulation. 2016;134:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F, Gams E, Harjula A, Jones MT, Pintor PP, Salamon R, Thulin L. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–822, discussion 822–823. [DOI] [PubMed] [Google Scholar]
- 4. George JN. Platelets. Lancet. 2000;355:1531–1539. [DOI] [PubMed] [Google Scholar]
- 5. Claushuis TA, van Vught LA, Scicluna BP, Wiewel MA, Klein KP, Hoogendijk AJ, Ong DS, Cremer OL, Horn J, Franitza M, Toliat MR, Nurnberg P, Zwinderman AH, Bonten MJ, Schultz MJ, van der Poll T. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127:3062–3072. [DOI] [PubMed] [Google Scholar]
- 6. Drews RE, Weinberger SE. Thrombocytopenic disorders in critically ill patients. Am J Respir Crit Care Med. 2000;162:347–351. [DOI] [PubMed] [Google Scholar]
- 7. Dvir D, Genereux P, Barbash IM, Kodali S, Ben‐Dor I, Williams M, Torguson R, Kirtane AJ, Minha S, Badr S, Pendyala LK, Loh JP, Okubagzi PG, Fields JN, Xu K, Chen F, Hahn RT, Satler LF, Smith C, Pichard AD, Leon MB, Waksman R. Acquired thrombocytopenia after transcatheter aortic valve replacement: clinical correlates and association with outcomes. Eur Heart J. 2014;35:2663–2671. [DOI] [PubMed] [Google Scholar]
- 8. Flaherty MP, Mohsen A, Moore JT, Bartoli CR, Schneibel E, Rawasia W, Williams ML, Grubb KJ, Hirsch GA. Predictors and clinical impact of pre‐existing and acquired thrombocytopenia following transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2015;85:118–129. [DOI] [PubMed] [Google Scholar]
- 9. Chung I, Lip GY. Platelets and heart failure. Eur Heart J. 2006;27:2623–2631. [DOI] [PubMed] [Google Scholar]
- 10. Kunishima S, Hattori M, Kobayashi S, Hattori H, Iwama Y, Imai Y, Ogawa K, Naoe T, Ohno R. Activation and destruction of platelets in patients with rheumatic heart disease. Eur Heart J. 1994;15:335–338. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JR, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TR, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 12. Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease: an evidence‐based guideline. Nat Rev Cardiol. 2012;9:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 14. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16. Milovanovic M, Fransson E, Hallert C, Jaremo P. Atrial fibrillation and platelet reactivity. Int J Cardiol. 2010;145:357–358. [DOI] [PubMed] [Google Scholar]
- 17. Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–964. [DOI] [PubMed] [Google Scholar]
- 18. Steele PP, Weily HS, Davies H, Genton E. Platelet survival in patients with rheumatic heart disease. N Engl J Med. 1974;290:537–539. [DOI] [PubMed] [Google Scholar]
- 19. Tse HF, Lau CP, Cheng G. Relation between mitral regurgitation and platelet activation. J Am Coll Cardiol. 1997;30:1813–1818. [DOI] [PubMed] [Google Scholar]
- 20. Makowski M, Smorag I, Makowska J, Bissinger A, Grycewicz T, Pasnik J, Kidawa M, Lubinski A, Zielinska M, Baj Z. Platelet reactivity and mean platelet volume as risk markers of thrombogenesis in atrial fibrillation. Int J Cardiol. 2017;235:1–5. [DOI] [PubMed] [Google Scholar]
- 21. Anfossi G, Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur J Clin Invest. 1996;26:353–370. [DOI] [PubMed] [Google Scholar]
- 22. Koenen RR. The prowess of platelets in immunity and inflammation. Thromb Haemost. 2016;116:605–612. [DOI] [PubMed] [Google Scholar]
