Abstract
Background
The optimal timing to administer non–vitamin K oral anticoagulants (NOACs) in patients with acute ischemic stroke and atrial fibrillation is unclear. This prospective observational multicenter study evaluated the rates of early recurrence and major bleeding (within 90 days) and their timing in patients with acute ischemic stroke and atrial fibrillation who received NOACs for secondary prevention.
Methods and Results
Recurrence was defined as the composite of ischemic stroke, transient ischemic attack, and symptomatic systemic embolism, and major bleeding was defined as symptomatic cerebral and major extracranial bleeding. For the analysis, 1127 patients were eligible: 381 (33.8%) were treated with dabigatran, 366 (32.5%) with rivaroxaban, and 380 (33.7%) with apixaban. Patients who received dabigatran were younger and had lower admission National Institutes of Health Stroke Scale score and less commonly had a CHA 2 DS 2‐VASc score >4 and less reduced renal function. Thirty‐two patients (2.8%) had early recurrence, and 27 (2.4%) had major bleeding. The rates of early recurrence and major bleeding were, respectively, 1.8% and 0.5% in patients receiving dabigatran, 1.6% and 2.5% in those receiving rivaroxaban, and 4.0% and 2.9% in those receiving apixaban. Patients who initiated NOACs within 2 days after acute stroke had a composite rate of recurrence and major bleeding of 12.4%; composite rates were 2.1% for those who initiated NOACs between 3 and 14 days and 9.1% for those who initiated >14 days after acute stroke.
Conclusions
In patients with acute ischemic stroke and atrial fibrillation, treatment with NOACs was associated with a combined 5% rate of ischemic embolic recurrence and severe bleeding within 90 days.
Keywords: acute stroke, anticoagulants, atrial fibrillation, secondary prevention
Subject Categories: Ischemic Stroke
Clinical Perspective
What Is New?
This study evaluated the rates of both recurrence and major bleeding within 90 days in patients with acute ischemic stroke and atrial fibrillation who were prescribed non–vitamin K oral anticoagulants for secondary prevention; the results showed a combined 5.2% rate of ischemic embolic recurrence (2.8%) and severe bleeding (2.4%).
Overall, 80% of the patients received non–vitamin K oral anticoagulants within 15 days of the index stroke.
What Are the Clinical Implications?
Non–vitamin K oral anticoagulants could be used within 2 weeks from stroke onset, given the seemingly acceptable risk of severe bleeding.
Introduction
Patients with acute ischemic stroke and nonvalvular atrial fibrillation (AF) are at high risk of early recurrence.1, 2 In these patients, anticoagulant therapy plays a major role in the prevention of recurrent ischemic stroke. Because early hemorrhagic transformation is a major concern, the optimal time to start anticoagulant therapy remains a controversial issue. Indeed, there are no comparative randomized studies on the optimal timing of the start of anticoagulation in patients with acute ischemic stroke and nonvalvular AF. Thus, such a decision hinges on the assessment of the competing risks of early thromboembolic recurrences and hemorrhagic transformation.3 Data from the RAF (Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation) study suggested that the optimal time for initiating anticoagulation treatment for secondary stroke prevention may be 4 to 14 days from stroke onset.4 Moreover, patients treated with oral anticoagulants alone had better outcomes compared with patients treated with low‐molecular‐weight heparins (LMWHs) alone or before oral anticoagulants. In the RAF study, with enrollment from January 2012 to March 2014, <10% of the patients were treated with non–vitamin K oral anticoagulants (NOACs).4 Observational studies reported that, if NOACs are started early after an index event, the risk of intracranial bleeding appears to be low.5, 6, 7
This international, prospective, observational, multicenter study in patients with acute stroke and AF treated with NOACs for secondary prevention evaluated, at 90 days from the acute event, (1) the rates of recurrent ischemic embolic event and severe bleeding (both intra‐ and extracranial) and their timing and (2) the risk factors associated with ischemic stroke recurrence, systemic embolism, symptomatic cerebral bleeding, and severe extracerebral hemorrhage.
Methods
The RAF‐NOACs (Early Recurrence and Major Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation Treated With Non–Vitamin K Oral Anticoagulants) study was a prospective observational study carried out between April 2014 and June 2016 that enrolled consecutive patients with acute ischemic stroke and known or newly diagnosed AF who did not have contraindications to anticoagulation with NOACs. As exclusion criteria, we considered 1) high risk of bleeding, defined as clinically significant liver disease (acute or chronic hepatitis, cirrhosis, or alanine aminotransferase level >3 times the upper limit of normal), creatinine clearance <30 mL/min (for apixaban, the threshold was 25 mL/min), 2) life expectancy of <3 to 6 months, 3) use of interacting medications, and 4) uncontrolled hypertension. The study was performed in 35 stroke units across Europe, the United States, and Asia. The study was approved by the local institutional review boards, if required. Patient consent was obtained from either the patient or a family member (e.g. in aphasic patient).
On admission, stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS). A noncontrast cerebral computed tomography (CT) or cerebral magnetic resonance imaging (MRI) scan was performed on admission in all patients to exclude intracranial hemorrhage. Thrombolysis treatment was given as per standard local protocol, if appropriate. Standard stroke unit care, monitoring, and treatment were provided by all participating centers according to current international recommendations for acute ischemic stroke.8, 9, 10 All patients were monitored for blood pressure, temperature, glucose level, and heart rate in the first days after stroke. Attending physicians were free to make decisions about the type of anticoagulant to be used for secondary prevention and the day on which to initiate it; however, in this article, we report only patients who received a NOAC.
Nonvalvular AF was classified as paroxysmal (episodes terminating spontaneously within 7 days), persistent (episodes lasting >7 days requiring pharmacologic and/or electrical stimulation), or permanent (persisting for >1 year, either because cardioversion failed or was not attempted).11
A second brain CT scan or MRI had to be performed 24 to 72 hours from stroke onset in all patients. Hemorrhagic transformation (HT) was defined on CT scan as any degree of hyperdensity within the area of low attenuation and was classified as either hemorrhagic infarction or parenchymal hematoma.12, 13 On MRI, HT was defined as hypointensity on axial T1‐ and T2‐weighted images. HT was considered symptomatic if associated with a decline in neurological status (an increase of ≥4 points in NIHSS score) in the absence of any bleeding evidence on the first CT.14 The sites and sizes of the qualifying infarcts were determined based on standard templates15, 16: (1) Small lesions were ≤1.5 cm in the anterior or posterior circulation; (2) medium lesions were in a cortical superficial branch of the middle cerebral artery (MCA), in the MCA deep branch, in the internal border zone territories, in a cortical superficial branch of the posterior cerebral artery, or in a cortical superficial branch of the anterior cerebral artery; (3) large anterior lesions involved the complete territory of the MCA, posterior cerebral artery, or anterior cerebral artery or were in 2 cortical superficial branches of the MCA, in a cortical superficial branch of MCA associated with the MCA deep branch, or in >1 artery territory (eg, MCA associated with anterior cerebral artery territory); (4) large posterior lesions were ≥1.5 cm in the brain stem or cerebellum.13
Risk Factors
Data on known stroke risk factors were collected, as described previously2: age, gender, history of hypertension (blood pressure ≥140/90 mm Hg at least twice before stroke or already under treatment with antihypertensive drugs), history of diabetes mellitus (fasting glucose level ≥126 mg/dL preprandial on 2 examinations, glucose level ≥200 mg/dL postprandial, HbA1c ≥6.5%, or under antidiabetic treatment), current cigarette smoking, past smoking (cessation <5 years earlier), hyperlipidemia (total cholesterol ≥200 mg/dL, triglyceride ≥140 mg/dL, or already under lipid‐lowering therapy), history of symptomatic ischemic heart disease (myocardial infarction, history of angina or existence of multiple lesions on thallium heart isotope scan, or evidence of coronary disease on coronary angiography), history of symptomatic peripheral arterial disease (intermittent claudication of presumed atherosclerotic origin; ankle/arm systolic blood pressure ratio <0.85 in either leg at rest; or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty), alcohol abuse (≥300 g/week), obesity (body mass index ≥30), or previous stroke or transient ischemic attack [TIA]). White matter changes (leukoaraiosis defined on the first CT [or MRI] examination as ill‐defined and moderately hypodense [or hyperintensity on T2‐weighted MRI] areas of ≥5 mm, according to published criteria) were investigated.17 Leukoaraiosis in the deep white matter was dichotomized into absent versus mild, moderate, or severe. Other baseline variables obtained at admission for all patients included fasting serum glucose, fasting serum cholesterol (total, high‐, and low‐density lipoprotein), platelet count, international normalized ratios, activated partial thromboplastin time, systolic blood pressure, and diastolic blood pressure.
Data on the use of any antiplatelet, anticoagulant, or thrombolytic agent before admission, at baseline, and during the follow‐up period were recorded.
The CHA2DS2‐VASc score was calculated for the periods before and after the index event.18
Evaluation of Outcome
Patients were followed up prospectively by face‐to‐face or telephone interviews. Study outcomes at 90 days were (1) recurrent ischemic cerebrovascular events (stroke or TIA) and symptomatic systemic embolisms and (2) symptomatic cerebral bleeding and major extracerebral bleeding.
The primary study outcome was the composite of stroke, TIA, symptomatic systemic embolism, symptomatic cerebral bleeding, and major extracerebral bleeding.4
HTs found on neuroimaging 24 to 72 hours after onset were not considered outcome events unless classified as symptomatic.
Stroke was defined as the sudden onset of a new focal neurological deficit of vascular origin in a site consistent with the territory of a major cerebral artery and categorized as ischemic or hemorrhagic. TIA was defined as a transient episode of neurological dysfunction caused by focal brain ischemia without acute infarction. Systemic embolism was defined as an acute vascular occlusion of an extremity or organ confirmed by imaging, surgery, or autopsy. Cerebral bleeding was considered symptomatic if associated with a decline in neurological status (an increase of ≥4 points in NIHSS score or leading to death). Major extracerebral bleeding was defined as a reduction in the hemoglobin level of at least 2 g/dL, requisite blood transfusion of at least 2 U, or symptomatic bleeding in a critical area or organ.19
Disability and mortality at 90 days were also assessed using the modified Rankin scale. Nondisabling functional outcome was defined as a modified Rankin scale score of 0 to 2.
Sample Size Calculation
To perform logistic regression analysis, at least 10 to 20 patients were needed for each variable included in the model. In this study, 60 variables were to be evaluated. Consequently, we anticipated the inclusion in the study of at least 1000 patients.
Statistical Analyses
Differences in the characteristics of patients with or without outcome events were tested using the χ2 test. Specifically, univariate tests were applied to compare both clinical characteristics on admission and preexisting risk factors for stroke. Logistic regression models were estimated to identify relevant predictors for outcome events20: combined ischemic and hemorrhagic, ischemic, and hemorrhagic. The variables included in the model were those reaching significance on univariate analysis <0.1 selected from risk factors, reperfusion therapies, admission NIHSS scores, admission antithrombotic treatment, CHA2DS2‐VASc scores, and sizes of the ischemic lesions. The day of starting anticoagulant treatment was inserted into the models as either a continuous or dichotomized categorical variable. To avoid separation in the data and biased parameter estimates in the standard logistic models, given the low number of outcome events recorded, the Firth method of penalized likelihood was performed.21 The confidence intervals (CIs) for the odds ratios (OR) were calculated using penalized likelihood profiles.
Survival function and empirical cumulative hazards function were estimated via the Kaplan–Meier estimator for various groups of patients; differences between functions were tested using the log‐rank test. Patients were censored at the time of an outcome event, at death, or if they were lost to follow‐up.22
Furthermore, we estimated additional multivariate models to investigate possible effects of predictor variables (eg, lesion size) on the occurrence of an outcome event, for different timing of NOAC administration (<3, 3–7, 7–14, ≥15 days). These results were reported as an OR with a 95% CI, for which a 2‐sided P<0.05 was considered significant.
All statistical analyses were performed using the software R version 3.0.3 (R Foundation for Statistical Computing).
Results
Overall, 1161 consecutive patients were included in the study (mean age 75.6±9.9 years). Of these, 34 patients were excluded for incomplete follow‐up data, leaving 1127 patients for the analysis (Table 1).
Table 1.