- 23. Golbasi Z, Ucar O, Keles T, Sahin A, Cagli K, Camsari A, Diker E, Aydogdu S. Increased levels of high sensitive C‐reactive protein in patients with chronic rheumatic valve disease: evidence of ongoing inflammation. Eur J Heart Fail. 2002;4:593–595. [DOI] [PubMed] [Google Scholar]
- 24. Guilherme L, Kalil J. Rheumatic heart disease: molecules involved in valve tissue inflammation leading to the autoimmune process and anti‐S. pyogenes vaccine. Front Immunol. 2013;4:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guilherme L, Kohler KF, Kalil J. Rheumatic heart disease: mediation by complex immune events. Adv Clin Chem. 2011;53:31–50. [PubMed] [Google Scholar]
- 26. Chow L, Aslam R, Speck ER, Kim M, Cridland N, Webster ML, Chen P, Sahib K, Ni H, Lazarus AH, Garvey MB, Freedman J, Semple JW. A murine model of severe immune thrombocytopenia is induced by antibody‐ and CD8+ T cell‐mediated responses that are differentially sensitive to therapy. Blood. 2010;115:1247–1253. [DOI] [PubMed] [Google Scholar]
- 27. Aslam R, Hu Y, Gebremeskel S, Segel GB, Speck ER, Guo L, Kim M, Ni H, Freedman J, Semple JW. Thymic retention of CD4+CD25+FoxP3+ T regulatory cells is associated with their peripheral deficiency and thrombocytopenia in a murine model of immune thrombocytopenia. Blood. 2012;120:2127–2132. [DOI] [PubMed] [Google Scholar]
- 28. Ekstrand C, Linder M, Cherif H, Kieler H, Bahmanyar S. Increased susceptibility to infections before the diagnosis of immune thrombocytopenia. J Thromb Haemost. 2016;14:807–814. [DOI] [PubMed] [Google Scholar]
- 29. Lopes CT, Dos ST, Brunori EH, Moorhead SA, Lopes JL, Barros AL. Excessive bleeding predictors after cardiac surgery in adults: integrative review. J Clin Nurs. 2015;24:3046–3062. [DOI] [PubMed] [Google Scholar]
- 30. Ranucci M, Baryshnikova EFTS. The interaction between preoperative platelet count and function and its relationship with postoperative bleeding in cardiac surgery. Platelets. 2017;28:794–798. [DOI] [PubMed] [Google Scholar]
- 31. Bilecen S, de Groot JA, Kalkman CJ, Spanjersberg AJ, Brandon BBG, Moons KG, Nierich AP. Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during high‐risk cardiac surgery: a randomized clinical trial. JAMA. 2017;317:738–747. [DOI] [PubMed] [Google Scholar]
- 32. Msaouel P, Lam AP, Gundabolu K, Chrysofakis G, Yu Y, Mantzaris I, Friedman E, Verma A. Abnormal platelet count is an independent predictor of mortality in the elderly and is influenced by ethnicity. Haematologica. 2014;99:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mojadidi MK, Galeas JN, Goodman‐Meza D, Eshtehardi P, Msaouel P, Kelesidis I, Zaman MO, Winoker JS, Roberts SC, Christia P, Zolty R. Thrombocytopaenia as a prognostic indicator in heart failure with reduced ejection fraction. Heart Lung Circ. 2016;25:568–575. [DOI] [PubMed] [Google Scholar]
- 34. Guo YK, Yang ZG, Shao H, Deng W, Ning G, Dong ZH. Right ventricular dysfunction and dilatation in patients with mitral regurgitation: analysis using ECG‐gated multidetector row computed tomography. Int J Cardiol. 2013;167:1585–1590. [DOI] [PubMed] [Google Scholar]
- 35. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61:2397–2405. [DOI] [PubMed] [Google Scholar]
- 36. Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre‐operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. [DOI] [PubMed] [Google Scholar]
- 38. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. [DOI] [PubMed] [Google Scholar]
- 39. Peres BD, Melot C, Lopes FF, Nguyen BV, Vincent JL. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002;28:1619–1624. [DOI] [PubMed] [Google Scholar]