Characteristics of the Treatment Groups
| Total (N=1161) | Dabigatran (n=395) | Apixaban (n=390) | Rivaroxaban (n=376) | |
|---|---|---|---|---|
| Age, y | 75.6±9.9 | 73.6±10.2a | 77.2±9.2 | 76.0±9.7 |
| Sex, male | 542 (46.8) | 209 (52.9)a | 171 (44.1) | 162 (43.1) |
| NIHSS on admission | 7.7±6.2 | 6.9±5.0a | 7.8±6.2 | 8.3±6.6 |
| Diabetes mellitus | 225 (19.4) | 74 (18.7) | 66 (17.0) | 85 (22.6) |
| Hypertension | 889 (76.5) | 302 (76.4) | 285 (73.1)a | 302 (80.3) |
| Hyperlipidemia | 410 (35.4) | 165 (41.8) | 113 (29.1)a | 132 (35.1) |
| Atrial fibrillationb | ||||
| Paroxysmal | 567 (50.3) | 184 (47.0) | 192 (51.3) | 189 (52.8) |
| Permanent | 391 (34.8) | 151 (38.3) | 119 (32.2) | 121 (33.8) |
| Persistent | 167 (14.9) | 58 (14.7) | 61 (16.5) | 48 (13.4) |
| History stroke/TIA | 304 (26.0) | 117 (29.6) | 97 (24.5) | 90 (23.9) |
| Current smoker | 129 (11.1) | 54 (13.7) | 31 (8.0) | 44 (11.7) |
| Alcoholism | 72 (6.2) | 29 (7.3) | 20 (5.2) | 23 (6.1) |
| History congestive heart failure | 181 (15.6) | 66 (16.7) | 57 (14.7) | 58 (15.4) |
| History myocardial infarction | 134 (11.5) | 39 (9.9) | 43 (11.1) | 52 (13.8) |
| History peripheral artery disease | 92 (7.9) | 29 (7.3) | 30 (7.8) | 32 (8.5) |
| Pacemaker | 65 (5.6) | 25 (6.3) | 22 (5.7) | 18 (4.8) |
| Lesion sizec | ||||
| Small | 448 (40.9) | 147 (40.5) | 162 (42.1) | 140 (40.0) |
| Medium | 388 (33.3) | 131 (36.1) | 138 (35.3) | 120 (31.9) |
| Large anterior | 180 (15.5) | 44 (12.1)a | 67 (17.4) | 70 (20.0) |
| Large posterior | 76 (6.6) | 33 (9.1) | 20 (5.2) | 21 (6.0) |
| Leukoaraiosis | 673 (58.0) | 196 (49.6)a | 258 (66.5) | 220 (58.5) |
| Atrial enlargementd | 703 (72.0) | 207 (65.5)a | 256 (76.0) | 241 (74.4) |
| Severe | 161 (16.5) | 45 (14.2) | 56 (17.0) | 58 (17.9) |
| Systemic thrombolysis (rtPA) | 317 (27.3) | 94 (23.8)a | 110 (28.1) | 114 (30.3) |
| Embolectomy | 47 (4.1) | 23 (5.8) | 14 (3.6) | 10 (2.6) |
| Combination rtPA plus thrombectomy | 69 (6.0) | 28 (7.0) | 16 (4.1) | 25 (6.6) |
| LMWH before oral anticoagulants | 111 (9.6) | 36 (9.1) | 34 (8.8) | 41 (10.9) |
| Hemorrhagic transformation at 24–72 h | 106 (9.1) | 37 (9.4) | 37 (9.5) | 32 (8.5) |
| Creatinine clearance, mL/min | 76.6±17.1 | 93.7±29.3a | 68.7±24.3 | 70.1±25.3 |
| CHA2DS2‐VASc score after index stroke | ||||
| 2 | 33 (2.8) | 16 (4.0)a | 10 (2.6) | 7 (1.9) |
| 3 | 87 (7.5) | 40 (10.7) | 24 (6.2) | 23 (6.1) |
| 4 | 177 (15.4) | 89 (22.5) | 48 (12.4) | 40 (10.6) |
| 5 | 311 (26.8) | 97 (24.5) | 114 (29.3) | 100 (26.6) |
| 6 | 358 (30.7) | 97 (24.5) | 126 (32.0) | 136 (36.2) |
| 7 | 152 (13.2) | 40 (10.2) | 54 (13.9) | 58 (15.4) |
| 8 | 35 (3.0) | 11 (2.8) | 13 (3.4) | 11 (2.9) |
| 9 | 7 (0.6) | 5 (1.3) | 1 (0.2) | 1 (0.3) |
| >4 | 863 (74.3) | 250 (63.3)a | 309 (78.9) | 306 (81.4) |
Data are shown as mean±SD or n (%).LMWH indicates low‐molecular‐weight heparin; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; TIA, transient ischemic attack.
P<0.05.
1123 patients.
1096 patients.
976 patients with transthoracic echocardiogram performed.
After the acute stroke, 395 patients (34.0%) were treated with dabigatran (189 with 300 mg/day), 390 (33.6%) with rivaroxaban (244 with 20 mg/day), and 376 (32.4%) with apixaban (261 with 10 mg/day). Before treatment with NOACs, 728 patients (62.7%) received antiplatelet agents, and 111 patients (9.6%) received full‐dose LMWH. In addition 25 patients (2.2%) were treated with NOACs concurrently with antiplatelet agents.
Patients who received dabigatran were significantly younger and had significantly lower NIHSS scores on admission and, less commonly, had CHA2DS2‐VASc scores >4 and reduced renal function (Table 1).
HT on neuroimaging performed 24 to 72 hours after stroke onset was identify in 106 patients (9.1%): 77 (6.6%) had hemorrhagic infarction, and 29 (2.5%) had parenchymal hematoma.
At 90 days, 312 (27.7%) patients were deceased or disabled (modified Rankin scale score ≥3); of those, 26 (2.3%) were deceased.
Rates of Recurrent Ischemic Events or Bleeding
Of the 1127 patients available for the final analysis, 59 (5.2%) had outcome events: 32 (2.8%) had ischemic stroke, TIA, or systemic embolism, and 27 (2.4%) had symptomatic intracranial bleeding or major extracerebral bleeding (Table 2 and Figure 1). Mean latency from index stroke to recurrent ischemic event (stroke, TIA, systemic embolism) was 23.2±27.4 days (median: 17 days; interquartile range [IQR]: 2–39 days) and to severe bleeding was 18.1±30.7 days (median: 7 days; IQR: −2 to 42 days). The characteristics of patients with and without outcome events are reported in Table 3.
Table 2.
Observed Study Outcome Events
| Total (N=1127) | Dabigatran (n=381) | Apixaban (n=380) | Rivaroxaban (n=366) | |
|---|---|---|---|---|
| Combined end pointa | 59 (5.2) | 11 (2.9) | 28 (7.4) | 20 (5.5) |
| Ischemic stroke | 22 (2.0) | 5 (1.3) | 10 (2.6) | 7 (1.9) |
| Symptomatic hemorrhagic transformation | 18 (1.6) | 3 (0.8) | 7 (1.9) | 8 (2.2) |
| Ischemic stroke, TIA, or systemic embolism | 32 (2.8) | 7 (1.8) | 16 (4.2) | 9 (2.4) |
| TIA | 7 (0.6) | 2 (0.5) | 3 (0.8) | 2 (0.5) |
| Systemic embolism | 3 (0.3) | 0 | 3 (0.8) | 0 |
| Symptomatic hemorrhagic transformation, severe extracranial bleeding | 27 (2.4) | 4 (1.0) | 12 (3.2) | 11 (3.0) |
| Serious extracranial bleeding | 10 (0.9) | 1 (0.2) | 5 (1.3) | 4 (1.1) |
| mRS ≥3 at 90 d | 312 (27.7) | 84 (22.0) | 111 (31.9) | 116 (31.7) |
| Mortality at 90 d | 26 (2.3) | 7 (1.8) | 9 (2.4) | 11 (3.0) |
Data are shown as n (%). mRS indicates modified Rankin Scale; TIA, transient ischemic attack.
Combined end point: symptomatic hemorrhagic transformation, ischemic stroke, transient ischemic attack (TIA), systemic embolism and severe extracranial bleeding.
Figure 1.
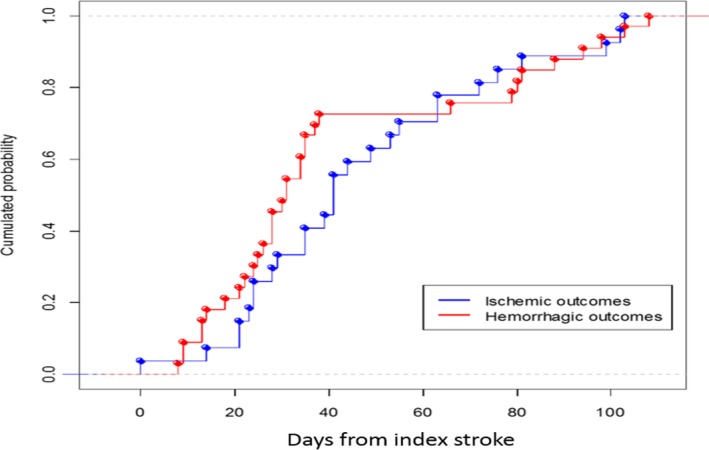
Cumulative probability of a first outcome event within 90 days from the index stroke.
Table 3.
Characteristics of Patients With and Without Outcome Events
| With Events (n=59) | Without Events (n=1068) | P Value | |
|---|---|---|---|
| Age, y | 75.5±9.9 | 76.8±9.1 | |
| Sex, male | 30 (46.8) | 483 (45.2) | |
| NIHSS on admission | 7.6±6.1 | 8.2±7.0 | |
| Diabetes mellitus | 18 (30.5) | 196 (18.3) | 0.02 |
| Hypertension | 49 (83.0) | 802 (75.1) | |
| Hyperlipidemia | 22 (37.3) | 378 (35.4) | |
| Atrial fibrillationa | |||
| Paroxysmal | 23 (39.0) | 520 (51.1) | |
| Permanent | 31 (52.5) | 340 (33.4) | 0.03 |
| Persistent | 5 (8.5) | 158 (15.5) | 0.05 |
| History stroke/TIA | 16 (27.1) | 275 (25.7) | |
| Current smoker | 10 (16.9) | 197 (18.4) | |
| Alcoholism | 4 (6.8) | 65 (6.1) | |
| History congestive heart failure | 10 (16.9) | 163 (15.3) | |
| History myocardial infarction | 9 (15.2) | 116 (10.9) | |
| History peripheral artery disease | 6 (10.2) | 78 (7.3) | |
| Pacemaker | 3 (5.1) | 58 (5.4) | |
| Lesion sizeb | |||
| Small | 19 (32.2) | 413 (38.7) | |
| Leukoaraiosis | 38 (64.4) | 600 (56.2) | |
| Atrial enlargementc | 37 (62.7) | 642 (68.2) | |
| Moderate/severe | 18 (30.5) | 361 (38.3) | |
| Systemic thrombolysis (rtPA) | 13 (22.0) | 293 (27.4) | |
| Embolectomy | 3 (5.1) | 44 (4.1) | |
| Combination rtPA plus embolectomy | 4 (6.8) | 64 (6.0) | |
| Antiplatelets on admission | 36 (61.0) | 663 (62.1) | |
| LMWH before oral anticoagulants | 13 (22.0) | 97 (9.1) | 0.003 |
| Hemorrhagic transformation at 24–72 h | 7 (11.9) | 95 (8.9) | |
| CHA2DS2‐VASc score after index stroke | |||
| >4 | 47 (79.7) | 791 (74.1) | |
Data are shown as mean±SD or n (%). LMWH indicates low‐molecular‐weight heparin; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; TIA, transient ischemic attack.
1018 patients.
1063 patients.
942 patients with trans‐thoracic echocardiogram performed.
Poststroke Anticoagulation and the Risk of Recurrent Ischemic Events or Bleeding
The analysis that evaluated the rates of the primary study outcome associated with the day of initiating anticoagulant treatment is reported in Figure 2. Primary outcome events were experienced by 12.4% (95% CI, 7.4–17.4%; 19/153) of patients who initiated NOACs within 2 days from the index stroke, 2.1% (95% CI, 1.1–3.1%; 15/710) who initiated NOACs between 3 and 14 days, and 9.1% (95% CI, 6.1–12.1%; 24/264) who initiated NOACs after 14 days (P<0.0001). Figure 3 reports the rates of ischemic or hemorrhagic outcome events associated with the day of initiating anticoagulant treatment. Despite the occurrences of outcome events having variability with respect to the timing of NOAC administration, the results from the multivariate model suggested no significant marginal effect regarding the timing of administration (within 3 days from index stroke as reference: between 3 and 7 days: OR: 1.30; 95% CI, 0.54–3.71; between 8 and 14 days: OR: 1.44; 95% CI, 0.36–3.02; >14 days: OR: 0.59; 95% CI, 0.15–1.95).
Figure 2.
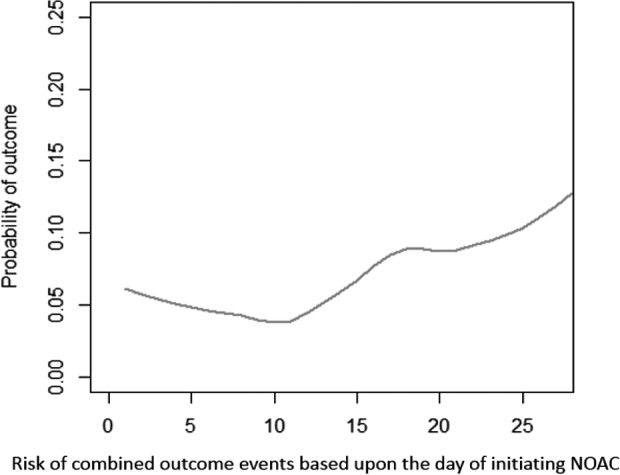
Combined risks of outcome events (ischemic and hemorrhagic) depending on the time between onset and initiation of therapy with non–vitamin K oral anticoagulants (NOACs). The lower risk of the combined outcome event was within 14 days.
Figure 3.
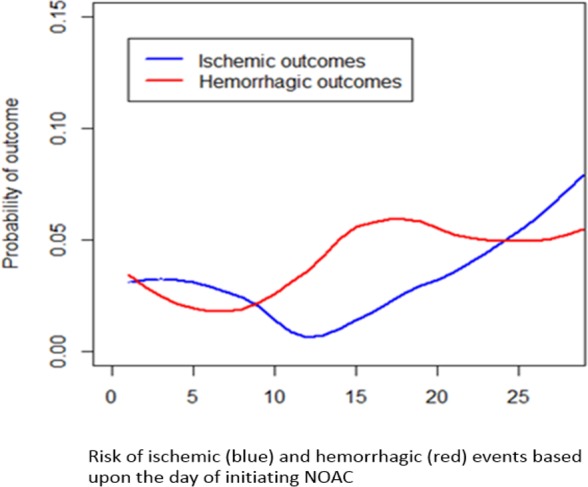
Risks of outcome events depending on the time between onset and initiation of therapy with non–vitamin K oral anticoagulants (NOACs).
Mean latencies from the start of NOAC treatment at different time points after stroke onset are reported in Table 4.
Table 4.
Mean Latencies (Days) From Start of NOAC Treatment at Different Time Points After Stroke Onset
| Time Intervals | Mean | Quartile 1 | Median | Quartile 3 |
|---|---|---|---|---|
| Combined (ischemic and hemorrhagic) outcome events, d | ||||
| <3 | 26.4 | 3.25 | 20.5 | 53.0 |
| 3–7 | 25.9 | 7.75 | 17.0 | 35.5 |
| 8–14 | 33.7 | 5.5 | 13.0 | 64.5 |
| ≥15 | 33.9 | 11.0 | 29.0 | 51.5 |
| Overall combined outcomes | 29.2 | 6.75 | 17.0 | 52.75 |
| Ischemic outcome events, d | ||||
| <3 | 28.8 | 4.0 | 31.0 | 52.0 |
| 3–7 | 32.4 | 17.0 | 20.0 | 43.5 |
| 8–14 | 30.0 | 5.5 | 11.0 | 45.0 |
| ≥15 | 26.3 | 20.0 | 29.0 | 34.0 |
| Overall ischemic outcomes | 30.4 | 12.0 | 22.5 | 45.75 |
| Hemorrhagic outcome events, d | ||||
| <3 | 31.0 | 7.75 | 33.0 | 56.25 |
| 3–7 | 15.9 | 5.0 | 7.0 | 12.0 |
| 8–14 | 35.1 | 6.25 | 27.5 | 59.75 |
| ≥15 | 33.8 | 11.0 | 11.0 | 64.0 |
| Overall hemorrhagic outcomes | 28.5 | 5.5 | 11.0 | 56.25 |
NOACs indicates non–vitamin K oral anticoagulants.
In Table 5 we reported the characteristics of the patients treated with low and high doses of direct oral anticoagulants; patients treated with low doses were older and had lower clearance of creatinine; furthermore, patients treated with low doses had more large lesions than patients treated with high doses. The risk of outcome events was similar in the groups treated with low and high doses of direct oral anticoagulants.
Table 5.
Characteristics of the Patients Treated With Low or High Dose of NOACs
| Low Dose (n=467) | High Dose (n=694) | P Value | |
|---|---|---|---|
| Age, y | 82.0 (71–93) | 74.0 (63–85) | 0.0001 |
| Sex, male | 190 (40.7) | 352 (50.7) | 0.001 |
| NIHSS on admission | 7.0 (−2 to 16) | 5.0 (−2 to 12) | 0.013 |
| Diabetes mellitus | 99 (21.2) | 125 (18.0) | |
| Hypertension | 387 (82.9) | 503 (72.5) | 0.0001 |
| Hyperlipidemia | 168 (36.0) | 242 (34.9) | |
| Current smoker | 32 (6.9) | 99 (14.3) | 0.0001 |
| Alcoholism | 28 (6.0) | 45 (6.5) | |
| Lesion sizea | |||
| Small | 161 (37.6) | 289 (43.3) | 0.06 |
| Large anterior | 82 (19.2) | 98 (14.7) | 0.05 |
| Leukoaraiosis | 299 (64.) | 377 (54.3) | 0.001 |
| Creatinine clearance, mL/min | 60.5 (27.5–93.5) | 74.0 (46–102) | 0.02 |
| End point events | |||
| Combined end point | 21 (4.5) | 38 (5.4) | |
| Ischemic recurrence | 13 (2.8) | 19 (2.7) | |
| Hemorrhagic event | 8 (1.7) | 19 (2.7) | |
Data are shown as median (interquartile range) or n (%). NIHSS indicates National Institutes of Health Stroke Scale; NOACs, non–vitamin K oral anticoagulants.
1094 patients.
Type of Anticoagulant Administered and Risk of Recurrent Ischemic Events or Bleeding
Nine of the 381 patients who received dabigatran had an outcome event after initiation of therapy (2.4%; 95% CI, 1.4–3.4%), as did 15 of the 366 (4.1%; 95% CI, 2.1–6.1%) who received rivaroxaban and 26 of the 380 (6.9%; 95% CI, 3.9–9.9%) who received apixaban (Table 6). The rates of early recurrence and major bleeding were 1.8% (95% CI, 0.8–2.8%) and 0.5% (95% CI, −0.2% to 0.7%), respectively, in patients who received dabigatran; 1.6% (95% CI, 0.6–2.6%) and 2.5% (95% CI 2.5–3.5%), respectively, in those who received rivaroxaban; and 4.0% (95% CI, 2.0–6.0%) and 2.9% (95% CI, 1.9–3.9%), respectively, in those who received apixaban (Figures 4 and 5). The mean times of starting treatment from the index events were 12.8±13.8 days (median: 8 days; IQR: 3–14 days) for patients treated with dabigatran, 11.1±11.7 days (median: 8 days; IQR: 4–14 days) for patients treated with rivaroxaban, and 11.0±12.5 days (median: 7 days; IQR: 3–14 days) for patients treated with apixaban (Figure 6). Overall, 80% of the patients received NOACs within 15 days from the index stroke.
Table 6.
Outcome Events in the Dabigatran, Rivaroxaban, and Apixaban Groups After Initiating Anticoagulants
| Dabigatran (n=381) | Apixaban (n=380) | Rivaroxaban (n=366) | |
|---|---|---|---|
| Combined end pointa | 9 (2.4) | 26 (6.9) | 15 (4.1.) |
| Stroke, TIA, or systemic embolism | 7 (1.8) | 15 (4.0) | 6 (1.6) |
| Symptomatic hemorrhagic transformation, severe extracranial bleeding | 2 (0.5) | 11 (2.9) | 9 (2.5) |
| Symptomatic hemorrhagic transformation | 2 (0.5) | 6 (1.6) | 5 (1.4) |
| Ischemic stroke | 5 (1.3) | 9 (2.4) | 4 (1.1) |
| TIA | 2 (0.5) | 3 (0.8) | 2 (0.5) |
| Systemic embolism | 0 | 3 (0.8) | 0 |
| Serious extracranial bleeding | 0 | 5 (1.3) | 4 (1.1) |
Data are shown as n (%). TIA indicates transient ischemic attack.
Combined endpoint: symptomatic hemorrhagic transformation, ischemic stroke, transient ischemic attack (TIA), systemic embolism and severe extracranial bleeding.
Figure 4.
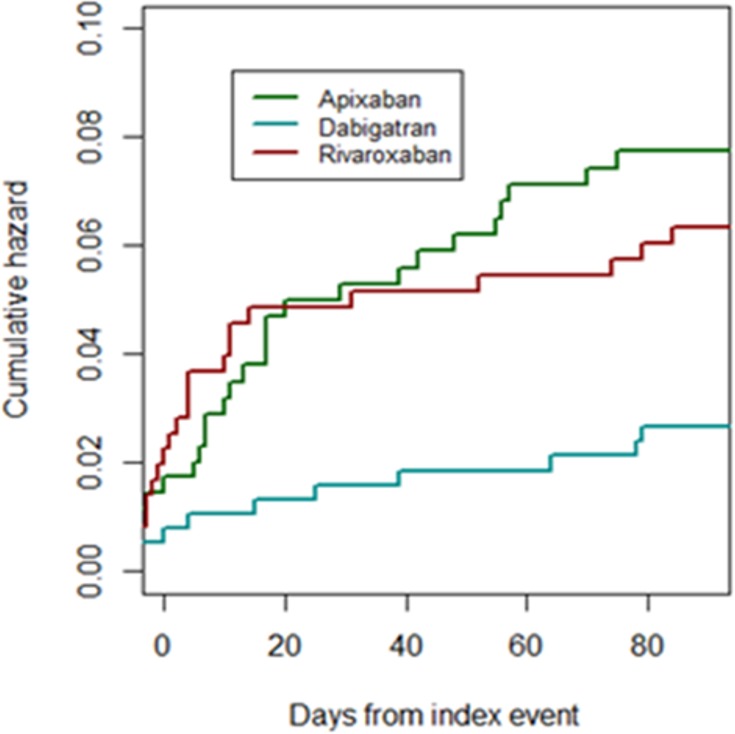
Cumulative risks of the combined end point (ischemic and hemorrhagic) for individual non–vitamin K oral anticoagulants.
Figure 5.
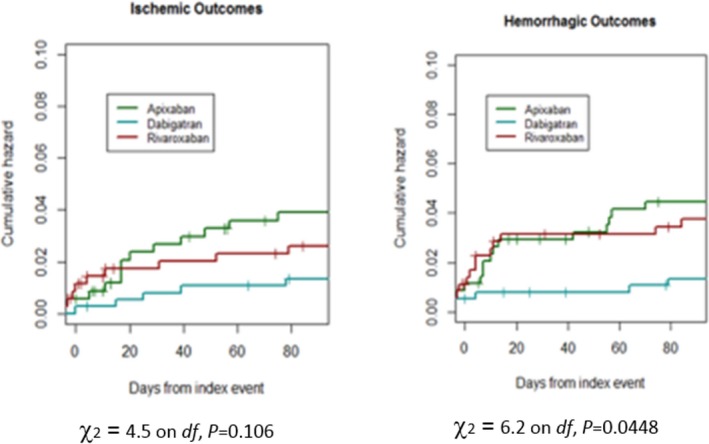
Cumulative risks of the ischemic or hemorrhagic end point for the non–vitamin K oral anticoagulants.
Figure 6.
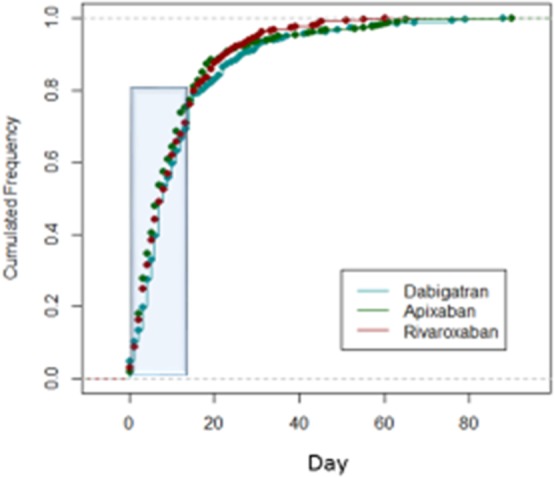
Time of initiating therapy for non–vitamin K oral anticoagulants.
Risk Factors Associated With the Risk of Recurrent Ischemic Events or Bleeding
The rates of outcome events (ischemic or hemorrhagic) within 90 days increased with rising CHA2DS2‐VASc scores: Event rates were 3.3% for patients with scores of 2 and 3, 3.0% for those with a score of 4, 4.0% for those with a score of 5, 6.0% for those with a score of 6, and 7.6% for those with scores of 7, 8, or 9 (P for trend 0.05).
About 12% of the patients treated with LMWH before oral anticoagulants had an outcome event compared with 4.4% of those treated with NOACs alone; the rates of bleeding events were 7.3% and 1.9% in the 2 groups, respectively (P=0.0001; Figure 7). The characteristics of the patients treated or not with LMWH before oral anticoagulants are reported in Table 7.
Figure 7.
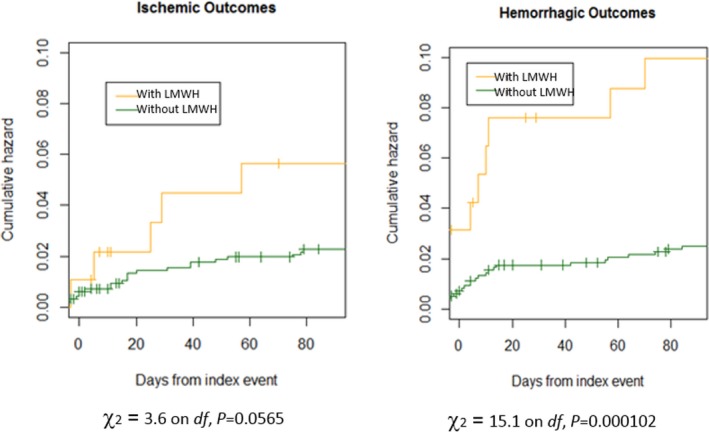
Cumulative risks of patients treated with low‐molecular‐weight heparin (LMWH) before non–vitamin K oral anticoagulants (NOACs) vs patients treated with NOACs alone.
Table 7.
Characteristics of the Patients Treated or Not With LMWH Before Oral Anticoagulants
| Not Treated With LMWH (n=1050) | Treated With LMWH (n=111) | P Value | |
|---|---|---|---|
| Age, y | 75.7±9.9 | 74.0±10.0 | |
| Sex, male | 473 (45.0) | 64 (57.6) | 0.012 |
| NIHSS on admission | 7.5±6.0 | 8.5±7.1 | |
| Diabetes mellitus | 201 (19.1) | 21 (18.9) | |
| Hypertension | 805 (76.7) | 79 (71.2) | |
| Hyperlipidemia | 369 (35.1) | 40 (36.0) | |
| Paroxysmal atrial fibrillation | 513 (48.9) | 52 (46.8) | |
| History stroke/TIA | 272 (25.9) | 31 (27.9) | |
| Current smoker | 117 (11.1) | 14 (12.6) | |
| Alcoholism | 66 (6.3) | 7 (6.3) | |
| History congestive heart failure | 164 (15.6) | 15 (13.5) | |
| History myocardial infarction | 119 (11.3) | 14 (12.6) | |
| Lesion sizea | |||
| Small | 409 (41.6) | 40 (36.7) | |
| Leukoaraiosis | 615 (58.6) | 60 (54.0) | |
| CHA2DS2‐VASc score after index stroke | |||
| >4 | 782 (74.5) | 75 (68.7) | |
Data are shown as mean±SD or n (%). LMWH indicates low‐molecular‐weight heparin; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
1063 patients.
There was a nonsignificant trend for small lesion size to be inversely associated with rates of study hemorrhagic outcome events within 90 days (Figure 8). Table 8 summarizes the study outcome events according to lesion size.
Figure 8.
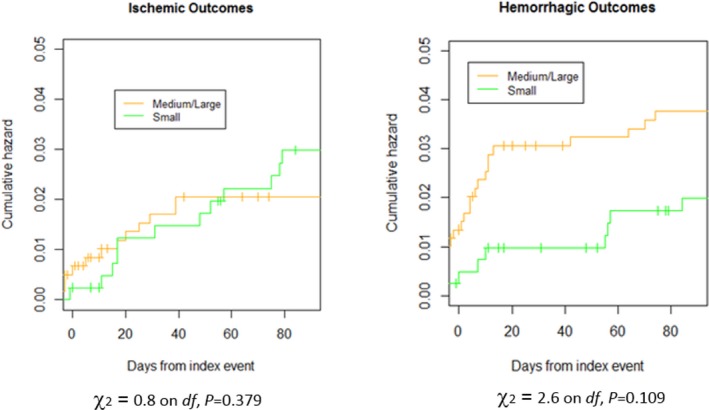
Cumulative risks of ischemic or hemorrhagic outcome events in patients with small vs medium/large lesions.
Table 8.
Outcomes Events According to Lesion Size
| Small (n=448) | Medium (n=388) | Large Anterior (n=180) | Large Posterior (n=76) | |
|---|---|---|---|---|
| Combined end pointa | 19 (4.2) | 22 (5.7) | 8 (4.4) | 2 (2.6) |
| Ischemic stroke | 10 (2.2) | 8 (2.1) | 1 (0.6) | 1 (1.3) |
| Symptomatic hemorrhagic transformation | 3 (0.7) | 10 (0.3) | 4 (2.2) | 0 |
| Stroke, TIA, or systemic embolism | 13 (2.9) | 10 (2.6) | 4 (2.2) | 1 (1.3) |
| Symptomatic hemorrhagic transformation, severe extracranial bleeding | 7 (1.6) | 13 (3.4) | 4 (2.2) | 1 (1.3) |
Data are shown as n (%). TIA indicates transient ischemic attack.
Combined end point: symptomatic hemorrhagic transformation, ischemic stroke, TIA, systemic embolism and severe extracranial bleeding.
Regarding the composite primary study outcome, the following variables were included in the multivariate analysis model: diabetes mellitus, history of hypertension, presence of leukoaraiosis, LMWH preceding oral anticoagulants, and CHA2DS2‐VASc score. LMWH preceding oral anticoagulants was predictive factor for the composite primary study outcome event (OR: 4.13; 95% CI, 1.73–8.96; P=0.0003). High poststroke CHA2DS2‐VASc score, as a continuous variable, was not significantly associated with the composite primary study outcome event (OR: 1.23; 95% CI, 0.96–1.60; P=0.09); neither was presence of leukoaraiosis (OR: 1.29; 95% CI, 0.61–2.5; P=0.5), diabetes mellitus (OR: 1.61; 95% CI, 0.80–3.23; P=0.12), or history of hypertension (OR: 1.45; 95% CI, 0.63–3.37; P=0.37).
Regarding the ischemic outcome events, the following variables were included in the multivariate analysis model: paroxysmal AF, LMWH preceding oral anticoagulants, and CHA2DS2‐VASc score. LMWH preceding oral anticoagulants was associated with the risk of ischemic events (OR: 3.73; 95% CI, 0.95–10.63; P=0.01); paroxysmal AF (OR: 0.62; 95% CI, 0.29–1.34; P=0.2) and CHA2DS2‐VASc score (OR: 1.32; 95% CI, 0.94–1.89; P=0.08) were not associated with ischemic events.
Regarding the hemorrhagic outcome events, the following variables were included in the multivariate analysis model: LMWH preceding oral anticoagulants, lesion size, leukoaraiosis, age, antiplatelet therapy on admission, and CHA2DS2‐VASc score. LMWH preceding oral anticoagulants was associated with the risk of symptomatic hemorrhagic transformation and severe extracranial bleeding (OR: 4.75; 95% CI, 1.60–12.32; P=0.0009). The presence of leukoaraiosis was not significantly associated with hemorrhagic events (OR: 1.24; 95% CI, 0.46–4.04; P=0.65); neither were age (OR: 1.03; 95% CI, 0.98–1.09; P=0.2), CHA2DS2‐VASc score (OR: 1.11; 95% CI, 0.80–1.59; P=0.5), the presence of a small lesion (OR: 0.71; 95% CI, 0.24–1.81; P=0.46), or therapy with antiplatelet agents on admission (OR: 1.96; 95% CI, 0.76–5.07; P=0.1).
Discussion
In the RAF‐NOACs study, patients with acute stroke and AF treated with NOACs had a 90‐day rate of 2.8% for recurrent ischemic stroke or TIA and 1.6% for symptomatic cerebral bleeding; the large majority of patients received NOACs within 15 days. In the current study, the rates of ischemic and hemorrhagic outcome events were 60% lower than in the RAF study that included mainly patients treated with vitamin K antagonists.4
A recent prospective cohort study reported that, despite the early start of NOACs (65% of the 155 included patients received therapy within 7 days), no intracerebral hemorrhage occurred and 4 patients had recurrent ischemic stroke during follow‐up.7 In the study by Seiffge et al,7 ≈11% of the patients had TIA as index event, and the median NIHSS score on admission was 4 compared with 6 in the RAF‐NOACs study, in which all patients had ischemic stroke. The differences in underlying stroke severity and inclusion criteria in our study may account for the discrepancies in the rates of hemorrhagic events between these 2 studies. It is noteworthy that the efficacy and safety of starting NOACs within 14 days after an acute stroke have not been evaluated in the existing pivotal randomized clinical trials because of their inclusion criteria: Apixaban was to be started at least 7 days after ischemic stroke, whereas rivaroxaban and dabigatran were to be initiated at least 14 days after stroke.23, 24, 25 The greatly elevated underlying rate of events likely partly explains the nonsignificantly higher event rates observed if NOACs were initiated within 2 days versus 3 to 14 days after stroke onset. Another reason for higher hemorrhage rates among patients starting anticoagulants early relates to the timing of routine repeated imaging: Evidence of asymptomatic bleeding will inevitably be detected by routine scans, which are conducted especially within the first 48 hours after the index stroke.
The optimal time to start anticoagulation is often chosen based on the size of the lesion, which is considered the major risk factor for HT. In fact, in the RAF study, multivariate analysis revealed that large lesions were associated with elevated rates of the composite of symptomatic cerebral bleeding and stroke recurrence but did not demonstrate a difference from smaller lesions in the relative proportions of recurrence and bleeding. In the current study, however, we found a trend toward fewer ischemic events but similar rates of hemorrhagic outcomes in patients with smaller lesions. This does not offer robust evidence to withhold or delay anticoagulation among patients with larger lesions, for whom risks and benefits may both be greater.
The results reported for the 3 NOACs in this study do not provide an unbiased comparison in terms of efficacy and safety. Consequently, the differences among NOACs are of very limited value because the analysis was not adjusted. The recent trend in analyzing the results of prospective nonrandomized studies is to avoid “sensitive” comparisons and to simply report the crude data, as we have done.26, 27, 28, 29
In the RAF‐NOACs study, ≈10% of the patients received LMWH before NOACs. We found that these patients had a significantly higher rate of bleeding events compared with patients treated with oral anticoagulants alone. Patients who received LMWH were likely patients with more severe stroke who were more likely to have dysphagia and perhaps to be at inherently greater risk of adverse outcomes. It also appears likely that LMWH may have been initiated earlier than NOACs, at a time when there was an inherently much higher rate of ischemic and hemorrhagic events, regardless of treatment allocation.30 We think that considering the similar pharmacokinetics of NOACs and LMWH, it is not recommended to start with LMWH before NOACs.
Our study had several limitations. First, the reported associations in our nonrandomized study were potentially influenced by numerous potential confounders. For this reason, the conclusions should not be applied in a generalized manner but instead used on a case‐by‐case basis. Second, the number of study outcome events was low, limiting the statistical power of our analyses. Most important, we cannot exclude the possibility that there might have been selection bias regarding the starting time of antithrombotic therapy. In fact, most patients who were either older adults or who had severe stroke were not given treatment or received treatment later compared with more stable patients. However, clinical decisions must be made for patients with nonvalvular AF after stroke, and our data may help inform choices on timing and agent and may prompt a more formal randomized study.
In conclusion, in patients with acute stroke and AF, treatment with NOACs was associated with a 5% combined rate for ischemic embolic recurrence and severe bleeding within 90 days. Furthermore, composite rates of recurrence and major bleeding were 12.4% in patients who initiated NOACs within 2 days after acute stroke, 2.1% in those who initiated NOACs between 3 and 14 days, and 9.1% in patients who initiated NOACs >14 days after acute stroke. Future randomized studies to assess timing of initiation and choice of agent in patients with acute stroke and AF are warranted.
Disclosures
Paciaroni received honoraria as a member of the speaker bureau of Sanofi‐Aventis, Boehringer Ingelheim, Bayer, Bristol Meyer Squibb, Daiiki Sankyo, and Pfizer. G. Agnelli received honoraria as a member of the speaker bureau of Boehringer Ingelheim and Bayer. Becattini received honoraria as a member of the speaker bureau of Bristol Meyer Squibb and Bayer. Caso received honoraria as a member of the speaker bureau and as consultant or advisory board of Boehringer Ingelheim. Putaala received honoraria for lectures related to atrial fibrillation and anticoagulants for Orion Pharma, Bristol Meyer Squibb, Pfizer, Bayer, and Boehringer Ingelheim. T. Tatlisumak received honoraria as consultant or advisory relationship by Lundbeck and Boehringer Ingelheim. Lees reports fees and expenses for data monitoring committee work and lectures from Boehringer Ingelheim. Ageno has received speaker's honoraria from, and participated in scientific advisory boards for Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer, and Daiichi Sankyo and has received research support from Bayer and Boehringer Ingelheim. Toni received honoraria as a member of speaker bureau and as advisory board of Boehringer Ingelheim, Pfizer, Bristol Meyer Squibb, and Bayer. The other authors have nothing to disclose.
(J Am Heart Assoc. 2017;6:e007034 DOI: 10.1161/JAHA.117.007034.)29220330
References
- 1. Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke. 1983;14:688–693. [DOI] [PubMed] [Google Scholar]
- 2. Kelley RE, Berger JR, Alter M, Kovacs AG. Cerebral ischemia and atrial fibrillation: prospective study. Neurology. 1984;34:1285–1291. [DOI] [PubMed] [Google Scholar]
- 3. Paciaroni M, Agnelli G, Ageno W, Caso V. Timing of anticoagulation therapy in patients with acute ischaemic stroke and atrial fibrillation. Thromb Haemost. 2016;116:410–416. [DOI] [PubMed] [Google Scholar]
- 4. Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, Rueckert C, Pezzini A, Poli L, Padovani A, Csiba L, Szabó L, Sohn SI, Tassinari T, Abdul‐Rahim AH, Michel P, Cordier M, Vanacker P, Remillard S, Alberti A, Venti M, Scoditti U, Denti L, Orlandi G, Chiti A, Gialdini G, Bovi P, Carletti M, Rigatelli A, Putaala J, Tatlisumak T, Masotti L, Lorenzini G, Tassi R, Guideri F, Martini G, Tsivgoulis G, Vadikolias K, Liantinioti C, Corea F, Del Sette M, Ageno W, De Lodovici ML, Bono G, Baldi A, D'Anna S, Sacco S, Carolei A, Tiseo C, Acciarresi M, D'Amore C, Imberti D, Zabzuni D, Doronin B, Volodina V, Consoli D, Galati F, Pieroni A, Toni D, Monaco S, Baronello MM, Barlinn K, Pallesen LP, Kepplinger J, Bodechtel U, Gerber J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Mosconi MG, Bubba V, Silvestri I, Lees KR. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175–2182. [DOI] [PubMed] [Google Scholar]
- 5. Arihiro S, Todo K, Koga M, Furui E, Kinoshita N, Kimura K, Yamagami H, Terasaki T, Yoshimura S, Shiokawa Y, Kamiyama K, Takizawa S, Okuda S, Okada Y, Nagakane Y, Kameda T, Hasegawa Y, Shibuya S, Ito Y, Nakashima T, Takamatsu K, Nishiyama K, Matsuki T, Homma K, Takasugi J, Tokunaga K, Sato S, Kario K, Kitazono T, Toyoda K. Three‐month risk‐benefit profile of anticoagulation after stroke with atrial fibrillation: the SAMURAI‐Nonvalvular Atrial Fibrillation (NVAF) study. Int J Stroke. 2016;11:565–574. [DOI] [PubMed] [Google Scholar]
- 6. Gioia LC, Kate M, Sivakumar L, Hussain D, Kalashyan H, Buck B, Bussiere M, Jeerakathil T, Shuaib A, Emery D, Butcher K. Early rivaroxaban use after cardioembolic stroke may not result in hemorrhagic transformation. A prospective magnetic resonance imaging study. Stroke. 2016;47:1917–1919. [DOI] [PubMed] [Google Scholar]
- 7. Seiffge DJ, Traenka C, Polymeris A, Hert L, Peters N, Lyrer P, Engelter ST, Bonati LH, De Marchis GM. Early start of DOAC after ischemic stroke. Risk of intracranial hemorrhage and recurrent events. Neurology. 2016;87:1–7. [DOI] [PubMed] [Google Scholar]
- 8. European Stroke Organisation (ESO) Executive Committee . Guidelines for management of ischemic stroke and transient ischemic attack. Cerebrovasc Dis. 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 9. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR; American Heart Association Stroke Council . 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. [DOI] [PubMed] [Google Scholar]
- 10. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri F, McMullan PW jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 11. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 12. Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, Del Zoppo GJ; for the rtPA Acute Stroke Study Group . Neuroradiologic evaluation of patients with acute stroke treated with rtPA. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 13. Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, Caso V, Micheli S, Bertolani L, Venti M, Palmerini F, Biagini S, Comi G, Previdi P, Silvestrelli G. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–2256. [DOI] [PubMed] [Google Scholar]
- 14. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 15. Tatu L, Moulin T, Bogousslavsky J, Duvemoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. [DOI] [PubMed] [Google Scholar]
- 16. Tatu L, Moulin T, Bogousslavsky J, Duvemoy H. Arterial territories of the human brain: brainstem and cerebellum. Neurology. 1996;47:1125–1135. [DOI] [PubMed] [Google Scholar]
- 17. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin A, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 18. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 19. Schulman S, Kearon C. Definition of major bleeding in clinical investigations and anti‐hemostatic medical products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 20. Reboldi G, Angeli F, Verdecchia P. Multivariable analysis in cerebrovascular research: practical notes for the clinician. Cerebrovasc Dis. 2013;35:187–193. [DOI] [PubMed] [Google Scholar]
- 21. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1983;80:27–28. [Google Scholar]
- 22. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 23. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 24. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 25. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 26. Campbell MJ. What is propensity score modelling? Emerg Med J. 2017;34:129–131. PMID: 28143814 [DOI] [PubMed] [Google Scholar]
- 27. Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report—Part II. Value Health. 2009;12:1053–1061. [DOI] [PubMed] [Google Scholar]
- 28. Papanikolaou PN, Christidi GD, Ioannidis JP. Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ. 2006;174:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim H, Gurrin L, Ademi Z, Liew D. Overview of methods for comparing the efficacies of drugs in the absence of head‐to‐head clinical trial data. Br J Clin Pharmacol. 2014;77:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abdul‐Rahim AH, Fulton RL, Frank B, Tatlisumak T, Paciaroni M, Caso V, Diener HC, Lees KR. Association of improved outcome in acute ischaemic stroke patients with atrial fibrillation who receive early antithrombotic therapy: analysis from VISTA. Eur J Neurol. 2015;22:1048–1055. [DOI] [PubMed] [Google Scholar]


